Abstract
Mild traumatic brain injury (mTBI) represents a major and increasing public health concern and is both the most frequent cause of mortality and disability in young adults and a chief cause of morbidity in the elderly. Albeit mTBI patients do not show clear structural brain defects and, generally, do not require hospitalization, they frequently suffer from long-lasting cognitive, behavioral, and emotional problems. No effective pharmaceutical therapy is available, and existing treatment chiefly involves intensive care management after injury. The diffuse neural cell death evident after mTBI is considered mediated by oxidative stress and glutamate-induced excitotoxicity. Prior studies of the long-acting GLP-1 receptor agonist, exendin-4 (Ex-4), an incretin mimetic approved for type 2 diabetes mellitus treatment, demonstrated its neurotrophic/protective activity in cellular and animal models of stroke, Alzheimer’s and Parkinson’s diseases, and, consequent to commonalities in mechanisms underpinning these disorders, Ex-4 was assessed in a mouse mTBI model. In neuronal cultures in this study, Ex-4 ameliorated H2O2-induced oxidative stress and glutamate toxicity. To evaluate in vivo translation, we administered steady-state Ex-4 (3.5 pM/kg/min) or saline to control and mTBI mice over 7 days starting 48 h prior to or 1 h post-sham or mTBI (30 g weight drop under anesthesia). Ex-4 proved well-tolerated and fully ameliorated mTBI-induced deficits in novel object recognition 7 and 30 days post-trauma. Less mTBI-induced impairment was evident in Y-maze, elevated plus maze, and passive avoidance paradigms, but when impairment was apparent Ex-4 induced amelioration. Together, these results suggest that Ex-4 may act as a neurotrophic/neuroprotective drug to minimize mTBI impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is occurring more commonly and, according to the World Health Organization, with an estimated 10 million people affected annually worldwide (Hyder et al. 2007), it will soon outstrip many common diseases as the major cause of death and disability (Zitnay 2005). With a high morbidity and mortality, and no specific therapeutic treatment (Garner and Brett 2007; Howard and Shapiro 2011), TBI has become a pressing public health and medical problem. The highest incidence of TBI is among young adults (15 to 24 years age) as well as in the elderly (75 years and older). Mild (m) to moderate TBI predominates and accounts for 80–95 % of cases, with severe TBI accounting the remainder (Tagliaferri et al. 2006). The elderly are particularly vulnerable to mTBI that, often associated with falls, carries an increased mortality and worse functional outcome following lower initial injury severity (Susman et al. 2002).
The pathology underpinning head injury is becoming increasingly better understood. As a consequence of mechanical forces inducing shearing and compression of neuronal and vascular tissue at the time of impact, a cascade of pathological events may then follow leading to further brain injury. This ensuing secondary injury may be amenable to intervention and is worsened by secondary physiological insults. Specific risk factors for poor outcome after TBI have been recognized. Some of these are established at the time of injury, such as age, gender, mechanism of injury, and presenting signs, whereas others, such as hypoxia, hypotension, and hyperglycemia, are potential areas for medical intervention (Moppett 2007). Although prompt and specialist neurocritical care is associated with improved outcome, to date, no drug treatments have proved to be successful in improving the outcome (Moppett 2007).
Irrespective of the type of induction, the resulting mTBI leads to a broad spectrum of neurological deficits, including cognitive impairments (Levin 1998; Schultz et al. 2011; Hesdorffer et al. 2009) that may significantly influence quality of life even after recovery from physical disabilities (Oddy et al. 1978, 1985; Oddy and Humphrey 1980). Recently, clinical and experimental publications demonstrate that mTBI-induced cognitive changes manifest, in large part, as deficits in hippocampal-dependent functions of learning and memory, including spatial information processing (Bramlett et al. 1997; Dixon et al. 1999; Levin 1998). The underlying mechanisms for these effects have remained elusive, although a variety of pathophysiological processes including oxidative stress, neuroinflammation, ischemia, neuronal degeneration, elevated excitatory neurotransmitters (e.g., glutamate excitotoxicity), loss/disruption of synaptic connections, or altered synaptic physiology have been implicated (Lyeth et al. 1990; McIntosh et al. 1998; Raghupathi et al. 2000; Tweedie et al. 2007; Frankola et al. 2011). Whether a single process or a combination of several processes, it is possible that effective mechanism-based treatment strategies that have proven effective in other disorders in which similar cascades of biochemical and cellular events are controlled could be assessed and, if useful, repositioned for mTBI.
Prior studies of the long-acting GLP-1 receptor agonist, exendin-4 (Ex-4), an incretin mimetic approved for treatment of type 2 diabetes mellitus (T2DM) (Gallwitz 2011; Lovshin and Drucker 2009), demonstrated its neurotrophic/protective activity in cellular and animal models of stroke, and Alzheimer’s and Parkinson’s diseases (Perry et al. 2002, 2003; Bertilsson et al. 2008; Harkavyi et al. 2008; Li et al. 2009, 2010b; Holscher 2010; Salcedo et al. 2012), and, in line with the commonality in mechanisms underpinning these disorders (Zipp and Aktas 2006), Ex-4 is studied in the present communication in cellular and mouse models associated with mTBI. We demonstrated that Ex-4 fully ameliorates the toxicity associated with oxidative stress and glutamate toxicity in neuronal cultures, events reported to occur in mTBI. In vivo translation was subsequently assessed in a classic mouse model of mTBI, involving a weight drop of 30 g under anesthesia, in which prior and post-administration of Ex-4 proved to be well tolerated and fully ameliorated mTBI-induced impairments.
Methods
Cellular studies
Cultures
Human SH-SY5Y neuroblastoma cells: SH-SY5Y cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in a 1:1 mixture of Eagle’s Minimum Essential Medium and Ham’s F12 Medium supplemented with 10 % heat-inactivated fetal bovine serum (FCS) and 100 U/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). These cells were maintained at 37 °C in a humidified incubator (5 % CO2 and 95 % air). Their culture medium was changed every other day and cells were split in a 1:3 ratio every 5 days (0.25 % trypsin, 0.53 mM EDTA solution) or when they reached approximately 80 % confluence.
Rodent primary cerebral cortical neurons: An area of cerebral cortex was removed from embryonic day 15 Sprague–Dawley rats and dissociated by mild trypsinization. Equal numbers of cells were initially seeded onto 96-well plates in media [DMEM-12 media containing 2 % B27 supplement (Invitrogen), 10 % HI-FBS, 0.5 mM l-glutamine, and 25 μM l-glutamate] at a density of approximately 6 × 104 cells/well. From day 3 in vitro (DIV), cultures were maintained in feeding media [neurobasal medium containing 2 % B-27 supplement (Invitrogen) and 0.5 mM l-glutamine] in a 5 % CO2/21 % O2 atmosphere at 37 °C. In parallel, cultures of human SH-SY5Y neuroblastoma cells were grown to 80 % confluence (in 1:1 Eagle’s minimal essential media and Hams’s F12 medium + 10 % fetal bovine serum).
