Abstract
Purpose
The critical coagulation concentration (CCC) is considered as one of the most important parameters to evaluate the particles aggregation and sedimentation behaviors in the environment. Even though there are a few methods for its measurement, each method has its limitations especially as those methods to be applied to polydisperse systems like soil. Thus, the purpose of this research is to establish a new and more reliable method for its determination.
Materials and methods
Two types of polydisperse colloidal materials were adopted: soil and humus in the experimental studies. The dynamic light scattering technique was employed to determine the effective hydrodynamic diameter of the particles or aggregates changing with time under different pH and electrolyte concentrations of CaCl2 or KCl. In addition, the fractal dimension of aggregates was also detected with the static light scattering technique.
Results and discussion
A new aggregation rate total average aggregation rate (TAA rate) was defined firstly. We found the defined TAA rate increased linearly with the increase of electrolyte concentration at electrolyte concentrations lower than the CCC value, and the TAA rate stayed approximately constant at electrolyte concentrations higher than the CCC value; an intersection point of the two straight lines therefore could be observed, and the electrolyte concentration at the intersection point will theoretically be the CCC value. The experimental results for the two materials under different pH conditions indeed meet those theoretical predictions, which imply that the CCC value can be determined through the measurement of the TAA rate under different electrolyte concentrations. The comparison of the CCC values obtained between our new method and the widely applied stability ratio method showed that the new method was much better than the stability ratio method for the two polydisperse materials. In addition, the static light scattering measurements also showed that the variations of the fractal dimensions of the aggregates with electrolyte concentrations could be well explained by the CCC values obtained by the new method, which once again verified the applicability of the new method.
Conclusions
A new theory and a corresponding new method for the CCC estimation, which can be applied to polydisperse colloidal suspensions, were developed, and the new method has been demonstrated to be much better than the widely applied method for polydisperse materials in environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Organic, mineral, or biological colloidal particles with huge specific surface areas and usually negatively charged play crucial roles in natural environments. The aggregation behavior of the colloidal suspension in the soil controls the fate, transport, and bioavailability of nutrients and contaminants, which may result in disease spread, soil erosion, and water eutrophication (Chen 2012; Wang et al. 2012; Nadeu et al. 2011; Borda et al. 2011; Rick and Arai 2010; Zhou et al. 2011; Liang et al. 2010; Perdriala et al. 2010). Those types of colloidal particles in the environment are often polydisperse in suspension.
The critical coagulation concentration (CCC), a minimum electrolyte concentration of fast aggregation or diffusion limited cluster aggregation (DLCA) (Hiemenz 1986), is used to estimate the stability of colloidal suspension in electrolyte solution. There are a few approaches for CCC estimation:
-
1.
Approaches based on measuring the aggregation rate constant and the stability ratio (W) include direct counting of aggregates with ultramicroscope or particle counter (Matthews and Rhodes 1970; Gedan et al. 1984; Cahill et al. 1986; Pelssers et al. 1990; Broide and Cohen 1992), zeta potential measurement (Mantegazza et al. 1995), turbidity measurements (Ottewil and Shaw 1966; Reerink et al. 1954; Lichtenbelt et al. 1974a, b), static light scattering (SLS) (Lips et al. 1971; Lips and Willis 1973; Giles and Lips 1978; Zeichner and Schowalter 1979), and dynamic light scattering (DLS) measurements (Novich and Ring 1985; Virden and Berg 1992; Einarson and Berg 1993; Amal et al. 1990). However, these methods still have their own limitations. For example, the particle counting measurement could just partly investigate the determination areas of the large aggregate, while the zeta potential measurement requires the Hamaker constant for calculating the stability ratio, which, so far as we know, is usually difficult to determine accurately, even though there have been a few approaches for its estimation (Bergstrom 1997). The static/dynamic light scattering technique provides an effective way to study the aggregation kinetics of colloidal suspensions; Holthoff and cooperators (Holthoff et al. 1996; Berka and Rice 2004) simultaneously used static and dynamic light scattering techniques to determine the coagulation rate constant. However, all these experiments were based on the assumption that the aggregates’ growth was a second-order dynamic reaction process and the aggregation of singlet particles to doublets was dominant in the initial aggregation stage (Holthoff et al. 1996; Berka and Rice 2004; García-García et al. 2007). Besides, the experimental data were usually treated to fit a straight line in the initial stage of aggregation and then its slope was used to obtain the aggregation rate constant (He et al. 2008; Chen et al. 2006; Adachi et al. 2005; Hanus et al. 2001; Heidmann et al. 2005; Smith et al. 2009). Nevertheless, according to the previous experimental results (He et al. 2008; Chen et al. 2006; Adachi et al. 2005; Hanus et al. 2001; Heidmann et al. 2005; Smith et al. 2009), the growth of aggregates was actually not linear but followed a power law.
