Abstract
Purpose
This life cycle assessment evaluates and quantifies the environmental impacts of renewable chemical production from forest residue via fast pyrolysis with hydrotreating/fluidized catalytic cracking (FCC) pathway.
Methods
The assessment input data are taken from Aspen Plus and greenhouse gases, regulated emissions, and energy use in transportation (GREET) model. The SimaPro 7.3 software is employed to evaluate the environmental impacts.
Results and discussion
The results indicate that the net fossil energy input is 34.8 MJ to produce 1 kg of chemicals, and the net global warming potential (GWP) is −0.53 kg CO2 eq. per kg chemicals produced under the proposed chemical production pathway. Sensitivity analysis indicates that bio-oil yields and chemical yields play the most important roles in the greenhouse gas footprints.
Conclusions
Fossil energy consumption and greenhouse gas (GHG) emissions can be reduced if commodity chemicals are produced via forest residue fast pyrolysis with hydrotreating/FCC pathway in place of conventional petroleum-based production pathways.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aromatics and olefins are two main types of petrochemicals and have a wide range of applications. Aromatics can be used to produce a number of petrochemical intermediates including styrene, phenol, purified terephthalic acid (PTA), and phthalic anhydride (PA) (ICIS 2013a). The most important commercial olefins are ethylene, propylene, and butadiene, all of which can be used to manufacture rubbers, polymer resins, and other chemical intermediates (ICIS 2013b). In 2012, the aromatics production capability of the US petroleum refining sector reached 296,911 barrels per day (EIA 2012b). The demand for light olefins in the USA is expected to increase at a compounded annual growth rate (CAGR) of 3.4 % over the period 2010–2020, reaching approximately 68.4 million tons by 2020 (GBI Research 2012). Petrochemicals are generally produced from fossil feedstocks such as the refining of crude oil or the processing of natural gas. Global petrochemical feedstock consumption is projected to rise through 2025 with the greatest proportion coming from naphtha and coal, increasing about 100 million tons and 70 million tons by 2025, respectively (CHEMSYSTEMS 2013). Among all the regions, North America leads in the feedstock consumption, accounting for 17 % of world feedstock consumption in 2010 (CHEMSYSTEMS 2013). The US Energy Information Administration (EIA) has projected a 2.4 % annual increase for crude oil prices from 2010 to 2035 (EIA 2012a). The growing greenhouse gas (GHG) emissions along with projected growth in consumption of fossil feedstocks drive an increasing interest in investigating alternative feedstocks for commodity chemical production. A variety of alternative feedstocks have been under consideration, including coal, unconventional natural gas, plastic waste, tar and heavy oil, and biomass (Ren and Patel 2009; McFarlane and Robinson 2007). Feedstocks of nonfossil resources (lignocellulosic biomass, etc.) within the petrochemical industry have also gained increasing interest (Brehmer et al. 2009; Christensen et al. 2008; Dale 2003; Gavrilescu and Chisti 2005; Schilling 1995).
Biomass can be converted into basic chemical compounds through thermochemical conversion (gasification, pyrolysis, and liquefaction/hydrothermal upgrading) or biochemical conversion (fermentation and anaerobic digestion) (Fahmi and Cremaschi 2012). The biochemical process is a complex process, and there are technical issues and challenges, such as low bulk density feedstock, high viscosity substrate, high enzymes cost, and low fermentability of some substrates (Stephanopoulos 2007; Lin and Tanaka 2006; Chen 2012). The energy use and CO2 emissions for basic chemical production from biomass are fully examined by Ren and Patel (2009). The pathways examined in that study include steam cracking of naphtha derived from the Fischer-Tropsch processing (FT) of syngas derived from biomass gasification, dehydration of ethanol (ethanol is derived from biomass via syngas fermentation or via direct fermentation), and the processing of methanol to olefins (methanol is derived from biomass via syngas). For thermochemical conversion, the process of chemical production from biomass gasification is relatively slow and typically requires large, complicated, and expensive equipment (Xu et al. 2008; Salge et al. 2006).
