Abstract
Background, aim and scope
Forest fires can result in severe economic and environmental consequences, and little is known about the ecological patterns and processes that may lead to the recovery of burnt areas. In the last decades, Portugal has been the Southern European country with the highest number of fire events and with the highest burnt area per hectare. With this work, we proposed to study the effect of a forest fire on the terrestrial ecosystem. More specifically, this work intended to evaluate the short-term recovery of several soil chemical, biochemical (microbial enzymatic activities) and biological (edaphic macro-arthropod community) variables in a burnt pine tree forest area.
Methodology
Soil and macro-arthropod sampling was carried out in a burnt area (transects BI, BII and BIII) and in a neighbouring unburnt area (U) 3 and 8 months after the fire, coinciding with autumn and spring. Soil was collected for the determination of physical (pH and conductivity) and chemical parameters (moisture and organic matter) and soil enzymes (cellulase, acid phosphatase and nitrogen mineralisation rate). Edaphic macro-fauna was captured using pitfall traps.
Results
Univariate and multivariate statistics revealed, overall, that burnt sites displayed lower acid phosphatase and cellulase activities and higher conductivity and pH values than the unburnt area. There was a recovery in the measured soil parameters between autumn and spring in the most interior parts of the burnt areas (BII and BIII), but the outer transect (BI, close to a road) still displayed considerable differences to the remaining burnt transects as well as to the unburnt area. A total of 47 macro-arthropod taxa were captured in both seasons, with Linyphiidae spiders (20.2%) and insect families Formicidae (13.4%) and Staphylinidae (11.9%) being the most abundant. Dominance by some taxa was overall stronger in the burnt than in the unburnt area, although dominant taxa varied between seasons. In autumn, the burnt area was dominated by ants and had also a high abundance of scavengers, carrion feeders and some ground active hunters. In spring, there was a general increase in taxa diversity, richness, and total catches; in the burnt area, there was a re-colonisation by several organisms sensitive to litter quality, such as isopods and pseudoscorpions, particularly in the outer transect (closest to the unburnt area).
Discussion
Differences in soil parameters between burnt and unburnt areas were most likely due to the deposition of nutrient-rich alkaline ashes. However, low cellulase activity in the outer part of the burnt area (BI) indicated compromised microbial activity in both sampling seasons. Recovery of soil functional parameters was delayed in the outer zone of the burnt area because of (i) fire intensity in that area or (ii) proximity to the road (enhancing erosion and exposure to contaminants). The pattern of arthropod re-colonisation of the burnt area followed the inverse recovery pattern (from the outer zone to the inner zone), stressing the primary role of the adjacent unburnt area as a source of potentially colonizing organisms.
Conclusions
Direct and indirect effects of fire on soil parameters (soil alkalinisation and nutrient enrichment) and edaphic fauna had a short-term persistence in the burnt area, and signs of recovery were evident 8 months after the fire (spring). The adjacent unburnt area seemed to act as an important source of arthropod colonisers.
Recommendations and perspectives
More prolonged studies on the recovery of soil functional parameters and arthropod community structure are required to understand long-term re-colonisation patterns. Researchers and authorities should also endeavour in the implementation of measures that favour and protect survivors and new indigenous colonisers (microbes, plants and animals) after a forest fire.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim and scope
Forest soils can be biologically, chemically and physically affected by forest fires. The occurrence and extension of these effects are mostly determined by the intensity of the fire and the resulting soil temperature. The most immediate effects of a fire are the loss of organic matter by combustion, mainly of the litter layer, the increase of soil temperature and a decrease in water content. An increase in the soil’s pH and a larger exposure to the effects of erosion and landslides is generally also registered. Forest fires can also cause serious damage to ecosystem dynamics due to changes on the amount of nutrients and other elements, as well as their recycling processes (Macadam 1989; Tolhurst et al. 1992; Hall 1994; DeBano et al. 1998; Wichmann et al. 1999).
Edaphic arthropods can also be affected by forest fires; however, the responses of these organisms to fire are very difficult to predict (Friend 1994). Their responses to fire depend upon a variety of factors, including the species studied, their stage of development at the time of fire and their responses to a variety of habitat and community alterations. Differences between studies concerning the impact of forest fires can also be due to differences in intensity and duration of the fires, study methods, or differences in the habitats where the studies have taken place (Coy 1994; Friend 1994; Norris and Conroy 1999). The recovery of the burnt areas takes place by the activity of surviving individuals, along with re-colonisation from non-burnt adjacent areas, and both these processes begin immediately after the fire (Wikars and Schimmel 2001; Bess et al. 2002). Furthermore, Panzer (2003) has shown that recovery times for 22 arthropod species was not compromised by the existence of an obstacle (e.g. road or railway line) between the burnt area and the unburnt refuges.
