Abstract
Aging and stroke alter the composition of the basement membrane and reduce the perivascular distribution of cerebrospinal fluid and solutes, which may contribute to poor functional recovery in elderly patients. Following stroke, TGF-β induces astrocyte activation and subsequent glial scar development. This is dysregulated with aging and could lead to chronic, detrimental changes within the basement membrane. We hypothesized that TGF-β induces basement membrane fibrosis after stroke, leading to impaired perivascular CSF distribution and poor functional recovery in aged animals. We found that CSF entered the aged brain along perivascular tracts; this process was reduced by experimental stroke and was rescued by TGF-β receptor inhibition. Brain fibronectin levels increased with experimental stroke, which was reversed with inhibitor treatment. Exogenous TGF-β stimulation increased fibronectin expression, both in vivo and in primary cultured astrocytes. Oxygen-glucose deprivation of cultured astrocytes induced multiple changes in genes related to astrocyte activation and extracellular matrix production. Finally, in stroke patients, we found that serum TGF-β levels correlated with poorer functional outcomes, suggesting that serum levels may act as a biomarker for functional recovery. These results support a potential new treatment strategy to enhance recovery in elderly stroke patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is the leading cause of acquired disability in the elderly worldwide (Feigin et al. 2019). Progressive cognitive and functional decline commonly occurs after stroke in aged individuals, although the mechanism underlying this progressive impairment is understudied (Ivan et al. 2004; Reitz et al. 2008). Following stroke, transforming growth factor-β (TGF-β) levels rise within the ischemic penumbra and activate astrocytes, which contributes to increased glial scarring within the aged brain (Krupinski et al. 1996; Doyle et al. 2010; Manwani et al. 2011). Prior studies indicate that TGF-β plays a neuroprotective role in the early stages of both ischemic and hemorrhagic stroke in young animals (Cekanaviciute et al. 2014; Taylor et al. 2017). However, chronic activation of astrocytes by TGF-β has been shown to be detrimental and may contribute to progressive functional decline after stroke in the aged population (Wyss-Coray et al. 1997; Buckwalter et al. 2002). In support of this hypothesis, constitutive overexpression of TGF-β has been shown to increase the production of extracellular matrix proteins and promote functional decline in animal models of Alzheimer’s disease (Wyss-Coray et al. 1995, 1997, 2000; Buckwalter et al. 2002). While multiple studies have characterized similar changes in the basement membrane after stroke and other types of brain injury, it is not clear whether TGF-β signaling to astrocytes mediates these changes after stroke (Hamann et al. 2002; Jullienne et al. 2014; Parham et al. 2016).
The basement membrane serves several established functions in the brain, including regulation of the perivascular distribution of cerebrospinal fluid (CSF) (Engelhardt and Sorokin 2009; Thomsen et al. 2017; Howe et al. 2018). Perivascular CSF distribution is thought to facilitate the clearance of waste products from the brain parenchyma (Morris et al. 2016). Briefly, CSF is transported from the subarachnoid space (SAS) along the basement membrane (Morris et al. 2016; Albargothy et al. 2018). Once there, solutes from the CSF and interstitial fluid (ISF) intermix and facilitate clearance by multiple potential mechanisms (Iliff et al. 2012; Albargothy et al. 2018). Currently, the mechanisms for CSF-ISF solute exchange are a matter of debate, and at least four different mechanisms have been postulated: (i) Para-arterial influx of the CSF, CSF and ISF intermix in the brain parenchyma driven by convection, and then paravenous efflux (Iliff et al. 2012, 2013, 2014; Xie et al. 2013; Kress et al. 2014); (ii) peri-arterial CSF influx through the vascular basement membrane and peri-arterial ISF efflux (Hawkes et al. 2011, 2012; Albargothy et al. 2018); (iii) CSF retention in the paravascular spaces on the surface of the brain (Ma et al. 2019); and (iv) CSF distribution through all vessel types (Lochhead et al. 2015; Pizzo et al. 2018). Thus, while all of the above studies support the observation that perivascular CSF distribution occurs, the kinetics and physiological mechanisms that produce this phenomenon remain unclear. Therefore, to negate the effect of such kinetic movements of CSF and ISF solutes, we limited the scope of the present study to only focus on the distribution of CSF tracers at a single time-point after stroke.
Prior work has shown that stroke impairs the perivascular distribution of CSF and promotes the focal trapping of CSF-derived solutes (Wang et al. 2017), which may lead to the sequestration of waste products within fibronectin-rich segments of the basement membrane (Howe et al. 2018). Disruption of perivascular CSF distribution has been associated with poor functional recovery after stroke and other types of brain injury, although it is unclear whether or not impairment of perivascular CSF distribution is reversible under disease conditions (Iliff et al. 2014; Howe et al. 2018). The current study tested the hypothesis that TGF-β signaling to aged astrocytes promotes remodeling of the basement membrane, leading to impairment in perivascular CSF distribution and poor functional recovery after stroke. We first evaluated the responses of astrocytes to ischemic injury, modeled in vivo by distal middle cerebral artery occlusion (DMCAO) in young and aged mice, as well as in vitro by oxygen-glucose deprivation (OGD) of primary astrocyte cultures. We treated aged mice with a TGF-β receptor-1 antagonist after DMCAO and examined the impact on astrogliosis, basement membrane composition, functional recovery, and perivascular CSF distribution. We then investigated the effects of TGF-β signaling to astrocytes on the production of basement membrane components both in vitro and in vivo. Finally, we explored whether serum TGF-β levels predicted recovery of neurological function in human stroke patients. Overall, our results indicate that TGF-β is a major regulator of stroke-induced changes in basement membrane composition and perivascular CSF distribution and provides a potential new treatment strategy to improve recovery in elderly stroke patients.
