Abstract
Purpose
Nitrification is a key process in the global nitrogen cycle, of which the first and rate-limiting step is catalyzed by ammonia monooxygenase. Root cap cells are one of substrates for microorganisms that thrive in the rhizosphere. The degradation of root cap cells brings about nitrification following ammonification of organic nitrogen derived from the root cap cells. This study was designed to gain insights into the response of ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) to mineralized N from root cap cells and the composition of active bacterial and archaeal ammonia oxidizers in rice soil.
Materials and methods
Rice callus cells were used as a model for root cap cells, and unlabelled (12C) and 13C-labelled callus cells were allowed to decompose in aerobic soil microcosms. Real-time quantitative polymerase chain reaction (PCR), DNA-based stable isotope probing (SIP), and denaturing gradient gel electrophoresis (DGGE) were applied to determine the copy number of bacterial and archaeal amoA genes and the composition of active AOB and AOA.
Results and discussion
The growth of AOB was significantly stimulated by the addition of callus cells compared with the growth of AOA with a much lesser extent. AOB communities assimilated 13C derived from the callus cells, whereas no AOA communities grew on 13C-callus. Sequencing of the DGGE bands in the SIP experiments revealed that the AOB communities belonging to Nitrosospira spp. dominated microbial ammonia oxidation with rice callus amendment in soil.
Conclusions
The present study suggests that root cap cells of rice significantly stimulated the growth of AOB, and the active members dominating microbial ammonia oxidation belonged to Nitrosospira spp. in rice rhizosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrification, the microbial oxidation of ammonia (NH3) to nitrite (NO2 −) and then to nitrate (NO3 −), is the only oxidative process that links the reduced and oxidized pools of inorganic nitrogen to sustain the global nitrogen cycle (Gruber and Galloway 2008). It affects the form of mineral nitrogen (N) (ammonium, NH4 +, or nitrate, NO3 −) in soil and thus the amount and form of mineral N taken up by plants. The amoA gene which encodes the ammonia monooxygenase (AMO) enzyme, responsible for catalyzing the first and rate-limiting step of the nitrification process, has been used extensively as a molecular marker for cultivation-independent studies of ammonia oxidizers in a diverse range of environments including marine, sediment, paddy field, and grassland (Venter et al. 2004; Francis et al. 2005; He et al. 2007; Di et al. 2009). Ammonia oxidation is carried out by both ammonia-oxidizing bacteria (AOB) belonging to β- and γ-Proteobacteria and ammonia-oxidizing archaea (AOA) belonging to Thaumarchaeota (Kowalchuk and Stephen 2001; Spang et al. 2010). There is evidence that addition of inorganic ammonia to soil does not stimulate archaeal ammonia oxidation (Verhamme et al. 2011). Levičnik-Höfferle et al. (2012) reported that oxidation of ammonia generated from native soil organic matter or added organic N, but not added inorganic N, was accompanied by increases in abundance of the AOA amoA gene. The stimulative effect of NH4 +–N on the AOB abundance was greater than that of NO3 −–N (Tian et al. 2014). Recent studies indicated that AOB and AOA communities prefer different nitrogen conditions for their growth, and thus their importance in dominated microbial ammonia oxidation varies under different nitrogen soil conditions (Di et al. 2010; Zhang et al. 2010).
Plant roots supply various organic substances to rhizospheric microorganisms. It has been estimated that 30–60 % of net photosynthesized carbon is allocated to roots, with 40–90 % of which enters soil in the form of root exudates, sloughed-off cells, and decaying roots (Lynch and Whipps 1990). On the other hand, the rhizodeposited N was reported to account for between 22 and 46 % of the total below-ground plant N budget (Sawatsky and Soper 1941; Hetier. 1986). These root-derived substrates are an important source of labile C and N to soil microbes in rhizosphere. Root cap cells are one of the substrates for microorganisms that thrive in the rhizosphere, representing 5–10 % of the total carbon deposited (Iijima et al. 2000). Many cells are generated in the root cap meristem and are sloughed into the rhizosphere (Clowes 1976). Incorporation of plant residues into soil brings about nitrification when ammonification of organic nitrogen derived from plant residues proceeds in the soil (Cabrera et al. 2005). Watanabe et al. (2011) demonstrated that carbon derived from 13C-labelled plant residues flowed into both AOB and AOA communities in aerobically incubated soil. In analogy, we speculate that assimilation of root cap cells also brings about nitrification following ammonification of organic nitrogen derived from the root cap cells. However, no attention has been paid to the response of ammonia oxidizer communities, AOB and AOA, to mineralized N from root cap cells and the composition of active bacterial and archaeal ammonia oxidizers in rice rhizosphere.
