Abstract
Purpose
Changes in the behavior of earthworms (for example avoidance of a particular substrate) can influence the soil ecosystem. Our aim was to determine whether the earthworms Eisenia fetida and Lumbricus terrestris are able to avoid ivermectin (a veterinary endectocide belonging to the avermectins). A standard avoidance test with earthworms was conducted using standardized Lufa 2.3 soil (Speyer, Germany) and sandy soil provided by Cinkarna Celje (Slovenia).
Materials and methods
A two-chamber system of avoidance test as described in ISO guideline 17512-1 (ISO 2008) was applied. We investigated the substrate preference of E. fetida and L. terrestris after 48 h at ivermectin concentrations of 8, 32, 64, and 256 mg kg−1 dry soil.
Results and discussion
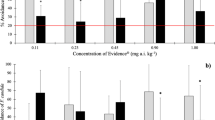
No deterrent effects were observed for either species and either soil types. E. fetida in Lufa 2.3 soil was clearly attracted to ivermectin. More than 70% of the earthworms preferred soil with ivermectin concentrations of 8, 64, and 256 mg kg−1. No observed effect concentration and lowest observed effect concentration, 2-day exposure, for effects on the avoidance of both species are >256 mg kg−1 dry soil in two different soils.
Conclusions
High percentage of earthworms in the test soil was observed in the experiment with E. fetida in Lufa 2.3 soil treated with 8, 64, and 256 mg ivermectin kg−1. We can conclude that earthworms, as it was observed in E. fetida in Lufa 2.3 soil treated with ivermectin, may be attracted by some chemicals. The percentage of L. terrestris in soil from Cinkarna Celje treated with ivermectin ranged from 39% to 52% and of E. fetida from 33% to 55%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Avermectins (AVMs), such as ivermectin, abamectin, and doramectin, are widely used antiparasitic drugs in veterinary medicine. They are derivatives of fermentation products of Streptomyces avermitilis (McKellar and Benchaoui 1996). Ivermectin is usually a product of the combination of two homologs: 22, 23-dihydro AVM B1a and 22, 23-dihydro AVM B1b (Campbell et al. 1983) and it efficiently controls parasitic gastrointestinal and pulmonary nematodes, mites, lice, warble flies, and ticks at a low dosage (Kaneda et al. 2005).
Soil may become contaminated when veterinary medicines are dispersed via slurry or deposited on land by grazing animals (Kolar et al. 2008). Ivermectin undergoes little metabolic change and most of the dose given to the animal is excreted, relatively unaltered, primarily in the feces (Halley et al. 1989). AVMs in the feces of cattle treated with sustained-release bolus, pour-on or subcutaneous injection formulations are lethal for dung-breeding invertebrates and can also negatively affect earthworms. Concentrations of ivermectin above 18.5 mg kg−1 in the dung can lead to death of earthworms (Sun et al. 2005). Halley et al. (1989) reported the 28-day lethal concentration (LC50) of ivermectin mixed in artificial soil (50 g bentonite clay, 100 g peat, 5 g cow manure, and quartz sand, pH 7) to be 315 mg kg−1 for earthworms Eisenia fetida.
Earthworms are often used in standardized ecotoxicological tests (e.g., ISO 1993; ISO11268-2 1998). An avoidance test reflects behavioral properties of earthworms, which is based on the stimulation of sensory cells (Schaefer 2003). Such a test is quick and easy to perform, and is known to be sensitive to a wide range of chemicals.
The aim of this study was to determine the avoidance behavior of the earthworm species E. fetida and L. terrestris when exposed to different concentrations of ivermectin.
2 Materials and methods
E. fetida (Savigny 1826) was obtained from a private company Biobrazda (Dragomer, Slovenia). Healthy adults with a body weight of 0.2–0.5 g and developed clitellum were used in the avoidance test. Earthworms of the species L. terrestris were obtained from Andreas Schentz, owner of retail and wholesale private company (Ückermünde, Germany). Healthy adults with a body weight of 4–6 g and developed clitellum were used.
The avoidance test was performed using natural Lufa 2.3 soil, a standardized soil, and soil provided by Cinkarna Celje, with properties listed in Table 1. We decided to compare natural Lufa soil to sandy soil from Cinkarna Celje because in nature earthworms are more likely to be found in soils similar to Lufa 2.3 than in sandy soils. The results obtained with Lufa 2.3 soil are therefore easier to extrapolate to field conditions. We chose sandy soil from Cinkarna Celje because of its low clay content comparing to Lufa 2.3 soil. Ivermectin (primarily ivermectin B1a, obtained from Sigma Aldrich) solution in acetone was mixed with quartz sand and left in a fume hood until the acetone evaporated. The portion of quartz sand was mixed in with the soil. The moisture content was adjusted to 60% of soil water holding capacity (water content was determined gravimetrically). Control soil was also spiked with acetone treated quartz sand. Soils were spiked with different concentrations of ivermectin (8, 32, 64, and 256 mg kg−1 soil dry weight). The choice of ivermectin concentrations which we used in the avoidance test was based on the results of reproduction tests reported by Gunn and Sadd (1994). The reference toxicant was boric acid. Avoidance behavior was obtained at 450 mg kg−1. The test with control soil on both sides of the test vessel was performed. The soil was used for the avoidance test after 5 days of aging in order to allow for sorption of the chemical.
