Abstract
Purpose
Attachment of bacteria on soil particles is ubiquitous and governs the transformation of nutrients and degradation of pollutants in soil and associated environments. The nature on the binding of bacteria by soil particles has remained unclear. The objectives of the present study were to investigate the adsorption of Pseudomonas putida on particle size fractions from an Ultisol as influenced by solution chemistry and organic matter.
Materials and methods
An Ultisol was collected from a forest land. One part of the soil was oxidized by H2O2 to remove organic matter. The other part was without such oxidization. Each part of the soil was separated into four size classes: coarse sand (200–2,000 μm), fine sand (20–200 μm), silt (2–20 μm), and clay (<2 μm). The corresponding organic matter-left fractions (OM-left) and organic matter-removed (OM-removed) fractions were obtained. Meanwhile, P. putida was grown in beef extract peptone medium at 28°C to the stationary growth phase. Cells were harvested by centrifugation, washed in deionized water, and resuspended in 10 mM acetate buffer (pH 5.5). Batch experiments were carried out to analyze equilibrium adsorption of bacteria and the effects of pH and electrolyte concentrations on bacterial adsorption.
Results and discussion
The adsorption isotherms of P. putida on the size fractions conformed to the Langmuir equation. The maximum amount of P. putida adsorbed by clay fraction was 4.3 and 62.3 times as great as that by silt and sand fractions, respectively. The number of P. putida attached to OM-removed fractions was significantly larger than that to OM-left fractions. P. putida adsorption on OM-left fractions with increasing pH from 4.0 to 9.0 was reduced by 44.0–78.8%. At the same time, further decreases (7.5–21.1%) were observed in the adsorption for OM-removed ones. Mg2+ was much more effective than Na+ in enhancing P. putida attachment. Na+ and Mg2+ ions more strongly promoted P. putida adsorption on OM-left fractions than on OM-removed fractions.
Conclusions
Clay fraction presented the largest adsorption capacity for bacteria, followed by soil silt and sand fractions. As compared with silt and sand fractions, it is likely that the greater amounts of bacteria adsorbed by clay fractions were attributed to their higher content of clay minerals and iron oxides. Soil organic matter plays a suppressive role in the interfacial processes occurring during the initial bacterial attachment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bacteria are ubiquitous in soils, sediments, and associated environments (Whitman et al. 1998). In soils, the bacterial count is approximately 1010 cells per gram soil (Torsvik and Øvreås 2002; Young and Crawford 2004). Most of these bacteria are attached to soil particles, including soil mineral fractions and organic matter (Nannipieri et al. 2003). Interactions of bacteria with soil particles have enormous impacts on mineral weathering (Kim et al. 2004), aggregation formation, decomposition of organic matter and nutrient release, transformation and ultimate fate of contaminants (Huang et al. 2005), and transport of pathogens (Morrow et al. 2005).
Studies have shown that the attachment of bacteria to soil/sediment particles is affected by the particle sizes. Fontes et al. (1991) have observed that the percent of the strain W6, a Gram-negative coccus, retained by fine-grained sand (330–400 μm) can be as high as 85.5% at an ionic strength of 0.89 mM. Guber et al. (2007) have reported that the attachment of fecal coliforms to soil silt particles (2–50 μm) and clay particles (<2 μm) in the absence of manure colloids is much higher than that to sand particles (62.5–500 μm) without organic coating, whereas there is no notable difference of the attachment between coated sand and silt or clay particles. Jeng et al. (2005) have found that approximately 18% of attached Escherichia coli cells are associated with the clay fraction (0.45–5 μm) in fresh stormwater, 80% with the silt fraction (5–53 μm), and 2% with the sand fraction (>53 μm). Soupir et al. (2010) have observed that at least 60% of attached E. coli and enterococci are associated with suspended particles within an 8–62 μm particle size category, and the remaining bacteria are attached to particles with a size larger than 63 μm. In their study, Bengtsson and Ekere (2001) used batch experiments of the attachment of indigenous groundwater bacteria to six size fractions and two mixtures of a sandy soil. They have found that there are no linear correlations between the partitioning coefficient and particle size or specific surface area. Previous studies have shown that the majority of bacteria are attached to soil fine size fractions. However, few studies have attempted to give sufficient explanations with regard to these phenomena. The underlying mechanisms of the attachment to soil fractions are not yet fully understood.
