Abstract
Ageing is accompanied by alterations to T-cell immunity and also by a low-grade chronic inflammatory state termed inflammaging. The significance of these phenomena is highlighted by their being predictors of earlier mortality. We have recently published that the proinflammatory cytokine TNFα is a strong inducer of CD4+ T-cell senescence and T-cell differentiation, adding to the growing body of literature implicating proinflammatory molecules in mediating these critical age-related T-cell alterations. Moreover, the inflammatory process is also being increasingly implicated in the pathogenesis of many common and severe age-related diseases, including cancer, cardiovascular diseases and type 2 diabetes. Furthermore, major age-related risk factors for poor health, such as obesity, stress and smoking, are also associated with an upregulation in systemic inflammatory markers. We propose the idea that the ensuing inflammatory response to influenza infection propagates cardiovascular diseases and constitutes a major cause of influenza-related mortality. While inflammation is not a negative phenomenon per se, this age-related dysregulation of inflammatory responses may play crucial roles driving age-related pathologies, T-cell immunosenescence and CMV reactivation, thereby underpinning key features of the ageing process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing is accompanied by a progressive, multidimensional, physiological degeneration with immune system alterations thought to play a key role in regulating these declines (suggested by Gorczynski and Terzioglu 2008). This decrease in immune cell activity is in part mediated by the accumulation of factors in the serum of old animals and humans (Gomez et al. 2006; Bagnara et al. 2000). In particular, the in vitro addition of exogenous interleukin (IL)-2 can rejuvenate many lymphocyte functions of the aged (Haynes et al. 2000; Haynes and Eaton 2005). The complex immune system remodelling observed during ageing includes a well-characterised profound modification of the cytokine network. A key feature of this phenomenon is a decrease in IL-2 plasma levels alongside an increase in proinflammatory molecules, particularly tumour necrosis factor α (TNFα) (Bruunsgaard et al. 2003a), IL-6 (Cohen et al. 1997) and C-reactive protein (CRP) (Ballou et al. 1996). These circulating inflammatory parameters can be positively correlated with each other, suggesting a generalised activation of the entire inflammatory network (Bruunsgaard et al. 2003a). However, this increase in proinflammatory agents is much smaller than that attained during an acute phase response, and ageing is thus said to be associated with a low-grade chronic proinflammatory condition that has been termed ‘inflammaging’ (Franceschi et al. 2000). Although inflammation is critical for dealing with infections and tissue damage, inflammaging appears to be physiologically deleterious and is predictive of all-cause mortality in multiple elderly cohorts (Bruunsgaard et al. 2003a, b). This age-related inflammatory activity is composed of local events and systemic activation of both the innate and adaptive immune system. The role of the innate immune system has been reviewed elsewhere (see Licastro et al. 2005), and as T-cell functional alterations are recognised as the most significant and best-characterised feature of immunosenescence (Pawelec et al. 2009), this article will concentrate on the role of T cells in age-related inflammation.
Age-related remodelling of the T-cell compartment
Although peripheral T-cell numbers do not diminish with age, the T-cell pool undergoes a striking age-associated remodelling, exhibiting an inverted CD4/CD8 T-cell ratio alongside a diminution in naïve T cells and accumulation of more differentiated memory cells, most profoundly observed within the CD8+ T-cell compartment (Pawelec et al. 2009). Ageing is associated with an accumulation of CD8+ T cells lacking expression of the lymphoid homing receptor CCR7 and the costimulatory molecules CD27 and CD28 alongside re-expressing CD45RA, termed T effector memory cells re-expressing CD45RA (TEMRA). These TEMRA produce large amounts of proinflammatory cytokines allowing them to potentially participate in immune pathology (Zhang et al. 2002). The significance of these age-related phenotypic changes is highlighted by their inclusion in the immune risk phenotype (IRP), a cluster of immune parameters associated with poor immune function and predictive of earlier mortality (Wikby et al. 2002), characterised by an inverted CD4/CD8 ratio, increased levels of CD28−CD8+ T cells, poor T-cell mitogen responses, low B-cell counts and cytomegalovirus (CMV) seropositivity.
CMV is associated with global changes to the host’s immune profile, which are particularly well documented in the peripheral T lymphoid pool (van de Berg et al. 2008), being associated with lymphocyte phenotype alterations very similar to those published as age-associated (Weinberger et al. 2007). Proinflammatory cytokines have been substantially implicated in these age- and CMV-related alterations to T-cell immunity. Indeed, accelerated immune ageing is observed in many inflammatory conditions including rheumatoid arthritis (RA), whereby patients have prematurely aged immune systems by over 20 years (Goronzy et al. 2010). Moreover, TNFα, which is augmented by ageing and CMV infection (Geist et al. 1997), induce CD28 expression loss and T-cell differentiation, in RA and ankylosing spondylitis, where these immune alterations are reversible following anti-TNFα therapy (Parish et al. 2009; Bruns et al. 2009; Bryl et al. 2005). Interferon-alpha (IFNα), secreted at high levels in response to CMV (Fletcher et al. 2005), is a component of inflammaging (Giunta 2008) and induces T-cell differentiation (Fletcher et al. 2005). Indeed, IFNα induces production of IL-15 (Zhang et al. 1998), which induces CD45RA re-expression on CD45RO+ EBV- and CMV-specific and bulk CD8+ T cells (Dunne et al. 2005).