The avoidance test was performed as described in ISO guideline 17512-1 (ISO 2008). Briefly: the avoidance test (two-chamber system) ran over 48 h (temperature: 20°C, relative humidity: 80%). The test soils were in direct contact with the control soils. The containers (1.2 L, 19 × 9 × 7 cm, for the avoidance test with E. fetida and 7 L, 26 × 17.5 × 15.5 cm, for the avoidance test with L. terrestris) were filled with 600 and 2,400 g of fresh soil. The depth of the soil layer for the avoidance test with E. fetida was 6 cm and 8 cm for the avoidance test with L. terrestris.
At the beginning of the test, the vessels were divided into two equal sections by a vertically introduced divider (thin metal). The divider was then removed, and ten adult earthworms were placed on the separating line of the vessel. To prevent the worms from escaping, the vessels were covered with a fine net, permeable to light and air (Hund-Rinke et al. 2005). After 48 h, the dividers were re-introduced and the worms on each side were counted. Five replicates were applied for each concentration.
The results of the avoidance test were presented as the mean number (±SD) of living earthworms in the test soil and the percentage of remaining worms in the test soil. Two procedures described in the ISO guideline 17512 (ISO 2008) were applied: a 20% threshold value and a statistical calculation (χ 2 test). Soil is considered to be toxic if more than 80% of the earthworms migrate to control soil. The avoidance test is valid if the mortality per each treatment does not exceed 10%. Second validity criteria is 60:40 distribution ratio in the control assay. No observed effect concentration (NOEC) and lowest observed effect concentration (LOEC) for both types of soil and both earthworm species were determined. To estimate NOEC and LOEC, χ 2 test was used and 20% threshold value was also applied.
3 Results
The mortality of both species did not exceed 10%. The highest average mortality was 6.8% in the experiment with L. terrestris. In the experiment with E. fetida in both soils, average mortality was 5.2%. Table 2 shows the avoidance behavior of E. fetida in the soil from Cinkarna Celje treated with ivermectin and in the control soil. In all concentrations, more than 20% of the earthworms remained in the test soil spiked with 8, 32, 64, and 256 mg kg−1 dry soil. Using χ 2 test, statistically significant differences (p < 0.05) were determined for the concentration 8 mg kg−1. In the control assay, distribution ratio was 65.2:34.8. With E. fetida exposed to Lufa 2.3 soil, more than 50% of the earthworms remained in the test soil (Table 3). No deterrent effect was observed. In this experiment, statistically significant differences (p < 0.001) between the control and the test soil were determined at all concentrations, except for the treatment with 32 mg ivermectin kg−1. This result was unexpected and indicates that for some reason, the earthworms were attracted to the soil containing ivermectin. In the experiment (Table 4) with L. terrestris in soil from Cinkarna Celje, more than 30% of the earthworms remained in the test soil. No significant differences were observed between the control and the treated soils.
NOEC and LOEC values after 2 days of exposure in the two test soils were >256 mg kg−1 dry soil for both species.
4 Discussion
In a comparable study, Gunn and Sadd (1994) investigated the deterrent effect of the ivermectin formulation Oramec R. They found significantly more worms in the soil treated with 4 mg ivermectin kg−1 dry soil. However, the situation was reversed at 20 mg kg−1 of ivermectin formulation, in which significantly more worms (p < 0.05) were found in the ivermectin-free soil. There were some major differences between the study performed by Gunn and Sadd (1994) and our study. We used a different type of soil (natural Lufa 2.3 soil instead of OECD artificial soil which consisted of 10% peat, 20% caolin clay and 70% sand) and pure ivermectin instead of a complex ivermectin formulation containing other ingredients (stabilizers, emulsifiers). Presumably, the presence of these chemicals increases ivermectin availability and triggers avoidance.
