Abstract
Trophocytes and fat cells in honeybees (Apis mellifera) have served as targets for cellular senescence studies, but mitochondrial energy utilization with advancing age in workers is unknown. In this study, mitochondrial energy utilization was evaluated in the trophocytes and fat cells of young and old workers reared in a field hive. The results showed that (1) mitochondrial density increased with advancing age; (2) mitochondrial membrane potential (∆Ψm), nicotinamide adenine dinucleotide oxidized form (NAD+) concentration, adenosine triphosphate (ATP) concentration, and NAD+/nicotinamide adenine dinucleotide reduced form (NADH) ratio decreased with advancing age; and (3) the expression of NADH dehydrogenase 1 (ND1), ATP synthase, and voltage-dependent anion channel 1 (VDAC1) increased with advancing age, whereas ND1 and ATP synthase did not differ with advancing age after normalization to mitochondrial density and VDAC1. These results show that the trophocytes and fat cells of young workers have higher mitochondrial energy utilization efficiency than those of old workers and that aging results in a decline in mitochondrial energy utilization in the trophocytes and fat cells of worker honeybees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellular senescence is a complex process that involves decreasing cellular proliferation and increasing cellular deterioration, which gradually lead to aging and cause a progressive decline in biological functions and increased incidence of age-associated diseases. Accordingly, understanding the biology of aging will allow us to not only improve the health of organisms but also potentially extend their life span.
Honeybees (Apis mellifera) are an attractive model system for studying aging because queens and workers have the same genome, yet queens have a much longer life span. In addition, they live in large colonies, are easily manipulated, and their genome has been sequenced (Remolina et al. 2007; Neukirch 1982; Rueppell et al. 2007a, b). A variety of honeybee specimens, including spermathecae, muscle, ventriculi, hemolymph plasma, semen, brain, trophocytes, and fat cells, have been used to study oxidative stress and aging (Weirich et al. 2002; Seehuus et al. 2006a, b; Williams et al. 2008; Collins et al. 2004; Corona et al. 2005; Hsieh and Hsu 2011a, b).
Trophocytes and fat cells attach to one another to form a single layer of cells around each segment of the honeybee’s abdomen. The ease with which these cells can be isolated from the abdomen and manipulated makes them attractive targets for cellular senescence studies in honeybees. Recent studies have shown that trophocytes and fat cells do not divide during adulthood in workers or queens (Hsieh and Hsu 2011a, b). Moreover, assays of age-related molecules have shown that the trophocytes and fat cells of old workers and queens exhibit higher levels of senescence-associated beta-galactosidase (SA-β-Gal), more lipofuscin granules, and more extensive lipid peroxidation and protein oxidation compared to newly emerged workers and young queens (Hsieh and Hsu 2011a, b). Collectively, these studies have demonstrated the utility of trophocytes and fat cells for cellular senescence studies. Furthermore, trophocytes and fat cells are immersed in abdominal body fluid. Thus, antiaging drugs can be screened for their effects on target trophocytes and fat cells by microinjecting them into the body fluid (Hsieh and Hsu 2011a; Hsu and Chan 2011a), adding to the suitability of trophocytes and fat cells as target cells for cellular senescence studies.
Recent studies have shown that the expression of age-related molecules in the trophocytes and fat cells of young and old workers reared in field hive is similar to that of young and old workers reared in a thermostatic chamber at 34 °C throughout their lives (Hsieh and Hsu 2011a; Hsu and Chan 2011a). Age is the only difference between workers reared in a thermostatic chamber at 34 °C throughout their lives, indicating that the trophocytes and fat cells of young and old workers reared in a field hive are suitable for studying aging.
If trophocytes and fat cells are to be used for future cellular senescence studies, it is important that changes in mitochondrial energy utilization in young and old workers be explored. Such data could be used as reference values for evaluating cellular rejuvenation or senescence in future aging studies, especially antiaging drug screening studies. In the current study, mitochondrial energy utilization molecules were evaluated in the trophocytes and fat cells of young and old workers reared in a field hive to clarify the relationship between mitochondrial energy utilization and aging in worker honeybees.
Materials and methods
Honeybees
Honeybees (A. mellifera) were bred in the open environment of a bee-breeding room on the tenth floor of our institute in Taiwan. Workers could fly freely into or out of hives for foraging as they do in field hives. Sucrose solution and pollen grains mixed with sucrose solution were sometimes added to the hives as dietary supplements. As a previous study described (Hsieh and Hsu 2011a), 1-day-old and 50-day-old workers were selected from the same colony on the same dates to serve as young (hive worker) and old workers (old forager), respectively, for the following studies. Young workers have dense and light yellowish-brown fuzz and light yellowish-brown epidermis. However, old workers have sparse and dark brown fuzz and dark blackish-brown epidermis.
