Abstract
Purpose
Spartina alterniflora widely invades coastal wetland in China and might change nitrification in sediment. Both ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) are involved in nitrification in this environment. The objective of this study was to examine the effect of S. alterniflora invasion on abundance and composition of AOA and AOB.
Materials and methods
The abundance and composition of AOA, AOB, and total bacteria in the sediments from S. alterniflora-invaded native mangrove vegetated and unvegetated zones at two depths of 0–5 cm (O) and 5–20 cm (R) were investigated using quantitative real-time polymerase chain reaction and denaturing gradient gel electrophoresis. Relationships were also determined between sediment properties and the AOB and AOA population sizes.
Results and discussion
Compared with native mangrove vegetated zone, the archaeal amoA gene abundance was reduced by 11.3-fold (O) and 46.1-fold (R), but the bacterial amoA gene abundance was increased by 9.8-fold (O) and 1.8-fold (R), respectively, in the S. alterniflora-invaded zone. The AOA abundance was always higher than AOB, especially in the native mangrove zone. Both AOA and AOB population sizes in the upper layer (O) were bigger than those in the deeper layer (R). Little difference was found in the AOB community composition among different zones, while diversity of AOA community was increased by the presence of S. alterniflora.
Conclusions
This study demonstrated that the S. alterniflora invasion affects the abundance of both AOA and AOB, but only affects the community composition of AOA in the tidal sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrification is ubiquitous in the environment and plays a central role in the global nitrogen (N) cycle (Francis et al. 2005; Leininger et al. 2006; Prosser and Embley 2002). Ammonia oxidation, the first step in nitrification, is the reaction of NH +4 to NH2OH catalyzed by ammonia monooxygenase encoded by the amoA gene of ammonia-oxidizing bacteria (AOB) (Kowalchuk et al. 1999; Kowalchuk and Stephen 2001; Rotthauwe et al. 1997). Recently, studies have shown that ammonia-oxidizing archaea (AOA) are also involved in nitrification and often show higher abundance than AOB (Abell et al. 2010; Leininger et al. 2006). AOA may play an important role in N cycling on Earth (Könneke et al. 2005; Zhang et al. 2010). The amoA gene is commonly used as a molecular marker of both AOA and AOB for abundance and community diversity studies (Abell et al. 2010; Francis et al. 2007).
Nitrification is of particular significance in estuarine sediments because coupled nitrification/denitrification can remove a substantial percentage (10% to 80%) of anthropogenic N input into estuaries (Beman and Francis 2006; Seitzinger 1988). Studies have shown that the AOA and AOB communities are sensitive to many environmental factors, such as pH, carbon, nitrogen, sulfur, oxygen, temperature, and sediment depth. Vegetation is also an important factor controlling N removal in wetlands directly and indirectly (Ruiz-Rueda et al. 2009) and close interactions are observed between plants and belowground microbial communities (Burke et al. 2002; Kowalchuk et al. 2002).
Exotic plants threaten the integrity of agricultural and natural systems throughout the world (Callaway and Aschehoug 2000). Invasive plants can change the interaction between soil and plants (Callaway and Ridenour 2004; Klironomos 2002; Kourtev et al. 2002) and affect nutrient cycling through differences in rates of rhizodeposition and litter production (Dollhopf et al. 2005; Ehrenfeld 2003; Van der Krift et al. 2001). Soil microbes are considered to be one of the drivers of successful exotic plant invasion (Callaway et al. 2004; Kourtev et al. 2002), playing a central role between nutrient cycling and plant invasion (Hawkes et al. 2005). Previous work has shown that exotic plant invasion could clearly impact nitrifying bacteria (Hawkes et al. 2005), denitrifiers, and total bacterial communities (Angeloni et al. 2006). Spartina alterniflora is a typical exotic invasive plant in the coastal wetland of China (Liao et al. 2008a; Liao et al. 2008b). S. alterniflora rapidly dominates ecosystems and forms a monoculture by excluding native plants, which subsequently change the carbon, nitrogen (Liao et al. 2008a; Liao et al. 2008b), and sulfur (Zhou et al. 2009) cycles in the invaded ecosystem. However, to our knowledge, it is not clear yet whether the presence of S. alterniflora would result in changes of abundance and composition of AOA and AOB communities. Specifically, no previous studies have addressed the effects of S. alterniflora invasion on the AOA community composition.
We hypothesize that the abundance and composition of AOA or AOB could be changed in S. alterniflora-invaded coastal wetlands. A typical S. alterniflora-invaded coastal wetland was selected in Jiulong River estuary in Fujian Province, China. The monoculture of S. alterniflora was formed after successful invasion in 2007. This study focused on characterization of the abundance and composition of AOB and AOA at the spread stage of S. alterniflora invasion, as well as analyzing the total bacterial community, sediment properties, and potential nitrification rate.