Methods
Experimental stroke and tissue processing
Young (3-month-old) and aged (20-month-old) male C57/Bl6 mice (Charles River, National Institute of Aging) were used for all in vivo experiments. Permanent distal middle cerebral artery occlusion (DMCAO) was performed as previously described, according to the STAIRS criteria (Doyle and Buckwalter 2014). Briefly, mice were anesthetized with isoflurane, the dorsolateral cranium was incised, and a burr hole was drilled to expose the distal MCA. Following induction of ischemia by MCA cauterization, the burr hole was closed with dental cement, and the incision sutured. Sham surgeries were performed without cauterization. All surgeries were performed under aseptic conditions, and mice were periodically monitored for signs of pain, infection, or weight loss following the procedure. At the time of sacrifice, mice were then deeply anesthetized with Avertin (250 mg/kg) and perfused with heparinized PBS. For studies requiring fresh tissue, the brain was immediately extracted and placed on ice. The cortex was isolated by blunt dissection and snap-frozen on dry ice. For histological studies, mice were perfused with 4% PFA and whole brains extracted. Mice were group housed and fed standard dry chow ad libitum and kept on a 12-h light-dark cycle. All protocols were approved by the UTHealth IACUC and carried out in an AAALAC-approved facility. Randomization and blinding were maintained for all experiments.
Primary cortical astrocyte culture
P1 mixed-sex pups (C57/Bl6) were bred in-house for the generation of primary glial cultures. P1 pups were anesthetized on ice and decapitated, and then cortices were dissected for isolation of primary cells. Cortical tissue was dissected and placed in HBSS (Ca2+/Mg2+-free). The meninges and subcortical tissue were removed, and the remaining cortices were placed in enzymatic digestion buffer. Following incubation, cells were then re-suspended in culture media (DMEM, 10% FBS), then plated on poly-D-lysine-coated culture vessels. The following day, the media was replaced, which continued once weekly until the completion of experiments. The remaining microglia were then depleted at 14 days in vitro (DIV) with 50 mM solution leucine methyl ester as previously described (Hamby et al. 2006). Oxygen-glucose deprivation (OGD) and in vitro stimulation experiments were carried out at 19–21 DIV in balanced salt solution (BSS), supplemented with 10 mM glucose for normoxic (NO) controls. Cultures were washed with NO or OGD media three times prior to the experiment to fully remove the culture media. In treated cells, BSS was supplemented with recombinant human TGF-β1 (3 ng/mL) and Aβ1-40 (10 μM). Immediately prior to OGD, media was equilibrated with 5% CO2 balanced with nitrogen. Following the addition of equilibrated media, cells were placed in a warmed hypoxic chamber and subjected to OGD for 6 h. Cells were then harvested (6-h time-point), or supplemented with 10 mM glucose and incubated for 18 h at 37 °C (24-h time-point).
TGF-β receptor antagonist treatment and gait analysis
The TGF-β receptor antagonist, GW788388 Hydrate, was reconstituted in 50% DMSO, 42.5% water, and 7.5% ethanol to a concentration of 10 mg/mL. Alzet osmotic pumps were loaded with GW788388 or vehicle alone. At 7 DPI, mice were anesthetized and the pump implanted subcutaneously over the dorsal back musculature. Drug was infused at a rate of 10 mg/kg/day, as previously described (de Oliveira et al. 2012). Following 7 days of treatment, motor function was assessed using DigiGait (Mouse Specifics) at 14 DPI. Mice were first acclimated to the system, and then the speed was gradually increased to 10 cm/s in aged animals. Locomotion was recorded for 60 s, and then parameters of gait were analyzed with DigiGait software by a separate blinded investigator.
Intracisternal injection protocols
Fresh aCSF was prepared in dH2O. For individual experiments, aCSF was supplemented with vehicle, recombinant TGF-β1, 14C-Inulin, and/or TR-dextran (3 kD). The aCSF mixture was infused into the cisterna magna at a rate of 2 μL/min over 5 min, after which mice were transferred to a warmed cage for the remainder of the experiment. For studies involving fluorescent tracers, sections were imaged under fluorescence with a Leica DMi8 microscope at × 10 magnification. For all tracer uptake measurements and IHC quantification, a blinded investigator imaged the lateral cortex (0.38 mm anterior to Bregma, 2.5 mm lateral, 1.5 mm ventral). Fluorescence intensity was quantified in ImageJ by a separate blinded investigator as previously described (Howe et al. 2018).
Immunostaining
Fixed brain sections (24 μm thickness) or primary astrocytes were washed three times for 5 min in wash buffer. Heat-mediated antigen retrieval was performed in sodium citrate buffer. Slides were washed, blocked, and incubated with primary antibodies overnight (anti-GFAP-Cy3 [Mouse, 1:1000, Millipore Sigma, cat. C9205], Lycopersicon esculentum lectin [1:100, Vector Laboratories, cat. DL-1177], anti-AQP4 [Rabbit, 1:5000, Millipore Sigma, cat. HPA014784], anti-integrin-α5 [Rabbit, 1:100, Abcam, cat. EPR7854], or anti-fibronectin [Rabbit, 1:100, Abcam, cat. ab2413]). Slides were incubated with secondary antibodies (Goat, 1:1000, Abcam, Alexa-Fluor 488/594/647), washed and coverslipped. Specificity of fluorescent signals was confirmed with secondary-only controls. The use and standardization of antibodies for the fluorescent immunohistochemical studies were performed according to the manufacturer’s protocol and our previous study (Howe et al. 2018).
Protein isolation, SDS-PAGE, and Western blot
For in vivo experiments, the right cortex was thawed on ice and manually homogenized in NP-40 lysis buffer. For in vitro experiments, cells were washed, and then lysed within the culture dish using 1× RIPA buffer. Following sonication and centrifugation, the supernatant was removed and diluted to 4 mg/mL in Laemmli buffer supplemented with β-mercaptoethanol, then heated to 95 °C for 5 min. Then, 40 μg of protein was loaded into each gel lane, run and then transferred to a PVDF membrane. Blots were washed, blocked, and probed with primary antibody overnight (anti-fibronectin [Rabbit, 1:1000, Abcam, cat. ab2413], anti-vimentin [Rb, 1:1000, Abcam, cat. ab92547], anti-GFAP [Rabbit, 1:500, Cell Signaling, cat. 12389S], and anti-β-actin [mouse/HRP conjugated, 1:50,000, Sigma, cat. A3854]. Blots were then probed with secondary antibody (HRP-conjugated goat anti-rabbit secondary antibody [1:10,000, Vector Laboratories, cat. PI-1000]) and imaged using a Bio-Rad ChemiDoc Imager. The use and standardization of antibodies for western blotting were performed according to the manufacturer’s protocol and our previous study (Howe et al. 2018).