Recently, many works have been devoted to the clarification of the binding mechanisms of bacteria to clay minerals that serve as the major components of soil fine size fractions. For example, Pseudomonas putida cells preferred to be attached to goethite, followed by kaolinite and montmorillonite. In addition, the amount of attachment decreased with the increase of pH from 3.0 to 10.0 or the decrease of MgCl2 or NaCl concentrations ranging from 50 to 0 mM, suggesting an important role of electrostatic force among bacteria–clay mineral interactions (Jiang et al. 2007). Additionally, hydrogen bonding also contributed to the adsorption of P. putida on these clay minerals (Rong et al. 2008, 2010). The significant suppressive effects of low-molecular-weight organic ligands and phosphate on P. putida attachment to kaolinite, montmorillonite, and goethite were ascribed to the increased negative charges of adsorbed ligands and the competition of ligands for binding sites (Wu et al. 2011). The attenuated total reflectance Fourier transform infrared spectroscopy revealed the formation of P-OFe and COOH bond between phosphate and carboxylate groups on the surface of Shewanella oneidensis, Pseudomonas aeruginonsa, and Bacillus subtilis and hematite (Parikh and Chorover 2006). A series of studies has been carried out on Shewanella alga BrY cells attachment in order to understand the adhesion of dissimilatory Fe(III)-reducing bacteria to Fe(III) minerals. The largest adsorption of S. alga BrY cells on hematite possessing the smallest specific surface area among hematite, goethite, and hydrous ferric oxide (HFO) suggests that the adhesion of bacteria seems to be independent of the specific surface area (Das and Caccavo 2001). Analyses of cell surface chemical components, hydrophobicity, and charges have indicated that the initial, reversible adhesion of S. alga BrY to HFO is mediated by hydrophobic surface proteins (Caccavo et al. 1997; Das and Caccavo 2000, 2001). Meanwhile, the adhesion system involves multiple proteins, including but not limited to 50-, 60-, and 31-kDa peptides, that served as iron binding sites (Caccavo 1999). The flagellum of S. alga BrY cell acts as an adhesin to different ferric oxides, and the 59-kDa flagellin corresponds in size to the peptide of 60 kDa (Caccavo and Das 2002).
Although these publications have given initial insights into the mechanisms of the adsorption of bacteria on clay minerals, it is still not an easy way to extrapolate the mechanisms from pure minerals to soil particles which are extremely complex in their constitution. Furthermore, it is also difficult to fully comprehend whether or not mineral constituents and organic matter in soils regulates the attachment of bacteria to different soil particle size fractions. The aim of the present study was to test how P. putida adsorption responded to changes in the content of organic matter in soil particles of different sizes. The effects of pH and electrolytes on adsorption were also investigated.
2 Materials and methods
2.1 Soil particle size fractions
A Red soil (Ultisol) was collected from a depth of 0–20 cm in a forest land in Wuhan, Hubei province, China. After removing the organic residue, the soil was rinsed in deionized water and dispersed by adding 0.5 M NaOH solution dropwise to pH 7–8 together with sonication. The coarse sand fraction (200–2,000 μm) was collected by wet sieving, while fine sand fraction (20–200 μm), silt fraction (2–20 μm), and clay fraction (<2 μm) were separated by sedimentation after dispersion. The clay suspension was flocculated by the addition of CaCl2 solution and washed with deionized water and ethanol to remove Cl−1 ions. These soil samples were organic matter-left (OM-left) fractions. To obtain organic matter-removed (OM-removed) fractions, the soil suspension was treated by H2O2 (30%) with intermittent agitation to oxidize organic matter. The oxidation process was repeated until the suspension was free of air bubbles. The OM-removed fractions were separated to four size fractions following the same wet sieving and sedimentation procedures described above. All the soil particle size fractions prepared were oven-dried at 60°C.