Lee et al. (2011) reported that the bacterial populations involved in plant residue decomposition were dominated by Actinobacteria (35 %), Bacilli (20 %), and γ-Proteobacteria (11 %) during the 56-day incubation of dried rice callus, as a model of plant residue, in rice soil under aerobic conditions. In contrast, Li et al. (2011) prepared 13C-labelled rice plant callus cells as a model of rice plant root cap cells and identified the bacterial communities that decomposed root cap cells in rice soil using a DNA-SIP approach. Rice callus cells were decomposed by specific bacterial species, and they were phylogenetically different from those that incorporated C from plant residue. More recently, Li et al. (2013) traced the carbon flow from 13C-labelled callus cells to T4-type phages, which demonstrated the phage-mediated carbon flow of root cap cells in microbial loop in rice rhizosphere. These findings indicate that rice callus is a good model material to reveal microbial communities involving carbon and nitrogen dynamics in rice rhizosphere. Thus, this study used rice (Oryza sativa L. cv, Yukihikari) callus cells as a model of root cap cells, where harvested 13C-labelled callus cells were allowed to decompose in aerobic soil microcosm. Real-time quantitative PCR analysis and DNA-SIP approach were applied to determine active members of ammonia oxidizers and how these members would respond to callus-derived ammonia during rice callus decomposition. The results would improve our knowledge and understanding on the role of AOB and AOA in N cycling in the rhizosphere.
2 Material and methods
2.1 Setup of the experiment
Soil samples used in this study were the same as those used in the previous studies, which elucidated bacterial communities assimilating callus cells carbon during the decomposition under aerobic soil conditions (Li et al. 2011). In brief, soil collected from a paddy field (D2 field) at Aichi-ken Anjo Research and Extension Center (34°48′ N, 137°30′ E; Oxyaquic Dystrudept), Japan, in April 2009. The total C content, total N content, and pH (H2O) of the soils for incubation were 12 g kg−1, 1.3 g kg−1, and 5.8, respectively. Soil was adjusted to 55 % of the maximum water holding capacity and preincubated at 25 °C for 14 days. Rice (O. sativa L. cv, Yukihikari) callus cells were added to the preincubated soil at the rate of 20 g kg−1 soil. Ten grams of preincubated soil with 13C-labelled (78 at.%), non-labelled rice callus (referred to as 12C-callus), or without callus was put in a test tube (3 cm in diameter and 20 cm in height) and closed with a silicon plug. The test tubes were incubated in a dark room at 25 °C for 56 days. Three test tubes were subjected to analysis at each sampling time after incubating for 0 (immediately after mixing the soil with the rice calluses), 3, 7, 14, 21, 28, 42, and 56 days. Soil samples were stored at −30 °C until use. DNA was extracted from 0.5 g of soil periodically using a FastDNA SPIN kit for soil (MP Biomedicals; Solon, OH, USA) according to the manufacturer’s instructions. Density gradient centrifugation was performed on bulk DNA extracted from the 12C-callus and 13C-callus treatments as described by Lueders et al. (2004), with a minor modification in scale: 5 μg of DNA was fractionated into 16 equal fractions and DNA in each fraction was dissolved in 30 μL of TE buffer.
2.2 Nitrification activity
Soil samples for the treatment of no callus and with non-labelled (12C) callus were used for the determination of ammonium–nitrogen (NH4 +–N) and nitrate–nitrogen (NO3 −–N) at each sampling time. Duplicate soil samples were homogenized with 2 M KCl by shaking for 1 h at room temperature for ammonium and nitrate extraction, respectively. These were then passed through a filter paper (Whatman no. 42), and NH4 +–N concentration was analyzed by the indophenol blue method using an UV spectrophotometer (UV-2450, Shimadzu) and NO3 −–N was analyzed with an ion chromatograph (PIA-1000, Shimadzu) (Keeneg and Nelson 1982; Watanabe and Kimura 1999).
2.3 Quantification of AOB and AOA amoA in soil
Soil samples for the treatment of no callus and with non-labelled (12C) callus were used for the determination of abundance of amoA genes with duplicate. Abundance of archaeal amoA genes was determined on an Thermal Cycler Dice Real Time System (TaKaRa, Otsu, Japan) for bulk DNA using primer pairs amoA19IF (5′-ATG GTC TGG CTI AGA CG-3′) and CrenamoA616r (5′-GCC ATC CAT CTG TAT GTC CA-3′) (Tourna et al. 2008; Morimoto et al. 2011). Each reaction was performed in a 25-μL volume containing 12.5 μL SYBR Premix Ex Taq (TaKaRa), 1 μM of each primer, 0.2 mg/mL of bovine serum albumin (TaKaRa), and 1 μL of tenfold dilution DNA template (2–5 ng). Amplification conditions were as follows: 95 °C for 10 min, 40 cycles of 30 s at 94 °C, 30 s at 55 °C, 1 min at 72 °C, and followed by extension of 10 min at 72 °C. Bacterial amoA genes were quantified using the primers amoA1F (5′-GGG GTT TCT ACT GGT GGT-3′) and amoA2IR (5′-CCC CTC IGI AAA GCC TTC TTC-3′) (Rotthauwe et al 1997; Nicolaisen and Ramsing 2002) with the same reaction conditions except 0.4 μM of each primer, and the annealing temperature was 54 °C.