Assay of mitochondrial density
Mitochondrial density in trophocytes and fat cells in young and old workers was assayed by confocal microscopy and transmission electron microscopy (TEM). In the confocal microscopy approach, mitochondrial density was determined using the fluorescent dye, MitoTracker Green (MTG) (27674w; Invitrogen, Carlsbad, CA, USA), as previously described (Spodnik et al. 2002). Briefly, the abdomens of workers were dissected by scissors. Trophocytes and fat cells from abdomens were detached from the cuticle of worker in phosphate-buffered saline (PBS) by a knife and collected by centrifugation. Trophocytes and fat cells from one young and one old worker were fixed in 4 % paraformaldehyde for 15 min and washed three times with PBS (pH 7.4). Suspensions of fixed cells were incubated with 40 nM MTG for 20 min at room temperature in the dark, washed twice, and observed by confocal microscopy (Leica TCS SP2; Leica, Wetzlar, Germany). Confocal microscopic images were analyzed with QWin image processing and analysis software (version 2.5; Leica, Wetzlar, Germany) (Hsu and Chiu 2009). This experiment was biologically replicated five times and used a total of five young and five old workers.
For TEM analyses, trophocytes from one young and one old worker were fixed in 2.5 % glutaraldehyde in 0.1 M phosphate buffer containing 0.35 M sucrose (pH 7.4) for 30 min at 25 °C and were postfixed in 1 % osmium tetroxide in 0.1 M phosphate buffer with 0.35 M sucrose (pH 7.4) for 2 h. Trophocytes were dehydrated through an ethanol series and embedded in Spurr’s resin. Thin sections (60–90 nm in thickness) were cut with a diamond knife, stained with uranyl acetate and lead citrate, and then examined using a TEM system (JEOL JEM-2000EXII; Tokyo, Japan) operating at an accelerating voltage of 100 kV (Hsu and Chan 2011b). This experiment was biologically replicated ten times and used a total of ten young and ten old workers.
Assay of ∆Ψm
Mitochondrial membrane potential (∆Ψm) was assayed in the trophocytes and fat cells of one young and one old worker using the specific dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazole carbocyanine iodide (JC1) (T3168; Invitrogen, Carlsbad, CA, USA). Trophocytes and fat cells were stained with 10 μM JC1 for 30 min, washed three times with PBS, fixed in 4 % paraformaldehyde, washed three times with PBS, excited at a wavelength of 488 nm, and visualized using confocal microscopy (Leica TCS SP2; Leica, Wetzlar, Germany). ∆Ψm was determined by the ratiometric analysis of red fluorescence emitted by JC1 aggregates (590 nm) and green fluorescence emitted by the monomer (525 nm) using QWin image processing and analysis software (version 2.5; Leica, Wetzlar, Germany) (López-Lluch et al. 2006; Hsu and Chiu 2009). This experiment was biologically replicated five times and used a total of five young and five old workers. In addition, ∆Ψm was also assayed in the trophocytes and fat cells of one young and one old worker using the specific dye, tetramethylrhodamine methyl ester (TMRM) (T668; Invitrogen, Carlsbad, CA, USA) (Ward et al. 2000). Trophocytes and fat cells were stained with 200 nM TMRM for 10 min, washed three times with PBS, and visualized under a confocal microscopy (Leica TCS SP2; Leica, Wetzlar, Germany) at an excitation wavelength of 550 nm and an emission wavelength of 610 nm. Five confocal microscopy images of trophocytes and fat cells from one young and one old worker were analyzed using QWin image processing and analysis software (version 2.5, Leica, Wetzlar, Germany). This experiment was biologically replicated five times and used a total of five young and five old workers.
Assay of NAD+ and NADH concentration
Nicotinamide adenine dinucleotide oxidized form (NAD+) and nicotinamide adenine dinucleotide reduced form (NADH) concentration in the trophocytes and fat cells of young and old workers were determined using an NAD+/NADH Quantification Kit (K337-100; BioVision, Mountain View, CA, USA), as described by the manufacturer. Briefly, trophocytes and fat cells from 15 young and old workers were extracted with 1 ml of NADH/NAD extraction buffer; 0.5 ml of the extracted samples was heated at 60 °C for 30 min for NADH measurement. Protein concentration was determined using a commercial protein assay reagent (500-0006; Bio-Rad Laboratories, Hercules, CA, USA). After background luminescence was recorded, extracted samples and extracted, heated samples were measured spectrophotometrically at 450 nm at room temperature. The resultant luminescence was normalized to background. Dilutions of an NADH standard solution (0, 20, 40, 60, 80, and 100 pmol μl−1) were processed concurrently to generate a standard curve, which was used to obtain NAD+ and NADH values (expressed as micromoles per milligram protein) and NAD+/NADH ratio. This experiment was biologically replicated four times and used a total of 60 young and 60 old workers.
Assay of ATP concentration
Adenosine triphosphate (ATP) concentration in the trophocytes and fat cells of young and old workers was quantified using an ATP Determination Kit (A22066; Invitrogen, Carlsbad, CA, USA), as described by the manufacturer’s instructions. Briefly, trophocytes and fat cells from three young and old workers were homogenized in 1.0 ml 50 mM phosphate buffer (pH 7.5) containing protease inhibitors (leupeptin 10 μg/ml, pepstatin 1 μg/ml, and phenylmethylsulfonyl fluoride 40 μg/ml) by using a polytron and sonicator, and centrifuged at 5,000 g for 10 min to obtain the resulting supernatant. Protein concentration was determined using a commercial protein assay reagent (500-0006; Bio-Rad Laboratories, Hercules, CA, USA). After recording background luminescence, 10 μl of diluted ATP standard solution (1, 10, 100, 1,000, and 5,000 nM) or 10 μl of supernatant was added to the standard reaction solution and measured spectrophotometrically at 560 nm at room temperature. ATP values, expressed as picomoles per milligram protein, were obtained from the resultant luminescence (standardized to background) by reference to the ATP standard curve generated concurrently. This experiment was biologically replicated four times and used a total of 60 young and 60 old workers.