Every year, in Southern European countries—including Portugal—a large number of forest fires take place. In Portugal alone, more than 420,000 fires consumed 2,714,547 ha of forest between 1980 and 2004 (European Commission 2005), making Portugal the Southern European country with the highest number of fire events and with the highest burnt area per hectare (European Commission 2005; Pereira et al. 2006). These fires can have severe economic and ecologic consequences, and it is therefore essential to study the effects of these disturbances on all ecosystem components, as well as the way their recovery takes place. Several studies have been carried out on the short- and long-term effects of fires in forest soils in Portugal and Spain. Most of these studies focused on post-fire soil erosion, water dynamics and nutrient losses (e.g. Thomas et al. 1999; Coelho et al. 2004; Ferreira et al. 2005), but few have addressed enzyme activity in soils (e.g. Prieto-Fernandez et al. 1993). Microbial soil enzymes are important in organic matter decomposition and nutrient cycling because they catalyze the biochemical reactions involved. They are regulated by several environmental factors (e.g. Pereira et al. 2008), with temperature, moisture, soil pH and nutrient availability being the most important. Microbial activity mediates vital soil biochemical processes, and its quantification is an excellent tool for assessing soil quality (Nielsen and Winding 2002; Miralles et al. 2007), as well as recovery from such disturbing events as forest fires. We hypothesize that intense fires severely impair the edaphic microbiota (see e.g. Staddon et al. 1998); however, the rapid growth of microorganisms should allow a rapid recovery of functional features within a short period of time (months).
The few studies conducted so far on macro-arthropod post-fire recovery in the Iberian Peninsula have been centred only on specific taxa, such as beetles (Garcia-Villanueva et al. 1998; Fernandéz Fernandéz and Salgado Costas 2002) or ants (Garcia et al. 1995; Arnan et al. 2006). To our knowledge, no study has focused on the global recovery of the arthropod community of Iberian forests. The organisms living in the soil superficial layers and in the litter have diverse environmental requirements (e.g. Pereira et al. 2008), life histories, and behaviours, thus showing very different responses after a fire, making it important to study the post-fire activity of the arthropod community as a whole. This work intended to study the short-term recovery of some soil chemical and biochemical (microbial enzymatic activities) parameters and of the edaphic macro-arthropod community in a burnt Pinus pinaster forest in Portugal.
2 Material and methods
Study area and sampling strategy
This study was performed in the northern part of the ‘Mata Nacional de Leiria’. This area is located in central coastal Portugal, and it is one of the most important wood areas in this country. It was one of the first examples of maritime pine (P. pinaster Aiton) monoculture in Portugal and in Europe. Most of this area (approximately 8,700 ha) is used for wood production, but the trees close to the coast play an important role in dune fixation and in the protection of agricultural areas from maritime winds (Páscoa et al. 2004). The area has a Mediterranean climate, with mean annual rainfall of around 880 mm. Mean monthly temperatures for January and July are 9.4°C and 19.4°C, respectively. The soil is described as spodic podzols, derived from sand dunes of maritime origin (Carta de Solos 1978). It is fairly homogeneous, very poor in nutrient elements and with low potential for agriculture (Aguiar et al. 2003; Páscoa et al. 2004).
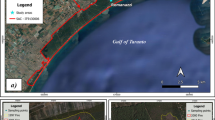
In August 2003, approximately 2,500 ha of forest burned after a wild fire. A small, unburnt area (approximately 3 ha) in Vieira de Leiria remained separated from the burnt forest by a paved road (Fig. 1). These areas were chosen for this study, since the unburnt area could be used as a reference and the re-colonisation of the burnt area by soil arthropods could be followed. The proximity between the burnt and unburnt areas also has the advantage that both areas are similar in terms of elevation, topography, soil type, plant cover and climatic conditions, eliminating any discrepancy that might arise from variations in these parameters when comparing both areas.
Localisation of the study area and sampling scheme. I, II and III represent transects parallel to the road, each comprising three sampling sites. Because of figure size constraints, the relative scale of the diagram is very coarse. To help the reader, we provide the actual distances between transects and sites, within each area (B and U)
Sampling was carried out in two different periods, approximately 3 and 8 months after the fire (November 2003 and April 2004), coinciding with autumn and spring. For both periods, nine sampling sites were chosen on the burnt area (B), along three transects (I, II and III) parallel to the road and nine sites on the unburnt area (U)—see scheme in Fig. 1. Fire direction in B was from III to I (see Fig. 1).