Brehmer et al. (2009) utilized a limited energetic and exergetic analysis with life cycle assessment (LCA) to evaluate the maximum fossil fuel feedstock replacement potential and concluded that the best potential for biomass to replace fossil fuel would be as an alternative feedstock source for the petrochemical industry. Hermann et al. (2007) analyzed the CO2 emissions and fossil energy use for bulk chemical production using industrial biotechnology and found that more than 100 % savings in nonrenewable energy use and greenhouse gas emissions would be possible with current state of the art biotechnology. Hipolito (2011) evaluated the environmental loads associated to the production of chemicals (phenolic compounds, acetone, polyhydroxybutyric acid, and polylactic acid) from lignocellulose biomass scenarios and compared these environmental loads to the petrochemical equivalents. Hipolito (2011) concluded that the production of chemicals from biomass could contribute to possible reductions between 37 and 48 % on greenhouse gas emissions, and up to 80 % fossil fuel can be saved while ecotoxicity indicators present much lower values for the production of chemicals from biomass. But chemicals from biomass may increase other impact categories such as eutrophication and acidification, human toxicity, photochemical oxidant formation, water depletion, and particulate matter formation. A number of other similar LCA studies discussed the environmental impacts of the production of biopolymers or bioplastics from biomass (Heyde 1998; Dornburg et al. 2003; Groot and Borén 2010; Piemonte 2011; Kim and Dale 2008; Lynd and Wang 2003). Yates and Barlow (2013) made a thoughtful review from these existing biopolymer production LCA studies especially for polylactic acid (PLA), polyhydroxyalkanoates (PHA), and starch-based biopolymers. It is demonstrated as well that reductions in nonrenewable energy use and GHG emissions can be achieved from biopolymer production, but higher impacts in other categories were commonly reported (Yates and Barlow 2013).
Although some LCA studies have been done on the intermediate chemicals or biopolymers, few LCA studies have been reported on basic commodity chemical (benzene, toluene, xylene, etc.) production from biomass. Recently, fast pyrolysis has attracted considerable interest as a means for converting biomass into valuable fuels or energy. With the growing interest in fast pyrolysis technology, the related economic and environmental impacts of pyrolysis-based energy systems are under extensive examination (Kauffman et al. 2011; Heracleous 2011; Zhong et al. 2010; Hsu 2012; Fan et al. 2011; Iribarren et al. 2012; Zhang et al. 2013b, c). Vispute et al. (2010) combined a two-stage hydrotreating process with FCC to form an integrated catalytic process for the high-yield production of valuable commodity chemicals (i.e., olefins and aromatics). Zhang et al. (2013c) compared this chemical production pathway with the transportation fuel production from the fast pyrolysis pathway and found that the internal rate of return (IRR) of the chemical production pathway is much higher than that of the transportation fuel production pathway. In this paper, life cycle assessment is conducted to evaluate and quantify the environmental impacts of renewable commodity chemical production via forest residue fast pyrolysis and hydrotreating/FCC upgrading. A variety of environmental impacts are evaluated, including the fossil energy input, GHG emissions, acidification, ozone depletion, ecotoxicity, smog, etc.
2 Description for the chemical production pathway
In the chemical production pathway, the bio-oil yield is assumed to be 52 % of the dry mixed wood, based on the previous techno-economic analysis study (Zhang et al. 2013c). Bio-oil generation from the raw feedstock includes five steps: biomass preprocessing, biomass fast pyrolysis, bio-oil recovery, solids removal, and heat generation. In the biomass preprocessing step, the raw biomass is assumed to be 25 wt% moisture and chopped to a 10-mm diameter. It is dried to 7 wt% moisture and ground to 3 mm diameter particles. Steam generated in the combustion area is used to dry the biomass, and a 1 % makeup is assumed to compensate for condensate losses. In the fast pyrolysis step, biomass particles are converted into noncondensable gases, bio-oil vapors, and solid char phases in a fluidized bed reactor operating at 500 °C and ambient pressure. Bio-oil vapors are recovered using a condenser and an electrostatic precipitator. In the solids removal step, the pyrolysis products are cleaned by removing 90 % of the entrained char and solid particles through cyclones. Finally, in the combustion step, the noncondensable gases are combusted to provide the heat for the pyrolysis process. The Aspen Plus model shows that there is enough heat available from the noncondensable gas combustion. The excess char is treated as a low-value coal substitute for local usage such as heat generation. Portions of the combusted noncondensable gases are compressed and sent to the pyrolyzer as the carrier gases.