RT–PCR
Total RNA was extracted from primary cerebral cortical neurons (DIV 10) as well as from SH-SY5Y cells with TRIzol reagent (Invitrogen). Specifically, cells from ten wells were pooled for RNA extraction, whose RNA quantity and quality were evaluated by spectrophotometer at 260- and 280-nM wavelength. Prior to RT–PCR, 1 μg of RNA was treated with DNase I (Ambion Inc.) in order to degrade genomic DNA. Thereafter, 50 ng of treated RNA was assessed for each one-step RT–PCR reaction (QIAGEN OneStep RT–PCR Kit). The primers used were as follows: rat GLP-1R, forward: 5′ AGTAGTGTGCTCCAAGGGCAT 3′ and reverse: 5′ AAGAAAGTGCGTACCCCACCG 3′, showing the expected PCR product of 190 bp; for rat GAPDH, forward: 5′ GACCTGCAGAGCTCCAATCAAC 3′ and reverse: 5′ CACGACCCTCAGTACCAAAGGG 3′, showing the expected PCR product of 214 bp. To provide a positive control, RNA was similarly extracted from a line of CHO cells permanently transfected with rat GLP-1R. The conditions for RT–PCR were similar for both GLP-1R and GAPDH, and were 50 °C for 30 min; 95 °C for 15 min followed by 35 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s; then 72 °C for 10 min. For the human SH-SY5Y neuroblastoma cells, the primers for the human GLP-1R were forward 5′ TCAAGGTCAACGGCTTATTAG 3′ and reverse 5′ TAACGTGTCCCTAGATGAACC 3′, showing the expected PCR product of 480 bp. Secondary primers (a second pair of human GLP-1R primers) were forward 5′ TTCTGCAACCGGACC 3′ and reverse 5′ CAAGTGCTCAAGCCG 3′, with an expected product size is 1.1 kb.
GLP-1R activation
To determine whether GLP-1Rs expressed on mouse primary cerebral cortical neurons were functional, cells (DIV 10) were exposed to Ex-4 (100 nM) or vehicle (culture media), and cAMP levels were quantified (EIA kit; Assay Designs, Ann Arbor, MI, USA).
GLP-1R mediated neuroprotection
As oxidative stress (Bauer and Fritz 2004) and glutamate toxicity (Faden et al. 1989; Hinzman et al. 2010) have been implicated in the pathophysiology of mTBI, SH-SY5Y neuronal cultures were challenged with either oxidative insult (H2O2) or excess glutamate to assess GLP-1R-mediated neuroprotective actions. Initial dose–response studies were undertaken to choose a concentration of glutamate or H2O2 and an exposure time to induce a significant but incomplete level of cellular toxicity. Based on these preliminary studies, cultures were treated with Ex-4 (10 or 100 nM) or vehicle (media) and exposed to 100 or 200 μM H2O2 for 2 h or to 100 mM glutamate. Twenty-four hours thereafter, cell viability was assessed by MTS (Promega) and LDH (Sigma) assays.
As assessed by translation from a human immortal cell line possessing neuronal characteristics to primary neuron cultures, rodent primary cerebral cortical neurons were exposed to excess glutamate (100 μM), a concentration likewise chosen from preliminary dose–response studies, in the presence and absence of Ex-4 (300 nM) to assess GLP-1R-mediated protective activity. Specifically, DIV11 cells were treated with B27 media lacking antioxidants (B27-AO; Invitrogen). On DIV 13, cells were treated with Ex-4 and challenged with glutamate (10 μM). Thereafter, cell viability was assessed 24 h later by lactate dehydrogenase (LDH) assay (Sigma, St Louis, MO, USA) or 48 h later using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega, Madison, WI, USA).
Mouse in vivo studies
Mice
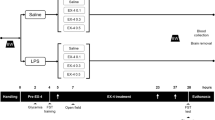
To elucidate translation to a relevant animal model, experimental mTBI was induced in mice with and without prior or post-administration of Ex-4 using a weight drop concussive head trauma device, described previously (Milman et al. 2005; Tweedie et al. 2007; Zohar et al. 2011). In this regard, male ICR mice weighing 30–40 g were maintained five per cage under a constant 12-h light/dark cycle at room temperature (22 ± 2 °C), and were provided food (Purina rodent chow) and water ad libitum. Each mouse was used in a single experiment and for one time point alone. The Ethics Committee of the Sackler Faculty of Medicine approved the experimental protocol (M-09-055), in compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW publication 85-23, revised, 1995).
Brain injury
In accord with published procedures (Milman et al. 2005; Tweedie et al. 2007; Tashlykov et al. 2009; Baratz et al. 2010b), mice were lightly anesthetized (isoflurane) and placed under this device. The device consisted of a metal tube (inner diameter 13 mm), which was vertically positioned over the mouse head. A metal weight (30 g) was dropped through the tube (80 cm length) to strike the skull on the temporal right side, between the corner of the eye and the ear. A sponge was positioned to support the head, which allowed antero-posterior motion without any rotational head movement at the moment of impact. Immediately after injury, mice were placed into their cages for recovery. As described previously, mice rapidly recovered from anesthesia following mTBI and, as assessed with a battery of general neurological test 24 h later (Milman et al. 2005; Tweedie et al. 2007), appeared both normal and indistinguishable from a sham group. The effect of the injury was then studied at 7 and 30 days following the trauma, using different groups of mice at each time point. Sham mice followed the same mTBI procedure, but the weight was not dropped.
Exendin-4 administration
Ex-4 was administrated via osmotic minipumps (model 1007D; Alzet, Cupertino, CA, USA) subcutaneously (s.c.), similar to the route of administration of this agent clinically. Specifically, mice were anesthetized using ketamine/xylazine. A small asceptic incision was made on the left side of the lower back to allow minipump s.c. implantation in a position that minimally affected the mice, and the incision was aseptically closed. Mice were kept under a warming lamp throughout the entire procedure in order to maintain body temperature. Two different protocols were followed for minipump implantation: (1) pumps were initially implanted and mTBI was induced 48 h later, and (2) pumps were implanted immediately after the trauma. In this manner, Ex-4 was administered continuously at a dose of 3.5 pM/kg/min (equivalent to 21 μg/kg/day) over seven consecutive days, achieving this level of administration some 6 h after pump insertion.
