Abstract
Purpose
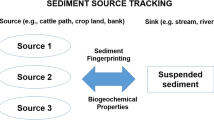
Sediment fingerprinting is a relatively recent research technique, capable of determining the origin of suspended sediment. In this study, we investigated sub-basins within a larger watershed we examined previously. The objectives were to determine if there was spatial variation in the origin of the suspended sediments and to test a streamlined fingerprinting approach which would reduce the cost, thereby paving the way for adoption by government agencies.
Materials and methods
Samples were collected from three tributaries, the outlet of the main stem, and at the middle of the main stem. Two methods to collect suspended sediment samples were compared: a mobile continuous-flow centrifuge and automated samplers. A relatively small initial tracer suite consisting of stable isotopes of nitrogen (N) and carbon (C) (15N and 13C), total N (TN), and total C (TC) was tested. Tracer concentrations were obtained through a single mass spectrometry analysis requiring <1 g of sediment.
Results and discussion
Multivariate discriminant analysis showed that three of the four tracers (δ 15N, δ 13C, and TC) from the initial pool were capable of accurate classification of the source samples. A multivariate mixing model showed that banks contributed the majority of sediment throughout all locations sampled and that in tributaries it was an even more dominant source. Despite variations in land use and stream order, the legacy sediments comprising the banks and floodplains were the main factor in impairment for suspended sediment. We found a small but statistically significant difference in δ 15N and δ 13C concentrations collected using automated samplers vs. the mobile centrifuge, but the effect on analysis of sediment source proportions was minimal.
Conclusions
The results of this study indicate that, at least in the study watershed, the majority of sediment in suspension was of streambank origin. A cost-effective tracer suite was identified as well as an attempt to make a streamlined approach to the technique. The streamlined approach cost much less ($7,550 US) than the conventional approach ($46,600 US) and should be suitable for total maximum daily loads analysis by state government agencies in the Southern Piedmont region of the USA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the USA, about 15 % of assessed stream miles are considered threatened or impaired with respect to suspended sediment according to the United States Environmental Protection Agency’s (USEPA) 2004 ATTAINS database (USEPA 2008). To reduce sediment loading, total maximum daily loads (TMDLs) and best management practices (BMPs) have been developed for sediment and runoff control. A TMDL is a calculation of the total amount of a pollutant that a waterbody can receive in a day and still meet water quality standards. The origin of the TMDL can be found in section 303(d) of the US 1972 clean water act. This law requires states, territories, and tribes to develop lists of impaired waters, then prioritize and develop TMDLs for them (USEPA 2011a). There are currently 40,235 impaired water bodies in the USA (USEPA 2011a).
The Southern Piedmont region had elevated rates of erosion during the intensive cotton farming era of 1830–1930 and, as a result, channels and floodplains were inundated with the estimated 9.7 km3 of soil that eroded from the uplands (Trimble 1974). In modern times, erosion rates in the Piedmont have waned to levels which are likely approaching, if not equal to, pre-European settlement rates because row-crop agriculture has decreased, soil conservation measures have been put in place, and much of the land is forested. It is apparent, however, that the effects of the cotton farming era are still being felt as fluvial processes continue the task of resuspending and transporting the legacy sediments deposited a century ago, aided by increased stream power due to urbanization (Carter et al. 2009).
Sediment fingerprinting is a relatively recent research technique capable of determining the source of suspended sediment in streams. There have been numerous sediment fingerprinting studies in the past 30 years, and the method has proven to be an effective tool in determining sediment source type and spatial origin (Gellis and Walling 2011). The technique involves the characterization of source types based on chemical, physical, and/or biological properties establishing individual source “fingerprints”. The tracers used must be measurable in both source soils and sediment, and must be conservative in that they do not undergo any chemical alterations between generation and delivery. Properties used include sediment color (Grimshaw and Lewin 1980), plant pollen (Brown 1985), mineral magnetic properties (Walden et al. 1997), rare earth elements (Kimoto et al. 2006), fallout radionuclides (Collins and Walling 2002; Nagle and Ritchie 2004; Walling 2005; Mukundan et al. 2010), stable isotopes of carbon (C) and nitrogen (N) (Papanicolaou et al. 2003; Fox and Papanicolaou 2007), and fatty acid methyl esters (Banowetz et al. 2006).
Whereas fallout radioisotopes rely on atmospheric deposition and elemental tracers rely on (in most cases) parent material, stable isotopes are based on biogeochemical cycling (Papanicolaou et al. 2003). During cycling, organisms exhibit a preference for the lighter isotopes. This preference leads to the enrichment of the heavier isotope in the soil and the “fractionation” or alteration of the isotopic ratios. The concentrations are usually expressed in terms of δ values which represent a difference from a standard (Peterson and Fry 1987). Plant cover, land use, and land management all affect the isotopic signature of soils (Fox and Papanicolaou 2007, 2008).
A previous study (Mukundan et al. 2010) described sediment sources in the North Fork Broad River (NFBR), a typical rural stream in the Piedmont region of Georgia, USA. The radioisotope caesium-137 (137Cs) and the stable isotopic ratio of nitrogen were selected as tracers using the tracer selection process described in Collins and Walling (2002). Three potential sources were identified from an initial pool of five (forests, pastures, unpaved roads and construction sites, row crops, and stream banks): (1) pastures, (2) exposed subsoil sources consisting of unpaved roads/construction sites, and (3) stream banks. Forests and row-crop agriculture were found not to contribute (row crop due to its very small land use percentage), and the study was unable to discriminate between unpaved roads and construction sites due to the inherent similarities in terms of tracer values (both are exposed subsoils). It was concluded that the origin of much of the suspended sediment was stream bank erosion. These banks largely consisted of floodplain deposits of previously eroded sediment from cotton agriculture in the nineteenth century termed “legacy sediment”. Although 137Cs could only distinguish pasture from other sources, distinct 15N signatures were apparent for all three principal sediment sources. Pasture soils exhibited the highest 15N value, followed by banks and then exposed subsoils. Overall, the study found that approximately 60 % of the suspended sediment in the main stem was of bank origin, 15 % of pasture origin, and 25 % from exposed subsoil sources.