Multiplex assays
The absolute level of TGF-β1 was quantified using a Bio-Plex Pro TGF-β Assay (Bio-Rad, cat. 171W4001M). Briefly, cortical lysates were diluted to 8 mg/mL in 1% NP40 buffer, then underwent acid-mediated antigen retrieval, and were further diluted in dH2O + BSA to a final concentration of 1 mg/mL. Subsequent steps were performed according to the manufacturer’s instructions. Plates were read with the Luminex-based Bio-Plex 200 using the manufacturer’s protocol.
RNA isolation, cDNA synthesis, and qPCR measurement of gene expression
RNA was harvested and purified using the RNEasy Mini Kit (Qiagen) and 25 μg of RNA was placed into a cDNA synthesis reaction (iScript). Gene expression was measured by qPCR (Table S1). All assays were performed according to the manufacturer’s instructions.
Liquid scintillation counting
Samples were thawed on ice, then placed in 5-mL glass vials and dissolved in 1 mL solvable (Perkin-Elmer) overnight at 37 °C. Homogenates were vortexed and mixed with 4 mL Hionic-Fluor scintillation cocktail (Perkin-Elmer). 14C-Inulin emissions were counted between 20 and 150 KeV with a Perkin-Elmer TriCarb LSC.
Correlation of clinical course with serum TGF-β1 levels
Our study cohort consisted of 41 imaging-confirmed ischemic stroke patients. All patients were part of a prospective stroke registry where relevant clinical data was catalogued and assessed throughout inpatient stay, which enrolled patients between 2013 and 2015 at a comprehensive stroke center (Hartford Hospital, Hartford CT). Serum samples were obtained from ischemic stroke patients 24 h after symptom onset, processed within 2 h, and stored at − 80 °C for future use. Patients or their families were consented for both sample collection and data registry participation, which was approved by the Institutional Review Board at Hartford Hospital. Our primary outcomes were the National Institutes of Health Stroke Scale (NIHSS) score at discharge, and the ΔNIHSS during inpatient stay (admission NIH-discharge NIH). Serum levels of TGF-β were quantified by multiplex ELISA using the TGF-β 3-PLEX (Bio-Rad).
Statistics
All statistical analyses were performed using Prism 7. Data are reported as mean (±) SEM with the number of biological replicates (n) per group. When only two groups were compared, Student’s t test (two-tailed) with or without Welch’s correction was applied where appropriate. When three groups were compared, one-way ANOVA with Tukey’s test for multiple comparisons was performed. When four or more groups were compared, two-way ANOVA with either Tukey’s or Dunnett’s test for multiple comparisons was utilized (denoted in figure legends). For analysis of clinical data, the relationship between TGF-β and discharge NIH or ΔNIH was analyzed by linear regression. Multivariate analysis was also performed, controlling for patient gender, age, and NIH admission score. All statistical analysis was performed using Prism 7 and Microsoft Excel. Asterisks indicate statistical significance levels based on the p value: *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
DMCAO produced widespread reactive astrogliosis that worsened with aging
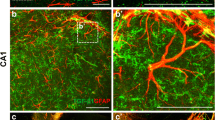
To assess how ischemia alters astrocyte activation in astrocytes adjacent to the cerebral vasculature, we first performed DMCAO on young (3-month-old) and aged (20-month-old) male mice. We examined astrocyte morphology in aged animals at 7 days post-injury (DPI) by IHC and counterstained with lectin to label the vascular basement membrane (Fig. 1a). Perivascular GFAP (t[7] = 3.1, p = 0.018) and lectin (t[7] = 9.7, p < 0.001) expression significantly increased in the aged brain after DMCAO, with no significant change in perivascular AQP4 expression (t[7] = 0.4, p = 0.677). To compare DMCAO-induced changes in reactive gliosis between young and aged mice, we dissected the ipsilateral cortex at 7 DPI and assessed the expression of both GFAP and vimentin, an additional marker of reactive gliosis, by Western blot of cortical lysates (Fig. 1b). Consistent with prior studies (Doyle et al. 2010; Manwani et al. 2011), we found that aging (F[1, 19] = 21, p < 0.001) and DMCAO (F[1, 19] = 50.8, p < 0.001) produced statistically significant increases in cortical GFAP expression, with a trending interaction between aging and DMCAO (Fig. 1b, (i), F[1, 19] = 3.5, p = 0.076). Similarly, we also found that both aging (Fig. 1b (ii) F[1, 18] = 11.2, p = 0.004) and DMCAO (F[1, 18] = 68.9, p < 0.001) increased vimentin expression within the cortex. A significant interaction effect of age and stroke on vimentin expression (F[1, 18] = 8.9, p = 0.008) was also found. We also found that DMCAO increased peri-infarct cortical TGF-β1 levels at 7 DPI (F[1, 18] = 74.05, p < 0.001), with no significant effect of aging (Fig. 1b (iii). Overall, these data indicate that DMCAO induces cortical astrocyte reactivity, which is potentiated by aging. Since DMCAO increased TGF-β1 levels similarly in both young and aged animals, further studies are needed to determine whether the aged astrocyte is more sensitive to TGF-β1, or if other molecular signaling pathways mediate increased gliosis in aged animals.