-
2.
Approaches based on measuring the sedimentation rate which was obtained from the determination of the percent transmittance of the supernatant after 24 h sedimentation (Saejiew et al. 2004; Burns et al. 1997; Frenkel et al. 1992; Hesterberg and Page 1990). The gravitational sedimentation methods have now been recognized to be complicated, cumbersome, and unreliable, and therefore have been recently replaced by the dynamic laser light scattering measurements.
Another issue is that almost all the previous experiments used relatively simple systems or monodisperse systems for the CCC measurement. The question that remains is whether the above-mentioned approaches for CCC estimation still work well for a polydisperse system, e.g., soil and humus colloidal suspension in the environment?
In this paper, we discuss a new approach for the estimation of the CCC value using complex colloidal systems of yellow earth colloids and natural humus suspensions, with the new approach being based on the measurement of the total average aggregation (TAA) rate of the colloidal particles under different electrolyte concentrations. We know that pH and ionic strength strongly influence the electrostatic repulsive force between two adjacent particles in suspension and therefore influence the stability of colloidal suspension. For variably charged particles with negative charges, e.g., humus, a decrease in pH will theoretically decrease the CCC value significantly through decreases in the surface charge as well as in the thickness of diffuse double layer. Therefore, the CCC determinations under different pH conditions for humus were carried out, and some interesting phenomena were observed.
2 Theory
According to the DLVO theory, the aggregation process of the colloidal particles mainly depends on the relative strength of van der Waals force (it is long range for colloidal-sized particles) which makes particles attract together and the electrostatic repulsive force of the diffuse double layer (DDL) that makes particles repel each other. When the van der Waals force is stronger than the electric repulsive force, or the kinetic energy of particles can overcome the repulsive potential (or activity energy), the particles will stick together to form aggregates. Similar to the collision theory of chemical reaction, the aggregation rate of the colloidal particles in suspension might be expressed as:
where t is time; f 0 is electrolyte concentration in bulk solution; v(t, f 0) (nanometers per minute) is the aggregation rate, which is defined as the growth rate of aggregate diameter; v′(t, f 0) is the cohesion rate of separate particles; d(t) is a size factor of separate particles in a suspension and the reason for introducing this factor is that the aggregation rate was defined as the growth rate of aggregate diameter; Z AB (t) is referred as the collision frequency, which is time dependent in aggregation; R is the gas constant; T is the absolute temperature; and ΔE(f 0) is the activity energy or the repulsive potential between two adjacent particles; for a given suspension under an isothermal condition, it is the function of electrolyte concentration in bulk solution.
Under isothermal conditions, the Z AB (t) value will be positively proportional to the concentration of separate particles in a suspension. Because the number of separate particles will decrease with time in aggregation process, the Z AB (t) value will decrease with time correspondingly, which will result in the decrease of v′(t, f 0). On the other hand, because the diameter of aggregated particles increases with time in aggregation, the d(t) value will increase with time correspondingly. For a given suspension under isothermal conditions, ΔE(f 0) will decrease with the increase of electrolyte concentration in bulk solution.
Figure 1a is a plot of the relationship of v(t, f 0) vs. t calculated from the experiment of soil colloid particles aggregation under the conditions of an electrolyte concentration of 1.8 mmol/L of Mg(NO3)2 and a temperature of 298 K (Zhu et al. 2009). In that experiment, ΔE(f 0) and RT were constant, so the decrease of Z AB (t) value was the sole source of decrease of v(t, f 0) based on Eq. (1).
From Fig. 1a, we can see that, in the initial 4 min of aggregation, the aggregation rate sharply decreased. Even though the aggregation rate decreased slowly after 4 min but the aggregation rate was low at this stage of aggregation. Therefore, using the rate values either in the initial 4 min or after the 4 min of aggregation, the measurement error of CCC will be large. To decrease the measurement error, here we introduce a new aggregation rate—TAA rate.
Based on Eq. (1), firstly we define an average aggregation rate from t = 0 to an arbitrary time moment t = t in the aggregation process under given conditions of temperature and electrolyte as below:
Where \( \widetilde{v}\left( {t,{f_0}} \right) \) is the average aggregation rate from t = 0 to an arbitrary time moment t = t in the aggregation process.