In addition to utilizing the fingerprinting technique, Mukundan et al. (2011) performed rapid geomorphic assessments (RGAs) to examine geomorphic stability of stream channels of NFBR. These assessments use the concept of a stream channel evolution model (Simon and Hupp 1986; Simon 1988). Geomorphic assessments are an important complement to fingerprinting because while land use data are generally available, little is known about channel conditions. The results of the RGAs were that both the main stem and several of the tributaries of the NFBR had incised and relatively unstable channels. Mukundan et al. (2012) proposed several measures to transform sediment fingerprinting from its current use as a research tool into an operational/management tool. In order to accomplish this, well-defined protocols must be developed for those wishing to adopt the technique. Suggestions included the use of small volume samples collected by automated samplers and extracted on filters as an alternative to traditional sampling methods and the use of Monte Carlo simulations for uncertainty analysis.
The objective of this study was to extend our earlier work on the NFBR in two ways. First, we wanted to examine several tributaries within the NFBR to identify spatial variations in the origins of suspended sediment. While the previous study sampled suspended sediment at the main stem outlet, it was not clear if there were variations in source contributions throughout the NFBR or if the same general trend would emerge. Second, we wanted to streamline the technique so that it could be adopted by government agencies developing and implementing sediment TMDLs. We wanted to make it as cost and time effective as possible, recognizing that the traditionally large cost of sediment fingerprinting used as a research tool made it impractical for widespread use as an operational tool by government agencies.
2 Materials and methods
2.1 Watershed
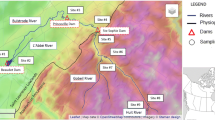
The North Fork Broad River (NFBR) drains a 182-km2 rural watershed in Northeast Georgia, USA (Fig. 1) (near the towns of Toccoa and Carnesville). Land use is predominantly forest (deciduous, evergreen, and mixed), occupying about 72 % of the watershed, followed by pasture (15 %) and row crops (7 %). In 1998, it was placed on the 303(d) list for impacted biota and habitat with sediment being the pollutant of concern. In 2004, the USEPA conducted a macroinvertebrate study on the watershed. Based on the results of the study, the watershed was removed from the 303(d) list; however, they reported that “habitat concerns are present but not to an extent impacting biota.” The primary source of sediment, the relative contribution of potential sediment sources, and their spatial variability remained unknown. Three tributaries of the NFBR were selected for this study: Tom’s Creek (61 % forest, 38 % pasture, 1 % urban) with an area of 50 km2, Clarke’s Creek (59 % forest, 39 % pasture, 2 % urban) with an area of 33 km2, and Davis Creek (80 % forest, 17 % pasture, 3 % urban) with an area of 7 km2 (see Fig. 1). The elevation of the NFBR watershed ranges from 200 m near the outlet to about 500 m in the headwaters. Ninety-eight percent of the watershed is comprised of Madison and Pacolet (Fine, kaolinitic, thermic Typic Kanhapludults) soils. The soils are moderately permeable and well drained. The average annual rainfall of the region is about 1,400 mm. While currently primarily forested, row-crop agriculture would have been much more prevalent during the cotton farming era.
2.2 Sampling
In our previous study (Mukundan et al. 2010), spatially distributed composite soil samples were taken from about 150 sites which represented the potential sediment sources within the watershed (Fig. 2). This sample data set was again utilized in the current study. Recapping briefly how these samples were collected, upland soil samples were collected from the upper 0–2 cm depth of potential sources. Bank samples were collected in areas of the channel visually identified as actively eroding by scraping the face of the bank and collecting samples from the surface of the stream to a height about 1 m above the stream.
The majority of suspended sediment is transported by streams during storms, and as such it is necessary to sample during storm events. Conventionally, fingerprinting has required pumping large volumes of water (100–400 l) for a sample and either centrifuging on-site using a mobile continuous-flow centrifuge (Mukundan et al. 2010) or transporting the samples to a laboratory for centrifugation (Walling et al. 1993). This has been due to the large mass requirements (20–100 g) of radionuclide tracers as well as the mass necessary for multiple tracer analyses and particle size analysis in a conventional tracer suite (10–50 g). In this study, suspended sediment samples were collected using two techniques: (1) pumping water out of the stream and passing it through a continuous-flow centrifuge collector mounted at the back of a pick-up truck, and (2) using automated ISCO samplers (Teledyne Isco, Inc., Lincoln, NE, USA) to collect water samples which were then filtered in the laboratory.
Automated samplers were used in our study for several reasons. First, the analysis for the tracers in our study only required 50–100 mg of sediment at most and therefore the large masses associated with analysis of fallout radionuclides, such as 137Cs, were unnecessary. Second, the use of automated samplers allowed multiple samples to be collected during different stages of a storm hydrograph at a site and simultaneous sampling on multiple tributaries, providing a more detailed picture of sediment dynamics within the watershed. Automated samples were taken at 1-h intervals after reaching a pre-determined stage threshold which was generally 15 cm above baseflow level. Samples were composited based on hydrograph stage and suspended sediment concentrations so that each section of the hydrograph was represented by an individual sample (rising, peak, recession) and that adequate sample was available for analysis. This was accomplished by combining 2–4 l of sample, enough to temporally integrate (0.75-l samples were taken every hour) as well as provide adequate sample mass for analysis. In lieu of particle-size analysis, samples were poured through a 0.05-mm sieve to remove sand so that tracer results could be expressed in terms of the fine fractions. Vacuum filtration and 0.45-μm glass-fiber filters were used to separate fine sediment. Sampling dates, locations, methods, rainfall, and turbidity are provided in Table 1. Turbidity values were measured in the field at the time of sampling for centrifuged samples and in the laboratory for ISCO samples using a HACH 2100 turbidimeter (HACH Co., Loveland, CO, USA).