Aging worsens reactive gliosis after DMCAO. a (i, ii) Representative fluorescent mosaic micrographs from aged mice showed increased levels of lectin and GFAP staining throughout the injured hemisphere after DMCAO (7 DPI). Scale Bar = 750 μm. (iii–viii) Three-dimensional reconstruction showed altered astrocyte morphology within the ipsilateral perivascular region following DMCAO in aged animals, identified by AQP4 staining. Scale bar = 50 μm. b Quantification of perivascular GFAP (i), lectin (ii), and AQP4 (iii) staining in aged animals at 7 DPI. n = 4–5 mice per group. Data were analyzed by Student’s t test. c (i, ii) Densitometry analysis of cortical lysates showed the highest levels of GFAP and vimentin expression in aged mice with DMCAO at 7 DPI. (iii) Multiplex ELISA showed increased expression of TGF-β1 in cortical lysates in both age groups at 7 DPI. n = 5–8 mice per group. Data were analyzed by Tukey’s post hoc test for multiple comparisons. Significance: ns, no significance, *p < 0.05; **p < 0.01; ***p < 0.001. Data are plotted as mean ± SEM
Treatment with a TGF-β receptor antagonist rescued DMCAO-induced impairments in motor function, reactive gliosis, and perivascular CSF distribution in aged animals
In order to directly determine if chronic TGF-β activation plays a detrimental role in post-stroke recovery, we performed DMCAO on aged animals and then treated them with continuous peripheral infusion of GW788388 Hydrate, a specific TGF-β receptor-1 (ALK5) antagonist, from 7 to 15 DPI (Gellibert et al. 2006; de Oliveira et al. 2012). This extended time-point was chosen to minimize the potential impact of our intervention on injury volume, as previous studies have shown that TGF-β is neuroprotective in the early phases of infarction (Cekanaviciute et al. 2014). We hypothesized that delayed treatment with an ALK5 antagonist would reduce gliosis and fibronectin/integrin-α5 expression following DMCAO, potentially normalizing the perivascular CSF distribution within the basement membrane (Fig. 2). Indeed, we confirmed that DMCAO increased GFAP expression in aged animals and newly found that this was significantly rescued by drug treatment (Fig. 2b (i), F[2, 12] = 6.2, p = 0.014). We also found that DMCAO increased fibronectin expression, which was reversed by drug treatment (Fig. 2b (ii) F[2, 12] = 5.8, p = 0.017). These changes in astrocyte reactivity and fibrosis correlated with alterations in perivascular CSF distribution (Fig. 2c, d). We found that DMCAO significantly inhibited perivascular CSF distribution (measured as the distribution of fluorescently-labeled 3 kDa dextran [TR-d3], a fluid-space marker), which was rescued by drug treatment (Fig. 2d (i), F[2, 12] = 5.4, p = 0.022). Overall, these data show that ALK5 activation induces reversible impairments in perivascular CSF distribution after DMCAO in aged animals.
TGF-β receptor antagonism reduces fibrosis and improves perivascular CSF distribution following DMCAO. Beginning at 7 DPI, mice were treated with continuous subcutaneous infusion of vehicle or GW788388 Hydrate, a TGF-β receptor antagonist, at a dosage of 10 mg/kg/day. a Representative images showing increased GFAP and fibronectin expression in the peri-infarct cortex following DMCAO (15 DPI), which is rescued with continuous subcutaneous infusion of GW788388 (10 mg/kg/day) by Alzet pump. Scale bar = 100 μm. b Fluorescence intensity quantification of GFAP and fibronectin expression. c Representative images showing decreased dextran distribution in the ipsilateral hemisphere following DMCAO (15 DPI), which is reversed by GW788388 treatment. Scale bar = 1 mm. Inset: magnified tracing showing distribution of tracer from the cortical surface.d Fluorescence intensity quantification of tracer distribution from the cortical surface. n = 4–5 per group. Data are analyzed by two-way ANOVA with Tukey’s post hoc test for multiple comparisons. *p < 0.05. Data are presented as mean ± SEM
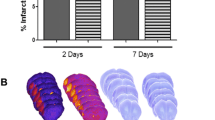
To determine whether improvements in perivascular CSF-ISF exchange correlated with improved functional recovery from DMCAO, we performed DigiGait analysis on mice at 14 DPI. We found that contralateral (injured) forelimb function was impaired by DMCAO, and that this was rescued with ALK5 antagonist treatment (Fig. 3a (i), F[2,11] = 5.7, p = 0.02). As an additional control, we did not observe any significant effect of DMCAO or treatment on the ipsilateral (uninjured) forelimb (Fig. 3a (ii), F[2,11] = 1.1, p = 0.355). Interestingly, these functional improvements in gait symmetry were not associated with reductions in histological damage following DMCAO, measured as hemispheric volume at 15 DPI (Fig. 3b, t[6] = 1.38, p = 0.23). As such, these findings show that delayed ALK5 inhibition can improve functional outcomes, without significantly affecting the severity of the initial injury.
TGF-β receptor antagonism improves functional outcomes after DMCAO in aged animals. a (i) Utilization of the contralateral (injured) forelimb during locomotion, measured on DigiGait as area per second at 14 DPI. (ii) Utilization of the ipsilateral (uninjured) forelimb during locomotion at 14 DPI. Data are analyzed by one-way ANOVA with Tukey’s post-hoc test for multiple comparisons, n = 4–5 per group. b (i) Representative cresyl violet-stained images at 15 DPI. (ii) Quantification of hemispheric atrophy (ipsilateral/contralateral hemisphere volume) at 15 DPI. Data are analyzed by Student’s t test, n = 4–5 per group. *p < 0.05. ns, no significance. Data are presented as mean ± SEM
TGF-β1 signals to astrocytes to increase fibronectin expression
To confirm our finding that TGF-β signaling increases fibronectin expression and impairment of perivascular CSF distribution, we intracisternally injected naive young mice with recombinant TGF-β1, and collected the cerebral cortex for Western blot 24 h later. We found that TGF-β1 stimulation significantly upregulated fibronectin expression (Fig. 4a, t[5] = 2.62, p = 0.047). We also measured CSF influx by intracisternal infusion of 14C-inulin, an additional fluid space marker, and found that treatment with TGF-β1 significantly reduced the distribution of 14C-inulin within the brain (Fig. 4a (ii), t[7] = 2.75, p = 0.029). Taken together with the preceding experiments, these findings further support the role of TGF-β1 as a negative regulator of perivascular CSF distribution.