2 Materials and methods
2.1 Sediment sampling
Sampling sites were located in the Mangrove Nature Reserve of the Jiulong River estuary (24°26′N, 117°54′ E) in Fujian, China. In 2007, S. alterniflora invaded the native mangrove habitat in the reserve and formed monoculture in the low tidal wetland. Three different habitats were selected to sample, i.e., unvegetated bare mudflat zone (B), S. alterniflora-invaded zone (S), and native mangrove zone (M). Sediment sample cores were collected with a PVC pipe (5 cm diameter, 20 cm length) on June 26, 2009, with five replicates in each zone. The sediment cores were placed in an ice box and transported to the laboratory within 2 h. Each core was then sliced into two layers, i.e., a shallow oxic layer (0–5 cm, O) and a lower reductive layer (5–20 cm, R). Therefore, it became 30 samples in total. Each core was homogenized and divided into four portions. One portion was used to determine moisture content, pH, total carbon, total nitrogen, and total sulfur; one fresh portion was used for analysis of potential nitrification rate (PNR), and the last two portions were stored at −80°C for DNA extraction after being freeze-dried.
2.2 Sediment properties and PNR analyses
Sediment pH was determined in a 1:2.5 sediment/water mixture. Total nitrogen (TN), total carbon (TC), and total sulfur (TS) were measured using C/N/H/S analyzer (Elementar Analysensysteme, Vario Max, Germany). PNR was measured using the chlorate inhibition method with minor modifications as previously described (Xia et al. 2007). KClO3 was used to inhibit NO -2 oxidation to NO -3 . Briefly, 5 g of fresh sediment was added to 50-ml centrifuge tubes containing 20 ml 1 mM phosphate-buffered saline (NaCl, 8.0 g L−1; KCl, 0.2 g L−1; Na2HPO4, 0.2 g L−1; NaH2PO4, 0.2 g L−1; pH 7.1) containing 1 mM of (NH4)2SO4; KClO3 was added to the tubes with final concentration of 100 mg L−1 to inhibit nitrite oxidation. The slurries were incubated at room temperature in the dark for 24 h after a 10 min shaking at 160 rpm. Five milliliters of 2 M KCl was added to the incubated tubes for extracting NO -2 -N, which was then determined by a flow injection analyzer (Lachat-QC8500, Lachat Instruments, Loveland, CO) at 540 nm with N-(1-naphthyl) ethylenediamine dihydrocholride.
2.3 DNA extraction
DNA was extracted from 0.5 g freeze-dried sediment samples using the FastDNA Spin Kit for Soil (Qbiogene, Carlsbad, CA). DNA extracts were suspended in 70 μl of DES solution (Qbiogene, USA) and quantified by Nanodrop spectrophotometer. A test of replicate extractions was performed to ensure reproducible yields from DNA extractions (data not shown).
2.4 PCR amplification for DGGE
For bacterial 16S rRNA gene polymerase chain reaction and denaturing gradient gel electrophoresis (PCR–DGGE) analysis, the 341fGC/534r primer was used (Muyzer et al. 1993; Table 1). PCR amplification was performed in a 50-μl reaction mixture, including 1 × PCR buffer, 3.0 mM MgCl2, 400 μM each dNTP, 2.5 U Taq DNA polymerase (Fermentas, Canada), 0.2 mg ml−1 bovine serum albumin (BSA) plus 0.2 mM of each primer, and 6 ng sediment genomic DNA as template. The thermal profile was conducted according to the previous method (Clark and Hirsch 2008) (see Table 1).
For amoA gene denaturing gradient gel electrophoresis (DGGE), amoA1F-GC/amoA2R (Rotthauwe et al. 1997) and arch-amoAF-GC/arch-amoAR (Francis et al. 2005) were primers for bacterial and archaeal amoA gene amplification, respectively (see Table 1). Amplifications were performed for AOA and AOB in 50-μl reaction mixtures including 1 × PCR buffer, 3.0 mM MgCl2, 400 μM each dNTP, 2.5 U Taq DNA polymerase (Fermentas, Canada), and 0.2 mg ml−1 BSA plus 0.2 mM of each primer; 120 ng sediment genomic DNA was added as template.
2.5 DGGE analysis
DGGE analysis of bacterial 16S rRNA gene fragments and amoA PCR products were performed with Dcode Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, USA). Eight microliters and 15 μl PCR products of 16S rRNA and both amoA genes were loaded into polyacrylamide gel wells, respectively. Denaturing gradient and electrophoresis conditions are given in Table 1. The gels were stained with 1:10,000 SYBR-Green I (Sigma, USA) for 30 min and documented using a GelDoc XR (BIO-RAD, USA). Unweighted pair group method with arithmetic mean (UPGMA) algorithms were used to cluster the DGGE banding patterns of bacterial 16S rRNA gene using the software Quantity One (Bio-Rad Laboratories, Hercules, USA).