After bio-oil production, a two-stage hydrotreating and FCC process is employed to upgrade the aqueous phase of the bio-oil to commodity chemicals. Process configurations are based on Vispute et al. (2010). A liquid-liquid (L-L) extractor is employed to separate the whole bio-oil into the aqueous phase and the water-insoluble fraction. The aqueous phase undergoes a two-stage hydrotreating process, which consists of a low-temperature hydrotreatment and a high-temperature hydrotreatment. The low-temperature hydrotreatment stage is carried out in a hydrotreater operating at 125 °C and 100 bar pressure, and the high-temperature hydrotreatment stage is carried out at 250 °C and 100 bar pressure. After the two-stage hydrotreating process, fluidized catalytic cracking is performed on the hydrotreated aqueous phase of the bio-oil over HZSM-5 catalyst at 600 °C. The FCC process converts the aqueous phase to commodity chemicals. Natural gas is steam-reformed to produce hydrogen via a two-stage catalytic process, and the hydrogen is used for the hydroprocessing steps. Because natural gas contains enough sulfur to poison the reforming catalysts, reforming is preceded by a desulfurization step. In the first stage of reforming, steam reacts with the natural gas in a steam methane reformer (SMR) at 700–1,100 °C to yield syngas. In the second stage, low-temperature water-gas shift (WGS) reaction occurs at about 250 °C to generate the hydrogen from the syngas. The hydrogen is separated from the syngas through a pressure swing adsorption (PSA) unit and the rest of the gas is treated as exhaust gas. The water-insoluble fraction is mainly the pyrolytic lignin, which is assumed to be a coal substitute and consumed locally.
3 LCA goal and scope definition
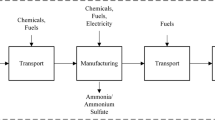
The goal of this LCA study is to analyze the environmental impacts of the chemical production from forest residue via fast pyrolysis with hydrotreating/FCC upgrading. The system for this LCA is divided into five unit processes: biomass production, biomass transportation, biomass preprocessing, bio-oil production, and bio-oil upgrading. The system boundary is illustrated in Fig. 1, which contains land, energy, and material resources consumptions as well as air emissions, solid waste, and water emissions in the process. Bio-oil production unit is a combination of biomass fast pyrolysis, bio-oil recovery, solids removal, and heat generation, which is shown as the sub-block in the system boundary diagram. The biomass fast pyrolysis and upgrading facility is assumed to be an integrated facility, where the biomass is preprocessed and converted to intermediate bio-oil that is ultimately upgraded to the commodity chemicals. Electricity usage is assumed to be medium voltage electricity produced in the USA. The indirect land-use change effects and chemical distributions are not considered in the LCA study. This study is a generic study for chemical production from forest residue fast pyrolysis and location-specific energy carriers associated with feedstocks are not considered.