Behavioral tests
A battery of behavioral tests focusing on cognition was conducted at 7 and 30 days in separate groups of identically treated mice with and without mTBI injury. Specifically, four behavioral tests were administered: Y maze, novel object recognition, elevated plus maze, and passive avoidance. The passive avoidance task was chosen as the final evaluation because of its aversive nature.
Novel object recognition paradigm
This task was used to evaluate visual recognition memory, as previously described for mice (Messier 1997), and is based on the innate tendency of rodents to explore new objects within their environment. Assessment of this natural tendency enables a determination of whether an animal is able to discriminate between a familiar object and a novel one. Mice were individually habituated to an open field plexiglass box (59 × 59 × 20 cm size) for 5 min, 48 h prior to the test. During the acquisition phases, two identical objects (A and B) were placed in a symmetric position within the arena for 5 min. These objects were suitably heavy and high to guarantee that mice could neither move nor climb over them. Twenty-four hours following this acquisition phase of training, one of the objects (either A or B, randomly) was substituted with a novel one (C), and the animal’s exploratory behavior was again evaluated over 5 min. Following each session, all objects were meticulously cleaned with 70 % ethanol to preclude odor recognition. Exploration of an object was defined as rearing on the object or sniffing it at a distance of less than 2 cm and/or touching it with the nose. Successful recognition was manifested by the preferential exploration of the novel object. A discrimination preference index was determined as follows: (time near the new object − time near the old object)/(time near the new object + time near the old object) (Dix and Aggleton 1999).
Y maze paradigm
This test was chosen to assess spatial memory. The maze consisted of three identical arms separated by a 120° angle and built from black plexiglass (Baratz et al. 2010a). Each arm was 8 × 30 × 15 cm and differed solely by the presence of unlike visual cues (a triangle, square, or a circle). One arm was randomly selected as the “start” arm. On the first trial, lasting for 5 min, each mouse was placed into the start arm and one of the two remaining arms was randomly blocked to limit access. In contrast, during the second trial, lasting for 2 min, all arms of the maze were open. These two trials were separated by a 2-min interval, during which the mouse was returned to its home cage. The time spent in each of the arms was measured during the two trial periods. Between trials, the maze was thoroughly cleansed using a 70 % ethanol solution and was then dried. A discrimination preference index was calculated as follows: (time in the new arm − time within the familiar arm)/(time in the new arm + time within the familiar arm) (Dix and Aggleton 1999).
Elevated plus maze
This paradigm assessed anxiety-like behavior of the animals (Zohar et al. 2011), utilizing a maze comprised of two identical open (30 × 5 × 0.25 cm) and two identical closed arms (30 × 5 × 15 cm) to form a “+” shape. Each two identical arms were placed against each other, and the entire apparatus was elevated 60 cm above the floor. To initiate the test, mice were placed at the center of the “+” shaped maze, facing an open arm. They were then allowed to explore the maze for 5 min. The apparatus was carefully cleaned between mice with 70 % ethanol and, thereafter, dried. The number of entries to the open/closed arms was quantified and the time that each mouse spent on the open arms was measured. The longer duration that animals were willing to spend within the open arms has been associated with a lower anxiety-like behavior (Belzung and Griebel 2001).
Passive avoidance paradigm
This task was used to assess simple non-spatial learning and memory abilities (Zohar et al. 2011). The passive avoidance apparatus (San Diego Instruments, San Diego, CA, USA) was constructed of black plexiglass (48 × 22 × 22 cm) and consisted of two separate chambers of equal size. One compartment was illuminated whereas the other remained dark. These two chambers were separated by a door that was closed at the beginning of each trial and could be opened by the experimenter. The test comprised two sessions separated by an interval of 24 h. Animals were placed in the illuminated chamber and, after 30 s, the door was opened. When the mouse crossed into the dark compartment, the door was closed and the mouse received a mild electric foot shock (1 mA for 3 s). Following this electric shock, mice were left for further 5 s within the apparatus and then removed and returned to their home cage. Twenty-four hours later, mice were tested for retention of the passive avoidance response to the shock by placing them into the illuminated compartment. Learning was operationally defined as a failure to enter the dark compartment within 3 min (Zohar et al. 2011). No shock was delivered during the second day.
Data analysis
All results are shown as mean ± standard error of mean. For cell culture studies, data was analyzed with SAS 9.1 (SAS Institute, Cary, NC, USA), and significant differences from controls or either glutamate/H2O2 challenge was determined by Dunnett’s multiple Student’s t test, with Bonferroni correction, as required (p < 0.05). Data from animal studies were analyzed using SPSS 17 software (Genius Systems, Petah Tikva, Israel). A one way ANOVA was used to analyze data for the behavioral paradigms: novel object recognition, Y maze, and elevated plus maze, and p values of post hoc tests were adjusted using Fisher’s least significant difference (LSD) test utilizing a nominal significance level of 0.05. When a comparison was made between “familiar” and “novel” objects within a specific group to show the level of recall within the group, a student's t test was used. Results of the passive avoidance test were analyzed using the Kaplan–Meier test and are presented as percentages.
Results
Rat primary cortical neurons and human SH-SY5Y cells possess a functional GLP-1R that mediates neuroprotection
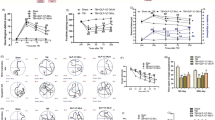
The existence of the GLP-1R was confirmed on both the primary cerebral cortical neurons (Fig. 1a) and SH-SY5Y cells (not shown) used in the current study by RT–PCR of GLP-1R mRNA. To evaluate functionality, both cell types were exposed to GLP-1 and, as shown for rat primary cortical neurons (Fig. 1b), Ex-4 elicited a 40-fold elevation in cAMP levels [increasing from 6.3 to 256.9 pM/mg protein (Student’s t test p < 0.001)].