TGF-β1 stimulation increases fibronectin expression and impairs perivascular CSF influx. a (i) In the brain, cortical lysates showed increased fibronectin expression by densitometry analysis by 24 h after intracisternal TGF-β1 infusion. (ii) The cortical distribution of CSF containing 14C-inulin was reduced in mice pre-treated with TGF-β1. n = 3–4 per group. b Stimulation of primary astrocytes with TGF-β increased fibronectin expression. (i) Quantification of fibronectin expression changes in primary murine astrocytes by western blot. n = 4–5 per group. (ii) ICC and image quantification showing increased fibronectin expression in primary murine astrocytes with stimulation. Quantitative data are analyzed by Student’s t test. Significance: *p < 0.05; **p < 0.01. Scale bar = 100 μm. Data are presented as mean ± SEM
The preceding experiments indicate that increased fibronectin after stroke is mediated by TGF-β. We next sought to identify the cellular target of TGF-β that mediates these phenotypes. We hypothesized that TGF-β signals to astrocytes to upregulate fibronectin expression. To test this hypothesis, we first treated primary murine astrocytes in vitro with recombinant TGF-β1 peptide for 24 h, and then measured fibronectin expression by Western blot and ICC. We found TGF-β1 significantly increased the expression of fibronectin in whole-cell lysates (Fig. 4b (i), t[6] = 4.91, p = 0.003), which was also observed with ICC quantification (Fig. 4b (ii), t[4] = 4.3, p = 0.012). Overall, these results indicate that astrocytes produce fibronectin in response to TGF-β1.
Exposure to OGD, TGF-β1, and Aβ1-40 impacts astrocyte phenotypes and the expression of extracellular matrix-related genes
Given the association between CSF influx and the drainage of amyloid-β (Aβ) along perivascular tracts identified in previous studies (Iliff et al. 2012; Howe et al. 2018), we next sought to characterize how hypoxia, TGF-β, and amyloid-β (Aβ) interact to influence astrocyte phenotypes and basement membrane composition. Previous work indicates that TGF-β promotes astrocyte activation after stroke (Cekanaviciute et al. 2014). Additionally, others have shown that stroke can increase amyloid plaque formation, which worsens inflammation and functional outcomes (Nguyen et al. 2018). Following activation after stroke, reactive astrocytes transition to an anti-inflammatory “A2” phenotype (Zamanian et al. 2012), which could change the complement of basement membrane components that they produce. In contrast, since the soluble form of Aβ (Aβ1-40) has been shown to provoke inflammatory cytokine production by astrocytes (Vincent et al. 2002; Smits et al. 2002; Zhang et al. 2017), we hypothesized that it would promote a pro-inflammatory “A1” activation state that may be associated with further changes in basement membrane composition. Since the mechanism of these transitions are not known, we first evaluated how these potentially opposing stimuli regulate the intrinsic transcription of activation genes in primary astrocytes under oxygen-glucose deprivation (OGD), an in vitro model of hypoxia. We found that Aβ1-40 and OGD induced opposing A1 and A2 gene expression profiles, respectively; neither of which were significantly impacted by TGF-β1 (Fig. 5a; Table S2).
Expression of cellular phenotype and basement membrane-related genes in primary astrocytes following acute stimulation. a Heat map showing variation in expression of genetic markers of astrocyte phenotype following 6 h of ischemia and/or co-stimulation (TGF-β1 [3 ng/mL]; Aβ1-40 [10 μM]) . ddCT values were plotted as Z-scores. b (i–viii) Expression (ddCT) of basement membrane-related genes. (ix) Expression of APP. n = 5–8 per group. Heat map data in panel a were analyzed by two-way ANOVA with Dunnet’s test for multiple comparisons to compare ddCT values of experimental groups against vehicle/NO control. All other expression data in panel b are analyzed by two-way ANOVA with Tukey’s post hoc test for multiple comparisons. Significance: *p < 0.05; **p < 0.01; ***p < 0.001. Abbreviations: AGRN, agrin; APP, amyloid precursor protein; COL16A1, collagen XVI; FBLN5, fibulin-5; FN1, fibronectin; HSPG2, perlecan; NO, normoxia; ischemia, oxygen-glucose deprivation; SDC2, syndecan-2; ITGA5, integrin-α5; ITGB1, integrin-β1. Astrocyte phenotyping criteria were based on previously published panels (Zamanian et al. 2012; Liddelow et al. 2017)
To further examine the role of TGF-β1 and OGD in regulating astrocyte function, we next broadly assessed how these stimuli impacted the production of basement membrane components by primary astrocytes, which may disrupt the perivascular microenvironment through the basement membrane. To accomplish this, we examined the mRNA levels of agrin (AGRN), collagen XVI (COL16A1), fibulin-5 (FBLN5), fibronectin (FN1), perlecan (HSPG2), syndecan-2 (SDC2), integrin-α5 (ITGA5), integrin-β1 (ITGB1), and amyloid precursor protein (APP) immediately following OGD, as well as with co-stimulation with TGF-β1 and Aβ1-40 (Fig. 5b). We found that exposure to OGD significantly reduced agrin expression (p < 0.001) but upregulated integrin-α5, a co-receptor for fibronectin (p = 0.001) (Huang et al. 2015; Su et al. 2016). Co-stimulation differentially impacted fibulin (p = 0.011) and syndecan-2 expression (p = 0.036). Finally, we also observed several statistically significant interaction effects between OGD and co-stimulation on the expression of agrin (p = 0.005), collagen XVI (p = 0.026), fibrillin (p = 0.018), and fibronectin (p = 0.037). There was no significant impact of OGD or co-stimulation on APP expression. Overall, these data indicate that A2 astrocytes produce a unique group of basement membrane proteins after stroke and suggest that TGF-β1 further modulates the production of these components without impacting APP expression.