Soil parameters and macro-arthropod community
Soil samples were collected in each of the nine sampling sites from U and B. Samples were gathered from the topsoil layer (upper 10 cm), with caution not to gather any plant material or litter. All collected samples were passed through a 2-mm sieve (discarding coarse materials), and an aliquot was kept frozen for at least 1 month for soil enzyme determination. In the laboratory, moisture, organic matter, pH, and conductivity were freshly determined for each soil sample, according to the procedures described in FAOUN (1984). The activities of the enzymes cellulase and acid phosphatase were determined in the thawed samples, as well as the nitrogen mineralisation rate, following methodologies proposed by Schinner et al. (1996), adapted to microplate reader.
The edaphic community was sampled in the same sampling sites as above in both areas. Sampling was performed using pitfall traps (a total of 18 pitfall traps—one for each sampling site), each one consisting of a cylindrical plastic container with an approximate diameter of 8 cm and a height of 12 cm. A mixture of water and formaldehyde was placed in the pitfall traps in order to capture and preserve the organisms, and some drops of detergent were added to break the superficial tension. Traps were covered with stones and small pieces of wood to minimise rainfall entrance, as well as prevent the capture of small vertebrates and human disturbance. Traps were maintained in the field during approximately 11 days; after this period, their contents were collected and traps were removed. The collected material was then sorted and identified to family level whenever possible, using the identification keys (Bland and Jacques 1978; Jones 1985; Barientos 1988; Chinery 1998). Only adult individuals were identified. Abundance, diversity (Shannon’s H′) and evenness (Pielou’s J′) were estimated for each sample (pitfall trap). Dominance was assessed using Lambshead et al. (1983) k dominance curves.
Data analysis
A comparison between the sampling areas and periods was carried out by means of a two-way analysis of variance (ANOVA), with site and period as factors, followed by a Tukey test. When a site × period interaction was observed, a one-way ANOVA was applied to assess statistical differences between sites within sampling seasons. A principal component analysis (PCA) was performed to address variation (due to site and period) in soil physical, chemical, and biochemical parameters, using CANOCO for Windows version 4.5 (ter Braak and Verdonschot 1995; ter Braak and Smilauer 1998). Before ordination, the environmental variables were standardised to reduce the relative influence of scale. A detrended correspondence analysis (DCA) was also performed, using the same software, in order to obtain a phenological comparison of the different sampling areas based on the taxa found in each of them. This ordination procedure was performed in the log-transformed arthropod community data matrix. Multivariate statistics (PCA and DCA) were used with the aim of reducing the multivariate data matrices to bi-dimensional representations, where patterns are more easily extracted.
3 Results
Soil parameters
There were significant differences between areas for all the soil (chemical and biochemical) parameters measured (Table 1, Fig. 2, and Fig. 3). Only organic matter, pH, and moisture showed significant differences among the two seasons (period), but there was also a significant site × period interaction for pH and moisture (see Table 1). In Fig. 2, it is possible to see, for both sampling periods, that the pH values were always significantly higher in the sites within the burnt area (B). In autumn, there were no significant differences between the B sites; however, in spring, all transects had significant differences from each other, with site BIII displaying a pH closer to the unburnt area (U). The moisture percentage in autumn was significantly higher in transect BIII than in all other sites. In spring, all the B transects had a significantly lower moisture percentage than the U. In addition, all the sampling areas had significantly higher values in autumn, overall (see Table 1, Fig. 2). Organic matter percentage was significantly higher in BIII than in the remaining areas for both seasons; there were no significant differences between the U and the other two B sites (BI and BII). Furthermore, its levels were lower in the spring for all sites. Conductivity values were significantly higher in BI and BIII, compared to U, for both seasons, in spite of large variation in the data.
Physical and chemical parameters of soils from the burnt (BI, BII and BIII) and unburnt (U) area in two periods (autumn and spring). Different letters (a–d) represent statistically significant differences between sites (Tukey test p ≤ 0.05) after one- (pH and moisture) or two-way (conductivity and organic matter) ANOVAs. See text and Table 1 for additional information
Microbiological (enzymatic) activity of soils from the burnt (BI, BII and BIII) and unburnt (U) area in two periods (autumn and spring). Different letters (a and b) represent statistically significant differences between sites (Tukey test p ≤ 0.05) after one- (N mineralisation) or two-way (cellulase and acid phosphatase) ANOVAs. See text and Table 1 for additional information
Relative to the soil enzymes (Fig. 3), the cellulase activity was significantly lower for both periods in transect BI than in the other areas, which had no significant differences between them. Acid phosphatase activity was, for both periods, significantly higher in the U than in all the B sites; there were no significant differences between the three B transects. Nitrogen mineralisation rate was, in spring, significantly higher in the U than in transects BI and BII but not significantly different from transect BIII. Extremely low values (null or close to zero) of this parameter were recorded in autumn.