It is not clear from our results why earthworms preferred ivermectin-contaminated Lufa soil over non-contaminated soil. An attraction response of earthworms was observed for fungicide carbendazim in OECD soil at 1 mg kg−1 dry soil. When interpreting an attraction behavior towards carbendazim, the difficult dose–response pattern has to be taken into account. (Garcia et al. 2008). Since fungicide benomyl and insecticide λ-cyhalothrin caused avoidance the researchers stated that OECD soil is not optimal for avoidance test. Floate (2007) observed preference of dung colonizing insects for dung from cattle treated with ivermectin. The most probable reason for the absence of a deterrent effect is the inability of earthworms to detect ivermectin with their chemoreceptors. Sousa et al. (2008) believe that, although earthworms are able to discriminate soils with similar contamination, it is nonetheless questionable whether the preference for some soils is determined by a reduced bioavailability of contaminants, the affinity of the species for organic matter-rich soils, or the inability of the chemoreceptors to detect some contaminants.
5 Conclusions
Our study showed that ivermectin, in the experimental conditions stipulated by ISO guideline 17512-1 (ISO 2008), does not have a deterrent effect on earthworms up to the highest concentration tested (256 mg kg−1).
In fact, E. fetida was attracted to ivermectin in Lufa 2.3 soil. We did not observe avoidance at the highest concentration (256 mg kg−1) which is close to the 28-day LC50 (315 mg kg−1 using artificial soil comprised of 100 g peat, 50 g bentonite clay, 5 g cow manure and 10 g calcium carbonate) reported by Halley et al. (1989).
References
Campbell WC, Fisher MH, Stapley EO, Albers-Schonberg G, Jacob TA (1983) Ivermectin: a potent new antiparasitic agent. Science 221:823–828
Floate KD (2007) Endectocide residues affect insect attraction to dung from treated cattle: implications for toxicity tests. Med Vet Entomol 21:312–322
Garcia M, Römbke J, De Brito MT, Scheffczyk A (2008) Effects of three pesticides on the avoidance behaviour of earthworms in laboratory test performed under temperate and tropical conditions. Environ Pollut 153:450–456
Gunn A, Sadd JW (1994) The effect of ivermectin on the survival, behaviour and cocoon production of the earthworm Eisenia fetida. Pedobiologia 38:327–333
Halley BA, Jacob TA, Lu AYH (1989) The environmental impact of the use of ivermectin: environmental effects and fate. Chemosphere 18:1543–1563
Hund-Rinke K, Lindemann M, Simon M (2005) Experiences with novel approaches in earthworm testing alternatives. J Soils Sediments 5:233–239
ISO (1993) ISO guideline 11268-1. Soil quality—effects of pollutants on earthworms (Eisenia fetida)—Part 2: Determination of acute toxicity using artificial soil substrate. International Organization for Standardisation, Geneva
ISO (2008) ISO guideline 17512-1. Soil quality—Avoidance test for determining the quality of soils and effects of chemicals on behavior—Part 1: Tests with earthworms (Eisenia fetida and Eisenia Andrei). International Organization for Standardisation, Geneva
ISO11268-2 (1998) ISO guideline 11268-2. Soil quality—effects of pollutants on earthworms (Eisenia fetida)—Part 2: Determination of effects on reproduction. International Organization for Standardisation, Geneva
Kaneda S, Yamashita N, Uchida T, Shimano S, Miyoshi N, Sasaki M, Enami Y (2005) Effects of ivermectin in dung pats on earthworm (Megascolecidae) populations and pat degradation in Japanese grassland. Appl Soil Ecol 31:280–285
Kolar L, Kožuh Eržen N, Hogerwerf L, Van Gestel CAM (2008) Toxicity of abamectin and doramectin to soil invertebrates. Environ Pollut 151:182–189
McKellar Q, Benchaoui HA (1996) Avermectins and milbemycins. J Vet Pharmacol Ther 19:331–351
Schaefer M (2003) Behavioural endpoints in earthworm ecotoxicology—evaluation of different test systems in soil toxicity assessement. J Soils Sediments 3:79–84
Sousa A, Pereira R, Antunes SC, Cachada A, Pereira E, Duarte AC, Gonçalves F (2008) Validation of assays for the screening assessment of soils under different anthropogenic disturbances. Ecotox Environ Safe 71:661–670
Sun Y, Diao X, Zhang Q, Shen J (2005) Bioaccumulation and elimination of avermectin B1a in the earthworms (Eisenia fetida). Chemosphere 60:699–704
Acknowledgments
This work was supported by the Slovenian Research Agency, Grant J4-9277. Our thanks go to Erik Zidar and Katarina Babnik for technical support. Special thanks to Dr. Jörg Römbke who provided numerous scientific advices during our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Kerstin Hund-Rinke
Rights and permissions
About this article
Cite this article
Torkhani, A.L., Eržen, N.K., Kolar, L. et al. Does ivermectin attract earthworms?. J Soils Sediments 11, 124–128 (2011). https://doi.org/10.1007/s11368-010-0284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-010-0284-5