High serum TGF-β1 predicts poor functional recovery in human stroke patients
Finally, we verified the relevance of our studies to humans by examining how serum TGF-β1 levels correlate with functional deficits in patients with ischemic stroke (Table 1). Linear regression analysis showed a trend towards higher levels of serum TGF-β1 in patients with more severe neurological deficits at discharge, as measured by the NIH Stroke Scale (NIHSS; p = 0.09). Similarly, there was also a trend towards higher levels of serum TGF-β1 and reduced recovery of neurological function during hospital stay in our patient cohort (p = 0.07) (Fig. 6). Multivariate analysis was then performed, controlling for patient age, sex, and initial neurological deficits. This analysis revealed that elevated serum TGF-β1 was a significant, independent predicter of greater neurological deficits at discharge (p = 0.03) and diminished improvement in neurological deficits during hospital stay (p = 0.03) (Table 1, Table S3). While these data suggest a detrimental role for TGF-β1 in the days following stroke, further studies are required to assess basement membrane fibrosis and long-term functional recovery in stroke patients. In addition, it is unclear to what degree peripheral TGF-β1 levels reflect signaling within the ischemic brain, necessitating additional studies examining CSF cytokine levels.
Discussion
While aging has been shown to increase glial scarring around the initial injury site after experimental stroke (Doyle et al. 2010), we found that this enlarged glial scar also extends along the peri-infarct vessels, which form a continuous network throughout the ipsilateral cortex. Previous studies have shown that TGF-β signaling drives glial scar formation after stroke (Cekanaviciute et al. 2014), as well as small vessel disease in animal models of Alzheimer’s disease (Wyss-Coray et al. 1995, 1997, 2000; Buckwalter et al. 2002). The present study identifies novel effects of TGF-β signaling to astrocytes on the production of basement membrane components, and their physiological correlates in aging and after stroke. It also indicates that peripheral TGF-β levels may provide a clinically useful biomarker to identify patients who are at risk for functional decline after stroke. Taken as a whole, these findings are significant, because they provide a new potential pathophysiological mechanism that links acute macrovascular insults to chronic microvascular changes, with the potential to improve functional recovery in millions of elderly stroke survivors worldwide (Gorelick et al. 2011; Feigin et al. 2019).
The current study examines how TGF-β signaling influences perivascular CSF distribution after an ischemic insult to the aged brain. Previous work has shown that perivascular CSF influx contributes to the clearance of macromolecules, including toxic waste products, from the brain parenchyma (Iliff et al. 2012, 2013). However, perivascular CSF influx becomes disrupted in aging, stroke, and other types of brain injury (Kress et al. 2014; Wang et al. 2017; Howe et al. 2018). Our previous study identified the basement membrane as a potential mediator of the abnormal perivascular distribution of fluid and solutes after stroke, with increased expression of fibronectin specifically promoting the binding of soluble Aβ1-40 within the basement membrane (Howe et al. 2018). The present study builds on these findings and shows that TGF-β signaling induces basement membrane fibrosis and chronically impairs perivascular CSF distribution after stroke in aged animals, providing a new mechanism by which brain injury can lead to prolonged functional impairment in the elderly (Feigin et al. 2019). While these findings are exciting, further studies are required to determine whether other TGF-β-responsive molecules, in addition to fibronectin, contribute to the observed derangements in perivascular CSF distribution after stroke. Furthermore, additional studies are also required to determine whether these changes in basement membrane composition contribute to the formation of vascular amyloid plaques.
Given the relationship between basement membrane composition and TGF-β activation, we sought to determine whether TGF-β signaling could be antagonized to promote post-stroke recovery. To examine this relationship, we performed experimental stroke in mice and employed a selective pharmacologic inhibitor of TGF-β receptor-1 (ALK5) that was administered in the chronic phase of injury. In addition to its translational relevance, this strategy allowed us to avoid any significant effects on infarct size, as the stroke is histologically complete by day 3 in the DMCAO stroke model (Gellibert et al. 2006). We found that delayed ALK5 antagonism reduced gliosis, reduced the expression of basement membrane fibronectin to sham levels, and improved functional outcomes following DMCAO in aged animals. While prior work has suggested a beneficial role for TGF-β signaling in the early stages of ischemic injury in young animals (Cekanaviciute et al. 2014), the present study differs in that we utilized aged animals, and allowed for a delay in drug administration to avoid altering the volume of the initial injury. Taken together, these studies suggest a biphasic role for TGF-β signaling, where moderate activation may provide early neuroprotection, but later have a negative impact on functional recovery. The negative impacts of prolonged TGF-β1 exposure may be worse in aged animals, which is associated with increased glial scarring and impaired neurological function after stroke (Doyle et al. 2010; Manwani et al. 2011). This may provide an additional mechanism driving functional decline after stroke in the aged population, but more studies are needed to specifically evaluate the causal relationship between fibronectin over-expression in the basement membrane and poor functional recovery.
The current study also indicates that astrocyte activation after stroke is dependent on multiple factors within the brain microenvironment. Using in vivo mouse models, previous work showed that A1 astrocytes are induced by peripheral LPS stimulation, while A2 astrocytes are induced after MCAO (Zamanian et al. 2012). We newly found that OGD was sufficient to induce an A2 phenotype in primary astrocyte cultures. Additionally, while previous studies found that treatment with TGF-β inhibits A1 astrocyte activation (Liddelow et al. 2017), we found that the A2 phenotype remained intact even with TGF-β co-stimulation under OGD conditions. We also newly found that Aβ1-40 stimulated classical A1 astrocyte activation and inhibited the transition to A2 that is seen after OGD. In addition, we also found that astrocyte phenotypes are associated with different genetic expression of basement membrane components that have been implicated in the pathogenesis of small vessel disease and vascular cognitive impairment (Thomsen et al. 2017). The present findings suggest that OGD and Aβ1-40 interact to modulate astrocyte phenotypes, which could influence recovery from stroke in vivo due, in part, to modulation of fibronectin expression and CSF flow within the basement membrane. Additional studies should be undertaken to further characterize the role of astrocyte polarization in the regulation of solute flux in the basement membrane, as well as to identify additional potential changes in other targets, including laminin and collagen subtypes.