The PCA biplot (Fig. 4) obtained using the chemical parameters and soil enzymes data matrix separated the sampled areas and the two sampling periods (more distinctly). Site U was associated with high values of acid phosphatase and cellulase and low levels of conductivity and pH, comparatively to the B sites, in both sampling periods. A distinction between autumn and spring is visible on the diagram, for both areas (burnt/unburnt), with autumn samples being associated with lower values of N mineralisation and higher levels of organic matter and soil moisture; the opposite was found for spring samples. The PCA biplot also shows a spatio-temporal pattern, as viewed by the differential scatter of the points: Autumn scores are more dispersed in the diagram than the spring scores, suggesting reduced dissimilarity between sampling sites in the latter period.
Macro-arthropod community
A total of 764 individuals were identified, 226 captured in autumn and 538 in spring, representing 47 different taxa. From these, Linyphiidae spiders (20.2%) and insect families Formicidae (13.4%) and Staphylinidae (11.9%) were the most abundant (see Table 2 for complete list of taxa and respective taxonomical position and abbreviations).
There was a general increase in taxa diversity, richness and total catches, and a general decrease in evenness, between autumn and spring in all sites (Fig. 5), as shown by a significant site-independent effect of period (Table 1). Differences between sites were found for all parameters, except for evenness (Fig. 5, Table 1). Diversity in BII was significantly lower than in U and BI, while total abundance was higher in BI when compared to U. All sites were comparable in terms of richness within each season, with the exception of sites BI (highest richness: autumn, eight taxa; spring, 13 taxa) and BII (lowest richness: autumn, four taxa; spring, eight taxa).
Overall, dominance was stronger in B than in U (although no differences were found in evenness—see above) and dominant taxa varied between seasons (Fig. 6; see also Table 2 for codes). In the autumn, dominant taxa in U were Staphylinidae, Agelenidae, Phalangiidae and Julida, while the most abundant taxa in spring were Formicidae and Linyphiidae. In the burnt area (B), Formicidae was the second dominant taxa in all transects (above 18% of relative abundance) during autumn. During this period (Fig. 6), Silphidae was the most abundant taxon in BI, similarly to Porcellionidae and Formicidae in BII; in BIII, Pseudocaeliciidae displayed strong dominance (about 43%). In spring (also in Fig. 6), however, Linyphiidae and Formicidae were the dominant taxa in all sampling sites (>33% of relative abundance), except for BI, where ant abundance was low. In addition, Staphylinidae was always one of the three most abundant taxa in all B sites.
To address variation (due to season and site) in the arthropod community data, a DCA was carried out with the log-transformed data matrix; the resulting diagram (Fig. 7) shows the first two axes, which together explain 19.4% of the total variation (with lengths of gradient of 2.9 and 2.7, respectively). The DCA biplot showed spatial and temporal segregation of site scores, although this is less clear than in the PCA (see Figs. 4 and 7). Similar to the PCA, autumn scores seem to be more dispersed in the diagram than spring scores, suggesting reduced dissimilarity between sampling sites in the spring.
DCA biplot of species data and sampling sites (stars U, circles BI, triangles BII, squares BIII) in autumn (black symbols) and spring (grey symbols). Taxa legends (abbreviations) appear at the corresponding species scores coordinates. See Table 1 for taxa abbreviations
4 Discussion
Soil parameters
After a forest fire, the soil is enriched with alkaline ashes containing inorganic elements and metallic ions (Macadam 1989; Tolhurst et al. 1992; DeBano et al. 1998). A direct consequence of such an enrichment is the relatively high pH of soils exposed to fire, as observed in this study. A second effect of this ion release is a high conductivity of the soil, as detected here in the B transects in autumn. The variation in the soil’s moisture content between the two periods is directly related with the climatic conditions at the time of sampling; in the days before the autumn sampling, it rained in the study area, thereby resulting in a high moisture percentage. Only in spring was it possible to perceive the effects of the fire in the soil’s permeability and water retention capacity, as all the B transects had significantly lower moisture percentages than the unburnt area (U), indicating that the fire had probably disturbed the soil structure, increasing its permeability. The results also indicated that the soil’s water retention capacity is dependent on its organic matter content, since it is possible to observe a correspondence between the pattern of the organic matter and moisture values along the B transects. This has been previously reported by other authors (e.g. Hall 1994), who noticed that in sandy soils—as the one in this work—much of the water holding capacity and cation exchange capacity are associated with the soil’s organic matter fraction. Organic matter content was unexpectedly high in BIII, despite the fire, and some other soil variables may be associated with this (see below).