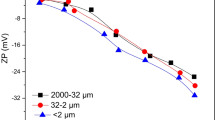
The mineralogy of the soil particle size fractions was determined by X-ray diffraction using CuKα radiation. Organic matter (OM), free iron oxides, point of zero charge (PZC), zeta potentials (ζ), and specific surface area were analyzed by K2Cr2O7 digestion, dithionite–citrate method, acid-base potentiometric titration (Xiong 1985), Zeta Potential Analyzer (ZetaPlus 90, Brookhaven Instruments Corporation) and N2 adsorption (Beijing Analytical Instrument Company), respectively. Some properties of the soil particle size fractions are listed in Table 1.
2.2 Bacteria
P. putida is a Gram-negative aerobic species ubiquitously found in soils. P. putida was grown in beef extract peptone medium at 28°C and 200 rpm to a stationary (21 h) growth phase. Cells were also harvested by centrifugation at a stationary growth phase, and then washed several times in deionized water to remove residual medium. The washed cells were suspended in 10 mM acetate buffer (pH 5.5). Afterwards, the cell concentration of the washed cell suspension was determined by spread plate method. Briefly, serial decimal dilutions of the washed cells were made using sterile acetate buffer. One-tenth milliliter of the dilutions was spread uniformly over the surface of a plate containing 15 mL of beef extract peptone medium. The plates were incubated for 36 h at 25°C. At the end of the incubation period, colonies were counted and the number of colony-forming units per milliliter was calculated. The final cell concentration used for adsorption assays was 2 × 108 cells mL−1. PZC and ζ of P. putida were measured by the same methods as those used for soil particles.
2.3 Adsorption of bacteria by soil particle size fractions
In a 50-mL centrifuge tube, 1,200 mg of sand (90 mg of silt or 35 mg of clay fraction) was mixed with 20 mL of acetate buffer (pH 5.5) containing 0~4 × 109 cells of P. putida. The bacteria–soil particle mixtures were agitated at 200 rpm and 25°C for 1 h. Preliminary experiments indicated that equilibrium time for these systems was less than 1 h. Adsorption was conducted in the range of pH from 4.0 to 9.0, in which the corresponding aforementioned amounts of soil particle size fractions and 20 mL of acetate buffer (pH 5.5) containing 3 × 109 cells of bacteria were used. Similar experiments were carried out in acetate buffer (pH 5.5) with the presence of 0–100 mM NaCl or 0–50 mM MgCl2.
Separation of unattached bacteria from the fraction containing soil particle size fractions and attached bacteria was accomplished by injecting 3 mL of sucrose solution (60% by weight) into the bottom of the soil particle–bacteria suspensions, after which the mixtures were centrifuged at 3,202×g for 15 min. The unattached bacteria were transferred to the plastic tube and 2 mL of 6.25 M NaOH was added, and then 10 mM of acetate buffer (pH 5.5) was supplied to yield a final volume of 25 mL. The mixture was placed in water bath at 100°C for 30 min. After cooling action, the mixture was filtered and the protein in the filtrate was determined by the method developed by Bradford (1976). Meanwhile, the content of bacterial protein was converted into the number of bacterial cells according to the linear correlation between them (Jiang et al. 2007). The amount of adsorbed bacteria was calculated by subtracting the amount of unattached bacteria from the total amount of bacteria.
2.4 Statistical analysis
The statistical analysis of adhesion data was performed by analysis of variance and p ≤ 0.05 was considered significant.
3 Results
3.1 Equilibrium adsorption of P. putida
The concentration-dependent adsorptions for P. putida on soil particles of different sizes are shown in Fig. 1. The adsorption data fitted well to a Langmuir equation and the parameters are given in Table 2. On average, the maximum amount of P. putida adsorbed by clay fraction was 4.3 and 62.3 times as great as that by silt and sand fractions, respectively. Overall bacterial adsorption on OM-removed clay, silt, and sand fractions was significantly larger than that on the corresponding OM-left fractions. The estimated affinity constant (K) for OM-removed fractions were 1.8–2.5 times as much as those for OM-left fractions, indicating that P. putida cells are adsorbed more tightly by OM-removed fractions than by OM-left fractions. The larger K values for silt fractions than for clay fractions may suggest higher adsorption affinity of bacteria toward silt fractions regardless of OM-left and OM-removed.