The plot of the relationship of \( \widetilde{v}\left( {t,{f_0}} \right) \) vs. t is shown in Fig. 1b based on the data in Fig. 1a. From this plot, we can see that, in the initial 4 min of aggregation, the decrease rate of \( \widetilde{v}\left( {t,{f_0}} \right) \) was much slower than that of v(t, f 0), and after 4 min the values of \( \widetilde{v}\left( {t,{f_0}} \right) \) were much higher than that of v(t, f 0). Therefore, it would be much better to use \( \widetilde{v}\left( {t,{f_0}} \right) \) instead of v(t, f 0) for estimating CCC values. To further decrease the measurement error, however, a TAA rate from t = 0 to a given time t = t 0 (t 0 >0) in aggregation can be defined based on the \( \widetilde{v}\left( {t,{f_0}} \right) \):
where \( {\widetilde{v}_T}\left( {{f_0}} \right) \) is the TAA rate from t = 0 to a given time t = t 0.
The following discussion will show that, just because of the defined TAA rate, a new and much more reliable approach for CCC estimation can be established.
Introducing Eq. (1) into Eq. (3) we get:
Considering Z AB (t) will be negatively related to f 0 and d(t) will be positively related to f 0, we thus suppose that, for a given t 0 value, under different f 0 conditions there might be:
Certainly, this assumption needs to be verified by experiment.
Thus Eq. (4) can be rewritten as:
From Eq. (6), we can see that, (1) at electrolyte concentrations higher than the CCC value, the ΔE(f 0) will be approximately 0, so we have \( {\widetilde{v}_{_T}}\left( {{f_0}} \right) = {\mathrm{constant}} \); (2) at electrolyte concentrations (f 0) lower than the CCC value, because ΔE~ρφ~ln(f 0/f), \( {\widetilde{v}_T}\left( {{f_0}} \right) \) will approximately linearly increase with the increase of f 0 (φ~ln(f 0/f) is based on the Boltzmann distribution equation; here φ is the potential in DDL, f is the concentration of ion in DDL, and ρ is the charge density of colloidal particle). Therefore, if we determine the \( {\widetilde{v}_T}\left( {{f_0}} \right) \) values under different electrolyte concentrations and then plot the relationship curve of \( {\widetilde{v}_T}\left( {{f_0}} \right) \) vs. f 0, a turning point could be expected, as shown in Fig. 1c, and the f 0 value at the turning point will be the CCC value.
3 Materials and methods
3.1 Materials
The colloidal particles <200 nm were extracted from a yellow earth soil and humus, respectively, adopting both sedimentation (Xiong et al. 1985) and International Humic Substances Society (IHSS) (Kuwatsuka et al. 1992) methods in suspension.
For the yellow earth soil sample, after being air-dried and sieved through a 0.25 mm mesh sieve, 50.0 g was weighed up into a 500 mL beaker, and organic matter was removed with 30 % H2O2. Then, 500 mL ultrapure water was added and the pH of the suspension was adjusted to 7.5 ± 0.1 with 0.01 mol/L KOH solution. After 15 min intensive sonication, the suspension was transferred into a 5,000 mL beaker and diluted to volume with ultrapure water. After 2 min stirring for fully mixing the suspension, standing for a given period of time calculated from the Stokes equation under given temperature (298 K) and particles size (200 nm in diameter here), the <200 nm size faction was extracted and collected by sucking out the supernatant suspension of a given depth (Xiong et al. 1985). The concentration of particles with diameter <200 nm in the collected suspension was approximately 0.95 g/L, and the concentration of KCl in bulk solution was less than10−5 mol/L. The suspension then was diluted 16 times for the DLS measurement. Here, we would like to note that the correct concentration of particles in suspension is very important in the DLS experiment. A statistically significant sample size will be critical for obtaining precise and stable mean size values of particles in DLS experiments. Too low a concentration of particles means too small a sample size in the statistical calculations of the mean size measurement, which might result in imprecise and unstable DLS data. On the other hand, if the concentration of particles is too high, the aggregation rate for the slow aggregation process with few effective collisions will reversely be fast because of the high collision frequency. In this case, the detection of the slow aggregation process becomes difficult. For each dilution operation, the suspension should be well mixed.