Generally, the soil’s acid phosphatase activity is high when the levels of inorganic P in the soil are low, converting organic into inorganic phosphorous (Tabatabai 1994; Staddon et al. 1998). In a fire, the levels of inorganic P, as of other nutrients, are expected to be high due to ash enrichment; therefore, an inhibition of this enzyme is expected under a fire scenario. In this study, low acid phosphatase activity values were found at B transects in autumn. Furthermore, there was no significant variation in phosphatase activity between autumn and spring. After a fire, runoff and leaching losses of some elements, such as Mg2+, Na+ or K+, frequently take place, although this might not occur for P, since it has been reported that the losses of this element due to runoff and leaching can be relatively low (Tolhurst et al. 1992; Hall 1994). Thus, the expected high amount of P remaining in the soil until spring, combined with the high pH, must have contributed to the low phosphatase activity in both seasons.
The cellulase activity in the BII and BIII transects was, already in the autumn sampling, similar to the U, indicating a regular microbial activity. These results showed that either the soil’s decomposing microorganisms were not heavily affected by the fire or that their recovery was considerably faster. It has been reported that the breakdown of cellulose can start shortly after the fire event in a Mediterranean Pinus halepensis forest (Radea and Arianoutsou 2000), and the results from the present work indicate that the same can occur for P. pinaster forests. However, cellulase activity remained clearly compromised in site BI for both seasons.
Null, or close to zero, mineralisation rates in autumn in all sampling sites might have arisen due to disturbances in the enzymatic composition of the soil and/or by unavailability of suitable substrate. In forest soils, organic N availability depends on several factors, such as temporal changes in organic substrates associated with variation in litter production, soil organic matter quality and environmental conditions (Hossain et al. 1995). Furthermore, organic N can be associated with a great variety of complex molecules, and each of these N pools is accessed by specific enzymatic systems, unlike other extracellular enzymes such as phosphatases, which typically have wide substrate preferences (Sinsabaugh 1994). In spring, the N mineralisation rate increased in all the sampled sites, indicating enzymatic recovery and the availability of suitable substrate. Of all sites in the burnt transect, site BIII was the only one that had mineralisation rate values not inferior to U. This could be related to the high organic matter content of BIII.
In general, it was possible to recognise a recovery in the measured soil parameters and enzymatic activities in the interior parts of the burnt area (BII and BIII)—the most distant from the road. Site BIII, in particular, was favoured because some organic matter remained after the fire. However, at the time of the last sampling, approximately 8 months after the fire, transect BI still had considerable differences to the remaining burnt area and, more noticeably, to the unburnt area. Even though the fire had an impact in the entire burnt area, it is likely that the barrier effect caused by the road has probably extended the permanency of the fire in this site, causing more drastic effects in the soil nearby. After the fire, the proximity of the road may have enhanced erosion effects, through the formation of an open area, more exposed to rain and wind action, and organic pollutants from the traffic are a likely cause to the low soil enzyme activities recorded in site BI of the burnt area.
Macro-arthropod community
Although there was no pre-fire sampling in this work to allow a comparison, we assumed that the arthropod community of the two areas was similar before the fire. We therefore hypothesise that the fire in the Pinhal de Leiria reduced the diversity of edaphic arthropods in B, even though this could not be proven in the present study. Our results suggest that post-fire recovery was very fast. However, some evidences provide indications that re-colonisation was still in progress at the stime of the last sampling. In autumn, there was an overall dominance of Formicidae in the entire burnt area. Ants are known to be among the first organisms to colonise areas exposed to some kind of disturbance, as in the case of forest fires, since they are a generalist and opportunist taxon that can efficiently exploit space and resource opportunities created by the disturbance. Several studies in different habitats and fire conditions have recorded higher ant abundances in burnt areas (Garcia et al. 1995; Neville 1999; Bess et al. 2002; Niwa and Peck 2002; Andersen and Müller 2000) and have even detected reduced ant abundance in the absence of prescribed burning in an Australian tropical savannah (Andersen and Muller 2000). In addition, although invasion from neighbouring unburnt areas can occur, their underground habits can offer protection from the increased heat, allowing their in situ survival (Bess et al. 2002; Garcia et al. 1995).