Western blotting
Trophocytes and fat cells were isolated from three young or old workers, homogenized in 100 μl of radioimmunoprecipitation (RIPA) buffer containing protease inhibitors (11697498001; Roche Applied Science, Indianapolis, IN, USA), and centrifuged at 5,000 g for 10 min at 4 °C. The protein concentration of resulting supernatant was determined using a protein assay reagent (500-0006; Bio-Rad Laboratories, Hercules, CA, USA). Proteins (30 μg) from the supernatant were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) on 10–15 % polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking for 1 h at 25 °C, membranes were first incubated with primary antibodies against ATP synthase (1:1,000; produced in-house), NADH dehydrogenase 1 (ND1) (h00004535-A01, 1:1,000; Abnova, Neihu, Taipei, Taiwan), or voltage-dependent anion channel 1 (VDAC1) (GTX114187, 1:1000; GeneTex, Irvine, CA, USA), tubulin (ab6046, 1:10,000; Abcam, Cambridge, MA, USA) and then probed with the appropriate horseradish peroxidase-conjugated secondary antibody (1:10,000). Immunoreactive proteins were detected using a chemiluminescence method (PerkinElmer, Covina, CA, USA) and analyzed using Image J software (NIH, Bethesda, MA, USA). The protein expression levels were normalized to tubulin. This experiment was biologically replicated five times and used a total of 15 young and 15 old workers.
Statistical analysis
The differences in mean values between the two age groups were examined using two-sample t tests. A p value less than 0.05 was considered statistically significant.
Results
Mitochondrial density
To examine the relationship between mitochondrial energy utilization and aging in workers, we assayed the mitochondrial density in the trophocytes and fat cells of young and old workers reared in a field hive. Mitochondrial density in trophocytes and fat cells, determined by assessing green staining with the mitochondrial-specific fluorescent marker, MitoTracker Green (MTG) via confocal microscopy, was low in young workers and high in old workers (Fig. 1a). Statistical analyses revealed that mitochondrial area in old workers was greater than that in young workers (n = 5, P < 0.05) (Fig. 1b). To confirm the differences in mitochondrial density observed by confocal microscopy, we evaluated mitochondria in trophocytes by TEM. The results also showed that mitochondrial density in trophocytes and fat cells was lower in young workers than in old workers (Fig. 1c). Statistical analyses of TEM results showed that the mitochondrial area/cellular area ratio increased with advancing age (n = 10, P < 0.01) (Fig. 1d). These results indicate that mitochondrial density increases with advancing age in workers.
Mitochondrial density in the trophocytes and fat cells of workers. a Mitochondrial density was determined with MTG by confocal microscopy using the mitochondria-specific fluorescent marker, MTG. Scale bar, 75 μm. b The area of MTG fluorescence was normalized to that in young workers. The results are presented as means ± SEMs, expressed as percentages. c Mitochondrial density in trophocytes was determined using TEM, Scale bar, 1 μm. d Mitochondrial area/cellular area ratio was normalized to that in young workers. The results are presented as means ± SEMs, expressed as percentages. In all cases, asterisks indicate statistically significant differences (* P < 0.05, ** P < 0.01; two-sample t test)
∆Ψm
To investigate the relationship between mitochondrial energy utilization and aging in workers, we assayed ∆Ψm in the trophocytes and fat cells of young and old workers reared in a field hive. ∆Ψm was assayed by calculating the ratio of monomeric (green fluorescence) and J-aggregate (red fluorescence) forms of JC1 stain, indicative of compromised versus intact functioning mitochondria, respectively (Fig. 2a). The resultant red/green ratios showed that ∆Ψm was higher in young workers than in old workers (n = 5, P < 0.05) (Fig. 2b), indicating that ∆Ψm decreased with advancing age in workers. To confirm the results of ∆Ψm, we further stained the cells with TMRM. The trophocytes and fat cells of young workers expressed higher red fluorescence than those of old workers (Fig. 2c). The ratio of red fluorescence/cell area represented for ∆Ψm. The statistical analysis showed significantly higher ∆Ψm levels in the young workers than in the old workers (n = 5, P < 0.01) (Fig. 2d), further confirming that ∆Ψm decreased with advancing age in workers.