2.3 Tracers
Because our previous study had shown the ability of 15N and TC to discriminate sources, we decided to expand their use in this study. The analysis used (isotope mass spectrometry) requires a small enough mass of sediment (<50 mg) so that 1-l stream samples from an automated sampler suffice. The analysis is relatively inexpensive making this an excellent candidate for government agency adoption. Because the analysis also yields 13C and total N (TN) data, these tracers were included in the initial pool and the selection process outlined in Collins and Walling (2002) was utilized. Each individual tracer was subjected to the Kruskal–Wallis H test. This is a non-parametric test equivalent to analysis of variance and is used to test the ability of each tracer to discriminate sources. Tracers from the initial suite unable to discriminate were excluded. The remaining tracers were then normalized by dividing by the maximum value for the group. Then stepwise discriminant analysis (DA) was employed to determine the final tracer suite. The DA can be thought of as a regression analysis where the dependent variable is group membership. By finding a linear combination of variables that maximizes between-group variance while minimizing within-group variance, observations can be classified into groups (Lachenbruch 1975).
To test the ability of the tracer suite in a second watershed, the South Fork Broad River (SFBR) watershed, which is located about 30 mi south of the NFBR, also was sampled and analyzed using DA. The SFBR has an area of about 563 km2 and its land use is predominantly forested (55 %), followed by pasture (43 %) and urban areas (2 %). A total of 40 source samples were collected in the summer of 2010 with 10 samples each collected from stream banks, forests, pastures, and exposed sub-soils (unpaved roads and road ditches). The sampling method was the same as with the NFBR with composited samples taken to a depth of about 2 cm, air dried, sieved, and analyzed for particle size.
Values for 15N and 13C were calculated in the same manner. The stable isotope was expressed relative to isotope standard in δ notation:
where δX is expressed in parts per thousand, R sample is the sample isotopic ratio, and R std is the ratio in the standard (Hayes 2004).
Stable isotope analysis was performed by the analytical chemistry laboratory at the Odum School of Ecology, University of Georgia. The laboratory used a Carlo Erba NA 1500 CN Analyzer coupled to a Thermo-Finnigan Delta V Mass Spectrometer via Thermo-Finnigan Conflo III Interface. The standards used in the calculation of delta values were air for δ 15N and the Vienna Pee Dee Belemnite for δ 13C. For source samples, the analysis was performed using a few milligrams out of 5 to 10 g of fine soil that was ground and homogenized in a ball mill. The same process was utilized for centrifuged sediment samples. For filtered samples, the sediment was removed from the filter and ground with a mortar and pestle before analysis.
Review of the tracer data obtained during the previous study showed that total C (TC) was positively correlated with 137Cs (R 2 = 0.67). This, in addition to it being able to correctly discriminate between sources, led to it being used in lieu of 137Cs. In addition to being less expensive, TC has the same low mass requirement for analysis as 15N (<50 mg). Total N also was used in the analysis. A known issue in fingerprinting studies is the variance associated with soil and sediment sample particle size distributions when samples are collected in a range of geographic locations. In order to account for this, textural analysis was performed on all soil and centrifuged sediment samples using the hydrometer method (Gee and Or 2002). Following the analysis, the elemental tracers (TN and TC) were expressed in terms of the fines (clay plus silt fractions) by multiplying the tracer value by the inverse of the fine fraction. This ensured that the sediment samples and the soil samples collected from the banks and uplands were comparable. In our case, only TC and TN needed particle size correction. The stable isotopes δ 13C and δ 15N are ratios (isotopic ratios do not pose the same issues with linear mixing models) and therefore not dependent on particle size (Mukundan et al. 2010). There may, however, be issues with particle size and the sampling method employed, and this will be discussed further below.
2.4 Mixing model and uncertainty
Relative source contribution of suspended sediment was estimated by using a multivariate mixing model (Collins et al. 1998; Owens et al. 1999; Walling et al. 1999). The method of least squares was used for deriving the source proportions by minimizing the residual sum of squares for the n tracers and m sources (Collins et al. 1998):
where RSS = the residual sum of squares; C sed,i = the concentration of the tracer property i in the sediment; C s,i = the mean concentration of the tracer property i in the source group s; and P s = the relative proportion from source group s (a value between 0 and 1) and is achieved by estimating the relative proportion from the source group (P s ) such that the calculated sum of squared residuals is minimized. This is accomplished by iteratively changing P s (a value between 0 and 1) and thereby the sum of the products of P s and tracer concentration value for each source. The residual with respect to the measured tracer concentration in the collected suspended sediment is used to determine the goodness of fit (RSS, the residual sum of squares) of the model. The constraint that the source proportions (P s ) must be equal to 1 is used to ensure the sources account for 100 % of the sediment. This can be accomplished using the SOLVER plug-in in Microsoft Excel.
In order to examine uncertainty in both source tracer values and sediment tracer values, Palisades @RISK optimizer software (Pallisades Corp., Ithaca, NY, USA) was used. Rather than using a mean or median value for tracer values in the model, this approach uses a distribution. The software was used to fit a distribution to each group of tracer values for each source. Then a distribution was fit for each stream sampling site’s sediment tracer values. These fitted distributions were selected based on the chi-squared goodness of fit (GOF). Probability–probability and quantile–quantile plots were also available for examination and allowed for visual inspection of how well the generated plots agreed with the data. Following this, a Monte Carlo simulation was performed and the model was solved 10,000 times with each solution being generated from a different randomly selected set of tracer values from the distributions. Some solutions to the model can be unrealistic and in order to retain only robust solutions, a GOF criteria of >0.80 was utilized (GOF = 1 − RSS) following the procedure used in Motha et al. (2003).