Real-time PCR standard curve was generated as described by Morimoto et al. (2011). Briefly, a standard curve for AOA amoA was generated from tenfold serial dilutions (102–106 copies μL−1) of clone S1001 (Hayatsu et al., unpublished) containing an archaeal amoA fragment (DDBJ accession number AB569307) derived from agricultural soil. Similarly, for AOB amoA, tenfold serial dilutions of pGEM-T easy (Promega, Madison, WI, USA) containing an amoA fragment derived from Nitrosospira multiformis ATCC25196 (GenBank accession number U91603) were used for generating a standard curve. High efficiencies of 88.5–109 % were obtained for AOB amoA amplification, with the R value ranging between 0.983 and 0.995, and efficiencies of 95.1–103 % were obtained for AOA amoA amplification, with the R value ranging between 0.991 and 0.994.
2.4 PCR amplification
The amoA genes of AOB and AOA were amplified with the primer sets amoA-1 F/amoA-2R (Rotthauwe et al 1997; Avrahami and Conrad 2003) and crenamoA23f/crenamoA616r (Tourna et al. 2008), respectively. A guanine cytosine (GC) clamp was attached to the primer amoA-1 F, but not to the primer for AOA, according to the procedure by Tourna et al. (2008). The PCR program for bacterial amoA genes had an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation (45 s, 95 °C), primer annealing (30 s, 57 °C) and primer extension (60 s, 72 °C), and a final extension step of 600 s at 72 °C. The program for archaeal amoA genes had an initial denaturation at 95 °C for 90 s, followed by 35 cycles of denaturation (30 s, 95 °C), primer annealing (30 s, 55 °C) and primer extension (90 s, 72 °C), and a final extension step of 360 s at 72 °C. The reaction mixture (50 μL) contained 0.5 μL of each primer (50 pmol each), 5 μL of 2.5 mM dNTP mixture, 5 μL of 10 × Ex Taq DNA buffer (20 mM Mg2+; TaKaRa), 0.5 μL of Ex Taq DNA polymerase (TaKaRa), 1 μL of DNA template, and 37.75 μL milli-Q water. The amplicons were checked by agarose gel electrophoresis with ethidium bromide. PCR amplification was conducted to the DNA before isopycnic centrifugation (bulk DNA) and the DNA after the centrifugation (fractionated DNA).
2.5 DGGE of PCR products
Four microliters of PCR product was applied to an 8 % (w/v) acrylamide gel with denaturant gradient range from 30 to 70 % and from 20 to 50 % for the bacterial and archaeal amoA genes, respectively, in which 100 % denaturing gradient is defined as 7 M urea and 40 % (v/v) deionized formamide. Electrophoresis was run in 1 × TAE buffer at 60 °C and 100 V for 14 and 15 h for bacterial and archaeal amoA genes, respectively, with a Dcode Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, the gel was stained in 1:10,000 (v/v) SYBR™ Green I nucleic acid staining solution (CAMBREX, Rockland, ME, USA) for 30 min and photographed under UV light.
DGGE was performed for PCR products from the bulk DNA (DNA before density gradient centrifugation) to evaluate the community structure of ammonia oxidizers during the incubation period and for the fractionated DNA to identify ammonia oxidizers that assimilated labelled C (13C) from rice callus cells.
2.6 Statistical analysis
To estimate the succession of ammonia oxidizers during the decomposition of callus cells over the 56-day incubation period, cluster analysis was performed for the DGGE band patterns of amoA gene fragments before the isopycnic centrifugation. The analysis was based on the intensity of the DGGE bands (0, no band; 1, weak; 2, moderate; 3, strong) using the PAST Program (http://folk.uio.no/ohammer/past/). Diversity and evenness of the communities were estimated by using Shannon’s diversity index with the formula, H = −∑Piln(Pi), for the calculation of the index, where Pi is the relative intensity of band i (=Ni/N; Ni, intensity of band i; N, sum of the intensities of all bands in a lane sample). Evenness was calculated by using the formula, E = H/lnR, where R is the total different number of DGGE bands.
2.7 Sequencing and phylogenetic analysis
Nucleotide sequences of bacterial amoA genes that were recovered from DGGE gels were determined by direct sequencing method (Watanabe et al. 2004). A clone library was constructed by using the PCR products obtained from the control treatment on day 0 to assess AOB community in the soil. Purified PCR products were cloned into pT7 Blue Vector (Novagen, Darmstadt, Germany) and transformed into competent cells of Escherichia coli XL I-blue (Toyobo, Osaka, Japan). About 50 clones were chosen from white colonies and PCR-amplified with the primers amoA-1 F-GC/amoA-2R. The PCR program was the same as described earlier except for the reduction of cycle number to 20. Four microliters of the PCR product of positive clones was used for DGGE according to the previously described method. Bands with the same mobility on the DGGE gel were considered to be the same clones. Plasmid DNA from different clones was harvested from overnight cultures of the transformants, and about 300 ng of the DNA was subjected to cycle sequencing reactions.