The GLP-1 receptor is both expressed and functional on cultured mouse primary cortical neurons, and its activation provides neuroprotection. a One step RT–PCR demonstrating GLP-1R mRNA expression in neuronal cell cultures. The predicted RT–PCR product size is 190 bp. The standard, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was evident across all lanes (not shown). Lane 1 positive control; 2 negative control, respectively, which for the former RNA was extracted from Chinese hamster ovary cells stably transfected with rodent GLP-1R; 3 RNA extracted from primary neuron cultures. b Ex-4 (100 nM) stimulated cAMP release from primary cortical neurons that was markedly elevated (40-fold) versus resting (unstimulated) cells (N = 3, *p < 0.001; Student’s t test). c, d Pretreatment with Ex-4 protected primary cortical neuron cultures from glutamate (10 μM) and SH-SY5Y cells from glutamate (100 mM) and oxidative stress (H2O2 100 and 200 μM)-induced cellular toxicity, as assessed in (c) by MTS assay at 48 h [N ≥ 3 per treatment group, *p < 0.05 Dunnett’s t test vs. cellular insult; N.S. not significantly different from control (no treatment group)], in (d) by MTS assay at 24 h (N ≥ 3 per treatment group, *p < 0.05, ***p < 0.001, Dunnett’s t test with Bonferonni correction), and in (e) by LDH assay at 24 h (N ≥ 3 per treatment group, *p < 0.05, **p < 0.01, and ***p < 0.001, Dunnett’s t test with Bonferonni correction)
SH-SY5Y cells proved susceptible to excess glutamate and oxidative stress. For glutamate (100 mM), this resulted in a loss of cellular viability of 54 % that was significantly mitigated by Ex-4 (Fig. 1d) rescuing up to 30 % of this loss. Likewise, SH-SY5Y cells proved vulnerable to oxidative stress (Fig. 1e). Cellular loss of 20 % and 40 % was evident following challenge with H2O2 100 and 200 μM, respectively, which coincided with an elevation in LDH levels to 134 % and 165 % of control values (Fig. 1e). Treatment with Ex-4 (100 nM) ameliorated this toxicity and provided significant protection. In a parallel study, primary cortical neurons proved highly vulnerable to excess glutamate (10 μM), inducing a 67 % loss of cell viability (Fig. 1c) that was fully mitigated by 300 nM Ex-4.
Novel object recognition
The novel object recognition test was used in order to examine visual recognition memory of the mice, and was performed 7 and 30 days following either mTBI or a sham procedure. As shown in Fig. 2a–d, mTBI mice demonstrated a deficit in visual memory, as compared to sham mice, and spent less time near the new object, thereby providing a preference index of close to zero value. Importantly, Ex-4, in the absence of mTBI, was well tolerated and did not modify the animal’s behavior.
mTBI induces impairment in visual memory as assessed by the novel object recognition paradigm that was fully ameliorated when Ex-4 was administered pre-trauma (a, b) and post-trauma (c, d). a Seven days post-trauma: one-way analysis of variance (ANOVA) showed that mTBI animals had a deficit in visual memory compared to all other groups [F (3, 55) = 4.93, p = 0.004 LSD Fisher’s LSD post hoc, p < 0.05]. b Thirty days post-trauma: although the reduction of visual memory in the untreatd mTBI group did not reach statistical significance, restoration by Ex-4 is clearly seen. c Seven days post-trauma: one-way analysis of variance (ANOVA) revealed that mTBI animals had a deficit in visual memory compared to all other groups [F (3, 52) = 4.456, p = 0.007 Fisher’s LSD post hoc, p < 0.01]. d Thirty days post-trauma: one-way analysis of variance (ANOVA) showed that mTBI animals had a deficit in visual memory compared to all other groups [F (3, 41) = 5.296, p = 0.004 Fisher’s LSD post hoc, p < 0.01]. Performance of mice was quantitatively assessed 7 and 30 days following mTBI as a preference index, calculated as (time near the new object − time near the old object)/(time near the new object + time near the old object). Values are mean ± SEM
Ex-4 Pre-mTBI administration
In studies involving initiation of Ex-4 administration prior to mTBI and examination of mice 7 days following trauma, the Ex-4 + mTBI group demonstrated complete protection of visual memory, and mice performed on par with the sham group. In contrast, the untreated mTBI mice exhibited a lower visual memory ability compared with all other groups (Fig. 2a). One-way ANOVA revealed a significant effect of group [F (3, 55) = 4.93, p = 0.004]. Fisher’s LSD post hoc analysis revealed that the preference index of the mTBI mice was significantly decreased compared to all other groups. Separate groups of mice were examined 30 days post-trauma to characterize the long-term influence of Ex-4 administration (Fig. 2b). Albeit that the untreated mTBI group demonstrated a strong trend to a reduced preference index, the Ex-4 + mTBI group manifested a visual memory that compared favorably with sham mice. When the visual memory of the mice was assessed within the groups, all mice apart from mTBI animals showed a significant preference to the “novel” object compared to the “familiar” one (Student’s t test p < 0.01, data not shown).
Ex-4 post-mTBI administration
In further studies, mice were administered Ex-4 following mTBI and examined 7 days post-trauma. The untreated mTBI group demonstrated a deficit in visual memory compared with sham mice (Fig. 2c). In contrast, Ex-4 + mTBI mice displayed complete recovery. One-way ANOVA revealed a significant effect of group [F (3, 52) = 4.456, p = 0.007]. Fisher’s LSD post hoc analysis revealed that the preference index of the mTBI mice was significantly reduced compared with all other groups (Fig. 2c). When visual memory was assessed within the groups, all groups with the exception of the untreated mTBI one showed a significant preference to the “novel” compared to the “familiar” object (Student’s t test p < 0.01, data not shown).
As above, separate groups of mice involving post-mTBI administration of Ex-4 were evaluated 30 days post-trauma. As shown in Fig. 2d, untreated mTBI animals displayed a significant visual memory impairment compared to all other groups. In contrast, Ex-4 + mTBI mice demonstrated full recovery and were no different from the sham group. One-way ANOVA revealed a significant effect of group [F (3, 41) = 5.296, p = 0.004]. Fisher’s LSD post hoc analysis revealed that the preference index of the untreated mTBI group was significantly reduced compared to all other groups. As before, visual memory assessment within the groups determined that all groups, apart from the untreated mTBI one, showed a significant preference to the “novel” versus the “familiar” object (Student’s t test p < 0.01, data not shown).
In summary, the novel object recognition paradigm demonstrated an ability to differentiate untreated versus treated mTBI injured mice. Ex-4 provided both protection when administered prior to and full amelioration when given post-injury, as assessed at 7 and 30 days.
Y maze
Spatial memory was examined using the Y maze at 7 and 30 days following injury. However, in contrast to prior studies in which control (unchallenged) mice performed with a preference index of greater than 0.4 for the new versus the old arm (Baratz et al. 2010a, b), this value was generally not achieved in the current study.
Ex-4 pre-mTBI administration
All mice across groups and times performed with a Y maze preference index of 0.3 or less following either mTBI or sham injury, with the exception of Ex-4-treated sham mice assessed on day 7 (Fig. 3a, b). No impairment was evident in mTBI challenged animals, and Ex-4-treated animals performed similar to sham controls.