2.6 Cloning and sequencing of amoA gene
Selected DGGE bands were excised for cloning and sequencing. Excised bands were crushed and incubated overnight at 4°C in 30 μl of sterilized water to elute the DNA and then reamplified with the primers amoA1F/amoA2R and arch-amoAF/arch-amoAR. Reamplified PCR products were purified and ligated into the PMD™ 19-T Vector (TaKaRa Code: D102A, Japan). The resulting ligation mixture transformed into Escherichia coli DH5α competent cells following the instructions of the manufacturer. Positive clones were amplified using the above primers with GC clamp and cross-checked by DGGE for migration behavior.
2.7 Real-time PCR assay for bacterial 16S rRNA and amoA genes
Quantitative real-time polymerase chain reaction (qPCR) assay methods were slightly modified according to previous studies (Dang et al. 2010; Shen et al. 2008; Peng et al. 2010; see Table 1). Gene copy numbers were determined for all samples in triplicates using Applied Biosystems 7500 Real-Time PCR System (ABI, USA). Total bacteria population size was quantified using primers BACT1369F/PROK1492R and oligonucleotide probe TM1389F (see Table 1, Suzuki et al. 2000; Peng et al. 2010). Each reaction was performed in a 25-μl volume containing 1 μl of DNA extract (containing 6 ng sediment genomic DNA) as template, 0.2 mg ml−1 BSA, 0.4 mM of each primer and probe (Takara, Japan), and 12.5 μl Premix Ex taq™ (Takara, Japan). The quantification of amoA gene was based on the fluorescent dye SYBR-Green I. A total of 40 cycles were run with annealing temperatures using primers listed in Table 1. Each reaction was performed in a 25-μl volume containing 1 μl of DNA extract as template (containing 60 ng of sediment genomic DNA), 0.2 mg ml−1 BSA, 0.2 mM of each primer, and 12.5 μl of FastStart Universal SYBR Green Master (Rox; Roche, Switzerland). Product specificity was confirmed by melting curve analysis and visualization in 1.2% agarose gels. Specific product was seen at the expected size for the bacterial and archaeal amoA genes ca. 491 and 635 bp, respectively.
Standard curves for qPCR were developed as previously described (Dang et al. 2010; He et al. 2007) Bacterial 16S rRNA gene fragments were obtained from extracted DNA with primers 27F/1492R (Lane 1991). Archaeal and bacterial amoA and bacterial 16S rRNA gene fragments were cloned as described in section 2.6, and clones that had the right gene inserts were chosen as standards for real-time PCR. Plasmid DNA was extracted with Plasmid Kit (TaKaRa, Japan), and the plasmid concentration was measured with a spectrophotometer (Nanodrop). As the sequences of the vector and PCR inserts were known, the copy numbers of amoA were calculated directly from the concentration of extracted plasmid DNA (Okano et al. 2004). Tenfold serial dilutions of a known copy number of plasmid of the gene clone from sediments were generated to produce the standard curve over seven orders of magnitude (4.97 × 103 to 4.97 × 109 copies of template for archaeal amoA, 4.83 × 103 to 4.83 × 109 copies of template for bacterial amoA, and 1.17 × 104 to 1.17 × 1010 copies of template for bacterial 16S rRNA gene) per assay, respectively. High amplification efficiencies of 96.8–115.4% were obtained for archaeal and bacterial amoA quantification with R 2 values 0.99 and slopes from −3.0 to −3.4. No template DNA negative control was present in each qPCR assay procedure. The inhibitor of DNA extract was tested by the method of previous study (Dumonceaux et al. 2006); no significant inhibition at any dilution (1–120 ng μl−1 DNA extract) was found. Data analysis was carried out with the 7500 System software (ABI, USA).
2.8 Phylogenetic analysis
Phylogenetic analysis was carried out using methods previously described (Chen et al. 2008; Shen et al. 2008). Sequences were compared with GenBank database sequences using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/), and the closest matches were included in the alignment. The neighbor-joining trees were constructed using MEGA 4 [Molecular Evolutionary Genetics Analysis (http://megasoftware.net)] and bootstrapped 1,000 times to calculate linear distances. The Shannon diversity index H and Evenness index E were calculated for the band pattern diversity of AOB and AOA based on the following equations:
where Ni is the abundance of the ith ribotype, N is the total abundance of all ribotypes in the sample (lane of DGGE gels), and S is the number of ribotypes.
The definition of the amoA sequence clusters was based on the previous study (Hunter et al. 2006).