The impact of aging on the expression of TGF-β after stroke remains understudied. Microglia and monocyte-derived macrophages are thought to be major cellular sources of TGF-β within the brain after stroke (Yeo et al. 2019). Previous studies have shown that TGF-β expression increases in the brain (Krupinski et al. 1996) and blood (Jiang et al. 2017) after stroke, leading to increased Smad signaling and reactive gliosis, which is further increased by aging (Doyle et al. 2010; Cekanaviciute et al. 2014). While the current study also showed increased glial scarring in the aged brain after stroke, surprisingly, it did not find a significant effect of aging on the absolute concentration of TGF-β in the brain. One explanation for this discrepancy is that the aged brain may exhibit increased sensitivity to TGF-β, such that similar infarct volumes and TGF-β levels produce increased glial scarring compared to that seen in the young brain. Further studies should address how aging alters expression of the TGF-β receptor complex, downstream signaling proteins and epigenetic changes in TGF-β-responsive regions of the genome that occur with aging (Reviewed in Koellhoffer et al. 2017; Nickel et al. 2018). Such studies may help to develop more specific therapies that target pathologically increased gliosis after stroke, without affecting TGF-β signaling throughout the body.
In summary, the present study supports the hypothesis that TGF-β induces gliosis and is a major negative regulator of perivascular CSF distribution, which is mediated, in part, by its effects on basement membrane composition. Astrocyte-specific knockouts, including TGF-β receptor-1/2, or the downstream Smad proteins may be of further use in understanding how aging increases gliosis after stroke. Additionally, further studies are needed to better characterize the cellular sources of TGF-β after stroke. Longitudinal studies with transgenic models of Alzheimer’s disease would be useful to correlate these durable changes in physiology with the chronic development of small vessel disease and to assess the long-term impact of basement membrane remodeling and altered perivascular CSF distribution on brain function. These changes in basement membrane composition and function could impair neurovascular coupling after stroke, which is hypothesized to play a major role in the development of both gait disturbances and cognitive impairment (Ungvari et al. 2017; Tarantini et al. 2017). Overall, targeting TGF-β signaling could provide a novel therapeutic strategy to improve basement membrane composition, perivascular CSF distribution, and the overall function of the neurovascular unit in the ischemic brain, which may eventually lead to strategies to augment functional recovery in human stroke patients.
References
Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO (2018) Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136:139–152. https://doi.org/10.1007/s00401-018-1862-7
Buckwalter M, Pepper J-P, Gaertner RF, von Euw D, Lacombe P, Wyss-Coray T (2002) Molecular and functional dissection of TGF-beta1-induced cerebrovascular abnormalities in transgenic mice. Ann N Y Acad Sci 977:87–95
Cekanaviciute E, Fathali N, Doyle KP, Williams AM, Han J, Buckwalter MS (2014) Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia 62:1227–1240. https://doi.org/10.1002/glia.22675
de Oliveira FL, Araújo-Jorge TC, de Souza EM, de Oliveira GM, Degrave WM, Feige JJ, Bailly S, Waghabi MC (2012) Oral Administration of GW788388, an Inhibitor of transforming growth factor beta signaling, prevents heart fibrosis in Chagas disease. PLoS Negl Trop Dis 6:e1696. https://doi.org/10.1371/journal.pntd.0001696
Doyle KP, Buckwalter MS (2014) A mouse model of permanent focal ischemia: distal middle cerebral artery occlusion. Methods Mol Biol 1135:103–110. https://doi.org/10.1007/978-1-4939-0320-7_9
Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS (2010) TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7:62. https://doi.org/10.1186/1742-2094-7-62
Engelhardt B, Sorokin L (2009) The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511. https://doi.org/10.1007/s00281-009-0177-0
Feigin VL, Nichols E, Alam T et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Gellibert F, de Gouville A-C, Woolven J, Mathews N, Nguyen VL, Bertho-Ruault C, Patikis A, Grygielko ET, Laping NJ, Huet S (2006) Discovery of 4-{4-[3-(pyridin-2-yl)-1 H-pyrazol-4-yl]pyridin-2-yl}-N-(tetrahydro-2 H - pyran-4-yl)benzamide (GW788388): a potent, selective, and orally active transforming growth factor-β type i receptor inhibitor. J Med Chem 49:2210–2221. https://doi.org/10.1021/jm0509905
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 42:2672–2713. https://doi.org/10.1161/STR.0b013e3182299496
Hamann GF, Liebetrau M, Martens H, Burggraf D, Kloss CU, Bültemeier G, Wunderlich N, Jäger G, Pfefferkorn T (2002) Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab 22:526–533. https://doi.org/10.1097/00004647-200205000-00004
Hamby ME, Uliasz TF, Hewett SJ, Hewett JA (2006) Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods 150:128–137. https://doi.org/10.1016/j.jneumeth.2005.06.016
Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO (2011) Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121:431–443. https://doi.org/10.1007/s00401-011-0801-7
Hawkes CA, Sullivan PM, Hands S, Weller RO, Nicoll JA, Carare RO (2012) Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 Allele. PLoS One 7:e41636. https://doi.org/10.1371/journal.pone.0041636
Howe MD, Atadja LA, Furr JW, Maniskas ME, Zhu L, McCullough L, Urayama A (2018) Fibronectin induces the perivascular deposition of cerebrospinal fluid–derived amyloid-β in aging and after stroke. Neurobiol Aging 72:1–13. https://doi.org/10.1016/J.NEUROBIOLAGING.2018.07.019
Huang Q, Chen B, Wang F, Huang H, Milner R, Li L (2015) The temporal expression patterns of fibronectin and its receptors-α5β1 and αvβ3 integrins on blood vessels after cerebral ischemia. Restor Neurol Neurosci 33:493–507. https://doi.org/10.3233/RNN-140491
Iliff JJ, Wang M, Liao Y et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111. https://doi.org/10.1126/scitranslmed.3003748
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33:18190–18199. https://doi.org/10.1523/JNEUROSCI.1592-13.2013
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34:16180–16193. https://doi.org/10.1523/JNEUROSCI.3020-14.2014