With respect to the extraction of humus colloid, the air-dried soil sample was first sieved through a 0.25 mm mesh sieve. Then about 500 g of the sieved soil sample was weighed into a 5,000-mL beaker and mixed well with ultrapure water to remove plant fine roots. After that, a mixed solution of 0.1 mol/L NaOH and 0.1 mol/L Na4P2O7 of 4.0 L was added into the beaker and stirred with a mixer for 4 h. After standing for 24 h, the upper part of the suspension was sucked out and collected. After the extraction was repeated three times, all the suspension collected was treated following the IHSS method to separate the humic sediment. The pH of the humics was adjusted to 9.5 ± 0.5 by adding 0.1 mol/L KOH solution, and after 15 min intensive sonication, the humus suspension was diluted with ultrapure water to volume. The suspension then was diluted 50 times to give a concentration of 78.6 mg/L for the dynamic light scattering measurement.
Figure 2 shows the size distribution of the collected soil and humus particles, showing that actual size distribution of the collected particles was 50–450 nm for both soil and humus, but the average diameters of the primary soil and humus colloidal particles were 176.0 and 169.9 nm, respectively. The difference between the theoretical calculation and the experimental measurement of particle size would mainly come from the neglecting of particle sharp in calculation.
3.2 The dynamic/static light scattering measurement
The BI-200SM multi-angle laser light scattering instrument with an autocorrelator of BI-9000AT was used in this study for the measurement of the particle mean size based on the assumption of a Gaussian distribution, and the autocorrelation function was used to treat the light intensity signal. The power of laser beam was 15 mW, polarized vertically with wavelength of 532 nm. In the measurement of intensity of scattered light vs. scattering angle for fractal dimension calculation of aggregates, the Rayleigh–Gans–Debye theory was involved. Particle size from 1 to 10,000 nm can be evaluated by this device.
The humus colloidal suspension was then adjusted to different pHs in each experiment, but only pH = 8.0 for the yellow earth colloidal suspension where it is the most stable in suspension. The results from DLS measurement showed that the average effective diameter of both humus suspensions above pH 3.0 and soil colloidal particles kept constant in 90 min, indicating a relative thermodynamic stable state of the suspension. However, at pH <3, humus suspension became unstable. For humus suspensions, experiments were carried out at pH = 3.0, 5.0, 6.5, and 8.0, respectively. The bulk electrolyte concentrations of CaCl2 and KCl were up to 200 mmol/L and 1.0 mol/L, respectively. Each suspension was prepared with a 2 min sonication before adding the electrolyte. After mixing, the information about particles size and the size distribution was recorded automatically by DLS measurement. The temperature of all measurements was kept at 297 ± 0.5 K. After the dynamic light scattering measurement, the time course of the scattered light intensity was recorded by a static SLS for all suspensions under the same aggregation conditions with DLS measurements; thus, the fractal dimension of the aggregates could be obtained.
4 Results and discussion
4.1 The growth of aggregates
The fast and slow aggregation processes of yellow earth and humus colloidal particles were measured at 2.0–150 mmol/L KCl and 0.1–10 mmol/L CaCl2 concentrations at a scattering angle of 90°. The previous study had indicated that the average effective hydrodynamic diameter determined at a certain angle by dynamic light scattering technique will be related to the true average hydrodynamic diameter through a constant factor (Derrendinger and Sposito 2000), and the Brownian motion dominated aggregation process can be expressed by the variation of the average effective hydrodynamic diameter with time, under the conditions that the autocorrelation function curve can decay smoothly to the baseline and the scattered light intensity shows no evidence of decline. Based on those mentioned, we chose the first 60 min aggregation data for the analysis.
The variation of the average effective diameter of soil and humus colloids with time at different electrolyte concentrations, and pH values are shown in Figs. 3 and 4, respectively. It can be seen that, for the soil colloidal suspensions, the diameter growth of the aggregated particles fitted straight lines at low electrolyte concentrations of 2.0 to 5.0 mmol/L KCl and 0.1 to 0.3 mmol/L CaCl2 but showed power law growth as the electrolyte concentration increased. After the electrolyte concentration increased to a certain value, the growth curves of the particles approximately overlapped.
Compared to the soil colloidal suspensions, the humus suspensions need a much higher electrolyte concentrations (both KCl and CaCl2) for aggregation under a given pH condition. It was also interesting that the aggregation phenomenon of humus suspensions in KCl solution can only be detected at pH 3.0. Above pH 3.0, the humus suspensions maintain a stable state, even if the KCl concentration was as high as 1.0 mol/L in bulk solution. To explain this phenomenon, further research will be required. One of the possible reasons may be that with the increase of pH the surface charge increased correspondingly, which could result in an increase of repulsive forces between two adjacent particles. We determined the surface charge density of the humus at pH = 7; it was −0.651 C/m2, a value approximately four times higher than that for montmorillonite. However, the surface charge density under other pH conditions could not be determined at this stage.