2.9 Data statistical analysis
All statistical analyses were performed using SPSS version 16.0 and one-way analysis of variance followed by Duncan test which was used to check for quantitative differences between treatments. P < 0.05 was considered to be statistically significant.
2.10 Sequence accession numbers
All amoA gene sequences from DGGE bands have been deposited in the GenBank nucleotide sequence database under accession numbers HM235899 to HM235913 for AOB and HM235889 to HM235898 for AOA.
3 Results
3.1 Sediment properties
Sediment characteristics were altered by S. alterniflora invasion. Sediment pH ranged from 6.12 to 6.99 with some variations among the different sediment samples (Table 2). The highest pH was found in the 5–20 cm sample of the unvegetated zone (BR), followed by the S. alterniflora-invaded and unvegetated zones in the shallow layer. Sediment pH in the S. alterniflora-invaded zone was significantly higher than in the native mangrove zone (P < 0.05).
TC and TN in the unvegetated and S. alterniflora zones were significantly lower than those in native mangrove zones. The lowest ratio of TC to TN was found in S. alterniflora zones, and significant differences of the C/N ratio were found between the native mangrove and S. alterniflora-invaded zones. Conversely, the lowest TS was found in native mangrove zones. Significant differences in TS were also found between the native and S. alterniflora-invaded zones. In addition, positive correlations were observed between TC and TN (r = 0.92, n = 30, P < 0.01) and between TS and pH (r = 0.86, n = 18, P < 0.01), respectively.
PNR provides an independent estimate of the abundance of ammonia oxidizers (see Table 2). PNR was lower in the S. alterniflora-invaded zones than in the native mangrove zones at both depths.
3.2 Bacterial 16S rRNA gene abundance in sediments
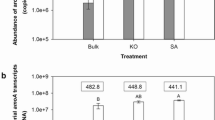
Bacterial 16S rRNA gene abundance was affected by S. alterniflora invasion (Table 3). The real-time PCR assays showed that bacterial 16S rRNA gene copy numbers per gram of dry sediment ranged from 3.49 × 1011 to 1.46 × 1012. In the mangrove zone, 16S rRNA gene was significantly higher than in both invaded and unvegetated zones at two layers. Bacterial abundance positively related to TC (r = 0.84, n = 18, P < 0.01) and TN (r = 0.74, n = 18, P < 0.01), respectively, but negatively related with pH (r = −0.63, n = 18, P < 0.01) and TS (r = −0.57, n = 18, P < 0.01), respectively. Bacterial 16S rRNA gene abundance positively correlated with AOA abundance (r = 0.79, n = 18, P < 0.01), but no evident correlation was found between bacterial 16S rRNA gene and AOB abundance.
3.3 amoA gene abundance in sediments
Average abundance of AOA genes in the S. alterniflora-invaded zones were 3.23 × 108 (O) and 4.01 × 107 (R) copy numbers per gram dry sediment and were reduced by 11.3-fold (O) and 46.1-fold (R) in the S. alterniflora-invaded zones, compared with native mangrove zone (3.72 × 109 (O) and 1.85 × 109 (R) copy numbers per gram dry sediment; see Table 3). However, AOB abundance in S. alterniflora zone was 1.61 × 108 (O) and 5.14 × 106 (R) copy numbers per gram dry sediment, higher than in the native mangrove zone (9.8-fold (O) and 1.8-fold (R), respectively). AOB abundance in S. alterniflora zone was significantly higher than in the native and unvegetated zones in the 0–5-cm layer, while no evident difference was observed among zones in the 5–20-cm layer.
Similar to total bacteria, positive correlations were obtained between AOA abundance, TC (r = 0.82, n = 18, P < 0.01), and TN (r = 0.77, n = 18, P < 0.01), respectively, but negative correlations were obtained with pH (r = −0.83, n = 18, P < 0.01) and TS (r = −0.83, n = 18, P < 0.01), respectively. No significant correlation was found between PNR and AOA or AOB abundance.
AOA was more abundant than AOB in all sediments (see Table 3). The ratios especially AOA/AOB in mangrove zones were 227 (O) and 639 (R), much higher than the others. The ratio of AOA/bacterial 16S rRNA gene in native zone (MO, 0.254%; MR, 0.284%) was also higher than those in the invaded (SO, 0.0561%; SR, 0.00968%) and unvegetated zones. However, the ratio AOB/bacterial 16S rRNA gene in native zone (MO, 0.00112%; MR, 0.000445%) was lower than that of invaded zones (SO, 0.0279%; SR, 0.00124%).