∆Ψm in the trophocytes and fat cells of workers. a Representative confocal images of the stained JC1-monomeric form (upper panel) and JC1-aggregated form (lower panel). Scale bar, 50 μm. b ∆Ψm values, presented as means ± SEMs, were measured by calculating the red/green ratio. c Representative confocal images of the stained TMRM. Scale bar, 50 μm. d ∆Ψm values were measured by calculating the red fluorescence/cell area ratio. The red fluorescence/cell area ratio was normalized to that in young workers. The results are presented as means ± SEMs, expressed as percentages. Asterisk indicates a statistically significant difference (* P < 0.05, ** P < 0.01; two-sample t test)
NAD+, NADH, and ATP
To study the relationship between ∆Ψm and the expression of NAD+ and ATP, we assayed NAD+, NADH, and ATP concentration in the trophocytes and fat cells of young and old workers. The mean values for the NAD+ concentration in trophocytes and fat cells were 7.5 ± 0.9 and 3.9 ± 0.8 μmol mg−1 of protein in young and old workers, respectively (n = 60, P < 0.05) (Fig. 3a). The mean values for the NADH concentration in trophocytes and fat cells were 5.7 ± 0.7 and 6.6 ± 1.0 μmol mg−1 of protein in young and old workers, respectively (n = 60, P > 0.05) (Fig. 3b). The mean values for NAD+/NADH ratio in trophocytes and fat cells were 1.4 ± 0.2 and 0.6 ± 0.1 in young and old workers, respectively (n = 4, P < 0.05), indicating that young workers have higher NAD+/NADH ratio than old workers (Fig. 3c). The mean ATP concentration in trophocytes and fat cells were 3.0 ± 0.2 and 0.6 ± 0.2 pmol mg−1 of protein in young and old workers, respectively (n = 60, P < 0.01) (Fig. 3d). These results show that NAD+ and ATP concentration and NAD+/NADH ratio decreased with age in workers.
ND1 and ATP synthase
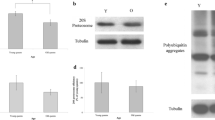
To extend our evaluation of NAD+ and ATP, we assayed ND1 and ATP synthase expression in the trophocytes and fat cells of young and old workers. ND1 expression was lower in young workers than in old workers (Fig. 4a), an observation confirmed by statistical analyses showing that ND1 expression was significantly less in young workers than in old workers (n = 15, P < 0.05) (Fig. 4b). Likewise, ATP synthase expression was also low in young workers and high in old workers (Fig. 4c), a difference that was statistically significant (n = 15, P < 0.01) (Fig. 4d). Because ND1 and ATP synthase are located in mitochondria, their expression levels were normalized to mitochondrial density. Therefore, VDAC1 was assayed in the trophocytes and fat cells. VDAC1 expression was low in young workers and high in old workers (Fig. 4e), a difference that was statistically significant (n = 24, P < 0.01) (Fig. 4f). The expression levels of ND1 and ATP synthase were normalized to mitochondrial density and VDAC1. Normalized ND1 and ATP synthase levels to mitochondrial density were not significantly different between young and old workers, respectively (n = 5, P > 0.05) (n = 5, P > 0.05) (Fig. 5a, b). Likewise, normalized ND1 and ATP synthase levels to VDAC1 were not significantly different between young and old workers, respectively (n = 5, P > 0.05) (n = 5, P > 0.05) (Fig. 5c, d).
The expression of ND1, ATP synthase, and VDAC1 in the trophocytes and fat cells of workers. ND1 (a), ATP synthase (c), and VDAC1 (e) were analyzed by western blotting. Tubulin served as a loading control, and rat muscle was used as a positive control. Y young workers, O old workers, P positive control. The expression of ND1 (b), ATP synthase (d), and VDAC1 (f) were normalized to that in young workers. The results are presented as means ± SEMs, expressed as percentages. In all cases, asterisks indicate statistically significant difference (* P < 0.05, ** P < 0.01; two-sample t test)
ND1 and ATP synthase expression normalized to mitochondrial density and VDAC1. a The ND1/mitochondrial density ratio; b the ATP synthase/mitochondrial density ratio; c the ND1/VDAC1 ratio; and d the ATP synthase/VDAC1 ratio. Ratios were expressed relative to those in young workers. The results are presented as means ± SEMs, expressed as percentages
Discussion
In this study, we evaluated mitochondrial energy utilization in trophocytes and fat cells of young and old workers reared in a field hive. Compared to old workers, young workers had lower mitochondrial density, higher ∆Ψm, elevated NAD+ and ATP concentration, and raised NAD+/NADH ratio. Although ND1 and ATP synthase levels were lower in young workers, these differences became statistically insignificant following normalization to mitochondrial density and VDAC1. Collectively, these results show that young workers utilized mitochondrial energy more efficiently than old workers and that aging results in a decline in mitochondrial energy utilization in worker honeybees.