4.2 The average growth rate of aggregates under different electrolyte concentrations and pH values
Considering v(t, f 0) = dD(t)/dt, according to Eq. (2) we have:
where D(t) is the effective hydrodynamic diameter of aggregates at time t, and D 0 is the effective hydrodynamic diameter of the primary particles.
Using the data shown in Figs. 3 and 4, the data of \( \widetilde{v}\left( {t,{f_0}} \right) \) at different time t can be calculated from Eq. (7). Further, using the data of \( \widetilde{v}\left( {t,{f_0}} \right) \) at different time t, the fitting curve of \( \widetilde{v}\left( {t,{f_0}} \right) \) vs. t can be obtained, and the results are shown in Tables 1, 2, and 3, respectively.
From the functions shown in Tables 1, 2, and 3, we see that: (1) when the electrolyte concentration was low, the average aggregation rate was maintained constant with an increase of time in aggregation, as was the aggregation rate according to Eq. (2), which implies that the product of d(t) and Z AB (t) remained constant with time according to Eq. (1); (2) when the electrolyte concentration increased to a given value, the aggregation rate decreased with time in aggregation process, which implies that the product of d(t) and Z AB (t) decreased with time according to Eq. (1).
4.3 The TAA rate and the estimation of the CCC value
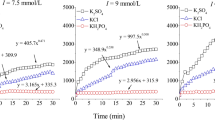
Based on the average aggregation rate functions in Tables 1, 2, and 3, the TAA rate can be easily obtained, by introducing the \( \widetilde{v}\left( {t,{f_0}} \right) \) function into Eq. (3) for a given period of time t = 0 to t = t 0. Figures 5 and 6 show the TAA rates change with electrolyte concentrations as given t 0 = 60 min for all experiments. The results showed that, when the electrolyte concentration increased, the TAA rate firstly increased approximately linearly and then stayed almost constant as theoretically predicted in Fig. 1c; thus, an intersection point emerged. Therefore, the experimental results have verified that the definite integral values of Eq. (5) under different f 0 conditions remain approximately constant.
According to the above theoretical analyses, the corresponding concentration at the turning point shown in Figs. 5 and 6 should be the CCC. The estimated CCC values of KCl and CaCl2 were 27.5 and 2.35 mmol/L, respectively, for the soil colloidal suspensions at pH = 8.0, while the CCC value of KCl was 414.0 mmol/L for the humus colloid suspensions at pH = 3.0, and the CCC values of CaCl2 increased with the increase of pH for the humus colloid suspensions; they were 15.43, 44.89, 47.16, and 56.26 mmol/L as pH = 3.0, 5.0, 6.5, and 8.0, respectively. Those results imply that the CCC value would be dependent not only on the type of colloidal particles but also on the surface charge density of colloidal particles.
The following discussions will show that our new approach for CCC estimation will be more reliable than the stability ratio approach. Currently, researchers employed the stability ratio (W) and the electrolyte concentration (f 0) to plot the logarithm chart of W vs. f 0 (Chen et al. 2006; Adachi et al. 2005; Hanus et al. 2001; Heidmann et al. 2005; Smith et al. 2009), thus the corresponding two linear regions would appear theoretically, and the intersection of the two linear regions was typically considered as the CCC. Because the aggregation rate constant k is proportional to the slope of the aggregate growth curve during the initial stage of aggregation, the CCC can be estimated by dynamic light scattering measurement. When we employed the slopes of growth curves for the soil and humus colloidal particles of the initial 2–10 min to the above CCC estimation method, the CCC values of KCl and CaCl2 obtained were 10.66 and 0.60 mmol/L, respectively, for the soil colloidal suspensions at pH = 8.0, while the CCC value of KCl was 329.4 mmol/L for the humus colloid suspensions at pH = 3.0, and the CCC values of CaCl2 for the humus colloid suspensions were 9.79, 38.55, 31.49, and 37.79 mmol/L as pH = 3.0, 5.0, 6.5, and 8.0, respectively. Comparing those results with the results by our approach, we found that all the CCC values obtained by the stability ratio approach were much less than the CCC values by the TAA rate approach. This raises the question of which approach gave correct results for the CCC estimation.