Nucleotide sequences were determined with an ABI PRISM® 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The obtained nucleotide sequences were translated to deduced amino acid sequences using the EMBOSS Transeq program at the European Bioinformatics Institute website. All amoA genes were checked for the closest relatives at the amino acid level by Basic Local Alignment Search Tool (BLAST) search within the National Center for Biotechnology Information (NCBI) website. The amino acid sequences were aligned with ClustalX 1.81 (Thompson et al. 1997). A neighbour-joining tree was constructed by Molecular Evolutionary Genetic Analysis software (MEGA4.1) (Kumar et al. 2004) with 1,000-fold bootstrap support. The DNA sequences of the amoA genes were deposited in the DDBJ database under the accession numbers AB813878–AB813915, among which AB813892–AB813915 were 13C-labeled AOB.
3 Results
3.1 Nitrification activity and amoA abundance in soil
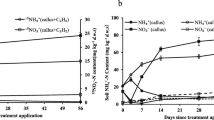
Following callus amendment, NH4 +–N concentrations increased rapidly, reaching a peak of 55.18 μg g−1 dry soil by day 3, and thereafter decreased to their initial concentrations of 10.49 μg g−1 dry soil on day 42 (Fig. 1). However, NO3 −–N concentrations in soil without callus cells ranged from 34.22 to 44.80 μg g−1 dry soil, whereas the NO3 −–N concentration with callus cells increased rapidly from 34.28 on day 0 to a peak of 180.45 μg g−1 dry soil on day 56 (Fig. 1). The application of the callus cells significantly increased the nitrification activity in the soil, resulting in higher NO3 −–N concentrations (Fig. 1).
Growth of archaeal and bacterial ammonia oxidizers during nitrification was estimated by quantification of amoA genes and is expressed as gene abundance μg−1 DNA to facilitate comparison between microcosms (Levičnik-Höfferle et al. 2012). The AOA amoA gene copy numbers were greater than those of the AOB in the soils both with and without addition of callus cells (Fig. 2a, b). The amoA gene copy numbers of AOB ranged from 8.9 × 104 μg−1 DNA in the soil without callus cells on day 56 to 1.8 × 106 μg−1 DNA in the soil with callus cells on day 21, whereas that of AOA ranged from 1.1 × 106 μg−1 DNA in the soil without callus cells on day 28 to 3.9 × 106 μg−1 DNA in the soil with callus cells on day 21 (Fig. 2). The ratios of the AOA to AOB amoA gene copy numbers ranged from 2.1 to 9.4 and from 6.1 to 16.3 for the soils with and without callus cells during the incubation time, respectively.
Although the AOB populations in the control soil (no callus amendment) remained relatively stable over the 56-day incubation period, they increased 5.6-, 7.5-, 10.1-, 7.4-, 5.7-, and 10.1-fold following the application of callus cells on days 7, 14, 21, 28, 42, and 56, respectively (Fig. 2a). In contrast, the amoA gene copy number of AOA in the callus treatment increased only 2.9- fold than those in the control at the peak on day 14 (Fig. 2b).
3.2 DGGE analysis of AOB and AOA between 12C-callus and 13C-callus treatments
Figure 3a shows that DGGE band patterns of AOB community during soil incubation. In total, 25 bands were observed with different mobility. The DGGE band patterns changed slightly during the incubation and some bands (e.g., shown with arrows in Fig. 3a) became stronger in intensity for soil samples with 12C- and 13C-callus cells than for those without callus from day 7 onward. Cluster analysis showed that variation of the patterns for soil samples without callus was smaller than that with callus (Fig. 4a). The samples with callus showed lower valuables (2.97 vs 3.03) for diversity at the beginning of the incubation. In contrast, they had higher values of index than those without callus amendment from day 7 onwards. Callus amendment brought out the highest index value for diversity (3.07) at day 7 while for evenness (0.97) at the later incubation stage (days 42 and 56).
Denaturing gradient gel electrophoresis (DGGE) band patterns of bacterial amoA genes (a) and archaeal amoA genes (b) obtained from the soil with incubation time before cesium chloride (CsCl) centrifugation. N, 12, and 13 in the figure indicate no callus treatment, 12C-callus treatment, and 13C-callus treatment, respectively
Cluster analysis of DGGE banding patterns of bacterial amoA genes (a) and archaeal amoA genes (b) during incubation time. YYY-xxd at the left-side column: YYY before the hyphen indicates the treatment (No the treatment without callus, 12 C the treatment with 12C-calluses, and 13 C the treatment with 13C-calluses); xx indicates the days after incubation
In the AOA community, bigger changes of the patterns for the soil samples without callus than those with callus were observed from the DGGE fingerprint and cluster analysis (Figs. 3b and 4b). The samples amended with callus on days 7 and 14 formed a different sub-cluster from those on days 28, 42, and 56 (Fig. 4b).