Jiang C, Kong W, Wang Y, Ziai W, Yang Q, Zuo F, Li F, Wang Y, Xu H, Li Q, Yang J, Lu H, Zhang J, Wang J (2017) Changes in the cellular immune system and circulating inflammatory markers of stroke patients. Oncotarget 8:3553–3567. https://doi.org/10.18632/oncotarget.12201
Jullienne A, Roberts JM, Pop V, Paul Murphy M, Head E, Bix GJ, Badaut J (2014) Juvenile traumatic brain injury induces long-term perivascular matrix changes alongside amyloid-beta accumulation. J Cereb Blood Flow Metab 34:1637–1645. https://doi.org/10.1038/jcbfm.2014.124
Koellhoffer E, McCullough L, Ritzel R (2017) Old Maids: Aging and Its Impact on Microglia Function. Int J Mol Sci 18:769. https://doi.org/10.3390/ijms18040769
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. https://doi.org/10.1002/ana.24271
Krupinski J, Kumar P, Kumar S, Kaluza J (1996) Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke 27:852–857. https://doi.org/10.1161/01.str.27.5.852
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG (2015) Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab 35:371–381. https://doi.org/10.1038/jcbfm.2014.215
Ma Q, Ries M, Decker Y, Müller A, Riner C, Bücker A, Fassbender K, Detmar M, Proulx ST (2019) Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137:151–165. https://doi.org/10.1007/s00401-018-1916-x
Manwani B, Liu F, Xu Y et al (2011) Functional recovery in aging mice after experimental stroke. Brain Behav Immun 25:1689–1700. https://doi.org/10.1016/j.bbi.2011.06.015
Morris AWJ, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO (2016) Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol 131:725–736. https://doi.org/10.1007/s00401-016-33-z
Nguyen T-VV, Hayes M, Zbesko JC et al (2018) Alzheimer’s associated amyloid and tau deposition co-localizes with a homeostatic myelin repair pathway in two mouse models of post-stroke mixed dementia. Acta Neuropathol Commun 6:100. https://doi.org/10.1186/s40478-018-0603-4
Nickel J, ten Dijke P, Mueller TD (2018) TGF-β family co-receptor function and signaling. Acta Biochim Biophys Sin (Shanghai) 50:12–36. https://doi.org/10.1093/abbs/gmx126
Parham CL, Shaw C, Auckland LD, Dickeson SK, Griswold-Prenner I, Bix G (2016) Perlecan domain V inhibits amyloid-β induced activation of the α2β1 integrin-mediated neurotoxic signaling cascade. J Alzheimers Dis 54:1629–1647. https://doi.org/10.3233/JAD-160290
Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, Abbott NJ, Meyerand ME, Sorokin L, Stanimirovic DB, Thorne RG (2018) Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol 596:445–475. https://doi.org/10.1113/JP275105
Smits HA, Rijsmus A, van Loon JH, Wat JW, Verhoef J, Boven LA, Nottet HS (2002) Amyloid-beta-induced chemokine production in primary humamacrophages and astrocytes. J Neuroimmunol 127:160–168
Su Y, Xia W, Li J et al (2016) Relating conformation to function in integrin α5β1. Proc Natl Acad Sci 113:E3872–E3881. https://doi.org/10.1073/pnas.1605074113
Tarantini S, Yabluchanksiy A, Fülöp GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O'Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z (2017) Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. GeroScience 39:601–614. https://doi.org/10.1007/s11357-017-0003-x
Taylor RA, Chang C-F, Goods BA, Hammond MD, Mac Grory B, Ai Y, Steinschneider AF, Renfroe SC, Askenase MH, McCullough L, Kasner SE, Mullen MT, Hafler DA, Love JC, Sansing LH (2017) TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest 127:280–292. https://doi.org/10.1172/JCI88647
Thomsen MS, Routhe LJ, Moos T (2017) The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 37:3300–3317. https://doi.org/10.1177/0271678X17722436
Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A (2017) Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience 39:33–42. https://doi.org/10.1007/s11357-017-9964-z
Vincent VAM, Selwood SP, Murphy GM (2002) Proinflammatory effects of M-CSF and A beta in hippocampal organotypic cultures. Neurobiol Aging 23:349–362
Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, Nedergaard M (2017) Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci 37:2870–2877. https://doi.org/10.1523/JNEUROSCI.2112-16.2017
Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L (1995) Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol 147:53–67
Wyss-Coray T, Masliah E, Mallory M et al (1997) Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature 389:603–606. https://doi.org/10.1038/39321
Wyss-Coray T, Lin C, Sanan DA et al (2000) Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 156:139–150
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377. https://doi.org/10.1126/science.1241224
Yeo H-G, Hong JJ, Lee Y et al (2019) Increased CD68/TGFβ Co-expressing microglia/macrophages after transient middle cerebral artery occlusion in rhesus monkeys. Exp Neurobiol 28:458–473. https://doi.org/10.5607/en.2019.28.4.458
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. https://doi.org/10.1523/JNEUROSCI.6221-11.2012
Zhang J-X, Xing J-G, Wang L-L et al (2017) Luteolin inhibits fibrillary β-Amyloid1–40-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules 22:334. https://doi.org/10.3390/molecules22030334
Funding
This study was partially supported by funding provided to the Texas Alzheimer’s Research and Care Consortium (TARCC) by the state of Texas, through the Texas Council on Alzheimer’s Disease and Related Disorders (to A.U.), and NIH/NIA RF1AG057576 (to A.U.), and by the NIH/NINDS R01NS094543 (to L.D.M.). Fellowship support was provided by the American Heart Association via AHA17PRE33410369 (to M.D.H.) and the NIH via 4TL1TR000369-10 (to M.D.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Please see the Online Supplement for additional supplemental data. Table S1 provides information on the primer sequences used for all qPCR data. Table S2 shows additional statistical values that pertain to the qPCR data. Table S3 contains detailed results of the multivariable modeling of serum TGF-β on neurological deterioration and function at discharge in human stroke patients.
ESM 1
(DOCX 47 kb).
About this article
Cite this article
Howe, M.D., Furr, J.W., Munshi, Y. et al. Transforming growth factor-β promotes basement membrane fibrosis, alters perivascular cerebrospinal fluid distribution, and worsens neurological recovery in the aged brain after stroke. GeroScience 41, 543–559 (2019). https://doi.org/10.1007/s11357-019-00118-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-019-00118-7