3.4 Genetic profiling of bacterial 16S rRNA and amoA genes
Bacterial 16S rRNA gene, AOB, and AOA composition were analyzed by PCR–DGGE (Figs. 1a, 2, and 3). Reproducibility of triplicates DGGE profiles from sediment DNA extracts was found to be high (data not shown). Duplicate results are shown in this study. For bacterial 16S rRNA gene, profiles from the native mangrove zones clustered separately from the unvegetated or S. alterniflora-invaded zones, and it exhibited 64% similarity between mangrove zone and others (see Fig. 1b).
a Denaturing gradient gel electrophoresis profiles of bacterial 16S rRNA gene in sediments with different overlying vegetation. Duplicate sample results using 1 and 2 were shown in each treatment. b Cluster analysis of the bacterial DGGE profiles based on 16S rRNA gene. The dendrogram was calculated on the basis of the UPGMA. The analysis was based on the presence or absence of bands at positions in each lane of the gel. Duplicate sample results using 1 and 2 were shown in each treatment
Phylogenetic relationship among bacterial amoA gene sequences retrieved from the Jiulong River estuary sediments. Designation of the clones in bold includes the following information: accession number in the GenBank with DGGE band label. Bootstrap values (≥50%) for 1,000 replicates are shown at the branch points. The scale bar represents 5% estimated sequence divergence
Shannon index and Evenness were used to analyze microbial community diversity (Table 4). The Shannon index of bacterial 16S rRNA gene in mangrove zone was higher than that in other zones. Shannon diversity was significantly negatively correlated with pH (r = −0.91, n = 18, P < 0.01) and TS (r = −0.75, n = 18, P < 0.01) but was positively correlated with TC (r = 0.81, n = 18, P < 0.01) and TN (r = 0.69, n = 18, P < 0.01). Conversely, diversity of AOA in the S. alterniflora-invaded and unvegetated zones was higher than in the native mangrove zone. AOA abundance negatively correlated with AOA Shannon index (r = −0.59, n = 18, P < 0.01) and Evenness (r = −0.47, n = 18, P < 0.05), respectively. AOA Shannon index also negatively correlated with TC (r = −0.56, n = 18, P < 0.05) and TN (r = −0.61, n = 18, P < 0.01), but positively correlated with pH (r = 0.85, n = 18, P < 0.05) and TS (r = 0.81, n = 18, P < 0.01). For AOB, the diversity in upper layer (0–5 cm) was higher than those of deeper layer (5–20 cm). However, no difference in AOB diversity was found among different zones.
Fifteen bands of AOB were sequenced and used to construct a phylogenetic tree (Fig. 4). AOB sequences were grouped into the environmental cluster, distinct from previously identified Nitrosomonas and Nitrosospira amoA clades. Ten AOA bands were sequenced and grouped into cluster S (soil/sediment) of Crenarchaeota (Fig. 5).
Phylogenetic relationships among archaeal amoA gene sequences retrieved from the Jiulong River estuary sediments. Designation of the clones in bold includes the following information: accession number in the GenBank with DGGE band label. Bootstrap values (≥50%) for 1,000 replicates are shown at the branch points. The scale bar represents 10% estimated sequence divergence
4 Discussion
This study shows that exotic S. alterniflora invasion affects the abundance and composition of AOA and AOB and provides some evidence on the linkage between exotic plants invasion and ecosystem function in Jiulong River estuarine sediment. In this study, changes in sediment properties and potential nitrification rate were explained in part by changes to AOA and AOB communities caused by S. alterniflora establishment and spread, and these alterations may directly affect nitrification in estuarine sediment.
Our experiments showed that pH was increased during the invasion and had a negative impact on AOA abundance, which was consistent with a previous study in acid soil (Nicol et al. 2008). Also, total sulfur concentration was found to be higher in S. alterniflora-invaded zones than in those with native plant and unvegetated zones which is consistent with previous studies (Stribling 1997; Zhou et al. 2009). AOA abundance increased with decreasing concentration of sulfur; this may due to AOA abundance being negatively correlated to sulfur concentration as demonstrated by other researchers (Caffrey et al. 2007; Dollhopf et al. 2005). Moreover, AOA abundance was found to be positively correlated with total carbon and total nitrogen which is in agreement with previous studies (Abell et al. 2010; Mosier and Francis 2008). PNR has been shown to be related to AOA abundance (Caffrey et al. 2007; Beman and Francis 2006) or AOB abundance (Nicol et al. 2008; Shen et al. 2008; Ying et al. 2010). In this study, both PNR and AOA abundance significantly decreased in S. alterniflora zone, while AOB abundance increased. However, in contrast to previous studies, our results show that PNR was not strongly correlated to the amoA gene copy numbers of AOB or AOA; this might be due to the number of samples analyzed in this study. From our results, it appears that S. alterniflora invasion have an impact on the sediment properties. However, it was difficult to attribute these changes to S. alterniflora only, as ecosystems are complex systems, and further studies are required.