The Aspen Plus model for the chemical production process is based on a previous process model of chemical production via fast pyrolysis (Brown et al. 2012; Zhang et al. 2013c). In this study, the bio-oil recovery modeling has been updated. The modified Aspen Plus model assumes an nth plant facility with mature technology processing 2,000 metric tons/day of dry mixed wood feedstock. SimaPro 7.3 is used to develop and link the primary unit processes related to the current chemical production pathway. Materials and energy associated with the unit processes are collected as the inventory data which is set as primary materials and energy in SimaPro 7.3. The built-in Ecoinvent v.2.2 database in SimaPro 7.3 provides life cycle inventories for secondary materials and energy required in those primary unit processes. The required primary materials and energy inputs associated with the unit processes of the LCA are derived from this modified Aspen Plus model in combination with a greenhouse gases, regulated emissions, and energy use in transportation (GREET) model (Argone National 2011). The functional unit for interpreting the LCA results is 1 kg of chemicals. The IPCC 2007 GWP 100a method is employed to calculate the GWP. The cumulative energy demand (CED) method is used to estimate the fossil energy input for the chemical production pathway. Other potential environmental impacts (smog, acidification, ecotoxicity, etc.) are evaluated with the TRACI 2 method.
4 Inventory analysis
4.1 Biomass collection and biomass transportation
The biomass feedstock in this analysis is assumed to be forest residue. The input data for the biomass collection is detailed in Table 1 (based on data from Fan et al. (2011)). In this study, forest residue is treated as a waste product. The inputs for biomass collection include the fuel consumption associated with the forwarding and the grinding of the biomass and the equipment involved (Fan et al. 2011).
The wet forest residue, which contains 25 wt% moisture, is transported by 40-ton trucks. The total weight of the transported wet forest residue is 2,670 metric tons and the transportation distance is 150 miles one way, based on the GREET model developed by Argone National (2011). Detailed input data for the biomass transportation are summarized in Table 2.
4.2 Biomass preprocessing
Steam is employed in the dryer to remove water from the wet forest residue. We assume a makeup of steam for biomass drying process, which is 1 % of the recycling steam. For the inventory analysis of biomass preprocessing, electricity and steam usage are considered as the material inputs. Water vapor from the drying process is treated as an emission to the environment. Detailed inventory data are shown in Table 3.
4.3 Bio-oil production
The inventory analysis for bio-oil production is shown in Table 4. Noncondensable gases generated during fast pyrolysis process are combusted to provide sufficient heat for the pyrolyzer. Char generated in pyrolysis is treated as coal substitute with an assumed heating value that is 50 % of the displaced coal. Electricity, air, and process water are considered to be inputs, while bio-oil and char are considered to be outputs. Process water is mainly used in bio-oil recovery and air is employed to support combustion of noncondensable gases. Emissions from bio-oil production include carbon oxides, hydrocarbons, nitrogen oxides, sulfide, and ash. Ash separated from the cyclones is used in sanitary landfills as waste treatment.
4.4 Bio-oil upgrading
The final products from the hydrotreating/FCC pathway are aromatics and olefins. The total yield of chemicals is 243 metric tons/day. Pyrolytic lignin generated from bio-oil separation is treated as coal substitute with an assumed heating value that is 50 % of the displaced coal. The inventory inputs involved in this step are as follows: air, catalysts, process water, electricity, natural gas, and bio-oil. Natural gas is used to produce the hydrogen for the two-stage hydrotreating processes. Process water includes the water used for gas cooling and separation processes. Wastewater from bio-oil upgrading is assumed to be sent to a wastewater treatment plant. Details of the inventory analysis are shown in Table 5. Chemical products include aromatics and olefins like benzene, toluene, and xylene. Table 6 details the composition of the commodity chemical.
5 Results and discussion
5.1 Fossil energy
Figure 2 includes the breakdown of fossil energy input for individual unit processes for chemical production. Fossil energy input is separated into two parts: required fossil energy and product credits (shown as negative fossil energy input). The bio-oil production and bio-oil upgrading steps consume the largest portion of the required fossil energy, each representing 28 % of the total fossil energy. Biomass transportation and biomass production consume relatively smaller portions, 11 and 7 % of the total required fossil energy input, respectively.