3.2 Effect of pH
As shown in Fig. 2, the adsorption of P. putida on OM-left coarse sand, fine sand, silt, and clay fractions with the increase of pH values from 4.0 to 9.0 was reduced by 78.8%, 69.2%, 63.1%, and 44.0%, respectively. Further decreases (7.5–21.1%) in bacterial adsorption were observed for OM-removed fractions with increasing pH values. These results showed that less bacterial cells were adsorbed by soil particles in neutral and alkaline soils.
3.3 Effect of electrolyte
The adsorption of bacteria on soil particles increased significantly (p ≤ 0.05) with increasing Na+ concentrations from 0 to 60 mM and remained constant when Na+ concentration was higher than 60 mM (Fig. 3). In the case of the OM-left fractions, P. putida cells attached to coarse and fine sands increased by 6.6 and 3.9 times with the increase of Na+ concentrations from 0 to 60 mM, while that by silt and clay fractions was 2.5 and 1.4 times, respectively. In contrast to OM-left fractions, the binding of bacterial cells to OM-removed coarse and fine sands was enhanced by 1.8- and 1.3-fold, while that to OM-removed silt and clay was 0.9- and 0.8-fold, respectively. Similar patterns were found for the influence of Mg2+ on bacterial adsorption by soil particles of different sizes (Fig. 4). Mg2+ was much more effective than Na+ in promoting P. putida adsorption. Both Na+ and Mg2+ ions enhanced more strongly the bacterial attachment to OM-left fractions than to OM-removed fractions.
4 Discussion
Our results indicated that P. putida adsorption on soil particle size fractions was notably affected by the surface characteristics of particles, including size, mineral composition, and content of organic matter. The largest number of bacteria adsorbed by clay fraction was 4.3 and 62.3 times as large as that by silt and sand fractions, respectively. The mineral compositions of clay fraction are illite, kaolinite, vermiculite, and iron oxides (see Table 1). The major mineral is quartz for silt fraction and the minorities are clay minerals and iron oxides. Sand fraction basically consists of quartz. Previous studies showed that clay minerals and iron oxides have greater abilities than quartz in bacterial adsorption. The maximum amount of P. putida bound to kaolinite was 4.1 × 1010 cells g−1 at pH 7.0 and that to goethite was 4.8 × 1010 cells g−1 (Jiang et al. 2007). The highest number of Bacillus polymyxa adsorbed by hematite was approximately 2 × 109 cells g−1 at pH 6.0, while that by quartz was about 1.6 × 109 cells g−1 (Shashikala and Raichur 2002). The cells of Paenibacillus polymyxa were shown to be preferentially adsorbed by hematite than by quartz (Deo et al. 2001). In contrast to silt and sand fractions, the higher content of clay minerals and iron oxides in clay fractions are considered to be responsible for their larger capacities in bacterial adsoprtion. It is interesting to note that the affinity of bacteria to soil particle size fractions followed the sequence of silt > clay > fine sand > coarse sand. This phenomenon may indicate that bacteria are apt to adhere to silt size particles at the initial attachment. However, the mineral composition of silt fractions may limit their capacity for bacterial adsorption.
The data in this study also showed that OM-removed fractions had greater adsorption capacity and affinity for bacteria than OM-left fractions. Therefore, organic matter in red soils was found to be unfavorable to bacterial attachment. Our findings seem to be in disagreement with the previous observations about the spatial distribution of bacteria in soil. Gray et al. (1968) revealed that 60% of the soil bacteria were located on organic particles in a sand soil, although these particles contributed only 15% to the total particle surface. The discrepancy may be explained by assuming that most microorganisms attached to organic matter are resulted from growth in situ rather than from the soil water. Upon surface colonization, soil microorganisms most likely form microcolonies or biofilms. High nutrient availability and water-holding capacity may provide these attached bacterial populations with a favorable microenvironment to live.