Mitochondrial density
Mitochondrial density increased with advancing age in the trophocytes and fat cells of workers. This result is consistent with previous studies showing that senescent human diploid fibroblasts have a high mitochondrial density (Martinez et al. 1991; Xu and Finkel 2002) and that mitochondrial density increased in the lungs and brains of old humans (Lee et al. 1998; Barrientos et al. 1997; Lee et al. 2000); livers, hearts, and brains of old rats (Gadaleta et al. 1992); and the livers of old mice, rats, guinea pigs, rabbits, sheep, cows, hamsters, and humans (Passos et al. 2007). This phenomenon principally reflects the low turnover of mitochondria and the accumulation of modified lipids, proteins, and DNA during the aging process, which leads to mitochondrial dysfunction and a decline in mitochondrial efficacy (Papa 1996; Wei 1998; López-Lluch et al. 2008; Navarro and Boveris 2007; Choksi et al. 2007). This interpretation is supported by a recent study showing that lipid peroxidation and protein oxidation increase with advancing age in the trophocytes and fat cells of workers reared in a field hive (Hsieh and Hsu 2011a). To compensate for reduced mitochondrial function with advancing age, cells increase mitochondrial density. This postulate is supported by previous studies showing that respiratory chain deficiency of mice induced the increase of mitochondrial mass (Wredenberg et al. 2002) and the loss of ∆Ψm induced mitochondrial biogenesis (Passos et al. 2007). In addition, this phenomenon may reflect the lower function of cellular degradation system with advancing age. Cellular degradation system can remove cytoplasmic materials and organelles by lysosomal degradation (Ciechanover 2005; Wolf and Hilt 2004; Mizushima et al. 2004; Cuervo et al. 2005). The accumulation of lipofuscin in lysosome may slow down autophagocytosis and impair the degradation of mitochondria, leading to the accumulation of dysfunction of mitochondria in old workers. This interpretation is supported by a recent study showing that SA-β-Gal and lipofuscin increase with advancing age in the trophocytes and fat cells of workers reared in a field hive (Hsieh and Hsu 2011a).
In addition, a previous study showed that shorter-lived mammals have high mitochondrial density, whereas animals with longer life span generally have fewer mitochondria (Passos et al. 2007). Furthermore, temperature reduction (TR) can extend the life span of organisms, a life span extension that is associated with low mitochondrial density (Hsu and Chiu 2009). The fact that mitochondrial density increased with advancing age in the trophocytes and fat cells of workers is reconfirmed by the expression levels of ND1, ATP synthase, and VDAC1.
∆Ψm
∆Ψm controls respiratory rate, ATP synthesis, and reactive oxygen species (ROS) production and is, in turn, controlled by electron transport and proton leakage (Nicholls 2004). In this study, ∆Ψm decreased with advancing age in the trophocytes and fat cells of workers. This result is in accord with previous studies showing that ∆Ψm decreases with advancing age in the hepatocytes of rats and mice (Hagen et al. 1997; Kokoszka et al. 2001), in the lymphocytes of mice (Wikowski and Micklem 1985; Rottenberg and Wu 1997), and hearts of rats (Savitha and Panneerselvam 2006). This phenomenon is the result of mitochondrial dysfunction during the aging process (Ames et al. 1995; Trifunovic and Larsson 2008; Artal-Sanz and Tavernarakis 2008; López-Lluch et al. 2008; Chanséaume and Morio 2009). In addition, the extended life span that accompanies TR, noted above, is associated with high ∆Ψm (Hsu and Chiu 2009). Thus, the high ∆Ψm in the trophocytes and fat cells of young workers may have cellular functions similar to the high ∆Ψm observed with TR.
NAD+ and NADH concentration
NAD+ concentration decreased with advancing age in the trophocytes and fat cells of workers. This result is in agreement with previous studies showing that NAD+ levels decrease with advancing age in pancreatic β cells and neurons of mice (Ramsey et al. 2008; Imai 2009), as well as in the livers, hearts, kidneys, and lungs of rats (Braidy et al. 2011; Son et al. 2012). NADH concentration was not significantly different with advancing age in the trophocytes and fat cells of workers. The NAD+/NADH ratio was decreased with advancing age in the trophocytes and fat cells of workers. This finding is consistent with previous studies showing that the NAD+/NADH ratio decreases with advancing age in pancreatic β cells and neurons of mice (Ramsey et al. 2008; Imai 2009), as well as in the livers, hearts, kidneys, and lungs of rats (Braidy et al. 2011; Son et al. 2012). These findings are also related to the differences in ∆Ψm level observed in the current study. Protons from NADH are pumped out of the mitochondrial matrix, creating a proton gradient that forms ∆Ψm. A decrease in this action results in a decrease in ∆Ψm and a subsequent decrease in NAD+ concentration. Calorie restriction (CR), which can also extend the life span of organisms, is associated with high NAD+ levels (Lin et al. 2002; Qin et al. 2006; Ziegler and Niere 2004). Thus, high NAD+ levels may play a role in the trophocytes and fat cells of young workers similar to that in CR.
ATP concentration
ATP concentration also decreased with advancing age in the trophocytes and fat cells of workers. Notably, ATP concentration in trophocytes and fat cells have also been shown to decrease with advancing age in worker bees reared in a thermostatic chamber at 34 °C (Hsu and Chan 2011a), providing further support for the idea that young and old workers reared in a field hive can be used for studying aging. This decrease in ATP concentration in trophocytes and fat cells of older workers is also consistent with previous studies showing that ATP levels decrease with advancing age in the gastric mucosa and muscle of humans (Kawano et al. 1991; Conley et al. 2000; Petersen et al. 2003; Gurd et al. 2008), the blood and brains of mice (Jayachandran et al. 2005; Joo et al. 1999), the erythrocytes of rabbits (Subasinghe and Spence 2008) and cows (Bartosz et al. 1982), and the hearts of rats (Guerrieri et al. 1996). This finding is also consistent with the results of ∆Ψm measurements reported in this study; as was the case for NAD+, a decrease in ∆Ψm can result in a decrease in ATP concentration.