2.5 Rapid geomorphic assessments
Rapid geomorphic assessments (RGAs) are used to determine the stage of channel evolution and overall stability. The RGAs carried out in this study followed the channel stability ranking scheme of Klimetz and Simon (2007). There are nine criteria used in performing an RGA. These are primary bed material, bed/bank protection, degree of channel incision (%), degree of downstream constriction (%), dominant bank erosion type (fluvial vs. mass wasting), percentage of each bank failing, established riparian woody buffer (%), occurrence of bank accretion (%), and the stage of channel evolution from Simon’s model (Simon 1988). A score >20 indicates a very unstable reach; a score <10 indicates a reach is quite stable. The first stage of the model is the pre-modified stream. This stage is the result of natural processes and banks are generally stable with very little mass wasting. The second stage is the constructed stage. This stage involves restructured banks or channel repositioning. It is considered the transition stage to more unstable stages. The third stage is the degradation stage. In this stage, there is a rapid erosion of the stream bed resulting in incision and an increase in the height of channel banks. Widening has not yet begun as the stream is still in the process of steepening the angle of the banks to the point where they exceed their critical angle. The fourth stage is the threshold stage. In this stage, the banks have met their critical height and angle threshold and are beginning to widen and experience mass wasting. Bank faces may be near vertical due to erosion of bank toes. The fifth stage is the aggradation stage. In this stage, the channel bed has begun to aggrade. In addition to bed aggradation, banks surfaces will often have sands deposited on them. Widening is still occurring in the upper bank; however, down slope of the upper bank, failed material is slumped and forming a distinguishable lower bank with a much less severe angle. The sixth stage is the re-stabilized stage, representing a new equilibrium in terms of sediment.
Davis Creek is located in the upper half of the NFBR (see Fig. 1). Rapid geomorphic assessments (RGAs) were carried out on five reaches of Davis Creek on 4/26/2010. RGAs were performed on Tom’s Creek and Clarke’s Creek in 2008. It is preferable to perform an assessment prior to the spring growth of grasses and leaves; however, the assessments at Davis Creek were performed under these conditions and required more attention and time to perform. Reaches were chosen to be representative and varied in length from 300 to 400 m. Spatial coordinates were recorded and each reach photographed for documentation. Also, bed samples were collected for later particle size distribution analysis. Primary bed material was determined visually, as were the presence of bed/bank protection. The degree of incision was determined by measuring the depth of the stream at the thalweg and dividing that by the average height of the bank from the top to the toe. Constriction was determined by measuring channel width at the upstream and downstream ends of the reach and determining their relative differences. Dominant stream bank erosion processes were determined visually for both the left and right bank as well as the percentage of failing banks. These may be either fluvial (undercutting) or mass wasting (movement of large amounts of bank sediment at once). In order to classify a bank as dominated by mass wasting, 50 % or more of the faces must exhibit this process. Vegetative cover was determined by judging the percentage of each bank with established woody vegetation. Grasses tend to be annual and provide no protection during winter months (Klimetz and Simon 2007). Final index values were determined by tallying each of the scores from the nine categories.
3 Results
3.1 Tracers
Tracer statistics, distributions of the tracer data sets (along with the associated chi squared statistic), and results of the Kruskal–Wallis H test (which illustrated the ability of the individual tracers to discriminate between source types within the NFBR) are provided in Table 2. The distributions listed describe the best possible fit to the data using @RISK software and were selected based on their chi-squared statistic and P–P plots. Table 3 displays the tracer statistics and results of the Kruskal–Wallis H test from the SFBR, and illustrates the general similarity of the tracer values in terms of land use between the NFBR and SFBR. The δ 15N and TC values were found to be highest in pastures, followed by banks and then subsurface in the NFBR (see Table 2). The δ 13C and TN values also were found to be highest in pastures, but values in the subsurface were higher than the banks. The results for the SFBR were similar in terms of relative magnitude and suggest the portability of the tracer suite (see Table 3).
All four tracers in the initial pool for the NFBR displayed an ability to discriminate; therefore, all four were used in the subsequent step in the selection process. In order to select the most effective composite fingerprint from this pool, multivariate discriminant analysis was used and the results are shown in Table 4. These results indicated that three of the four tracers (δ 15N, δ 13C, and TC) from the initial pool were capable of accurate classification of 95 % of the source samples (number of observations = 73). Discriminant analysis results of the SFBR tracer comparison study are shown in Table 5 and highlight the ability of the suite to work in a similar watershed. In the SFBR, all four tracers (δ 15N, δ 13C, TN, and TC) were selected and were able to classify 98 % of the samples correctly. Sources listed in the tables are in the order that they were selected, from most effective to least effective in terms of discrimination.
3.2 Sampling method comparison
To compare using automated samplers for suspended sediment collection to our previous, significantly more expensive, technique of utilizing a mobile continuous-flow centrifuge, we used both methods at two sites (the outlet of the main stem and the Tom’s Creek tributary) and compared them using ANOVA (Table 6). The sampling method employed at each site for each event is listed in Table 1. There were no significant differences between the mean TC and TN values measured using the two methods. However, the mean δ 15N and δ 13C were significantly higher when the samples were collected using automated samplers. This discrepancy may lie in the fact that the centrifuge did not capture some of the finer particles that were retained in the samples collected with the automated samplers and filtered in the laboratory. It has been shown (Billings and Richter 2006) that there is an inverse relationship between the size of soil organic matter (SOM) and the values of δ 15N and δ 13C. Therefore, the filtered samples could have been enriched in finer particles which exhibited greater isotopic enrichment. These results imply that although isotopic ratios need not be corrected for particle size, loss of a certain fine fraction from a sample can affect the δ 13C and δ 15N values. This means that sampling methods which may bias the sample towards larger size particles (e.g., mobile centrifuge, passive time-integrated samplers) may be inferior to filtration (which collects all particles in solution) with respect to results obtained using stable isotopic ratios.
Figure 3 shows the individual event source proportions using the deterministic solution to the mixing model for the main stem outlet of the NFBR and the middle stem positions. For the main stem outlet, the results using samples collected with ISCO automated samplers (Fig. 3a) can be compared to the results using samples collected with the mobile centrifuge (Fig. 3b). Both methods showed that the source was predominately bank erosion in most events. Results for the middle stem position using samples collected with ISCO samplers also showed that bank erosion was the predominant source.