Effect of mTBI on animal performance in a Y maze preference index paradigm. a, b At 7 and 30 days following mTBI in the presence or absence of prior Ex-4 administration, no significant difference was evident across groups. c. Seven days after injury when Ex-4 was given post-trauma, one-way analysis of variance (ANOVA) showed that mTBI animals had a deficit in spatial memory compared to all other groups [F (3, 64) = 3.439, p = 0.022 Fisher’s LSD post hoc, p < 0.05]. d At 30 days, no significant difference was evident across mTBI and sham groups in the presence or absence of post-mTBI Ex-4 administration. Performance of mice was quantitatively assessed by Y maze paradigm as a preference index, calculated as (time new − time old)/(time new + time old). Values are mean ± SEM
Ex-4 post-mTBI administration
A deficit in spatial memory in the mTBI alone cohort was apparent 7 days post-injury versus sham controls, with mTBI mice spending less time within the “novel” arm of the Y maze (preference index 0.1). Ex-4 treatment fully ameliorated this, and all treated animals performed similar to sham controls (preference index >0.3). One-way ANOVA revealed a significant effect of group [F (3,64) = 3.439, p = 0.022] (Fig. 3c). Fisher’s LSD post hoc analysis revealed that the preference index of the mTBI mice was significantly less than all other groups. At 30 days, all groups performed with a preference index of 0.2 to 0.3, and no significant difference between groups was manifest (Fig. 3d).
Elevated plus maze
Anxiety-like behavior following mTBI was evaluated by use of the elevated plus maze. As illustrated in Fig. 4a–b, mice examined 7 days post-mTBI or sham injury with or without Ex-4 pre- or post-treatment spent relatively little time in the open arm of the maze and could not be differentiated from one another. In contrast at 30 days, a statistically significant difference became apparent in one study (Fig. 4d) in mice subjected to mTBI alone. One-way ANOVA revealed a significant effect of group [F (3,45) = 3.421, p = 0.024], and Fisher’s LSD post hoc analysis revealed that the time spent within the open arm was significantly reduced compared to all other groups.
Assessment of mTBI mice by elevated plus maze demonstrated a significant measure of anxiety at 30 days: a, c At 7 days following injury, no statistically significant differences were evident between mTBI and sham control cohorts of mice. b, d mTBI induced a significant reduction in time spent within the open arm in study (d) at 30 days following injury, which was ameliorated by post-trauma Ex-4 administration. One-way analysis of variance (ANOVA) revealed a group effect. mTBI animals in this group demonstrated a higher anxiety-like behavior compared to sham control mice and Ex-4 + mTBI animals [F (3,45) = 3.421, p = 0.024 Fisher’s LSD post hoc, *p < 0.05]. No statistical significance was evident across groups at 30 days post-trauma in study (c). Values are mean ± SEM
In summary, anxiety-like behavior was no different between cohorts at 7 days, was detected at 30 days in one of the mTBI challenged groups, and was fully ameliorated by Ex-4 in this group.
Passive avoidance
The passive avoidance task was utilized to provide a simple evaluation of non-spatial learning and memory abilities. In large part, this task revealed a weak trend towards lower memory and learning abilities in the mTBI alone group compared to the control sham group at 7 (Fig. 5a, c) and 30 days (Fig. 5d). Pair-wise comparisons revealed that the difference between the groups did not approach statistical significance and, in general, this trend was not apparent following either pre- or post-treatment with Ex-4.
mTBI induced a trend towards impairment in performance in a passive avoidance paradigm that was not apparent following Ex-4 administration. The entrance index was defined as the percentage of mice failing to enter the dark chamber within 3 min on the second day. Performance of mice was quantitatively measured at 7 and 30 days post-mTBI. a–d Although no significant differences in performance were found across cohorts at either 7 or 30 days post-injury, trends to a reduced performance of the mTBI alone vs. sham control groups were apparent (a, c, d), which were not evident in groups administered Ex-4 either pre- or post-mTBI
In summary, across the four behavioral paradigms studied, a general pattern emerged. The mTBI alone groups presented a reduced performance, as compared to the sham mice, reaching statistical significance or demonstrating a noticeable trend at either 7 or 30 days post-injury. Ex-4 proved to be well tolerated in mice challenged with mTBI both prior and post-injury. These mice performed at levels that were not statistically different from the sham group in all behaviors and ameliorated any apparent mTBI-induced changes.
Discussion
In the present study, we report the efficacy of s.c. administered Ex-4, an effective and increasingly used drug in the treatment of T2DM, to ameliorate the behavioral sequelae of mTBI in a mouse model that shares key characteristics of the clinical situation. Although mTBI represents a major and increasing public health concern, impacting some 1.7 million Americans annually (Faul et al. 2010), currently there is no effective pharmaceutical therapy. Existing treatment principally involves intensive care management to stabilize a patient following injury, followed by symptomatic treatment (Moppett 2007; Marshall et al. 2012), even though guidance on the treatment and management of those experiencing persistent symptoms is lacking (Berrigan et al. 2011). These patients may suffer detrimental physical, cognitive, and/or behavioral/emotional symptoms that persevere for years after injury, which substantially impact their quality and duration of life (Hesdorffer et al. 2009). Hence, effectual treatment strategies for mTBI are a significant medical need, and the repurposing of an efficacious currently prescribed drug could rapidly lead to positive translational studies.
The biochemical processes induced by TBI that underlie the associated behavioral, cognitive, and physical impairments are being increasingly characterized. TBI damage can be classified as (1) focal, which occurs locally and involves injury to surrounding brain tissue and vessels, and (2) diffuse, which occurs widely throughout the brain (Sivanandam and Thakur 2012). Mechanical stress generates compression as well as shearing of neuronal and vascular tissue at the time of impact, leading to axonal injury, brain swelling, and hypoxia (Laurer et al. 2000; Hellewell et al. 2010). The occurrence of cell death following TBI, which we have previously characterized in our mTBI weight drop model (Tashlykov et al. 2009), is likely a key cause of neurological deficits and mortality. This initial injury may subsequently instigate biochemical and cellular changes that contribute to the initiation of secondary injury involving a process of neurochemical events that time-dependently lead to additional damage. Indeed, in mTBI, unlike severe TBI, neurons are considered to be less affected by primary mechanical disruption and more susceptible to secondary events (Arundine et al. 2004). Whereas numerous neurons may become impaired and dysfunctional following mTBI, this is not necessarily an irremediable event. The status of the post-injury cerebral microenvironment together with compensatory responses to the initial and secondary damage create a setting in which the balance between pro- and anti-apoptotic biochemical cascades will ultimately promote either cell death or survival (Sivanandam and Thakur 2012).