Figure 7 is the logarithm chart of the stability ratio as a function of different CaCl2 concentrations for soil and humus at pH 8.0, which will be used as an example to explain why the stability ratio approach is not suitable for such polydisperse suspensions. From Fig. 7, the CCC values obtained were 0.60 and 37.79 mmol/L CaCl2 for the soil and humus colloid suspensions, respectively. Obviously, the CCC values obtained by this approach were much lower than the results by our approach, which were 2.35 and 56.26 mmol/L CaCl2, respectively. From the aggregate growth curves shown in Figs. 3 and 4 for those two suspensions, we can conclude that the CCC values of 0.60 and 37.79 mmol/L CaCl2 were incorrect. The reason is that, at electrolyte concentrations higher than the CCC value, the aggregation rate will not change when the electrolyte concentration increases further, which means that the curves of the relationship of D(t) vs. t will overlap each other for all the growth curves of f 0 ≥CCC, and obviously the corresponding curves in Figs. 3 and 4 did not exhibit this feature at solution concentration of CaCl2 higher than 0.60 and 37.79 mmol/L for soil and humus suspensions. On the contrary, the corresponding curves in Figs. 3 and 4 showed that, at solution concentration of CaCl2 higher than 2.35 and 56.26 mmol/L for the soil and humus suspension, respectively, all the D(t) vs. t curves approximately overlapped, indicating that our approach could give reliable results for CCC estimation.
In order to reconfirm that the CCC estimation by our new approach is much more reliable than the stability ratio approach mentioned above, the static light scattering technique was used to measure the fractal dimension of the aggregates, the magnitude of which has been believed to be a sensitive index for characterizing whether the growth of aggregate to be the DLCA or the reaction limited cluster aggregation (RLCA) (Weitz et al. 1991), under the same determination conditions with dynamic light scattering measurements. Theoretically, at electrolyte concentrations lower than CCC value, the aggregation process will be the RLCA (Hiemenz 1986), and for RLCA aggregation, the fractal dimension values will decrease with the increase of electrolyte concentration. On the other hand, at electrolyte concentrations higher than the CCC value, the aggregation process will be DLCA, and for DLCA aggregation, the fractal dimension values will keep a relative low constant as the electrolyte concentration further increase. The fractal dimension values change with electrolyte concentration for the suspensions were shown in Fig. 8. It can be seen that, when the electrolyte concentrations are higher than the CCC values, the fractal dimension of the aggregates maintain a relatively low constant value for each suspension. However, as the electrolyte concentrations were below the CCC value, the fractal dimension significantly increased with the decrease of electrolyte concentration. Thus, these results again illustrated that our new approach for the estimation of the CCC value should be feasible and reliable.
Why does the stability ratio approach not give reliable results for CCC estimation? Firstly, in the stability ratio approach, the experiment data of aggregate growth were treated to fit a straight line in the initial stage of aggregation and then its slope was used to obtain the aggregation rate constant (Chen et al. 2006; Adachi et al. 2005; Hanus et al. 2001; Heidmann et al. 2005; Smith et al. 2009). Nevertheless, according to the previous experimental results, the aggregates growth was actually not linear but followed a power law (He et al. 2008; García-García et al. 2007; Chen et al. 2006; Adachi et al. 2005; Hanus et al. 2001; Heidmann et al. 2005; Smith et al. 2009; Zhu et al. 2009). Secondly, the definition of initial aggregation stage is confused, and different researchers may choose a different aggregation time as the initial aggregate stage (Adachi et al. 2005; Hanus et al. 2001; Smith et al. 2009). Thirdly, collision of the particles may lead to triplets or even much larger aggregates simultaneously rather than to only doublets even in the initial aggregation stage, so the particle growth could not conform to a double molecular growth model and usually power law growth occurred at high electrolyte concentrations.
The above three limitations for the stability ratio approach do not exist for the new approach suggested here. Therefore, our new approach is not only simple for use, but also more reliable and accurate for CCC estimation.
However, from Figs. 5 and 6, we found for some cases, although not all, the experimental \( {\widetilde{v}_T} \sim {f_0} \) relationship was not exactly linear. Thus systematic errors occurred possibly for some cases. However, the above discussion indicated that the influence of possible systematic errors in the CCC estimation might be small.
5 Conclusions
A TAA rate was firstly defined in this research. The theoretical analysis indicated that, at electrolyte concentrations lower than the CCC value, the TAA rate will increase linearly with the increase of electrolyte concentration, and at electrolyte concentrations lower than the CCC value, the TAA rate will remain constant as the electrolyte concentration increases. Therefore, a turning point may theoretically be exhibited, and the electrolyte concentration at the turning point will be the CCC value. Our experimental results indeed showed that as the electrolyte concentration increased the TAA rate firstly increased approximately linearly and then became approximately constant as the electrolyte concentration increased further, and a distinct turning point was exhibited. Therefore, a new method for the estimation of CCC values which can be applied to polydisperse colloidal suspensions was developed. The studies demonstrated that our new approach for the CCC value estimation was much more reliable than the stability ratio approach.