Conventional chemicals are petroleum-based products. In this study, chemicals are produced from renewable feedstocks and treated as substitutes for petroleum-based chemicals. Biochar generated in pyrolysis contributes as a credit, which reduces fossil energy input by 12.1 MJ per kg chemical production. Pyrolytic lignin generated in bio-oil upgrading process also contributes as a credit, which reduces fossil energy input by 14.3 MJ per kg chemical production. Accounting for all fossil energy inputs and credits, net fossil energy input is 34.8 MJ per kg chemical production.
5.2 GHG emissions
The contributions of the unit processes to GWP are shown in Fig. 3. Contributions to GWP have two parts: positive GWP and negative GWP. Positive GWP contributions come from biomass production, biomass transportation, biomass preprocessing, bio-oil production, and bio-oil upgrading. Negative GWP contributions come from carbon fixation by biomass, char credit, and pyrolytic lignin credit. For biomass absorption, it is assumed that the atmosphere can take up 0.942 kg CO2 per kg of forest residue during the biomass growth (Hsu et al. 2010). Biochar and pyrolytic lignin are treated as credits since they are used as coal substitute.
Total positive GWP is 9.2 kg CO2 eq. per kg chemical production. Among all of the unit processes, bio-oil production step has the largest GHG emissions, contributing 57 % of the total positive GWP. Bio-oil upgrading and biomass preprocessing steps represent 23 and 14 % of the total positive GWP, respectively. Biomass transportation contributes 5 % of the total positive GWP, and biomass production makes the smallest contribution (0.6 %) to GHG emissions.
Total CO2 absorption by the forest residue is 8.33 kg CO2 eq. per kg of chemicals. Biochar and pyrolytic lignin save 0.076 and 1.33 kg CO2 eq. per kg chemicals, respectively. As a result, net GWP is −0.53 kg CO2 eq. per kg chemicals.
5.3 Other impact categories
Table 7 presents the potential environmental impacts evaluated with the TRACI 2 method, and the relative contributions of individual unit processes to potential environmental impacts are presented in Fig. 4. Biomass transportation has the largest impact on ozone depletion, contributing 86 % of the total impact. Bio-oil production has the greatest impact on eutrophication, contributing 85 % to the total impact. Bio-oil upgrading contributes almost the rest of the 15 % of the eutrophication. During the bio-oil production and bio-oil upgrading processes, nitrogen gases are emitted which results in the large eutrophication impacts. Char has larger contributions to reductions of ecotoxicity and carcinogenics compared to other environmental impacts due to offsetting fossil energy input.
5.4 Comparison to other chemical production pathways
Table 8 describes several pathways selected for comparison. Generally, steam cracking is considered the key petrochemical conversion pathway (IEA 2004). Naphtha steam cracking routes based on Ren and Patel (2009) are selected for comparison. In these pathways, a variety of energy sources, including crude oil, natural gas, coal, and lignocellulosic biomass, are considered for the chemical production. Table 9 compares the GHG emissions and fossil energy input in this study to the results from other production pathways.
As indicated in Table 9, among all of the pathways, coal-based pathways (coal FT naphtha SC I, coal FT naphtha SC II, and coal direct naphtha SC) have the greatest GHG emissions and fossil energy inputs. Coal-based pathways produce chemicals via steam cracking of coal-derived naphtha. Coal-based pathways can consume three to eight times more fossil energy and release five to eight times more GHG emissions than other petroleum-based (oil, natural gas, etc.) pathways. Lignocellulose biomass-derived chemicals have negative GHG emissions (included the carbon fixation from biomass growth) and negative fossil energy input. Ligno FT naphtha pathways produce chemicals via steam cracking of FT naphtha derived from biomass gasification. Ligno FT naphtha II represents the maximum co-generation of electricity and the minimum output of FT liquids, and it has the largest saving of GHG emissions and fossil energy input. Ligno FT naphtha I, on the other hand, represents the minimum co-generation of electricity and the maximum output of FT liquids. This indicates that electricity production from biomass-derived synthesis gas could save larger GHG emissions as well as fossil energy. Other petroleum-based pathways (oil, natural gas, etc.) have comparable smaller GHG emissions and fossil energy than coal-based pathways but larger GHG emissions and fossil energy than lignocellulose biomass-based pathways.