The activation of the GLP-1R, which is expressed widely within the central and peripheral nervous system, has been extensively described to provide neurotrophic actions and promote neural cell survival (Salcedo et al. 2012). The GLP-1R belongs to the glucagon subfamily of class B1 G protein-coupled receptors (GPCRs). Following ligand binding and activation, these seven transmembrane domain proteins undergo a conformational change from an inactive to an active state to trigger a Gαs-mediated increase in cAMP production (Hoare 2005). Downstream of cAMP, activation of PKA-, PI3K-, and MAPK-mediated pathways has been reported (Li et al. 2009, 2010a; Perry and Greig 2003). This has been associated with the suppression of proapoptotic caspase-3 activity and BAX protein levels, and the augmentation of anti-apoptotic Bcl-2 and activating transcription factor-4 (ATF-4) protein levels. Under normal physiological conditions, brain GLP-1R expression primarily is localized to dendrites and synaptic regions of neurons (Hamilton and Hölscher 2009) and, in accord with prior studies (Perry et al. 2003; Li et al. 2009), was present and functional in primary cortical neuron cultures (Fig. 1a, b). To elucidate whether GLP-1R activation could shift the balance between neuronal death and survival after insults relevant to mTBI, neurons were challenged with excess glutamate and oxidative stress.
The relevance of these insults is that immediately following TBI, an overwhelming disturbance in cellular ion homeostasis, especially calcium ions, occurs that is instigated by the excessive release of excitatory amino acids, in particular glutamate, with the subsequent activation of glutamate receptors. Such calcium ion cellular influx is regarded as a pivotal event early post-TBI and leads to mitochondrial damage and an uncoupling of mitochondrial ATP synthesis, an increase in free radical production, changes in gene expression, and activation of calcium-dependent proteases including caspases and calpains inducing cellular damage and promoting apoptosis (Marklund and Hillered 2011). Elevated extracellular glutamate is thus considered to play a primary role in mediating primary and secondary damage in mTBI as well as in cerebral ischemia (Arundine and Tymianski 2004; Hossmann 1994; Siesjo et al. 1995) and numerous other neurodegenerative disorders (Lau and Tymianski 2010). Altered glutamate receptor functioning (Zhang et al. 1996) has also been proposed to account for the vulnerability of neurons that undergo mTBI-related disruption and deformation after exposure to sub-lethal levels of extracellular glutamate (Arundine and Tymianski 2004). Such glutamate toxicity has long been linked to dose-dependent increases in free radicals and reactive oxygen species (Lafon-Cazal et al. 1993; Reynolds and Hastings 1995) whose overproduction can derive from numerous sources, including the mitochondrial respiratory chain, elevated free Fe2+ levels deriving from breakdown of extravasated hemoglobin, oxidation of catecholamines, breakdown of membrane phospholipids, infiltrating neutrophils, and activation of nitric oxide synthetase (Marklund and Hillered 2011), concomitantly occurring when endogenous antioxidant defense mechanisms are at their most challenged (Shohami et al. 1997; Marklund and Hillered 2011). As illustrated in Fig. 1c, d, and e, GLP-1R activation, achieved by Ex-4, provided neuronal cultures significant protection from glutamate and oxidative stress-induced toxicity, in accord with its previously reported anti-apoptotic actions following lethal insult with hypoxia, amyloid-β peptide (Aβ), 6-hydroxydopamine, Fe2+, and trophic factor withdrawal (Li et al. 2009, 2010a, b, 2012; Perry et al. 2002, 2003). Of translational relevance, the administration of Ex-4 (10 μg s.c.) to humans achieves plasma levels of 200 pg/mL (48 nM) (Calara et al. 2005), which compares favorably to the doses studied herein.
As Ex-4 and related GLP-1 analogues appear to readily cross the blood–brain barrier and freely enter the brain following their systemic administration (Kastin et al. 2002; Kastin and Akerstrom 2003; Banks et al. 2003; Hunter and Holscher 2012), Ex-4 was administered to rodents prior and post-mTBI to clarify whether neuroprotective actions associated with insults pertinent to mTBI in cellular studies translated in vivo. In accord with its route and extended-release administration in humans with T2DM (Lovshin and Drucker 2009; Barnett 2012), Ex-4 was administered to mice s.c. under steady-state conditions for 7 days. This duration covered both immediate and late biochemical responses to mTBI that, for glutamate and oxidative stress, can last over days (Hinzman et al. 2010, 2012). Our chosen Ex-4 dose [3.5 pM/kg/min (21 μg/kg/day)] was previously successfully utilized to ameliorate hyperglycemia and lower Aβ in 3xTg-AD mice (Li et al. 2009), to mitigate the symptoms and pathology associated with amyotrophic lateral sclerosis in SOD1 G93A mutant mice (Li et al. 2012), and to compare favorably with human studies focusing on glycemic control (once weekly exenatide LAR—2 mg/week provides 5.7 μg/kg/day for a 50-kg human; Drucker et al. 2008).
Although no single animal TBI model perfectly mimics the human condition (Marklund and Hillered 2011), our non-invasive weight drop mTBI model has been extensively characterized (Baratz et al. 2010a, b; Milman et al. 2005; Tashlykov et al. 2009; Zohar et al. 2011) and has features relevant to the human head injury that can occur in traffic accidents, sports injuries, and falls. Similar to the Marmarou weight drop model in the rat (Marmarou et al. 1994; Foda and Marmarou 1994), diffuse axonal injury occurs throughout the brain leading to diffuse neuronal loss and neuroinflammation, which are features predominant in the majority of human mTBI (Maas et al. 2007, 2008). Unlike open cranium and focal TBI animal models (Marklund and Hillered 2011), there is no contusion and focal site of tissue loss or lesion in our model (Tweedie et al. 2007). Furthermore, as animals are temporarily anesthetized during the procedure, there are no potential confounding effects associated with post-traumatic stress.
In accord with mTBI-induced behavioral changes previously reported in our weight drop model (Milman et al. 2005; Zohar et al. 2011; Baratz et al. 2010a, b), memory impairments were apparent following mTBI in both the novel object recognition and, albeit to a lesser extent, the Y maze paradigm at the early and late observation times of 7 and 30 days. These tasks provide information related to visual and spatial memory (Sharma et al. 2010). In addition, a trend towards impairment was apparent within the non-spatial passive avoidance learning task. These impairments were fully reversed by Ex-4, whether administered prior to mTBI as a protective strategy or after mTBI as an ameliorative strategy. In the latter scenario, pumps containing Ex-4 were implanted directly post-injury and it took a further 6 h to effectively initiate delivery (personal communication, Alzet Technical Support, Cupertino, CA, USA), thereby providing a post-injury treatment window of approximately 6-h duration. Anxiety levels following mTBI, assessed by the elevated plus maze paradigm (Alcalay et al. 2004), have been reported to be largely unchanged in our model between 7 and 90 days post-injury with weight drops of up to 30 g (Zohar et al. 2011). Our study was primarily in accord with this; however, a mTBI-induced increase in anxiety-like behavior was noted in one 30-day post-injury group (Fig. 4d), as has occurred previously (Baratz et al. 2010a), and this was fully ameliorated by Ex-4. In this regard, Ex-4 has not only been recently demonstrated to lack anxiety-inducing actions (Strawn et al. 2008; Erreger et al. 2012) but to decrease a clinical index of anxiety in humans (Grant et al. 2011).