Storm event source proportions for the North Fork Broad River (NFBR) main stem outlet and middle stem using the deterministic mixing model. For the main stem outlet, the results using samples collected with ISCO automated samplers (a) and the mobile centrifuge (b) are shown. For the main stem middle, samples were collected with the ISCO automated samplers only (c)
Figure 4 shows the individual event source proportions for tributaries of the NFBR. For Tom’s Creek, results are shown for samples collected with the ISCO samplers (Fig. 4a) and with the mobile centrifuge (Fig. 4b). Again, both methods showed that the source was predominately bank erosion. For Clarke’s Creek (Fig. 4c) and Davis Creek (Fig. 4d), only centrifuge samples were collected. The results for Clarke’s Creek indicated that bank erosion was the predominate source, but for Davis Creek, where only one storm (two samples) was monitored, a mixture of bank erosion and subsurface sources was indicated.
Storm event source proportions for tributaries of the North Fork Broad River (NFBR) using the deterministic mixing model. For Tom’s Creek, the results using samples collected with ISCO automated samplers (a) and the mobile centrifuge (b) are shown. For Clarke’s Creek (c) and Davis Creek (d), samples were collected using the mobile centrifuge only
Results using the centrifuge do appear to exhibit a higher relative proportion of subsurface-derived sediments. As mentioned earlier, the centrifuge samples were depleted in fines relative to the filtered samples. This may be skewing the results by allowing particles enriched in 15N and TC to pass through. This would cause an apparent relative increase in subsurface-derived sediment as it exhibits the most depleted 15N and TC values.
Figure 5 shows the temporal variability in sediment sources at different stages of the hydrograph during two storms where samples were collected using an ISCO automated sampler at the NFBR main stem outlet (four storms were sampled with ISCOs at this site, Table 1). In the storm on 3/06/2011 (Fig. 5a), the sources did not vary greatly as a function of hydrograph stage, and this was the pattern for two of the other storms at the main stem outlet (data not shown). In the storm on 2/02/2011 (Fig. 5b), there was a higher proportion of sediment from pasture and subsoil sources in the rising limb of the hydrograph. This may be due to runoff which reaches the stream during the rising limb before ceding predominance to interflow and groundwater during later stages. The three storms on Tom’s Creek tributary when ISCO samplers were used (2/02/2011, 3/06/2011, and 3/11/2011) also showed this pattern, but the differences were not as prominent (data not shown).
Figure 6 compares the mean sediment contributions over all storms from each site using both deterministic (mean tracer value for sources and suspended sediment) and stochastic (using Monte Carlo simulations) approaches. Results are shown based on samples collected using the ISCO samplers and the centrifuge for the NFBR main stem outlet (Fig. 6a, b) and for Tom’s Creek (Fig. 6c, d). For Clarke’s Creek (Fig. 6e) and the NFBR main stem middle (Fig. 6f), only centrifuge samples were collected. Based on the ISCO samples at the main stem outlet, the deterministic average source proportions (dashed lines) were 81 % bank, 8 % exposed subsoil, and 11 % pasture (Fig. 6a). The results using the centrifuge samples were slightly different: 68 % bank, 24 % exposed subsoil, and 8 % pasture. The differences are less when comparing the mean values from the stochastic results and have two possible explanations. The first and most likely is simply that while some of these events were sampled using both methods, some were not and there were differences in inputs between events. The second is that the particle size bias inherent with the centrifuge may be affecting our results. Although a different tracer suite was used in this study (15N, 13C, TC), the deterministic results are similar to our previous study (using 137Cs and 15N) where we sampled from this location using the mobile centrifuge (Mukundan et al. 2010): 60 % bank, 23–30 % exposed subsoil, and 10–15 % pasture. This confirms our previous results with a tracer suite which is both cheaper and requires less mass of suspended sediment. The mean source proportions from the stochastic Monte Carlo simulations were close to the deterministic proportions. However, the box and whisker plots of the Monte Carlo results provide more information in terms of variability of the distributions and the associated uncertainty in the model results. Observing the distance between the first and third quartiles allows for an appreciation of the variability which may exist in the model results. At all of the sites, the range between the first and third quartiles for any given source was between 10 % and 20 %, except Tom’s Creek (Fig. 6c) where the results from automated sampling varied by as much 38 % for the bank source. This information allowed us to see variability in possible contributions that a deterministic approach would not have disclosed.
Source proportions based on all samples for the main stem outlet using ISCO samplers (a), North Fork Broad River (NFBR) main stem outlet using centrifuge sampler (b), Tom’s Creek using ISCO samplers (c), Tom’s Creek using centrifuge sampler (d), Clarke’s Creek using centrifuge sampler (e), and NFBR middle stem using centrifuge sampler (f). Whisker plots (plus sign represents mean from simulations) are from Monte Carlo simulations and the dashed line indicates the value from using the deterministic mean in the mixing model. Whiskers illustrate minimum and maximum values and boxes are the quartiles. X-axis represent source and Y-axis represents percent source contribution
Within the NFBR, there was a striking similarity in source origin in terms of the spatial distributions. From the outlet to the middle of the main stem and from the tributaries we sampled, the suspended sediment was predominantly of bank origin (see Figs. 3, 4, 5, and 6). This seemed to indicate that at least in this watershed (which we consider typical of the Southern Piedmont), regardless of variations in land use and stream order, the legacy sediments comprising the banks and floodplains and the geomorphologic processes which are occurring as the stream channels evolve toward a stable stage should be considered the primary factor in impairment for suspended sediment. Furthermore, considering the nature of the problem now defined, it is inherently difficult to prevent or even mitigate such a problem at this scale. Identifying bank areas of particular concern is possible, but they will likely be numerous and expensive to restore. Time may be an important aspect of the solution as channels move towards equilibrium in terms of sediment transport.