Compared to the pathways in the literature and practice, the GHG emissions related to the current pathway are smaller than all the petroleum-based chemical production pathways (coal, natural gas, oil, etc.). This is because carbon fixation from biomass growth for current production pathway significantly reduces the GHG emissions compared to petroleum-based pathways. Also, the fossil energy input for the current pathway is smaller than the coal-based pathways. But compared to chemical production via steam cracking of biomass-derived naphtha pathways, the fast pyrolysis process has larger GHG emissions and fossil energy input.
5.5 Sensitivity analysis
We select bio-oil yield, chemicals yield, electricity consumption, biomass transportation distances, and catalysts as the operating parameters for sensitivity analysis according to the previous techno-economic analysis (Zhang et al. 2013a) as well as their contributions in the inventory input. Each of these operating parameters is changed by a prescribed amount in relation to the baseline operating conditions (expressed as a percentage of the baseline case ranging from 75 to 125 %) to analyze the impact on GHG emissions.
The overall GWP ranges from −2.1 to 2.1 kg CO2 eq., as indicated in Fig. 5. Commodity chemicals are the main products, so the yield of commodity chemicals has the most significant impact on the GHG emissions. The increase of bio-oil yield could increase the final yields of chemicals so it is also a significant parameter for GHG emissions. A variation of bio-oil yield from 125 to 75 % results in a −1.9- to 1.8-kg CO2 eq. kg chemical range of GWP. Electricity consumption for biomass pyrolysis and bio-oil preprocessing and coal substitution by pyrolytic lignin are the secondary important parameters for GHG emissions. This is because electricity consumption for biomass pyrolysis and preprocessing occupies the majority of inventory inputs. Pyrolytic lignin yield is considerable so the effect of coal substitute from pyrolytic lignin is important for GHG emissions. If electricity consumption for biomass pyrolysis or pyrolytic lignin has a variation of ±25 % on the baseline, GWP varies from −0.9 to −0.2 kg CO2 eq. per kg chemicals. Biomass upgrading electricity consumption and transportation distance have comparably smaller impact on the GHG emissions. A variation of ±25 % in biomass upgrading electricity consumption and transportation distance results in a −0.6- to −0.5-kg CO2 eq. per kg chemical range of GWP. Quantity of catalysts used has very little impact on GWP.
The results of sensitivity analysis show that chemical yield and bio-oil yield are the most sensitive GHG emission factors which indicates the increase of plant efficiency for the pyrolysis process or bio-oil upgrading to chemicals process could largely improve the GHG emissions. In addition, the reduction of electricity consumptions related to the production processes could also reduce the carbon footprints.
6 Conclusions
The life cycle assessment of chemical production via forest residue fast pyrolysis and hydrotreating/FCC upgrading is examined in this study. Renewable chemicals are evaluated as substitutes for fossil-based chemicals and char is treated as a substitute for coal. Among the production processes, bio-oil production is the key driver of the fossil energy input and GHG emissions. Bio-oil production contributes the largest GHG footprint (57 % of total positive GWP) and the largest fossil energy demand (28 % of the total required fossil energy) among all unit processes. Total fossil energy input is 34.8 MJ per kg chemicals and GWP is −0.53 kg CO2 eq. per kg chemicals. In contrast to conventional petrochemical production pathways, fossil energy input and GWP are smaller, which indicates that fossil energy consumption and GHG emissions could be reduced if commodity chemicals are produced via forest residue fast pyrolysis with hydrotreating/FCC upgrading processes. Biomass transportation makes the largest contribution to ozone depletion, and bio-oil production makes the largest contribution to eutrophication. Sensitivity analysis indicates that bio-oil yield and chemical yields play the most important roles in determining GHG emissions.