The decrease of ∆Ψm, NAD+ concentration, ATP concentration, and NAD+/NADH ratio in the trophocytes and fat cells of workers with advancing age indicated that aging results in a decline in mitochondrial energy utilization in the trophocytes and fat cells of worker honeybees. This inference is consistent with a previous study, showing age-associated decline in mitochondrial respiration and electron transport in the flight muscle of Drosophila melanogaster (Ferguson et al. 2005). The decline in mitochondrial energy utilization in the trophocytes and fat cells of workers most likely reflect the accumulation of oxidative damages. This hypothesis is consistent with previous studies, showing that flight behavior accelerated oxidative damages in house flies (Musca domestica) (Vance et al. 2009). Conversely, stopping flight decreases oxidative damages and increases longevity (Yan and Sohal 2000). In addition, honeybee foragers have higher flight metabolic rate in flight muscles and result in 43 % body mass loss compared to hive bees (Vance et al. 2009; Schippers et al. 2010). Likewise, orchid bee foragers also have higher flight metabolic rate in flight muscles (Suarez et al. 2005). This higher energy consumption increases oxidative damages (Ferguson et al. 2005).
ND1 and ATP synthase
ND1 expression increased with advancing age in the trophocytes and fat cells of workers. This finding is consistent with previous studies showing that NADH dehydrogenase expression increases with advancing age in the muscle of rats (Chang et al. 2007) and in lung fibroblasts of humans (Allen et al. 1999). ATP synthase expression also increased with advancing age in the trophocytes and fat cells of workers. Previous studies have reported similar findings, showing that ATP synthase expression increases with advancing age in the brains and muscle of rats (Nicoletti et al. 1995; Chang et al. 2007; Doran et al. 2008; Donoghue et al. 2010), and in muscle of humans (Gelfi et al. 2006). Although ND1 and ATP synthase expression measured in absolute terms increased with advancing age in the trophocytes and fat cells of workers, the relative expression of ND1 and ATP synthase normalized to mitochondrial density and VDAC1 was not significantly different. In contrast, the concentration of NAD+ and ATP decreased with advancing age, indicating that NADH dehydrogenase and ATP synthase efficiency decreases with advancing age in the trophocytes and fat cells of workers. These observations are consistent with previous reports that ATP synthase activity decreases with advancing age in the erythrocytes of cows (Bartosz et al. 1982) and the hearts of mice (Yarian et al. 2005). Similarly, NADH dehydrogenase activity has also been reported to decrease with advancing age in the kidneys, livers, and testes of rats (Napoleone et al. 1991; Torres-Mendoza et al. 1999; Vázquez-Memije et al. 2008), as well as in the livers and brains of mice (Navarro et al. 2002; Navarro 2004).
Taken together, our results indicate that young workers have lower mitochondrial density, higher ∆Ψm, higher NAD+ and ATP concentration, and higher NAD+/NADH ratio than old workers, showing that energy utilization efficiency decreases with advancing age in the trophocytes and fat cells of workers.
References
Allen RG, Tresini M, Keogh BP, Doggett DL, Cristofalo VJ (1999) Differences in electron transport potential, antioxidant defenses, and oxidant generation in young and senescent fetal lung fibroblasts (WI-38). J Cell Physiol 180:114–122
Ames BN, Shigenaga MK, Hagen TM (1995) Mitochondrial decay in aging. Biochim Biophys Acta 1271:165–170
Artal-Sanz M, Tavernarakis N (2008) Mechanisms of aging and energy metabolism in Caenorhabditis elegans. IUBMB Life 60:315–322
Barrientos A, Casademont J, Cardellach F, Estivill X, Urbano-Marquez A, Nunes V (1997) Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Mol Brain Res 52:284–289
Bartosz G, Grzelinska E, Wagner J (1982) Aging of the erythrocytes. XIV. ATP content does decrease. Experientia 38:575
Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R (2011) Age related changes in NAD+ metabolism oxidative stress and sirt1 activity in Wistar rats. PLoS One 6(4):e19194. doi:10.1371/journal.pone.0019194
Chang J, Cornell JE, van Remmen H, Hakala K, Ward WF, Richardson A (2007) Effect of aging and caloric restriction on the mitochondrial proteome. J Gerontol A Biol Sci Med Sci 62:223–234
Chanséaume E, Morio B (2009) Potential mechanisms of muscle mitochondrial dysfunction in aging and obesity and cellular consequences. Int J Mol Sci 10:306–324
Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J (2007) Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radical Biol Med 43:1423–1438
Ciechanover A (2005) Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol 6:79–87
Collins AM, Williams V, Evans JD (2004) Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Mol Biol 13:141–146
Conley KE, Jubrias SA, Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526:203–210