While similarities in the spatial distribution of sediment sources existed among sites, there were also dissimilarities. The largest tributaries (Clarke’s Creek, Fig. 6e, and Tom’s Creek, Fig. 6d) showed an increase in bank sediment relative to the main stem (Fig. 6b) when looking at results from centrifuged samples using the deterministic approach. This may be due to one of several reasons. Referring to the channel evolution model (Klimetz and Simon 2007), the RGAs (Table 7) performed on the NFBR showed a main stem which was predominantly at stage five, a stage where aggradation has begun and which directly precedes stage six or a stage of renewed equilibrium. However, many of the tributary reaches surveyed (Clarke’s Creek was an exception) were at stage three or four suggesting that while the main stem may have begun to stabilize, the tributaries might still be generating sediment due to degradation and channel widening. In the early 1900s, the lower section of the NFBR main stem was channelized under a program initiated by a State of Georgia drainage law passed in 1911 (Barrows and Phillips 1917). Note the straight reaches in the NFBR main stem downstream of Tom’s Creek in Fig. 1. This disturbance may have created a “knickpoint” of disturbance that has moved up from the lower main stem into the tributaries (Simon and Hupp 1986). Also, field gullies were present in floodplains in the tributaries. These gullies are comprised of the same legacy sediments as the banks and are likely indistinguishable from a tracer perspective (we did not sample the gullies as an erosion source). We believe it may be possible that the elevated levels of bank sediments could at least in part be originating from the headcuts of these gullies. More investigation is needed in that regard.
The smallest tributary, Clarke’s Creek (Fig. 6e), had the lowest percentage of bank contributions. This may be due to the fact that Clarke’s Creek had a channel stage which was predominantly stage five, the same as the main stem (Table 7).
There were dissimilarities between the two main tributaries as well. Tom’s Creek (Fig. 6c, d) exhibited less sediment of subsurface origin than Clarke’s Creek (Fig. 6e). Of the two, which are both quite rural, Clarke’s Creek appeared to have a larger number of residences. Also, while both sub-basins contained a number of unpaved roads and road ditches, Clarke’s Creek had several which were on steep gradients and had large incised road ditches. Tom’s Creek exhibited more sediment from bank origin. This was confirmed by RGA results which showed Tom’s Creek was predominantly stage three, while Clarke’s Creek was predominantly stage five. Also, both tributaries had several farm ponds and decades-old sediment detention ponds, and their effect on the results is unknown.
3.3 A streamlined approach
Traditional approaches to fingerprinting have been performed in the context of research and have not focused on the issues of cost or time. It was our intent to provide an outline of the steps necessary to conduct a fingerprinting study in the Southern Piedmont using the most cost-effective means available. The main benefit comes from the reduced costs associated with having the tracer selection process abbreviated and using a tracer suite which is suitable for automated samplers. Table 8 compares costs of the study of the NFBR by Mukundan et al. (2010) using 137Cs and 15N and the mobile centrifuge with the current costs using 15N, 13C, and TC and the ISCO automated samplers. The costs for the previous approach are much higher due to the need for a modified mobile centrifuge and the analysis costs associated with the tracer selection process. Using the latter method, costs were limited to an ISCO sampler and a single analysis. The following is an outline of that approach.
The first step in this streamlined approach is to determine contributing sub-basins and their respective land uses using GIS. This can be done with the USEPA BASINS (Better Assessment Science Integrating point and Non-point Sources) free software (USEPA 2011b). This software contains data for all watersheds in the USA and can be used for sub-basin delineations and land-use characteristics. Online tutorials are available on the EPA BASINS website.
The second step is to characterize the stream channels utilizing RGAs or some other stream classification system such as the Rosgen system (Rosgen 1985, 1994). Land-use data alone does not provide a complete picture in terms of potential sediment sources. RGAs are quickly and inexpensively performed (with training), and the stream stability index provides an effective method to compare streams in terms of bank erosion potential. It should be kept in mind that RGAs indicating eroding streambanks are expected as this is a natural process which occurs in all streams. The RGAs, however, allow for an understanding of where the stream is in terms of channel evolution and therefore the relative potential to generate sediment compared to the same channel at a different stage. RGAs and the Rosgen system are especially relevant in the Southern Piedmont because the disturbance posed by the deposition of the legacy sediments from the cotton farming era has caused the channels to undergo considerable change as they move towards equilibrium.
The third step is sampling. Using the methods outlined in this study and our previous study (Mukundan et al. 2010), source sampling can be accomplished in a matter of days (depending on watershed area), provided there is ample access in the areas of interest. Sample sizes should be large enough to ensure statistical power and accurate representation of sources. Bank sampling can be performed alongside RGA. Stream sampling is easily performed using automated samplers. The use of ISCO or other samplers equipped with pressure transducers or flow meters allows not only for automated composite samples during stormflow but also for the collection of flow data on ungauged streams, provided a stage–discharge relationship is developed. If automated samplers are found to be too expensive, an even less costly method exists. Time-integrated samplers (e.g., Phillips et al. 2000) consist of in situ sedimentation chambers made of commercially available polyvinylchloride. Small-diameter inlet and outlet tubes allow water to enter the chamber where it loses much of its velocity and allows for sedimentation. Samplers are placed horizontally in the stream channel and secured to metal posts. After an event, they are removed and a single sample representing the entire event is collected. Individual samplers can be constructed for <$25. Sample preparation and analysis should consist of air-drying and sieving source samples to 2 mm followed by particle size analysis, or sieving to 0.05 mm. Source samples need to be ball milled for isotope ratio mass spectrometry. Suspended sediment samples should be poured through a 0.05-mm sieve to remove sand and vacuum-filtered through a 0.45-μm filter for suspended sediment removal. Oven-drying and grinding using a mortar and pestle are all that is needed for sample preparation.
While automated sample collection has a number of advantages, several pitfalls should be considered. First, while we were able to composite samples by hydrograph position (generally consisting of two to six samples) using ISCO samplers, each individual sample represents only 1 l of water collected. Using a technique such as the centrifuge allows for hundreds of liters of water to be collected, and each sample therefore represents a better temporal integration. Also, our results indicated a small but statistically significant difference in isotopic values with respect to sampling methods. This was likely due to differences in minimum particle size recovery between the two methods. While the differences existed, their effect appeared minimal in terms of sediment source results.