The amoA and bacterial 16S rRNA gene abundance in sediments revealed important patterns (see Table 3). Firstly, discrepancy responding to exotic plant S. alterniflora was found between AOA and AOB. To our best knowledge, this is the first study revealing that AOA was significantly reduced in the presence of the invasive plant species. Of particular interest is the fact that the abundance of AOB was one to two orders of magnitude higher after S. alterniflora invasion (in 0–5 cm sediment layer), compared with other zones in this study, which was consistent with previous work (Hawkes et al. 2005), although A more recent study in paddy soil showed that AOA and AOB abundance were mainly determined by the soil types and not related to aboveground rice (Chen et al. 2010). Secondly, amoA and bacterial 16S rRNA gene abundance at shallow layer (0–5 cm) was higher than those at deeper layer (5–20 cm). This different vertical distribution of microbes among sediment layers may be due to bioavailability of nitrogen and organic carbon (Leininger et al. 2006). Finally, our results showed that archaeal amoA copies were more abundant than bacterial amoA copies in sediment for both depths, which was similar to other previous studies (Abell et al. 2010; Jia and Conrad 2009; Leininger et al. 2006). The ratios, especially, of AOA/AOB and AOA/total bacteria with the native mangrove zone were much higher than others. Conversely, the ratio of AOB/total bacteria was increased by the S. alterniflora invasion. In addition, the ratio in the deeper layer was lower than in the shallow layer, suggesting that archaeal amoA dominated over bacterial amoA in sediments, while high ratios of AOA to AOB and AOA to total bacteria in the native mangrove zone were dramatically decreased by the S. alterniflora invasion. A previous study demonstrated that AOA played an important role in nitrification using stable isotope probing method (Zhang et al. 2010). However, other studies have shown that the AOA population sizes were much higher than AOB; AOB rather than AOA functionally dominate ammonia oxidation in grassland soils (Di et al. 2009, 2010) and agricultural soil (Jia and Conrad 2009).
No significant difference in AOB composition was found among different zones (see Fig. 2 and Table 4), demonstrating that AOB was quite stable in relation to plant community composition. Some previous studies also showed that there was little response in AOB composition to long-term fertilization treatments (He et al. 2007), vegetations in terrestrial ecosystem (Ying et al. 2010), ammonium, and temperature (Avrahami and Conrad 2003), and the same type flooded paddy soils (Chen et al. 2010). High variation in overall bacterial composition in relation to a stable ammonia oxidizer community was also found in forest soil (Laverman et al. 2001) and freshwater sediments (Speksnijder et al. 1998). The slow-growing and specialized ammonia oxidizers are frequently more tolerant to these environmental changes than the fast-growing and changing bacterial community (Hawkes et al. 2005). However, the cDNA DGGE profile of AOB communities without rice plants were clearly different from those with planted rice (Chen et al. 2008). The AOB sequences recovered from DGGE bands were highly similar to the known sequences from the previous studies with a variety of marine-related or estuarine sediment environments, such as Jiaozhou Bay (Dang et al. 2010), Pearl River Estuary, marine sponge (Mohamed et al. 2010), San Francisco Bay (Mosier and Francis 2008), and Chesapeake Bay (Francis et al. 2003). These provide further evidence that this AOB group is ubiquitous in environments, suggesting that AOB found here are not specific in Jiulong River estuary of this study. These amoA sequences were not closely affiliated with any amoA gene from known cultured ammonia-oxidizing bacteria strains, but all phylotypes were affiliated with Nitrosospira spp.
AOA abundance was reduced by S. alterniflora invasion and positively related to bacterial abundance, while AOA diversity was higher in the invaded and unvegetated zones (see Fig. 3 and Table 4). It might imply that the change in AOA abundance could be partly due to the shifts of the community composition. More taxa of ammonia-oxidizing archaea may play functional role in the unvegetated and invaded zones than those in the native mangrove zone. The composition and population sizes of AOA were changed by plant invasion, which was similar to land utilization patterns (Ying et al. 2010). All AOA sequences fell in cluster S (soil/sediment) and are closely related to those found in the estuarine sediment from Australia (Abell et al. 2010) and China (He et al. 2007; Zhou et al. 2009), suggesting that these organisms are widely distributed in similar environments. Sequences originating from terrestrial sources suggested that some of AOA may be a result of the influx of particulate matter from land runoff, consistent with the high rainfall in this catchment (Abell et al. 2010).