3.3 Comparison of DGGE band patterns of 13C-enriched AOB and AOA communities
Based on the results of DGGE band patterns and cluster analysis, DNA extracted from callus-amended soil samples on days 7, 14, 28, and 42 were subjected to further analysis. Figure 5 compares the DGGE band patterns of the AOB community between the isopycnically fractionated DNA from the treatments with 13C- and 12C-callus cells. DGGE bands were solely observed in the heaviest fractions (fourth to fifth fractions) for 13C-callus treatment compared to the 12C-callus treatment, in which no discernible band in the heavy fractions (fourth to fifth fractions) appeared (Fig. 5). In contrast, the heaviest fractions in which DGGE bands were observed were sixth or seventh for both 13C- and 12C-callus treatments for AOA community (“Electronic supplementary material”, Fig. S1). Therefore, only the bands in the heaviest fractions (fourth to fifth fractions) for 13C-callus treatment of AOB were considered to be assimilators of 13C and subjected to the sequencing analysis.
Comparison of DGGE banding patterns of 13C-enriched AOB community. N, 12, and 13 in the figure indicate no callus treatment, 12C-callus treatment, and 13C-callus treatment, respectively; 4-11 indicate the fraction number; triangles indicate the sequenced AOB amoA. Clone names were assigned xdy-z in which x, y and z indicate the date of sampling, fraction number, and band number, respectively (example: 7d4-1 was the band of day 7, the fourth fraction, and the first band from the top). The values at the bottom of the lane indicate the mass density (g mL−1) of isopycnically isolated fractions
3.4 Phylogenetic analysis of 13C-enriched AOB communities
The DGGE bands with different mobility were excised for direct sequencing from the heaviest fractions for 13C-callus treatment (Fig. 5). Figure 6 shows the phylogenetic relationship of amino acid sequences among AOB clones obtained from the control soil and bands in the heaviest fractions of 13C-callus treatment and reference sequences. All these amino acid sequences obtained in the present study belonged to genus Nitrosospira except 0d13. Most of the bands observed in the heaviest fractions of 13C-callus treatment were affiliated with cluster 1 (Nicolaisen and Ramsing 2002), leaving three bands (7d4-1, 7d4-3, and 7d4-3) ungrouped.
4 Discussion
4.1 Nitrification rate and amoA abundance
The first increase and subsequent decrease in NH4 +–N concentration were likely due to the mineralization of callus nitrogen to NH4 + and subsequent nitrification to NO3 − (Fig. 1). This variation of NH4 +–N concentration corresponded to the shift of the soil pH during the incubation time, which increased to pH 6.2 during the first 7 days and then gradually decreased to near the initial pH 5.7 after 56 days of incubation (Li et al. 2011). The trend of the abundance of archaeal and bacterial amoA gene copies was consistent with that of pH at some degree during the incubation, while there was no significant correlation between amoA gene copies of AOA/AOB and soil pH. On the other hand, NO3 −–N concentration increased continually even though the NH4 +–N concentration decreased to their initial concentrations after 42 days of incubation (Fig. 1). Subsequent nitrification from day 42 onward appeared to be due to the oxidation of ammonium derived from native soil organic matter (Levičnik-Höfferle et al. 2012). Furthermore, the maximum of ammonia was smaller than the amount of nitrate produced, which was likely because ammonification and nitrification simultaneously proceeded from soil organic nitrogen.
AOA amoA copies were always more numerous than AOB amoA copies in both soils with and without addition of callus cells in this study. This finding was consistent with those of previous studies, which reported that AOA were numerically dominant than AOB in most arable soils (He et al. 2007; Nicol et al. 2008). For the control, the ratio of the AOA to AOB amoA gene copy numbers ranged from 6.1 to 16.3 during the 56-day incubation period. However, the ratio of the AOA to AOB amoA gene copy numbers decreased to between 2.1and 9.4 following the amendment of callus cells. These results indicated that the growth of AOB was significantly simulated by the addition of callus cells compared to that of AOA, resulting in over five- to tenfold increases in the AOB amoA gene copy numbers in the callus amendment treatment. Similarly, the ratio of AOA to AOB in soils treated with nitrogen fertilizers was smaller than that in soils not treated with nitrogen fertilizers in a long-term fertilization experiment using sandy loam soil in China (Shen et al. 2008). Di et al. (2009) reported that the number of AOB increased 3.2–10.4-fold in response to addition of urine–N substrate in six grassland soils in New Zealand. These studies suggest that the growth of AOB is favored at high ammonium concentrations, which is more typical in agricultural and grassland soils receiving high N inputs (Jia and Conrad 2009; Di et al. 2010; Verhamme et al. 2011). Ammonium concentration was deemed as a key factor determining the niche separation of AOA and AOB in both acidic soils and neutral or alkaline soils (Zhang et al. 2012). In this study, based on 24 % water content of the soil, the calculated ammonium concentration in the soil with addition of callus cells reached 13.23 μM at day 3, which is sufficiently high for the growth of AOB in pure culture (above 1 μM near neutral pH; (Nicol et al. 2008)). Therefore, the high ammonium concentration, resulting from mineralization of amended callus cells, significantly stimulated the growth of AOB rather than AOA.