Tracer selection is the next step. We have found that the tracer suite we used could distinguish three sources in the NFBR and in a similar watershed nearby. The mixture of elemental and isotopic tracers provides enough variety to insure a robust tracer suite. Furthermore, only one analysis (isotope ratio mass spectrometry) is required for all tracers. Mass spectrometry is available at numerous laboratories around the US (and worldwide) with turnaround times under a month. In contrast, radioisotope analysis is available at only a few laboratories. The mass necessary is small enough that sediment collected on filters is adequate and the analysis is quite inexpensive (<$15 per sample as compared to >$100 for radioisotopes). Any statistical package capable of performing the operations discussed above would be adequate for the tracer selection process.
The tracers selected here will likely prove quite portable in regional watersheds. The reason lies in the processes which give us such distinct values for the sediment sources investigated. The elemental tracers TN and TC are both dependent on inputs to the soil. When comparing pastures to the other two sources in this study, surficial inputs in the form of organic matter from grasses (TC) and chicken litter amendments (TN) are the cause for their higher relative magnitude. We would expect to see the slightly higher values for bank sediments (relative to upland subsurface soils) due to their proximity to the water table (which slows nitrification and organic matter decomposition) and indeed that is the case for TC but not TN in NFBR. There was, however, substantial variability in our NFBR TN values and the tracer was not selected during discriminant analysis.
While the elemental tracers are dependent on inputs, the isotopic tracers are dependent on biota (as well as inputs). In Mukundan (2010), it was found that distinct δ 15N signatures were apparent for the sediment sources in the study (stream banks, forests, pastures, and construction sites/dirt roads). Pasture soils exhibited the highest δ 15N value. Enrichment in pasture soils is due to plant preference for 14N, then the subsequent removal of biomass by both the harvesting and consumption of grasses, and the addition of manures. Banks exhibited the next highest δ 15N value. Enrichment in the banks is due primarily to landscape position. Their proximity to the water table leads to frequent anaerobic conditions. Anaerobic microbes prefer 14N during denitrification and therefore leave the soil enriched in 15N. Also, it has been observed that enrichment tends to increase with age and therefore depth in the profile for both 15N and 13C (Billings and Richter 2006). Unpaved roads and construction sites had relatively low enrichment due to less biological activity in these exposed sub-soils.
Finally, mixing model analysis can be performed using Microsoft Excel using the free Solver plug-in software. Results using uncertainty analysis should be desirable for agencies making decisions about how to implement TMDLs and BMPs. If there is a high degree of uncertainty regarding which are the main sources, then further analysis should be undertaken before implementing changes.
There are a number of factors to keep in mind during a study such as this. First, it would be pertinent to insure that the field agents performing the study have suitable training. While the RGAs provide useful information, failure to perform properly the analysis on the system may lead to erroneous conclusions. An example from this study would be the potential for floodplain field gully headcuts to contribute to the sediment load. Remediation would require a much different strategy from unstable banks; however, if they were ignored during the field study, the sediment generated from them would likely be classified as bank. Second are the limitations of the sampling method employed. With the automated samplers, care must be taken to retrieve the samples as quickly as possible (<24 h) as TC and TN values will likely be affected by microbial growth relatively quickly. Also, while it is possible to composite along the hydrograph, each sample taken only represents the 30 or so seconds necessary to pump that sample. This precludes true temporal integration and should be kept in mind when interpreting results. Also, as mentioned earlier in the paper, particle size is of some importance when considering tracer values. Sampling methods which bias (i.e., exhibit <100 % recovery) based on particle size may yield misleading results. Finally, should the tracer suite suggested here prove ineffective, the tracer selection process described is capable of producing alternate tracers which can be found in the literature. An excellent review can be found in Davis and Fox (2009).
Using this process and the numerous studies which have been discussed both here and in the literature, it should be possible to use sediment fingerprinting as an operational tool in TMDL planning and implementation.
4 Conclusions
In this study, we were able to extend our previous study on sediment sources in the NFBR in multiple ways. One of the questions we asked following the previous study was whether or not a single sample location at the outlet of the watershed was providing us with an accurate picture of sediment origin. It was possible that perhaps only the local area of the basin was contributing a majority of bank derived sediment, skewing our results. The current study showed that bank erosion is common throughout the entire system and that channel processes coupled with legacy sediments dominate sediment generation. The results of this study show the applicability of the fingerprinting technique as an operational tool in TMDL implementation. We identified a cost-saving regional tracer suite for the Southern Piedmont. In addition, we found that suspended sediment samples collected using automated water samplers were comparable with the more expensive continuous-flow centrifuge method for sediment source fingerprinting, thus allowing sampling of multiple locations during the same event. Finally, we discuss a streamlined sediment fingerprinting approach for use by government agencies, particularly agencies in the US Southern Piedmont. The methods outlined in this study may be applied in other watersheds of the region to develop an accurate picture of sediment origin in streams where sediment problems exist.