A high abundance of scavengers and carrion feeders was also observed in the burnt area, such as the two dominant beetle taxa, Silphidae and Lathridiidae. These organisms may have taken advantage of the animal mortality caused by the fire, increasing their resource opportunities. Other authors have also observed a high abundance of saprophagous and generalist beetles in burnt areas of P. pinaster (Fernandéz Fernandéz and Salgado Costas 2002), Pinus sylvestris (Wikars and Schimmel 2001) and Quercus pyrenaica (Garcia-Villanueva et al. 1998). The other dominant beetle taxon, Staphylinidae, consists mainly of ground layer predator organisms, which may have benefited from the habitat simplification following the fire: the fire reduced litter depth and other wood debris, creating a more open and structurally simplified area, more suitable for the hunting style of staphylinid beetles (Niwa and Peck 2002).
Two other dominant taxa in autumn were Atypidae and Agelenidae, and they might have survived in situ to the fire, due to their burrowing and hiding abilities as hunters. The taxa Aradidae and Aphididae were found in both sampling seasons only in BI; these apparently pyrophilous taxa were most probably taking advantage of the new food resources. Several species of the Aradidae family feed on the bark of dead trees (Chinery 1998), and the tree mortality caused by the fire would have increased their food resources. As for aphids, which were also a dominant taxon in autumn, these organisms are almost all sap-suckers and preferentially feed on young green plants, where it is easier to extract sap. In B, there were young plants, mainly ferns, already sprouting in autumn that would be a preferential target for aphids, in contrast with the relatively mature trees and shrubs of U.
In spring, 8 months after the fire, the recovery of the arthropod community of the area disturbed by the fire was already in an advanced state. There was an increase in Linyphiidae numbers in all the sampled areas, a taxon considered dependent on the restoration of the litter layer and characteristic of intact forests (Moretti et al. 2002) and, therefore, an indicator of recovery in B. Formicidae was still dominant in all B transects, but the fact that it was also dominant in U reveals that this could be an effect of the season. There was also a high abundance of Staphylinidae in all sites; these ground-layer predators (Garcia-Villanueva et al. 1998) would now have more opportunities to find suitable prey in B, due to the more diversified community.
The first transect of the burnt area, BI, displayed some faunal characteristics similar to the U in autumn: two of the dominant taxa in U—Julida (millipedes) and Phalangiidae (opilionids)—were also captured in BI; additionally, this was the only B transect where Armadillidiidae (isopods) were captured. In spring, there was a re-colonisation of the burnt area by two of these taxa—Phalangiidae and Armadillidiidae—together with others that in autumn only existed in U, such as Neobisiidae (pseudoscorpions). These last two taxa are sensitive to the water content and temperature of the litter (Raw 1967; Radea and Arianoutsou 2000), and their overall presence in B suggests that the forest litter layer had been recovering and was already capable of supporting such sensitive organisms. These evidences suggest that the closer the B transects were to the unburnt area, the higher their similarity relative to U, and thus, the higher their level of recovery. However, this is contrary to what we observed in terms of the physical and chemical characteristics of the burnt soil. Although the road (between U and B) might have delayed soil recovery (see discussion above), the above-mentioned pattern of arthropod colonisation supports strong reasons to believe that the adjacent unburnt area played an important role in the recovery of the arthropod fauna and of the community structure of B, acting as a source of potentially colonising organisms.
5 Conclusions
Soil recovery was fast and various soil functions seemed to be similar between burnt and unburnt areas. Recovery was more evident in spring (8 months after the fire) than in autumn (3 months after the fire). Recovery of soil functional parameters was delayed in the outer zone of the burnt area because of (1) fire intensity in that area or (2) proximity to the road (enhancing erosion and exposure to contaminants). However, in terms of macrofauna, this outer zone was favoured in terms of species re-colonisation, probably due to the proximity to the unburnt area (burnt and unburnt areas were separated by the road). The pattern of arthropod colonisation suggests a vital role of the adjacent unburnt area, in the recovery of edaphic arthropod fauna, acting as a source of potentially colonising organisms.
6 Recommendations and perspectives
Studies on the effect of forest fires on the abiotic and biotic framework of soils are vital to our understanding of the impacts on biodiversity. Particularly, it is important to assess how re-colonisation processes of edaphic fauna occur and the time frame in which they occur. Our case study provides some insight relative to the arthropod community structure, but more prolonged studies are required to understand the long-term re-colonisation patterns (and their relationship with the slow recovery of the vegetation cover). Furthermore, research should progress toward the development of measures that envisage the protection of burnt areas (from physical agents and from human usage), including the use of methodologies favouring insect colonisation from adjacent unburnt areas and protection of extant vegetation and new plant colonisers.