Corona M, Hughes KA, Weaver DB, Robinson GE (2005) Gene expression patterns associated with queen honey bee longevity. Mech Ageing Develop 6:1230–1238
Cuervo AM, Bergamini E, Brunk UT, Drӧge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1:131–140
Donoghue P, Staunton L, Mullen E, Manning G, Ohlendieck K (2010) DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J Proteomics 73:1441–1453
Doran P, O'Connell K, Gannon J, Kavanagh M, Ohlendieck K (2008) Opposite pathobiochemical fate of pyruvate kinase and adenylate kinase in aged rat skeletal muscle as revealed by proteomic DIGE analysis. Proteomics 8:364–377
Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS (2005) Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J 390:501–511
Gadaleta MN, Rainaldi G, Lezza AMS, Milella F, Fracasso F, Cantatore P (1992) Mitochondrial DNA copy number and mitochondrial DNA deletion in adult and senescent rats. Mutat Res 275:181–193
Gelfi C, Viganò A, Ripamonti M, Pontoglio A, Begum S, Pellegrino MA, Grassi B, Bottinelli R, Wait R, Cerretelli P (2006) The human muscle proteome in aging. J Proteome Res 5:1344–1353
Guerrieri F, Vendemiale G, Turturro N, Fratello A, Furio A, Muolo L, Grattagliano I, Papa S (1996) Alteration of mitochondrial F0F1 ATP synthase during aging. Ann N Y Acad Sci 786:62–71
Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM (2008) O2 uptake kinetics, pyruvate dehydrogenase activity, and muscle deoxygenation in young and older adults during the transition to moderate-intensity exercise. Am J Physiol Regul Integr Comp Physiol 294:R577–R584
Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN (1997) Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA 94:3064–3069
Hsieh YS, Hsu CY (2011a) Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp Gerontol 46:233–240
Hsieh YS, Hsu CY (2011b) The changes of age-related molecules in the trophocytes and fat cells of queen honeybees (Apis mellifera). Apidologie 42:728–739
Hsu CY, Chan YP (2011a) The use of honeybees reared in a thermostatic chamber for aging studies. Age. doi:10.1007/s11357-011-9344-z
Hsu CY, Chan YP (2011b) Identification and localization of proteins associated with biomineralization in the iron deposition vesicles of honeybees (Apis mellifera). PLoS One 6(4):e19088. doi:10.1371/journal.pone.0019088
Hsu CY, Chiu YC (2009) Ambient temperature influences aging in an annual fish (Nothobranchius rachovii). Aging Cell 8:726–737
Imai S (2009) The NAD world: a new systemic regulatory network for metabolism and aging-Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys 53:65–74
Jayachandran M, Karnicki K, Miller RS, Owen WG, Korach KS, Miller VM (2005) Platelet characteristics change with aging: role of estrogen receptor beta. J Gerontol A Biol Sci Med Sci 60:815–819
Joo HJ, Ma JY, Choo YG, Choi BK, Jung KY (1999) Age-related alteration of intracellular ATP maintenance in the cell suspensions of mice cerebral cortex. Mech Aging Dev 110:1–12
Kawano S, Tanimura H, Sato N, Nagano K, Tsuji S, Takei Y, Tsujii M, Hayashi N, Masuda E, Kashiwagi T, Fusamoto H, Kamada T (1991) Age-related change in human gastric mucosal energy metabolism. Scand J Gastroenterol 26:701–706
Kokoszka JE, Coskup P, Esposito LA, Wallace DC (2001) Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA 98:2278–2283
Lee HC, Lu CY, Fahn HJ, Wei YH (1998) Aging- and smoking-associated alteration in the relative content of mitochondrial DNA in human lung. FEBS Lett 441:292–296
Lee HC, Yin PH, Lu CY, Chi CW, Wei YH (2000) Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J 348:425–432
Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L (2002) Calorie restriction extends Saccharomyces cerevisiae life span by increasing respiration. Nature 418:344–348
López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103:1768–1773
López-Lluch G, Irusta PM, Navas P, de Cabo R (2008) Mitochondrial biogenesis and healthy aging. Exp Gerontol 43:813–819
Martinez AO, Over D, Armstrong LS, Manzano L, Taylor R, Chambers J (1991) Separation of two populations of old human fibroblasts by mitochondria (rhodamine 123) fluorescence. Growth Dev Aging 55:185–191
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophage in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Napoleone P, Bronzetti E, Amenta F (1991) Enzyme histochemistry of aging rat kidney. Mech Ageing Dev 61:187–195
Navarro A (2004) Mitochondrial enzyme activities as biochemical markers of aging. Mol Aspects Med 25:37–48
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C686
Navarro A, Sánchez Del Pino MJ, Gomez C, Peralta JL, Boveris A (2002) Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol 282:R985–R992
Neukirch A (1982) Dependence of the life span of the honeybee (Apis mellifera) upon flight performance and energy consumption. J Comp Physiol 146:35–40
Nicholls DG (2004) Mitochondrial membrane potential and aging. Aging Cell 3:35–40
Nicoletti VG, Tendi EA, Lalicata C, Reale S, Costa A, Villa RF, Ragusa N, Giuffrida Stella AM (1995) Changes of mitochondrial cytochrome c oxidase and F0F1 ATP synthase subunits in rat cerebral cortex during aging. Neurochem Res 20:1465–1470