The sediment overall bacterial composition and gene abundance in the sediments were impacted by S. alterniflora invasion. Interestingly, bacteria had no relationship with AOB, while it had a close correlation with AOA community. This may be due to the exotic plants′ root exudates (Callaway and Aschehoug 2000) inducing changes in sediment environmental parameters, such as pH, carbon, nitrogen, and sulfur, in turn, significantly shaping rhizospheric bacterial community structure (Haichar et al. 2008; Hunter et al. 2006; Berg and Smalla 2009). Our results demonstrated sediment properties had similar impacts on both total bacteria and AOA but not on AOB. Specific microbial community favors for specific environment. Therefore, this indicated that exotic plant–microbe interaction in sediment has a complex influence on ecosystem function.
The process of biological invasion has been artificially divided into four stages as transport, colonization, establishment, and spread (Theoharides and Dukes 2007). Indeed, knowledge of temporal patterns of ammonia oxidizers is important in the S. alterniflora invasion succession (Hawkes et al. 2005). To answer what the response is of ammonia oxidizers to exotic S. alterniflora invasions, the spread stage of S. alterniflora invasion was selected in this study. The samples were collected in the end of June at the growing peak of S. alterniflora. The environmental factor, such as temperature, was chosen to favor ammonia oxidizers and enhance potential nitrification rate (Urakawa et al. 2008; Tourna et al. 2008). Root exudation at the growing peak strongly influence sediment microenvironment. As we learned in this study, AOB composition was quite stable in sediments from the three different zones. Our results could be representative for growing season with a maximum response of ammonia oxidizers to the S. alterniflora invasion, although multiple time point studies may be ideal (Hawkes et al. 2005).
This study revealed the significant effects of S. alterniflora invasion on ammonia oxidizers in estuarine sediment. Since ammonia oxidizers play a critical role in N cycle and transformation in estuarine sediment, these shifts caused by S. alterniflora invasion may be of great ecological importance.
References
Abell GC, Revill AT, Smith C, Bissett AP, Volkman JK, Robert SS (2010) Archaeal ammonia oxidizers and nirS-type denitrifiers dominate sediment nitrifying and denitrifying populations in a subtropical macrotidal estuary. ISME J 4:286–300
Angeloni NL, Jankowsk KJ, Tuchman NC, Kelly JJ (2006) Effects of an invasive cattail species (Typha x glauca) on sediment nitrogen and microbial community composition in a freshwater wetland. FEMS Microbiol Lett 263:86–92
Avrahami S, Conrad R (2003) Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl Environ Microbiol 69:6152–6164
Beman JM, Francis CA (2006) Diversity of ammonia-oxidizing archaea and bacteria in the sediments of a hypernutrified subtropical estuary: Bahia del Tobari, Mexico. Appl Environ Microbiol 72:7767–7777
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure a function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Burke DJ, Hamerlynck EP, Hahn D (2002) Interactions among plant species and microorganisms in salt marsh sediments. Appl Environ Microbiol 68:1157–1164
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISMEJ 1:660–662
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Callaway RM, Thelen GC, Barth S, Ramsey PW, Gannon JE (2004) Soil fungi alter interactions between the invader Centaurea maculosa and North American natives. Ecology 85:1062–1071
Chen XP, Zhu YG, Xia Y, Shen JP, He JZ (2008) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ Microbiol 10:1978–1987
Chen X, Zhang LM, Shen JP, Xu Z, He JZ (2010) Soil type determines the abundance and community structure of ammonia-oxidizing bacteria and archaea in flooded paddy soils. J Soils Sediments 10(8):1510–1516
Clark IM, Hirsch PR (2008) Survival of bacterial DNA and culturable bacteria in archived soils from the Rothamsted Broadbalk experiment. Soil Biol Biochem 40:1090–1102
Dang H, Li J, Chen R, Wang L, Guo L, Zhang Z, Klotz MG (2010) Diversity, abundance, and spatial distribution of sediment ammonia-oxidizing Betaproteobacteria in response to environmental gradients and coastal eutrophication in Jiaozhou Bay, China. Appl Environ Microbiol 76:4691–4702
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Sherlock RR, Shen JP, He JZ, Winefield CS (2010) Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J Soils Sediments 10:943–954
Dollhopf SL, Hyun JH, Smith AC, Adams HJ, Brien SO, Kostka JE (2005) Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Appl Environ Microbiol 71:240–246
Dumonceaux TJ, Hill JE, Briggs SA, Amoako KK, Hemmingsen SM, Kessel AGV (2006) Enumeration of specific bacterial populations in complex intestinal communities using quantitative PCR based on the chaperonin-60 target. J Microbiol Meth 64:46–62
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Francis CA, O’Mullan GD, Ward BB (2003) Diversity of ammonia monooxygenase (amoA) genes across environmental gradients in Chesapeake Bay sediments. Geobiology 1:129–140
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
Francis CA, Beman JM, Kuypers MMM (2007) New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J 1:19–27