References
Aguiar A, Almeida MH, Borralho N (2003) Genetic control of growth, wood density and stem characteristics of Pinus pinaster in Portugal. Silva Lusit 11(2):131–139
Andersen AN, Müller WJ (2000) Arthropod responses to experimental fire regimes in an Australian tropical savannah: ordinal level analysis. Austral Ecol 25:199–209
Arnan X, Rodrigo A, Retan J (2006) Post-fire recovery of Mediterranean ground ant communities follows vegetation and dryness gradients. J Biogeogr 33:1246–1258
Barrientos JA (1988) Bases para un curso práctico de Entomologia. Associación Española de Entomologia, Dep. de Biologia Animal–Facultad de Biología Animal, Salamanca
Bess EC, Parmenter RR, McCoy S, Molles MC Jr (2002) Responses of a riparian forest-floor arthropod community to wildfire in the middle Rio Grande Valley, New Mexico. Environ Entomol 31(5):774–784
Bland RE, Jacques HE (1978) The pictured key nature series, 2nd edn. Brown, Dubuque, IA
Carta de Solos (1978) Unidades Pedológicas segundo o 1:1000000—Atlas de Ambiente. Comissão Nacional do Ambiente, Portugal
Chinery M (1998) Collins guide to the insects of Britain and Western Europe. Harper Collins, London
Coelho CDOA, Ferreira AJD, Boulet AK, Keizer JJ (2004) Overland flow generation processes, erosion yields and solute loss following different intensity fires. Q J Eng Geol Hydrogeol 37:233–240
Coy R (1994) The impact of fire on soil invertebrates in E. regnans forest at Powelltown, Victoria. In: Proceedings of the conference ‘Fire and biodiversity: the effects and effectiveness of fire management. Victorian National Parks Association, Melbourne, paper no 15
DeBano LF, Neary DG, Ffolliott PF (1998) Fire’s effects on ecosystems. Wiley, New York
European Comission (2005) Forest Fires in Europe 2004. Official publication of the European Commission S.P.I.05.147., European Communities
FAOUN (1984) Food and Agriculture Organization of the United Nations. Physical and chemical methods of soil and water analysis. Soils Bull 10:1–275
Fernandéz Fernandéz M, Salgado Costas JM (2002) Recolonization of a burnt pine tree forest (Pinus pinaster) by edaphic coleoptera. Entomol Gen 26(1):17–28
Ferreira AD, Coelho COA, Boulet AK, Leighton-Boyce G, Keizer JJ, Rietsema CJ (2005) Influences of burning intensity on water repellency and hydrological processes at forest and shrub sites in Portugal. Aust J Soil Res 43(3):327–336
Friend G (1994) Fire ecology of invertebrates—implications for nature conservation, fire management and future research. In: Proceedings of the conference ‘Fire and biodiversity: the effects and effectiveness of fire management. Victorian National Parks Association, Melbourne, paper no 13
Garcia JA, Ena V, Mediavilla G, Tarrega R (1995) Explotación post-fuego por hormigas (Hymenoptera, Formicidae) en ecosistemas de Quercus pyrenaica. Avances en Entomologia Iberica, pp 91–100
Garcia-Villanueva JA, Ena V, Tarrega R, Mediavilla G (1998) Recolonization of two burnt Quercus pyrenaica ecosystems by coleoptera. Int J Wildland Fire 8(1):21–27
Hall RG (1994) The effects of fuel reduction burning on forest soils. In: Proceedings of the conference Fire and biodiversity: the effects and effectiveness of fire management. Victorian National Parks Association, Melbourne, paper no 16
Hossain AK, Raison RJ, Khanna PK (1995) Effects of fertilizer application and fire regime on soil microbial biomass carbon and nitrogen, and nitrogen mineralization in an Australian subalpine eucalypt forest. Biol Fert Soils 19:246–252
Jones D (1985) Guia de campo de los aracnidos de España y de Europa. Ediciones Omega S.A., Barcelona
Lambshead PJD, Platt HM, Shaw KM (1983) The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J Nat Hist 17:859–874
Macadam A (1989) Effects of prescribed fire on forest soils. B.C. Min. For. Research Report 89001-PR, Victoria, Australia
Miralles I, Ortega R, Sánchez-Marañón M, Leirós MC, Trasar-Cepeda C, Gil-Stores F (2007) Biochemical proporties of range and forest soils in Mediterranean mountain environments. Biol. Fertil Soils 43:721–729
Moretti M, Conedera M, Duelli P, Edwards PJ (2002) The effects of wildfire on ground-active spiders in deciduous forests on the Swiss southern slope of the Alps. J Appl Ecol 39:321–336
Neville PJ (1999) The effects of prescribed burning and wildfire on epigaeic invertebrates of the Dandenong Ranges National Park, Victoria, Australia. In: Lunt I, Green DG, Lord B, editors. Conference Proceedings of the Australian Bushfire Conference ‘Bushfire 99’, Albury, pp 291–297