In the present study, three reasons may explain the inhibition of organic matter on bacterial attachment to soil particle size fractions. Firstly, free iron oxides coated by organic matter are suspected to be exposed after the removal of organic matter. The content of free iron oxides in OM-removed fractions was 50.47 g kg−1 in clay, 10.73 g kg−1 in silt, and 1.67 g kg−1 in fine sand (see Table 1). Fe–oxyhydroxide coating has been reported to enhance significantly the adsorption of B. subtilis and Pseudomonas mendocina on quartz surface (Ams et al. 2004). Fe(III)-coated quartz presented 100% adsorption of the added bacteria, while that by quartz was less than 75% at an ionic strength ranging from 1.16 to 57.8 mM (Mills et al. 1994). It is likely that the exposed iron oxides provide new sites for bacterial binding. Secondly, organic matter coating led to an alteration in the surface charge of soil fractions and then reduced bacterial attachment. OM-left fractions displayed more negative charges than OM-removed fractions, which was demonstrated by the reduced PZCs (0.43–0.82 units) and zeta potentials (3.32–5.66 mv) for OM-left particles (see Table 1). It is reasonable that OM-left fractions may present much stronger electrostatic repulsion with bacteria than OM-removed fractions. Further evidence from our results was that both monovalent and divalent salts displayed greater abilities in promoting bacterial attachment to OM-left fractions than to OM-removed fractions. Therefore, the presence of organic matter may increase the electrostatic repulsion between bacteria and soil size fractions, resulting in reduced bacterial adsorption. Thirdly, the occurrence of organic matter on soil fractions may increase steric hindrance between bacteria and soil fractions. Reduced colloidal or bacterial adhesions were also observed in the presence of humic acid through increased steric repulsion forces (Bob and Walker 2001; Mosley and Hunter 2003; Foppen et al. 2008). Based on these facts, the interfacial processes of the initial bacterial attachment in soils may be constrained by organic matter coating. Although soil particles are extraordinarily complex in their constitution, our work still adequately clarifies the effect of organic matter on bacterial attachment to soil fractions.
5 Conclusions
Clay fraction displayed the largest adsorption capacity for bacteria, followed by soil silt and sand fractions. The silt fractions showed the highest affinity for bacteria among all soil size fractions. The larger amounts of bacteria adsorbed by clay fractions were probably assigned to their higher content of clay minerals and iron oxides. Soil organic matter plays a suppressive role in the interfacial processes of the initial bacterial attachment to soil particles.
References
Ams D, Fein JB, Dong H, Maurice PA (2004) Experimental measurements of the adsorption of Bacillus subtilis and Pseudomonas mendocina onto Fe–oxyhydroxide-coated and uncoated quartz grains. Geomicrobiol J 21:511–519
Bengtsson G, Ekere L (2001) Predicting sorption of groundwater bacteria from size distribution, surface area, and magnetic susceptibility of soil particles. Water Resour Res 37:1795–1812
Bob MM, Walker HW (2001) Effect of natural organic coatings on the polymer-induced coagulation of colloidal particles. Colloids Surf A 177:215–222
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Caccavo F (1999) Protein-mediated adhesion of the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY to hydrous ferric oxide. Appl Environ Microbiol 65:5017–5022
Caccavo F, Das A (2002) Adhesion of dissimilatory Fe(III)-reducing bacteria to Fe(III) minerals. Geomicrobiol J 19:161–177
Caccavo F, Schamberger PC, Keiding K, Nielsen PH (1997) Role of hydrophobicity in adhesion of the dissimilatory Fe(III)-reducing bacterium Shewanella agla to amorphous Fe(III) oxide. Appl Environ Microbiol 63:3837–3843
Das A, Caccavo F (2000) Dissimilatory Fe(III) oxide reduction by Shewanella alga BrY requires adhesion. Curr Microbiol 40:344–347
Das A, Caccavo F (2001) Adhesion of the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY to crystalline Fe(III) oxides. Curr Microbiol 42:151–154