Extensive clinical and basic data suggests that the brain, and in particular the hippocampus and cerebral cortex, is highly vulnerable to trauma that can commonly instigate apoptosis (Tashlykov et al. 2009; Tweedie et al. 2007) and neuroinflammation (Zipp and Aktas 2006; Baratz et al. 2010b). In this regard, TBI is a strong epigenetic risk factor for Alzheimer’s disease (Fleminger et al. 2003; Magnoni and Brody 2010), which is characterized by the presence of extracellular senile amyloid plaques and intracellular neurofibrillary tangles (Sambamurti et al. 2002). There are many pathological features shared between TBI and AD, including Aβ deposition, tau hyperphosphorylation, neurite degeneration, synaptic loss, and reactive microgliosis (Ikonomovic et al. 2004; Uryu et al. 2007; Sivanandam and Thakur 2012), which can all be triggered by neuroinflammation (Frankola et al. 2011). Recent studies suggest that GLP-1R stimulation in brain provides pleiotropic actions, augmenting synaptic plasticity, long-term potentiation, and cognitive processes (During et al. 2003; Porter et al. 2012). This provision of neuromodulatory and anti-inflammatory activity (Harkavyi et al. 2008; Chaudhuri et al. 2012) may, in turn, protect synapses from Aβ-induced dysfunction, by both lowering Aβ and tau generation (Perry et al. 2003; Li et al. 2010a; Bomfim et al. 2012) and inducing neurogenesis (Bertilsson et al. 2008) across numerous cellular and animal models (Salcedo et al. 2012). Hence, it is likely that the beneficial actions of Ex-4 in our mTBI model are mediated at many levels.
In summary, the results of the current study support Ex-4 as a promising drug in the treatment of experimental mTBI that is worthy of further study. Administration pre- and post-injury resulted in full amelioration of behavioral deficits utilizing a clinically relevant dose and administration route. As GLP-1R stimulation has proved well tolerated and efficacious in the setting of human T2DM (Drucker et al. 2008; Lovshin and Drucker 2009; Gallwitz 2011), one can postulate that drugs targeting the GLP-1R may perform similarly in neurological disorders. Clearly, this hypothesis requires testing in humans, and phase II clinical trials of Ex-4 for the treatment of Alzheimer’s disease (clinicaltrial.gov identifier NCT01255163) and Parkinson’s disease (clinicaltrial.gov identifier NCT01174810) are ongoing. As the insulinotropic, but not neurotrophic/protective, actions of Ex-4 appear to be glucose dependent, risk of hypoglycemia is low (Garber 2011). In addition, Ex-4 is well tolerated in the elderly (Pawaskar et al. 2012), whose functional outcome and mortality are particularly poor following mTBI.
References
Alcalay RN, Giladi E, Pick CG, Gozes I (2004) Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci Lett 361:128–131
Arundine M, Aarts M, Lau A, Tymianski M (2004) Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci 24:8106–8123
Arundine M, Tymianski M (2004) Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci 61:657–668
Banks WA, During MJ, Niehoff ML (2004) Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. J Pharmacol Exp Ther 309:469–475
Baratz R, Rubovitch V, Frenk H, Pick CG (2010a) The influence of alcohol on behavioral recovery after mTBI in mice. J Neurotrauma 27:555–563
Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, Greig NH, Pick CG (2010b) Tumor necrosis factor-α synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem 118:1032–1042
Barnett AH (2012) The role of GLP-1 mimetics and basal insulin analogues in type 2 diabetes mellitus: guidance from studies of liraglutide. Diabetes Obes Metab 14:304–314
Bauer R, Fritz H (2004) Pathophysiology of traumatic injury in the developing brain: an introduction and short update. Exp Toxicol Pathol 56:65–73
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149
Berrigan L, Marshall S, McCullagh S, Velikonja D, Bayley M (2011) Quality of clinical practice guidelines for persons who have sustained mild traumatic brain injury. Brain Inj 25:742–751
Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Rönnholm H, Wikström L (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 86:326–338
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Invest 122:1339–1353
Bramlett HM, Green E, Dietrich WD (1997) Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res 762:195–202
Calara F, Taylor K, Han J, Zabala E, Carr EM, Wintle M, Fineman M (2005) A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic Exendin-4). Clin Ther 27:210–215
Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, Makdissi A, Dandona P (2012) Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 97:198–207
Dix SL, Aggleton JP (1999) Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res 99:191–200
Dixon C, Kochanek P, Yan H, Schiding J, Griffith R, Baum E, Marion D, DeKosky ST (1999) One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma 16:109–122
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L, and for the DURATION-1 Study Group (2008) Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372:1240–1250
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9:1173–1179
Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A (2012) Exendin-4 decreases amphetamine-induced locomotor activity. Physiol Behav 106:574–578
Faden AI, Demediuk P, Panter SS, Vink R (1989) The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244:798–800
Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta
Fleminger S, Oliver D, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74:857–862
Foda MA, Marmarou A (1994) A new model of diffuse brain injury in rats. Part II: morphological characterization. J Neurosurg 80:301–313
Frankola KA, Greig NH, Luo W, Tweedie D (2011) Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets 10:391–403
Gallwitz B (2011) Glucagon-like peptide-1 analogues for type 2 diabetes mellitus: current and emerging agents. Drugs 71:1675–1688
Garber AJ (2011) Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 34(Suppl 2):S279–S284
Garner J, Brett SJ (2007) Mechanisms of injury by explosive devices. Anesthesiol Clin 25:147–160
Grant P, Lipscomb D, Quin J (2011) Psychological and quality of life changes in patients using GLP-1 analogues. J Diabetes Complications 25:244–246
Hamilton A, Hölscher C (2009) Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport 20:1161–1166
Harkavyi A, Abuirmeileh A, Lever R, Kingsbury A, Biggs C et al (2008) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 5:19
Hellewell SC, Yan E, Bye N, Agyapomaa D, Morganti-Kossmann C (2010) Post traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J Neurotrauma 27:1997–2010
Hesdorffer DC, Rauch SL, Tamminga CA (2009) Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J Head Trauma Rehabil 24:452–459
Hinzman JM, Thomas TC, Burmeister JJ, Quintero JE, Huettl P, Pomerleau F, Gerhardt GA, Lifshitz J (2010) Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode array study. J Neurotrauma 27:889–899
Hinzman JM, Thomas TC, Quintero JE, Gerhardt GA, Lifshitz J (2012) Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J Neurotrauma 29:1197–1208
Hoare SR (2005) Mechanisms of peptide and nonpeptide ligand binding to class B G-protein-coupled receptors. Drug Discov Today 10:417–427
Holscher C (2010) Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer’s disease. Recent Pat CNS Drug Discov 5:109–117
Hossmann KA (1994) Glutamate-mediated injury in focal cerebral ischemia: the excitotoxin hypothesis revised. Brain Pathol 4:23–36