4.2 DGGE band pattern of 13C-enriched ammonia oxidizer
The most critical SIP approach for characterizing the ammonia oxidizers that incorporate callus C is to compare the DGGE band patterns in each fraction between 13C-labelled and non-labelled treatments and identify the DGGE bands that solely appeared in the heavy fractions of the 13C-labelled samples. In the AOB community, there was no discernible band in the heavy fractions (fourth to sixth fractions) for 12C-callus treatment at any sampling time, but bright bands appeared in the heavy fractions for 13C-callus treatment (Fig. 5). According to Rolfe and Meselson (1959), genomic DNA of microorganisms have 30–70 % of GC contents whose buoyant density (BD) ranged between 1.688 to 1.728 g mL−1 in isopycnic centrifugation. Nitrosospira spp., which were predominant members in the studied soil, had 53.2–55.4 % of GC content in their genomic DNA (Koops and Pommerening-Röser 2005), suggesting that their estimated BD ranges from 1.711 to 1.713 g mL−1 (Watanabe et al. 2011). Lueders et al. (2004) conducted CsCl density gradient centrifugation to fully separate 13C-labelled DNA from Methylobacterium extorquens and unlabelled DNA from Methanosarcina barkeri and found that the range of primary detection of their DNA in the precipitated gradient fractions was within the BD calculated from the bacterial GC content ± 0.02 (g mL−1). In this study, BD of the heaviest fractions ranged from 1.727 to 1.730 g mL−1 for the samples with 12C-callus cells (Fig. 5), which fell within the estimated BD ± 0.02 g mL−1 (1.709–1.733 g mL−1). Therefore, the presence of the bands in the heaviest fractions (fourth and fifth fraction) for 13C-callus amendment samples with BD of 1.746–1.757 g mL−1 was confirmed to be the AOB enriched with 13C derived from callus cells. In contrast, for AOA community, the BD (<1.736 g mL−1) of fractions in which DGGE bands were observed for 13C-callus amendment samples cannot rule out the incorporation of 12C and be judged as full 13C incorporator according to Lueders et al. (2004). However, the callus amendment increased the abundance of AOA to some extent (Fig. 2b). This discrepancy was probably due to the growth of AOA on 13C-callus which was too small to be detected by the DGGE fingerprinting. In addition, the bright bands in the heaviest fractions for 13C-callus amendment samples indicated AOB growth using carbon sources derived from callus, which was consistent with the abundance of AOB stimulated by the amendment of callus cells. These results indicated that AOB rather than AOA dominated ammonia oxidation in the test paddy soil with callus cells amendment.
4.3 Phylogenetic analysis of 13C-enriched AOB community
All the sequenced bands from the heaviest fractions were closely related to Nitrosospira spp. and no Nitrosomonas-like AOB was identified. Phylogenetic analysis of bacterial amoA genes retrieved from 13C-labelled heavy fractions in 13C-callus treatment revealed that active AOB responsible for ammonia oxidation in the tested soil predominantly belonged to amoA cluster 1 (Fig. 6). AOB communities belonging to cluster 1 were also shown to be associated with rice roots (Ikenaga et al. 2003) in surface layer of paddy soil (Murase et al. 2003; Bowatte et al. 2006b), associated with weeds in rice field (Bowatte et al. 2006a), and in paddy rhizosphere soil (Chen et al. 2008). Intriguingly, Watanabe et al. (2011) demonstrated that AOB community mostly consisting of clusters 1 and 9, members of Nitrosospira spp., assimilated carbon derived from 13C-labelled plant residues by DNA-SIP. However, Nitrosomonas-like AOB communities were also detected in rice roots under elevated atmospheric CO2 concentration (Bowatte et al. 2007) and in upland red soil and alkaline sandy loam under long-term fertilization practices (He et al. 2007; Shen et al. 2008). This might be due to the ability of Nitrosomonas to outcompete Nitrosospira-like AOB in the fields with high fertilizer input (Chen et al. 2008). On the other hand, it is difficult to detect active microbial populations that have slow rates of substrate turnover and growth due to low energy yield or that comprise only a tiny fraction of the total microbial community. AOB members affiliated with Nitrosomonas spp. accounted for a considerable proportion, up to 4.5 %, of the total 16S rRNA genes retrieved by pyrosequencing, but these AOB sequences could not be retrieved by clone library analysis of the 16S rRNA genes (Xia et al. 2011). Thus, the sequencing of DGGE bands in this SIP experiment revealed that the AOB communities belonging to Nitrosospira spp. dominated microbial ammonia oxidation with rice callus amendment in soil. Because the nitrifier Nitrosospira grows autotrophically using CO2 as a sole source of carbon (Aleem et al. 1965), the 13C-labelling of AOB DNA in the 13C-callus treatment indicates the flow of carbon from callus cells to AOB via CO2 (callus cells → CO2 → AOB). AOB members were reported to assimilate CO2 derived from 13CH3OH and 13CH4 during the methylotrophic carbon metabolism (Radajewski et al. 2002). Thus, the results presented here indicate that AOB assimilated callus-derived carbon, possibly through a nutritional association with the active callus-decomposing bacteria.