Haichar FZ, Christine M, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achoua W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Hawkes CV, Wren IF, Herman DJ, Firestone MK (2005) Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8:976–985
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Hunter EM, Mills HJ, Kostka JE (2006) Microbial community diversity associated with carbon and nitrogen cycling in permeable shelf sediments. Appl Environ Microbiol 72:5689–5701
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Kourtev PS, Ehrenfeld JG, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecol Lett 83:3152–3166
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Kowalchuk GA, Naoumenko ZS, Derikx PJ, Felske A, Stephen JR, Arkhipchenko IA (1999) Molecular analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in compost and composted materials. Appl Environ Microbiol 65:396–403
Kowalchuk GA, Buma DS, de Boer W, Klinkhamer PGL, van Veen JA (2002) Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Anton Leeuwint J G 81:509–520
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons, New York, pp 115–175
Laverman AM, Speksnijder A, Braster M, Kowalchuk GA, Verhoef HA, van Verseveld HW (2001) Spatiotemporal stability of an ammonia-oxidizing community in a nitrogen-saturated forest soil. Microb Ecol 42:35–45
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Liao CZ, Luo YQ, Fang CM, Chen JK, Li B (2008a) Litter pool sizes, decomposition, and nitrogen dynamics in Spartina alterniflora-invaded and native coastal marshlands of the Yangtze Estuary. Oecologia 156:589–600
Liao CZ, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B (2008b) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714
Mohamed NM, Saito K, Tal Y, Hill RT (2010) Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges. ISMEJ 4:38–48
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10:3002–3016
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, Lebauer D, Scow KM (2004) Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 70:1008–1016
Peng JJ, Cai C, Qiao M, Li H, Zhu YG (2010) Dynamic changes in functional gene copy numbers and microbial communities during degradation of pyrene in soils. Environ Pollut 158:2872–2879
Prosser JI, Embley MT (2002) Cultivation-based and molecular approaches to characterisation of terrestrial and aquatic nitrifiers. Anton van Leeuwenhoek 81:165–179
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Ruiz-Rueda O, Hallin S, Bañeras L (2009) Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol Ecol 67: 308–319
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnol Oceanogr 33:702–724
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Speksnijder AG, Kowalchuk GA, Roest K, Laanbroek HJ (1998) Recovery of a Nitrosomonas-like 16S rDNA sequence group from freshwater habitats. Syst Appl Microbiol 21:321–330
Stribling JM (1997) The relative importance of sulfate availability in the growth of Spartina alterniflora and Spartina cynosuroides. Aquat Bot 56:131–143
Suzuki MT, Taylor LT, DeLong EF (2000) Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl Environ Microbiol 66:4605–4614
Theoharides KA, Dukes JS (2007) Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol 176:256–273
Tourna M, Freitag TE, Nicol GW, Prosser J I (2008) Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10:1357–1364
Urakawa H, Tajima Y, Numata Y, Tsuneda S (2008) Low temperature decreases the phylogenetic diversity of ammonia-oxidizing archaea and bacteria in aquarium biofiltration systems. Appl Environ Microbiol 74:894–900
Van der Krift TAJ, Kuikman PJ, Mller F, Berendse F (2001) Plant species and nutritional-mediated control over rhizodeposition and root decomposition. Plant Soil 278:191–200
Xia Y, Zhu YG, Gu Q, He JZ (2007) Does long-term fertilization treatment affect the response of soil ammonia-oxidizing bacterial communities to Zn contamination? Plant Soil 301:245–254
Ying JY, Zhang LM, He JZ (2010) Putative ammonia-oxidizing bacteria and archaea in an acidic red soil with different land utilization patterns. Environ Microbiol Rep 2:304–312
Zhang LM, Offre PR, He JZ, Verhammea DT, Nicola GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil thaumarchaea. Natl Acad Sci USA 107:17240–17245
Zhou CF, An SQ, Deng ZF, Yin DQ, Zhi YB, Sun ZY, Zhao H, Zhou LX, Fang C, Qian C (2009) Sulfur storage changed by exotic Spartina alterniflora in coastal saltmarshes of China. Ecol Eng 35:536–543
Acknowledgments
We thank Professor Yongguan Zhu and Dr. Matthew Rodda for the valuable comments and improvements in English. This work was supported by the National Science Foundation of China (31070463), the Hundred Talent Program of Chinese Academy of Sciences (A0815), the Knowledge Innovative Project of Chinese Academy of Sciences (KZCX2-YW-Q02-04), and Ningbo University (RCL2009005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ji-Zheng He
Rights and permissions
About this article
Cite this article
Zhang, QF., Peng, JJ., Chen, Q. et al. Impacts of Spartina alterniflora invasion on abundance and composition of ammonia oxidizers in estuarine sediment. J Soils Sediments 11, 1020–1031 (2011). https://doi.org/10.1007/s11368-011-0369-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0369-9