References
Adachi Y, Koga S, Kobayashi M, Inada M (2005) Study of colloidal stability of allophane dispersion by dynamic light scattering. Colloids Surf A Physicochem Eng Asp 265(1/3):149–154
Amal R, Coury JR, Raper JA, Walsh WP, Waite TD (1990) Structure and kinetics of aggregating colloidal hematite. Colloids Surf 46(1):1–19
Bergstrom L (1997) Hamaker constants of inorganic materials. Adv Colloid Interface 70:125–169
Berka M, Rice J (2004) Absolute aggregation rate constants in aggregation of kaolinite measured by simultaneous static and dynamic light scattering. Langmuir 20(15):6152–6157
Borda T, Celi L, Zavattaro L, Sacco D, Barberis E (2011) Effect of agronomic management on risk of suspended solids and phosphorus losses from soil to waters. J Soils Sediments 11:440–451
Broide ML, Cohen RJ (1992) Measurements of cluster-size distributions arising in salt-induced aggregation of polystyrene microspheres. J Colloid Interface Sci 153(2):493–508
Burns JL, Yan Y, Jameson GJ, Biggs S (1997) A light scattering study of the fractal aggregation behavior of a model colloidal system. Langmuir 13(24):6413–6420
Cahill J, Cummins PG, Staples EJ, Thompson L (1986) Aggregate size distribution in flocculating dispersions. Colloids Surf 18(2/4):189–205
Chen G (2012) S. typhimurium and E. coli O157:H7 retention and transport in agricultural soil during irrigation practices. Eur J Soil Sci 63:239–248
Chen KL, Mylon SE, Elimelech M (2006) Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ Sci Technol 40(5):1516–1523
Derrendinger L, Sposito G (2000) Flocculation kinetics and cluster morphology in illite/NaCl suspensions. J Colloid Interface Sci 222(1):1–11
Nadeu E, de Vente J, Martínez-Mena M, Boix-Fayos C (2011) Exploring particle size distribution and organic carbon pools mobilized by different erosion processes at the catchment scale. J Soils Sediments 11:667–678
Einarson MB, Berg JC (1993) Electrosteric stabilization of colloidal latex dispersions. J Colloid Interface Sci 155(1):165–172
Frenkel H, Fey MV, Levy GJ (1992) Organic and inorganic anion effects on reference and soil clay critical flocculation concentration. Soil Sci Soc Am J 56:1762–1766
García-García S, Wold S, Jonsson M (2007) Kinetic determination of critical coagulation concentrations for sodium- and calcium-montmorillonite colloids in NaCl and CaCl2 aqueous solutions. J Colloid Interface Sci 315(2):512–519
Gedan H, Lichtenfeld H, Sonntag H, Krug H (1984) Rapid coagulation of polystyrene particles investigated by single-particle laser-light scattering. J Colloid Surf 11(1/2):199–207
Giles D, Lips A (1978) Light-scattering method for study of close range structure in coagulating dispersions of equal sized spherical-particles. J Chem Soc Faraday Trans 1 74:733–744
Hanus LH, Hartzler RU, Wagner NJ (2001) Electrolyte-induced aggregation of acrylic latex. 1. Dilute particle concentrations. Langmuir 17(11):3136–3147
He YT, Wan JM, Tokunaga T (2008) Kinetic stability of hematite nanoparticles: the effect of particle size. J Nanopart Res 10:321–332
Heidmann I, Christl I, Kretzschmar R (2005) Aggregation kinetics of kaolinite-fulvic acid colloids as affected by the sorption of Cu and Pb. Environ Sci Technol 39(3):807–813
Hiemenz PC (1986) Principles of colloid and surface chemistry, 2nd edn. Marcel Dekker: CRC, New York
Hesterberg D, Page AL (1990) Flocculation series test yielding time-invariant critical coagulation concentrations of sodium illite. Soil Sci Soc Am J 54:729–735
Holthoff H, Egelhaaf SU, Borkovec M, Schurtenberger P, Sticher H (1996) Coagulation rate measurements of colloidal particles by simultaneous static and dynamic light scattering. Langmuir 12(23):5541–5549
Kuwatsuka S, Watanabe A, Itoh K, Arai S (1992) Comparison of 2 methods of preparation of humic and fulvic-acids, IHSS method and NAGOYA method. Soil Sci Plant Nutr 38(1):23–30
Liang XQ, Liu J, Chen YX, Li H, Ye YS, Nie Z, Su MM, Xu ZH (2010) Effect of pH on the release of soil colloidal phosphorus. J Soils Sediments 10:1548–1556