References
Argone National Laboratory (2011) The greenhouse gases, regulated emissions, and energy use in transportation (GREET) model. http://greet.es.anl.gov/Accessed Aug 2013
Brehmer B, Boom RM, Sanders J (2009) Maximum fossil fuel feedstock replacement potential of petrochemicals via biorefineries. Chem Eng Res Des 87(9):1103–1119
Brown TR, Zhang Y, Hu G, Brown RC (2012) Techno-economic analysis of biobased chemicals production via integrated catalytic processing. Biofuels Bioprod Biorefin 6(1):73–87
CHEMSYSTEMS (2013) Feedstock options for the petrochemical industry. http://www.chemsystems.com/about/cs/news/items/PPE%20PCMD%20Feedstocks%202011.cfmAccessed Aug 2013
Chen Y (2012) Biomass to fuels: thermo-chemical or bio-chemical conversion? Ferment Technol 1 (2):e104. http://www.omicsgroup.org/journals/2167-7972/2167-7972-1-e104.pdf
Christensen CH, Rass-Hansen J, Marsden CC, Taarning E, Egeblad K (2008) The renewable chemicals industry. ChemSusChem 1(4):283–289
Dale BE (2003) ‘Greening’ the chemical industry: research and development priorities for biobased industrial products. J Chem Technol Biotechnol 78(10):1093–1103
Dornburg V, Lewandowski I, Patel M (2003) Comparing the land requirements, energy savings, and greenhouse gas emissions reduction of biobased polymers and bioenergy. J Ind Ecol 7(3–4):93–116
EIA (2012a) Annual energy outlook. http://www.eia.gov/Accessed Aug 2013
EIA (2012b) Production capacity of operable petroleum refineries. http://www.eia.gov/dnav/pet/PET_PNP_CAPPROD_A_(NA)_8PR_BSD_A.htmAccessed Aug 2013
Fahmi I, Cremaschi S (2012) Stage-gate representation of feedstock development for chemical process industry. Foundations of Computer-Aided Process Operations. http://focapo.cheme.cmu.edu/2012/proceedings/data/papers/012.pdf
Fan J, Kalnes TN, Alward M, Klinger J, Sadehvandi A, Shonnard DR (2011) Life cycle assessment of electricity generation using fast pyrolysis bio-oil. Renew Energy 36(2):632–641
Gavrilescu M, Chisti Y (2005) Biotechnology—a sustainable alternative for chemical industry. Biotechnol Adv 23(7–8):471–499
GBI Research (2012) Light Olefins Americas Market to 2020—cost effective ethylene from shale gas-derived ethane and on-purpose technology for propylene to promote industry growth. http://www.marketresearch.com/GBI-Research-v3759/Light-Olefins-Americas-Cost-Effective-6744067/Accessed Aug 2013
Groot W, Borén T (2010) Life cycle assessment of the manufacture of lactide and PLA biopolymers from sugarcane in Thailand. Int J Life Cycle Assess 15(9):970–984
Heracleous E (2011) Well-to-wheels analysis of hydrogen production from bio-oil reforming for use in internal combustion engines. Int J Hydrogen Energy 36(18):11501–11511
Hermann BG, Blok K, Patel MK (2007) Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ Sci Technol 41(22):7915–7921
Heyde M (1998) Ecological considerations on the use and production of biosynthetic and synthetic biodegradable polymers. Polym Degrad Stab 59(1–3):3–6
Hipolito MG (2011) Life cycle assessment of platform chemicals from fossil and lignocellulosic biomass scenarios. http://www.diva-portal.org/smash/get/diva2:458493/FULLTEXT01.pdf
Hsu DD (2012) Life cycle assessment of gasoline and diesel produced via fast pyrolysis and hydroprocessing. Biomass Bioenergy 45:41–47
Hsu DD, Inman D, Heath GA, Wolfrum EJ, Mann MK, Aden A (2010) Life cycle environmental impacts of selected U.S. ethanol production and use pathways in 2022. Environ Sci Technol 44(13):5289–5297