Howard PK, Shapiro SE (2011) Diagnosing and treating mild traumatic brain injury in children. Adv Emerg Nurs J 33:274–278
Hunter K, Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33
Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC (2007) The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22:341–353
Ikonomovic M, Uryu K, Abrahamson E, Ciallella J, Trojanowski J, Lee V, Clark R, Marion D, Wisniewski S, DeKosky S (2004) Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 190:192–203
Kastin AJ, Akerstrom V, Pan W (2002) Interactions of glucagon-like peptide-1 (GLP-1) with the blood–brain barrier. J Mol Neurosci 18:7–14
Kastin AJ, Akerstrom V (2003) Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27:313–318
Lafon-Cazal M, Pietri S, Culcasi M et al (1993) NMDA-dependent superoxide production and neurotoxicity. Nature 364:535–537
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542
Laurer HL, Lezlinger PM, Mclintoch TK (2000) Models of traumatic brain injury. Eur J Trauma 26:95–100
Levin HS (1998) Cognitive function outcomes after traumatic brain injury. Curr Opin Neurol 11:643–646
Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, Mattson MP, Wang Y, Harvey BK, Ray B, Lahiri DK, Greig NH (2012) Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One 7(2):e32008
Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK, Greig NH (2010a) GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis 19:1205–1219
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A 106:1285–1290
Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH (2010b) Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem 113:1621–1631
Lovshin JA, Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269
Lyeth BG, Jenkins L, Hamm J, Dixon C, Phillips L, Clifton G, Young H, Hayes R (1990) Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res 526:249–258
McIntosh TK, Juhler M, Wieloch T (1998) Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J Neurotrauma 15:731–769
Magnoni S, Brody DL (2010) New perspectives on amyloid-β dynamics after acute brain injury. Arch Neurol 67:1068–1073
Marklund N, Hillered L (2011) Animal modeling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol 164:1207–1229
Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K (1994) A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg 80:291–300
Marshall S, Bayley M, McCullagh S, Velikonja D, Berrigan L (2012) Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician 58:257–267
Maas AI, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A, Mushkudiani NA, McHugh GS, Murray GD (2007) Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma 24:303–314
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741
Messier C (1997) Object recognition in mice: improvement of memory by glucose. Neurobiol Learn Mem 67:172–175
Milman A, Rosenberg A, Weizman R, Pick CG (2005) Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma 22:1003–1010
Moppett IK (2007) Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth 99:18–31
Oddy M, Humphrey M (1980) Social recovery during the year following severe head injury. J Neurol Neurosurg Psychiatry 43:798–802
Oddy M, Humphrey M, Uttley D (1978) Subjective impairment and social recovery after closed head injury. J Neurol Neurosurg Psychiatry 41:611–616
Oddy M, Coughlan T, Tyerman A, Jenkins D (1985) Social adjustment after closed head injury: a further follow-up seven years after injury. J Neurol Neurosurg Psychiatry 48:564–568
Pawaskar M, Li Q, Reynolds MW (2012) Metabolic outcomes of elderly patient populations initiating exenatide BID versus insulin glargine in an ambulatory care setting. Curr Med Res Opin 28:991–997
Perry T, Greig NH (2003) The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci 24:377–383
Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH (2002) Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther 302:881–888
Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH (2003) Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 72:603–612
Porter D, Faivre E, Flatt PR, Hölscher C, Gault VA (2012) Actions of incretin metabolites on locomotor activity, cognitive function and in vivo hippocampal synaptic plasticity in high fat fed mice. Peptides 35:1–8
Raghupathi R, Graham DI, McIntosh TK (2000) Apoptosis after traumatic brain injury. J Neurotrauma 17:927–938
Reynolds IJ, Hastings TG (1995) Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15:3318–27
Salcedo I, Tweedie D, Li Y, Greig NH (2012) Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol 166:1586–1599
Sambamurti K, Greig NH, Lahiri DK (2002) Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med 1:1–31
Schultz BA, Cifu DX, McNamee S, Nichols M, Carne W (2011) Assessment and treatment of common persistent sequelae following blast induced mild traumatic brain injury. NeuroRehabilitation 28:309–320
Sharma S, Rakoczy S, Brown-Borg H (2010) Assessment of spatial memory in mice. Life Sci 87:521–536
Shohami E, Beit-Yannai E, Horowitz M, Kohen R (1997) Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab 17:1007–1019
Siesjo BK, Zhao Q, Pahlmark K, Siesjo P, Katsura K, Fol-bergrova J (1995) Glutamate, calcium and free radicals as mediators of ischemic brain damage. Ann Thorac Surg 59:1316–1320
Sivanandam TM, Thakur MK (2012) Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev 36:1376–1381
Strawn JR, D’Alessio DA, Keck PE, Seeley RJ Jr (2008) Failure of glucagon-like peptide-1 to induce panic attacks or anxiety in patients with panic disorder. J Psychiatr Res 42:787–789
Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D (2002) Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma 53:219–223
Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J (2006) Systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 148:255–268
Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, Zohar O, Pick CG (2009) Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci 37:16–24
Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH (2007) Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res 85:805–815
Uryu K, Chen X, Martinez D, Browne K, Johnson V, Graham D, Lee VM, Trojanowski JQ, Smith DH (2007) Multiple proteins implicated in neurodegenerative disease accumulate in axons after brain trauma in humans. Exp Neurol 208:185–192
Zhang L, Rzigalinski BA, Ellis EF, Satin LS (1996) Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science 274:1921–1923
Zipp F, Aktas O (2006) The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 29:518–527
Zitnay GA (2005) Lessons from national and international TBI societies and funds like NBIRTT. Acta Neurochir Suppl 93:131–133
Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG (2011) Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp (Wars) 71:36–45
Acknowledgments
This research was supported in part by the Intramural Research Program of both the National Institute on Aging, National Institutes of Health, and the Sackler School of Medicine, Tel-Aviv University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nigel H. Greig, Chaim G. Pick, and Barry J. Hoffer contributed equally to this work as senior authors.
About this article
Cite this article
Rachmany, L., Tweedie, D., Li, Y. et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. AGE 35, 1621–1636 (2013). https://doi.org/10.1007/s11357-012-9464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-012-9464-0