Nielsen MN, Winding A (2002) Microorganisms as indicators of soils health. National Environmental Research Institute, Denmark. Technical Report No 388
Niwa CG, Peck RW (2002) Influence of prescribed fire on carabid beetle (Carabidae) and spider (Araneae) assemblages in forest litter in southwestern Oregon. Environ Entomol 31(5):785–796
Norris P, Conroy B (1999) Fire ecology of soil and leaf litter invertebrates. In: Lunt I, Green DG, Lord B (eds), Conference Proceedings of the Australian Bushfire Conference ‘Bushfire 99’, Albury, pp 299–306
Panzer R (2003) Importance of in-situ survival, recolonization, and habitat gaps in the postfire recovery of fire-sensitive prairie insect species. Nat Area J 23(1):14–21
Páscoa F, Martins F, González RS, João C (2004) Estabelecimento simultâneo de equações de biomassa para o pinheiro bravo. In: II Latin American Symposium on Forest Management and Economics, Barcelona
Pereira JMC, Carreiras JMB, Silva JMN, Vasconcelos MJ (2006) Alguns conceitos básicos sobre os fogos rurais em Portugal. In: Pereira JS, Pereira JMC, Rego FC, Silva JMN, Silva TP (eds) Incêndios florestais em Portugal—Caracterização, impactes e prevenção. ISA Press, Lisbon, pp 133–161
Pereira R, Marques SM, Antunes SC, Marques C, Abrantes N, Pestana JL, Gonçalves F (2008) Comparison of Portuguese soils from different geographical regions using physicochemical, biological and biochemical parameters. J Soil Sediments 8(2):106–115
Prieto-Fernandez A, Villar MC, Carballas M, Carballa T (1993) Short-term effects of a wildfire on the nitrogen status and its mineralization kinetics in an Atlantic forest soil. Soil Biol Biochem 25(12):1657–1664
Radea C, Arianoutsou M (2000) Cellulose decomposition rates and soil arthropod community in a Pinus halenpensis Mill. forest of Greece after a wildfire. Eur J Soil Biol 36:57–64
Raw F (1967) Arthropoda (except Acari and Collembola). In: Burges A, Raw F (eds) Soil biology. Academic, London, pp 323–362
Schinner F, Kandeler E, Öhlinger R, Margesin R (1996) Methods in soil biology. Springer, Germany
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fert Soils 17:69–74
Staddon WJ, Duchesne LC, Trevors JT (1998) Acid phosphatase, alkaline phosphatase and arylsulfatase activities in soils from a jack pine (Pinus banksiana Lamb.) ecosystem after clear cutting, prescribed burning, and scarification. Biol Fertil Soils 27:1–4
Tabatabai MA (1994) Soil Enzymes. In: Weaver RW, Angle S, Bottomley P (eds) Methods of soil analysis, Part 2—Microbiological and biochemical properties, Chap. 37. Soil Sciences Society of America, Wisconsin
ter Braak CJF, Smilaeur P (1998) CANOCO reference manual and user’s guide to Canoco for Windows: Software for Canonical Community Ordination (version 4). Microcomputer Power, Ithaca, NY, USA, p 352
ter Braak CJF, Verdonschot PFM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289
Thomas AD, Walsh RPD, Shakesby RA (1999) Nutrient losses in eroded sediment after fire in eucalyptus and pine forests in the wet Mediterranean environment of northern Portugal. Catena 36(4):283–302
Tolhurst KG, Flinn DW, Loyn RH, Wilson AAG, Foletta I (1992) Ecological effects of fuel reduction burning in a dry sclerophyll forest—a summary of principal research findings and their management implications. Forest Research Centre, Department of Conservation And Environment, Melbourne
Wichmann H, Schmidt-Nädler C, Bahadir M (1999) Fast elemental analysis to assess dioxin contamination: a new tool for the investigation of damages caused by fire. Umweltwiss Schadst Forsch 11(1):33–37
Wikars L-O, Schimmel J (2001) Immediate effects of fire-severity on soil invertebrates in cut and uncut pine forests. Forest Ecol Manag 141:189–200
Acknowledgements
The authors wish to thank Dr. P.J. van Helsdingen from the National Museum of Natural History/Naturalis (The Netherlands), for helping in spider identification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Scott Chang
Rights and permissions
About this article
Cite this article
Antunes, S.C., Curado, N., Castro, B.B. et al. Short-term recovery of soil functional parameters and edaphic macro-arthropod community after a forest fire. J Soils Sediments 9, 267–278 (2009). https://doi.org/10.1007/s11368-009-0076-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0076-y