Rice callus cells are rich in water-soluble organic materials and poor in cellulose and lignin (major intercellular substances) (Lee et al. 2011). Callus cells are loose aggregates of plant cells due to the lack of these intercellular substances, and thus callus cells are easily disrupted by gentle pressing with a spatula. Root cap cells are similarly easy to disaggregate (Hawes and Pueppke 1986). Based on these properties of callus cells and root cap cells, callus cells were supposed to be an appropriate model of root cap cells (Li et al. 2011). The present study strongly suggests that the root cap cells of rice significantly stimulated the growth of AOB, and the active members dominating microbial ammonia oxidation belonged to Nitrosospira spp. in rice rhizosphere. Li et al. (2008) reported the strong nitrification promoted by rice root and active AOB in rice rhizosphere. In contrast, AOA was shown to be dominant in rhizosphere paddy soil, which was attributed to exudation from rice root (e.g., oxygen, carbon dioxide) (Chen et al. 2008). Our previous study showed that root cap cells were decomposed by specific bacterial species in rice rhizosphere (Li et al. 2011). The infection of these bacterial hosts by T4-type phages indicated active phage-mediated lysis of the bacteria and drove carbon flow of root cap cells in the microbial loop (Li et al. 2013). The present study elucidates the carbon flow from callus cells to autotrophic bacteria, ammonia-oxidizing bacteria, via CO2. Those studies indicate that CO2, the end product for degradation of rice callus, could be used further by autotrophs and could be expected to enter into the microbial loop in soil.
5 Conclusions
The present study demonstrated that callus cells significantly stimulated the growth of AOB community compared to the slightly stimulated effect on AOA, resulting from nitrification following ammonification of organic nitrogen derived from callus cells. The stimulation of the growth of AOB by callus amendment was further confirmed by DGGE fingerprinting analysis of amoA genes showing that the DGGE bands were solely located in the heaviest fractions (fourth and fifth fractions) for 13C-callus treatment samples. Many AOB clones within cluster 1 of Nitrosospira spp. assimilated carbon derived from 13C-labelled callus cells, possibly through a nutritional association with the active callus-decomposing bacteria. These results indicated that AOB rather than AOA dominated ammonia oxidation in the test paddy soil with callus cells amendment. The present study suggests that the root cap cells of rice significantly stimulated the growth of AOB, and the active members dominating microbial ammonia oxidation belonged to Nitrosospira spp. in rice rhizosphere. It also indicates that CO2, the end product for degradation of rice callus, could be used further by autotrophs.
References
Aleem MIH, Hoch GE, Varner JE (1965) Water as the source of oxidizing and reducing power in bacterial chemosynthesis. Proc Natl Acad Sci U S A 54:869–873
Avrahami S, Conrad R (2003) Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl Environ Microb 69:6152–6164
Bowatte S, Ishihara R, Asakawa S, Kimura M (2006a) Characterization of ammonia oxidizing bacteria associated with weeds in a Japanese paddy field using amoA gene fragments. Soil Sci Plant Nutr 52(5):593–600
Bowatte S, Jia Z, Ishihara R, Nakajima Y, Asakawa S, Kimura M (2006b) Molecular analysis of the ammonia oxidizing bacterial community in the surface soil layer of a Japanese paddy field. Soil Sci Plant Nutr 52(4):427–431
Bowatte S, Asakawa S, Okada M, Kobayashi K, Kimura M (2007) Effect of elevated atmospheric CO2 concentration on ammonia oxidizing bacteria communities inhabiting in rice roots. Soil Sci Plant Nutr 53:32–39
Cabrera ML, Kissel DE, Vigil MF (2005) Nitrogen mineralization from organic residues: research opportunities. J Environ Qual 34:75–79
Chen XP, Zhu YG, Xia Y, Shen JP, He JZ (2008) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ Microbiol 10:1978–1987
Clowes FAL (1976) Cell production by root caps. New Phytol 77:399–407
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010) Ammonia oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammoniaoxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Gruber N, Galloway JN (2008) An earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Hawes MC, Pueppke SG (1986) Sloughed peripheral root cap cells: yield from different species and callus formation from single cells. Am J Bot 73:1466–1473
He J, Shen J, Zhang L, Zhu Y, Zheng Y, Xu M, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Hetier JM (1986) Organic matter inputs to soil after growth of carbon-14–nitrogen-15 labeled maize. Soil Sci Soc Am J 50:76–80
Iijima M, Griffiths B, Bengough AG (2000) Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytol 145:477–482
Ikenaga M, Asakawa S, Muraoka Y, Kimura M (2003) Phylogenetic study on CTO primer-amplified ammonia-oxidizing bacteria and β-Proteobacteria associated with rice roots grown in a flooded paddy soil. Soil Sci Plant Nutr 49(5):719–727
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Keeneg DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL et al (eds) Methods of soil analysis: part 3. SSSA and ASA, Madison, pp 643–692