Lichtenbelt JW, Pathmama C, Wiersema PH (1974a) Rapid coagulation of polystyrene latex in a stopped-flow spectrophotometer. J Colloid Interface Sci 49(2):281–285
Lichtenbelt JW, Ras HJM, Wiersema PH (1974b) Turbidity of coagulating lyophobic sols. J Colloid Interface Sci 46(3):522–527
Lips A, Smart C, Willis E (1971) Light scattering studies on a coagulating polystyrene latex. J Chem Soc Faraday Trans 67(586):2979–2988
Lips A, Willis E (1973) Low-angle light-scattering technique for study of coagulation. J Chem Soc Faraday Trans 69(7):1226–1236
Mantegazza F, Giardini ME, Degiorgio V, Asnaghi D, Giglio M (1995) Electric birefringence study of reaction-limited colloidal aggregation. J Colloid Interface Sci 170(1):50–56
Matthews BA, Rhodes CT (1970) Studies of coagulation kinetics of mixed suspensions. J Colloid Interface Sci 32(2):332–338
Novich BE, Ring TA (1985) Photon-correlation spectroscopy of a coagulating suspension of illite platelets. J Chem Soc Faraday Trans 1 81:1455–1457
Ottewil RH, Shaw JN (1966) Stability of mono-disperse polystyrene latex dispersions of various sizes. Discuss Faraday Soc 42:154–163
Pelssers EGM, Cohen Stuart MA, Fleer GJ (1990) Single particle optical (SPOS): II. Hydrodynamic forces and application to aggregating dispersions. J Colloid Interface Sci 137(2):362–372
Reerink H, Th J, Overbeek G (1954) The rate of coagulation as a measure of the stability of silver iodide sols. Discuss Faraday Soc 18:74–84
Perdriala N, Perdriala JN, Delphinb JE, Elsassa F, Liewig N (2010) Temporal and spatial monitoring of mobile nanoparticles in a vineyard soil: evidence of nanoaggregate formation. Eur J Soil Sci 61:456–468
Rick AR, Arai Y (2010) Role of natural nanoparticles in phosphorus transport processes in ultisols. Soil Sci Soc Am J 75:335–347
Saejiew A, Grunberger O, Arunin S, Favre F, Tessier D, Boivin P (2004) Critical coagulation concentration of paddy soil clays in sodium–ferrous iron electrolyte. Soil Sci Soc Am J 68:789–794
Smith B, Wepasnick K, Schrote KE, Bertele AH, Ball WP, O’Melia C, Fairbrother DH (2009) Colloidal properties of aqueous suspensions of acid-treated, multi-walled carbon nanotubes. Environ Sci Technol 43(3):819–825
Virden JW, Berg JC (1992) The use photon-correlation spectroscopy for estimating the rate-constant for doublet formation in an aggregating colloidal dispersion. J Colloid Interface Sci 149(2):528–535
Wang DJ, Bradford CA, Paradelo M, Peijnenburg WJGM, Zhou DM (2012) Facilitated transport of copper with hydroxyapatite nanoparticles in saturated sand. Soil Sci Soc Am J 76:375–388
Weitz DA, Lin MY, Linday HM (1991) Universality laws in coagulation. Chemom Intell Lab 10(1/2):133–140
Xiong Y, Chen JF, Zhang JS (1985) Soil Colloid (Vol 2): methods for soil colloid research. Science, Beijing, pp 7–31, in Chinese
Zeichner GR, Schowalter WR (1979) Effects of hydrodynamic and colloidal forces on the coagulation of dispersions. J Colloid Interface Sci 71(2):237–253
Zhou DM, Wang DJ, Cang L, Hao XZ, Chu LY (2011) Transport and re-entrainment of soil colloids in saturated packed column: effects of pH and ionic strength. J Soils Sediments 11:491–503
Zhu HL, Li B, Xiong HL (2009) Dynamic light scattering study on the aggregation kinetics of soil colloidal particles in different electrolyte systems. Acta Phys Chim Sin 25:1225–1231, in Chinese
Acknowledgments
This work was supported by the National Natural Science Foundation of China (40971146), the National Basic Research Program of China (grant no. 2010CB134511), and the Natural Science Foundation Project of CQ CSTC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ying Ouyang
Rights and permissions
About this article
Cite this article
Jia, M., Li, H., Zhu, H. et al. An approach for the critical coagulation concentration estimation of polydisperse colloidal suspensions of soil and humus. J Soils Sediments 13, 325–335 (2013). https://doi.org/10.1007/s11368-012-0608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0608-8