Deo N, Natarajan KA, Somasundaran P (2001) Mechanisms of adhesion of Paenibacillus polymyxa onto hematite, corundum and quartz. Int J Miner Process 62:27–39
Fontes DE, Mills AL, Hornberger GM, Herman JS (1991) Physical and chemical factors influencing transport of microorganisms through porous media. Appl Environ Microbiol 57:2473–2481
Foppen JW, Liem Y, Schijven J (2008) Effect of humic acid on the attachment of Escherichia coli in columns of goethite-coated sand. Water Res 42:211–219
Gray TRG, Baxby P, Hill LR, Goodfellow M (1968) Direct observation of bacteria in soil. In: Gray TRG, Parkinson D (eds) The ecology of soil bacteria. University of Toronto Press, Toronto, Canada, pp 171–197
Guber AK, Pachepsky YA, Shelton DR, Yu O (2007) Effect of bovine manure on fecal coliform attachment to soil and soil particles of different sizes. Appl Environ Microbiol 73:3363–3370
Huang PM, Wang MK, Chiu CY (2005) Soil mineral–organic matter–microbe interactions: impacts on biogeochemical processes and biodiversity in soils. Pedobiologia 49:609–635
Jeng HC, England AJ, Bradford HB (2005) Indicator organisms associated with stormwater suspended particles and estuarine sediment. J Environ Sci Heal 40:779–791
Jiang D, Huang Q, Cai P, Rong X, Chen W (2007) Adsorption of Pseudomonas putida on clay minerals and iron oxide. Colloids Surf B Biointerfaces 54:217–221
Kim J, Dong H, Seabaugh J, Newell SW, Eberl DD (2004) Role of microbes in the smectite-to-illite reaction. Science 303:830–832
Mills A, Herman JS, Hornberger GM, Dejesús TH (1994) Effect of solution ionic strength and iron coatings on mineral grains on the sorption of bacterial cells to quartz sand. Appl Environ Microbiol 60:3300–3306
Morrow JB, Stratton R, Yang HH, Smets BF, Grasso D (2005) Macro- and nanoscale observations of adhesive behavior for several E. coli strains (O157:H7 and environmental isolates) on mineral surfaces. Environ Sci Technol 39:6395–6404
Mosley LM, Hunter KA (2003) Forces between colloid particles in natural waters. Environ Sci Technol 37:3303–3308
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Parikh S, Chorover J (2006) ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide. Langmuir 22:8492–8500
Rong X, Huang Q, He X, Chen H, Cai P, Liang W (2008) Interaction of Pseudomonas putida with kaolinite and montmorillonite: a combination study by equilibrium adsorption, ITC, SEM and FTIR. Colloids Surf B Biointerfaces 64:49–55
Rong X, Chen W, Huang Q, Cai P, Liang W (2010) Pseudomonas putida adhesion to goethite: studied by equilibrium adsorption, SEM, FTIR and ITC. Colloids Surf B Biointerfaces 80:79–85
Shashikala AR, Raichur AM (2002) Role of interfacial phenomena in determining adsorption of Bacillus polymyxa onto hematite and quartz. Colloids Surf B Biointerfaces 24:11–20
Soupir ML, Mostaghimi S, Dillaha T (2010) Attachment of Escherichia coli and enterococci to particles in runoff. J Environ Qual 39:1019–1027
Torsvik V, Øvreås L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5:240–245
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95:6578–6583
Wu H, Jiang D, Cai P, Rong X, Huang Q (2011) Effects of low-molecular-weight organic ligands and phosphate on adsorption of Pseudomonas putida by clay minerals and iron oxide. Colloids Surf B Biointerfaces 82:147–151
Xiong Y (1985) Soil colloids, 2nd vol. Science, Beijing
Young IM, Crawford JW (2004) Interactions and self-organization in the soil–microbe complex. Science 304:1634–1637
Acknowledgments
The study was financially supported by the National Natural Science Foundation of China (project no. 40825002) and the National High Technology Research & Development Program of China (“863” Program, 2009AA06Z302).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chengrong Chen
H. Wu and D. Jiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, H., Jiang, D., Cai, P. et al. Adsorption of Pseudomonas putida on soil particle size fractions: effects of solution chemistry and organic matter. J Soils Sediments 12, 143–149 (2012). https://doi.org/10.1007/s11368-011-0441-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0441-5