ICIS (2013a) Aromatics & aromatic chemicals information from ICIS. http://www.icis.com/v2/chemicals/petrochemicals_aromatics.aspxAccessed Aug 2013
ICIS (2013b) Olefins & olefin chemicals information from ICIS. http://www.icis.com/V2/chemicals/petrochemicals_olefins.aspxAccessed Aug 2013
IEA (2004) Energy statistic manual. http://www.iea.org/training/toolsandresources/energystatisticsmanual/Accessed Aug 2013
Iribarren D, Peters JF, Dufour J (2012) Life cycle assessment of transportation fuels from biomass pyrolysis. Fuel 97:812–821
Kauffman N, Hayes D, Brown R (2011) A life cycle assessment of advanced biofuel production from a hectare of corn. Fuel 90(11):3306–3314
Kim S, Dale BE (2008) Energy and greenhouse gas profiles of polyhydroxybutyrates derived from corn grain: a life cycle perspective. Environ Sci Technol 42(20):7690–7695
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69(6):627–642
Lynd LR, Wang MQ (2003) A product-nonspecific framework for evaluating the potential of biomass-based products to displace fossil fuels. J Ind Ecol 7(3–4):17–32
McFarlane J, Robinson S (2007) Survey of alternative feedstocks for commodity chemical manufacturing. Oak Ridge National Laboratory Report No. ORNL/TM-2007/28 http://info.ornl.gov/sites/publications/files/Pub8760.pdf
Piemonte V (2011) Bioplastic wastes: the best final disposition for energy saving. J Polym Environ 19(4):988–994
Ren T, Patel MK (2009) Basic petrochemicals from natural gas, coal and biomass: energy use and CO2 emissions. Resour Conserv Recycl 53(9):513–528
Salge JR, Dreyer BJ, Dauenhauer PJ, Schmidt LD (2006) New process to produce completely renewable fuels and chemicals. http://scitizen.com/future-energies/new-process-to-produce-completely-renewable-fuels-and-chemicals_a-14-235.htmlAccessed Aug 2013
Schilling LB (1995) Chemicals from alternative feedstocks in the United States. FEMS Microbiol Rev 16(2–3):101–110
Stephanopoulos G (2007) Challenges in engineering microbes for biofuels production. Science 315(5813):801–804
Vispute TP, Zhang H, Sanna A, Xiao R, Huber GW (2010) Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 330(6008):1222–1227
Xu Y, Hanna MA, Isom L (2008) “Green” chemicals from renewable agricultural biomass—a mini review. http://www.benthamscience.com/open/toasj/articles/V002/54TOASJ.pdf
Yates MR, Barlow CY (2013) Life cycle assessments of biodegradable, commercial biopolymers—a critical review. Resour Conserv Recycl 78:54–66
Zhang Y, Brown TR, Hu G, Brown RC (2013a) Comparative techno-economic analysis of biohydrogen production via bio-oil gasification and bio-oil reforming. Biomass Bioenergy 51:99–108
Zhang Y, Brown TR, Hu G, Brown RC (2013b) Techno-economic analysis of monosaccharide production via fast pyrolysis of lignocellulose. Bioresour Technol 127:358–365
Zhang Y, Brown TR, Hu G, Brown RC (2013c) Techno-economic analysis of two bio-oil upgrading pathways. Chem Eng J 225:895–904
Zhong ZW, Song B, Zaki MBM (2010) Life-cycle assessment of flash pyrolysis of wood waste. J Clean Prod 18(12):1177–1183
Acknowledgments
The authors would like to acknowledge the financial support of the Bioeconomy Institute and the Biobased Industry Center of Iowa State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ivan Muñoz
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, G. & Brown, R.C. Life cycle assessment of commodity chemical production from forest residue via fast pyrolysis. Int J Life Cycle Assess 19, 1371–1381 (2014). https://doi.org/10.1007/s11367-014-0745-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-014-0745-y