Papa S (1996) Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim Biophys Acta 1276:87–105
Passos JF, von Zglinicki T, Kirkwood TBL (2007) Mitochondria and ageing: winning and losing in the numbers game. BioEssays 29:908–917
Petersen FK, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754
Ramsey KM, Mills KF, Satoh A, Imai S (2008) Age associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7:78–88
Remolina SC, Hafez DM, Robinson GE, Hughes KA (2007) Senescence in the worker honey bee Apis mellifera. J Insect Physiol 53:1027–1033
Rottenberg H, Wu S (1997) Mitochondrial dysfunction in lymphocytes from old mice: Enhanced activation of the permeability transition. Biochem Biophys Res Commun 240:68–74
Rueppell O, Bachelier C, Fondrk MK, Page RE (2007a) Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.). Exp Gerontol 42:1020–1032
Rueppell O, Christine S, Mulcrone C, Groves L (2007b) Aging without functional senescence in honey bee workers. Curr Biol 17:R274–R275
Savitha S, Panneerselvam C (2006) Mitochondrial membrane damage during aging process in rat heart: potential efficacy of L-carnitine and DL α lipoic acid. Mech Ageing Dev 127:349–355
Schippers MP, Dukas R, McClelland GB (2010) Lifetime- and caste-specific changes in flight metabolic rate and muscle biochemistry of honeybees, Apis mellifera. J Comp Physiol B 180:45–55
Seehuus SC, Krekling T, Amdam GV (2006a) Cellular senescence in honey bee brain is largely independent of chronological age. Exp Gerontol 41:1117–1125
Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV (2006b) Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci USA 103:962–967
Son N, Hur HJ, Sung MJ, Kim MS, Hwang JT, Park JH, Yang HJ, Kwon DY, Yoon SH, Chung HY, Kim HJ (2012) Liquid chromatography-mass spectrometry-based metabolomic analysis of liver from aged rats. J Proteome Res 11:2551–2558
Spodnik JH, Wozniak M, Budzko D, Teranishi M, Karbowski M, Nishizawa Y, Usukura J, Wakabayashi T (2002) Mechanism of leflunomide-induced proliferation of mitochondria in mammalian cells. Mitochondrion 2:163–179
Suarez RK, Darveau CA, Welch KC, O'Brien DM, Roubik DW, Hochachka PW (2005) Energy metabolism in orchid bee flight muscle: carbohydrate fuels all. J Exp Biol 208:3573–3579
Subasinghe W, Spence DM (2008) Simultaneous determination of cell aging and ATP release from erythrocytes and its implications in type 2 diabetes. Analytic Chimica Acta 618:227–233
Torres-Mendoza CE, Albert A, de la Cruz Arriaga MJ (1999) Molecular study of the rat liver NADH: cytochrome c oxidoreductase complex during development and aging. Mol Cell Biochem 195:133–142
Trifunovic A, Larsson N-G (2008) Mitochondrial dysfunction as a cause of ageing. J Intern Med 263:167–178
Vance JT, Williams JB, Elekonich MM, Roberts SP (2009) The effects of age and behavioral development on honey bee (Apis mellifera) flight performance. J Exp Biol 212:2604–2611
Vázquez-Memije ME, Capin R, Tolosa A, El-Hafidi M (2008) Analysis of age-associated changes in mitochondrial free radical generation by rat testis. Mol Cell Biochem 307:23–30
Ward MW, Rego AC, Frenguelli BG, Nicholls DG (2000) Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 20:7208–7219
Wei YH (1998) Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med 217:53–63
Weirich GF, Collins AM, Williams VP (2002) Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 33:3–14
Wikowski JM, Micklem HS (1985) Decreased membrane potential of T lymphocytes in ageing mice: flow cytometric studies with a carbocyanine dye. Immunology 56:307–313
Williams JB, Roberts SP, Elekonich MM (2008) Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Exp Gerontol 43:538–549
Wolf DH, Hilt W (2004) The proteasome: a proteolytic nanomachine of cell regulation and waste disposal. Biochim Biophys Acta 1695:19–31
Wredenberg A, Wibom R, Wilhelmsson H, Graff C, Wiener HH, Burden SJ, Oldfors A, Westerblad H, Larsson NG (2002) Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci USA 99:15066–15071
Xu D, Finkel T (2002) A role for mitochondria as potential regulators of cellular life span. Biochem Biophys Res Commun 294:245–248
Yan LJ, Sohal RS (2000) Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associated oxidative damage to specific mitochondrial proteins. Free Radic Biol Med 29:1143–1150
Yarian CS, Rebrin I, Sohal RS (2005) Aconitase and ATP synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem Biophys Res Commun 330:151–156
Ziegler M, Niere M (2004) NAD+ surface again. Biochem J 382:e5–e6
Acknowledgments
This work was supported by a grant (CMRPD 1A0491) from Chang Gung Memorial Hospital, Taiwan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Chuang, YL., Hsu, CY. Changes in mitochondrial energy utilization in young and old worker honeybees (Apis mellifera). AGE 35, 1867–1879 (2013). https://doi.org/10.1007/s11357-012-9490-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-012-9490-y