References
Banowetz GE, Whittaker GW, Dierksen KP, Azevedo MD, Kennedy AC, Griffith SM, Steiner JJ (2006) Fatty acid methyl ester analysis to identify sources of soil in surface water. J Environ Qual 35:133–140
Barrows HH, Phillips JV (1917) Agricultural drainage in Georgia. p. 1–122. Bulletin No. 32. Geological Survey of Georgia, Atlanta, GA, USA
Billings SA, Richter DD (2006) Changes in stable isotopic signatures of soil nitrogen and carbon during 40 years of forest development. Oecologia 148:325–333
Brown AG (1985) The potential use of pollen in the identification of suspended sediment sources. Earth Surf Process Landforms 10:27–32
Carter T, Jackson CR, Rosemond A, Pringle C, Radcliffe D, Tollner W, Maerz J, Leigh D, Trice A (2009) Beyond the urban gradient: barriers and opportunities for timely studies of urbanization effects on aquatic ecosystems. J N Am Bentholl Soc 28:1038–1050
Collins AL, Walling DE (2002) Selecting fingerprint properties for discriminating potential suspended sediment sources in river basins. J Hydrol 261:218–244
Collins AL, Walling DE, Leeks GJL (1998) Use of composite fingerprints to determine the provenance of the contemporary suspended sediment load transported by rivers. Earth Surf Process Landforms 23:31–52
Davis MC, Fox JF (2009) Sediment fingerprinting: review of the method and future improvements for allocating nonpoint source pollution. J Environ Eng 135:7(490)
Fox JF, Papanicolaou AN (2007) The use of carbon and nitrogen isotopes to study watershed erosion processes. J Am Water Res Assoc 43:1047–1064
Fox JF, Papanicolaou AN (2008) Model of the spatial distribution of nitrogen stable isotopes for sediment tracing at the watershed scale. J Hydrol 358:46–55
Gee GW, Or O (2002) Particle size analysis. In: Dane JH, Topp GC (eds) Methods of soil analysis. Part 4. Physical methods. Soil Science Society of America, Madison, pp 255–293
Gellis AC, Walling DE (2011) Sediment-source fingerprinting (tracing) and sediment budgets as tools in targeting river and watershed restoration programs. In: Simon A, Bennett S, Castro JM (eds) Stream restoration in dynamic fluvial systems: scientific approaches, analyses, and tools. American Geophysical Union Monograph Series 194, pp 263–291
Grimshaw DL, Lewin J (1980) Source identification for suspended sediments. J Hydrol 47:151–162
Hayes JM (2004) An introduction to isotopic calculations. Woods Hole Oceanographic Institution. http://www.whoi.edu/cms/files/jhayes/2005/9/IsoCalcs30Sept04_5183.pdf. Accessed 12 January 2013
Kimoto A, Nearing M, Shipitalo MJ, Polyakov VO (2006) Multi-year tracking of sediment sources in a small agricultural watershed using rare earth elements. Earth Surf Process Landforms 31:1763–1774
Klimetz L, Simon A (2007) Suspended-Sediment Transport Rates for Level III Ecoregions of EPA Region 4: The Southeast. USDA–ARS National Sedimentation Laboratory Research Report. No. 55, 135 pp
Lachenbruch PA (1975) Discriminant analysis. Hafner, New York
Motha JA, Wallbrink PJ, Hairsine PB, Grayson RB (2003) Determining the sources of suspended sediment in a forested catchment in southeastern Australia. Water Resour Res 39:1056
Mukundan R, Radcliffe DE, Ritchie JC, Risse LM, Mckinley RA (2010) Sediment fingerprinting to determine the source of suspended sediment in a southern Piedmont stream. J Environ Qual 39:1328–1337
Mukundan R, Radcliffe DE, Ritchie JC (2011) Channel stability and sediment source assessment in streams draining a Piedmont watershed in Georgia, USA. Hydrol Process 25:1243–1253
Mukundan R, Walling DE, Gellis AC, Slattery MC, Radcliffe DE (2012) Sediment source fingerprinting: transforming from a research tool to a management tool. J Am Water Res Assoc 48:1241–1257
Nagle GN, Ritchie JC (2004) Wheat field erosion rates and channel bottom sediment sources in an intensively cropped northeastern Oregon drainage basin. Land Degrad Dev 15:15–16
Owens PN, Walling DE, Leeks GJL (1999) Use of floodplain sediment cores to investigate recent historical changes in overbank sedimentation rates and sediment sources in the catchment of the River Ouse, Yorkshire, UK. Catena 36:21–47
Papanicolaou AN, Fox JF, Marshall J (2003) Soil fingerprinting in the Palouse Basin, USA, using stable carbon and nitrogen isotopes. Int J Sediment Res 18:278–284
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Phillips JM, Russell MA, Walling DE (2000) Time-integrated sampling of fluvial suspended sediment: a simple methodology for small watersheds. Hydrol Process 14:2589–2602
Rosgen DL (1985) A stream classification system. In: Riparian ecosystems and their management. First North American Riparian Conference. Rocky Mountain Forest and Range Experiment Station, RM-120, pp 91–95
Rosgen DL (1994) A classification of natural rivers. Catena 22:169–199
Simon A (1988) A model of channel response in disturbed alluvial channels. Earth Surf Process Landforms 14:11–26
Simon A, Hupp CR (1986) Channel evolution in modified Tennessee channels. Proceedings of the Fourth Federal Interagency Sedimentation Conference March 24–27, 1986, Las Vegas, Nevada, USA
Trimble SW (1974) Man-induced soil erosion on the Southern Piedmont, 1700–1970. Soil Conservation Society of America, Ankeny
USEPA (2008) Water quality assessment and total maximum daily loads information. Available at http://www.epa.gov/waters/ir/index.html (verified 9 May 2011). USDA, Washington, DC, USA
USEPA (2011a) National Summary of Impaired Waters and TMDL Information [Online] Available at http://iaspub.epa.gov/waters10/attains_nation_cy.control?p_report_type=T (verified 10 May 2011)
USEPA (2011b) BASINS (Better Assessment Science Integrating point and Non-point Sources). Available online at http://water.epa.gov/scitech/datait/models/basins/index.cfm (verified 1 March, 2012)
Walden J, Slattery MC, Burt TP (1997) Use of mineral magnetic measurements to fingerprint suspended sediment sources: approaches and techniques for data analysis. J Hydrol 202:353–372
Walling DE (2005) Tracing suspended sediment sources in catchments and river systems. Sci Total Environ 344:159–184
Walling DE, Woodward JC, Nicholas AP (1993) A multi-parameter approach to fingerprinting suspended-sediment sources. In: Peters NE (ed) Tracers in hydrology. IAHS, Wallingford, p 215
Walling DE, Owens PN, Leeks GJL (1999) Fingerprinting suspended sediment sources in the catchment of the River Ouse, Yorkshire, UK. Hydrol Process 13:955–975
Acknowledgment
This study was supported by the USDA–NIFA grant no. 2007-51130-03869 (A New Approach to Sediment TMDL Watersheds in the Southern Piedmont).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Allen C. Gellis
Rights and permissions
About this article
Cite this article
Mckinley, R., Radcliffe, D. & Mukundan, R. A streamlined approach for sediment source fingerprinting in a Southern Piedmont watershed, USA. J Soils Sediments 13, 1754–1769 (2013). https://doi.org/10.1007/s11368-013-0723-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0723-1