Koops HP, Pommerening-Röser A (2005) Genus III. Nitrosospira. In: Garrity GM (ed) Bergey’s manual of systematic bacteriology, 2nd edn., vol. 2. The Proteobacteria, part C: the alpha-, beta-, delta-, epsilonproteobacteria. Springer, New York, pp 868–869
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Ann Rev Microbiol 55:485–529
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Br Bioinforma 5:150–163
Lee CG, Watanabe T, Sato Y, Murase J, Asakawa S, Kimura M (2011) Bacterial populations assimilating carbon from 13C-labelled plant residue in soil: analysis by DNA-SIP approach. Soil Biol Biochem 43:814–822
Levičnik-Höfferle S, Nicol GW, Ausec L, Mulec I, Prosser JI (2012) Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not inorganic nitrogen. FEMS Microbiol Ecol 80:114–123
Li YL, Fan XL, Shen QR (2008) The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Environ 31:73–85
Li Y, Lee CG, Watanabe T, Murase J, Asakawa S, Kimura M (2011) Identification of microbial communities that assimilate substrate from root cap cells in an aerobic soil using a DNA-SIP approach. Soil Biol Biochem 43:1928–1935
Li Y, Watanabe T, Murase J, Asakawa S, Kimura M (2013) Identification of major capsid gene (g23) of T4-type bacteriophages that assimilate substrate from root cap cells in aerobic and anaerobic soil conditions applying a DNA-SIP approach. Soil Biol Biochem 63:97–105
Lueders T, Manefield M, Friedrich MW (2004) Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ Microbiol 6:73–78
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Morimoto S, Hayatsu M, Takada-Hoshino Y, Nagaoka K, Yamazaki M, Karasawa T, Takenaka M, Akiyama H (2011) Quantitative analyses of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in fields with different soil types. Microbes Environ 26:248–253
Murase J, Itoh K, Kano M, Kimura M (2003) Molecular analysis of β-Proteobacterial ammonia oxidizer populations in surface layers of a submerged paddy soil microcosm. Soil Sci Plant Nutr 49(6):909–913
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Nicolaisen MH, Ramsing NB (2002) Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J Microbiol Meth 50:189–203
Radajewski S, Webster G, Reay DS, Morris SA, Ineson P, Nedwell DB, Prosser JI, Murrell JC (2002) Identification of active methylotroph populations in an acidic forest soil by stable-isotope probing. Microbiol 148:2331–2342
Rolfe R, Meselson M (1959) The relative homogeneity of microbial DNA. Proc Natl Acad Sci U S A 45:1039–1043
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Sawatsky N, Soper RJ (1941) A quantitative measurement of the nitrogen loss from the root system of field peas (Pisum avense L.) grown in the soil. Soil Biol Biochem 23:255–259
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, Spieck E, Streit W, Stahl DA, Wagner M, Schleper C (2010) Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol 18:331–340
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tian XF, Hu HW, Ding Q, Song MH, Xu XL, Zheng Y, Guo LD (2014) Influence of nitrogen fertilization on soil ammonia-oxidizers and denitrifiers abundance, microbial biomass and enzyme activities in an alpine meadow. Biol Fertil Soils 50(4):703–713
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10:1357–1364
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidizing archaea and bacteria in soil microcosms. ISME J 5:1067–1071
Watanabe A, Kimura M (1999) Influence of chemical properties of soils on methane emission from rice paddies. Comm Soil Sci Plant Anal 30:2449–2463
Watanabe T, Asakawa S, Nakamura A, Nagaoka K, Kimura M (2004) DGGE method for analyzing 16S rDNA of methanogenic archaeal community in paddy field soil. FEMS Microbiol Lett 232:153–163
Watanabe T, Lee GC, Murase J, Asakawa S, Kimura M (2011) Carbon flow into ammonia-oxidizing bacteria and archaea during decomposition of 13C-labeled plant residues in soil. Soil Sci Plant Nutr 57:775–785
Xia W, Zhang C, Zeng X, Feng Y, Weng J, Lin X, Zhu J, Xiong Z, Xu J, Cai Z, Jia Z (2011) Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5:1226–1236
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci U S A 107:17240–17245
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Acknowledgments
Yong Li extends his gratitude to the China Scholarship Council for their financial support in conducting this study. The authors also thank Professor Hongjie Di, Zhejiang University, for his insightful and helpful suggestions for improving the manuscript. This work is partially supported by the Natural Science Foundation of China (41301254), Specialized Research Fund for the Doctoral Program of Higher Education (20130101120182), Foundation of Zhejiang Educational Committee (Y201329798), and Fundamental Research Funds for the Central Universities (2014QNA6008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jizheng He
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Comparison of DGGE banding patterns of 13C-enriched AOA community. N, 12 and 13 in the figure indicate no callus treatment, 12C-callus treatment and 13C-callus treatment, respectively; 4-11 indicate the fraction number (PPTX 445 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Watanabe, T., Murase, J. et al. Abundance and composition of ammonia oxidizers in response to degradation of root cap cells of rice in soil microcosms. J Soils Sediments 14, 1587–1598 (2014). https://doi.org/10.1007/s11368-014-0910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0910-8