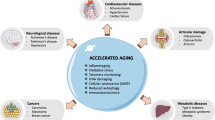
Ageing is also associated with impairment of lymphocyte telomerase upregulation and progressive telomere attrition (van de Berg et al. 2010), the significance of which being a strong correlation between shortened lymphocyte telomeres and a variety of age-associated pathologies (reviewed in Calado and Young 2009) and predictive of earlier mortality (Cawthon et al. 2003). TNFα and IFNα are also implicated in this age-related telomere shortening, as they inhibit lymphocyte telomerase activity (Reed et al. 2004; Parish et al. 2009). When telomeres reach a critically short length, they initiate a DNA damage response that can induce apoptosis or growth arrest termed replicative senescence, an increasingly recognised important factor in T-cell immunosenescence (reviewed in Akbar and Henson 2011). It has been shown that p38 MAP kinase is one of the key molecules that regulate both telomere dependent and independent senescence (Li et al. 2011a), with roles in cellular activation, proliferation, and cell cycle progression (Rincon and Pedraza-Alva 2003). Signalling through the p38 MAP kinase pathway can be reversed, inducing functional augmentation of CD4+ TEMRA (Di Mitri et al. 2011) and virus-specific CD8+ T cells (Henson et al., author communication). TNFα is further implicated in lymphocyte senescence by its ability to activate p38 (Di Mitri et al. 2011). p38 signalling is implicated in the production of IFNγ by CD4+ and CD8+ T cells (Merritt et al. 2000; Dodeller et al. 2005), a potent inducer of macrophage TNFα production (Williams et al. 1992), and p38 may also be directly involved in T-cell TNFα production (Schafer et al. 1999). Therefore, a vicious circle is set up, whereby ageing and CMV upregulate proinflammatory markers, driving T-cell differentiation and activating p38, pushing T cells towards senescence and inducing further inflammatory cytokine production (Fig. 1).
The vicious cycle of inflammaging. Ageing is associated with augmented ROS and antigenic stress that drives systemic macrophage activation. This, alongside increased adiposity and infection incidence, results in an upregulation in circulating inflammatory mediators that promotes the development of age associated inflammatory diseases, CMV reactivation and T-cell senescence. These in turn further exacerbate inflammaging in a positive feedback loop
Molecular mediators of age-related inflammation
Well-defined molecular inflammatory processes underpin inflammaging and age-related diseases (Chung et al. 2006; Franceschi et al. 2000). The transcription factor NF-κB, thought to be the master regulator of the inflammatory process, controls the transcription of proinflammatory molecules such as cytokines, chemokines, matrix metalloproteinases, adhesion molecules, COX2 and iNOS (Chung et al. 2009; Franceschi et al. 2007). The NF-κB pathway is activated by the PI3K/AKT, MAPK pathways and cytokines. Its activity can also be modulated by IκB kinase (IKK; reviewed in Li and Verma 2002). Activation of NF-κB is greatest amongst the TEMRA subset of CD8+ T cells (Gupta et al. 2006). However, unlike other organs where both MAPK and NF-κB activity increase with age (Kim et al. 2002), in lymphocytes, the activation of NF-κB is diminished amongst old subjects (Gupta et al. 2005). This is due to age-related alterations in T-cell signalling and CD28 loss, decreasing AKT phosphorylation and DNA-binding activity of the NF-κB complex (reviewed in Larbi et al. 2011).
The FoxO family of transcription factors link the AKT, MAPK and NF-κB signalling pathways to regulate the oxidative stress response (Kops et al. 2002). Phosphorylation of AKT directly interferes with FoxO binding to target DNA sequences causing the nuclear export and exclusion of FoxO (Birkenkamp and Coffer 2003). In the absence of AKT signalling, FoxO proteins promote transcription of catalase and MnSOD. The accumulation of ROS during ageing activates NF-κB through the phosphorylation of FoxO leading to the downregulation of MnSOD and catalase, further increasing intracellular ROS (Fig. 2). Once again while aged kidneys exhibit upregulated levels of phosphorylated FoxO (Kim et al. 2008), highly differentiated CD4+ T cells display low levels of phosphorylated FoxO, but rather than being protective, these cells are more prone to apoptosis (Riou et al. 2007).
Age-related pathologies
Inflammation is being increasingly implicated as a characteristic part of the pathological process of many age-related diseases, including RA (Feldmann and Maini 2003), cardiovascular diseases (Libby et al. 2010), cancer (Grivennikov and Karin 2011), osteoporosis (Lencel and Magne 2011), metabolic syndrome (Monteiro and Azevedo 2010), frailty syndrome (Ershler 2007) and Alzheimer’s disease (Morales et al. 2010). Suffering from an inflammatory disease acts as a risk factor for other pathologies of old age, setting up a positive feedback loop whereby the systemic inflammatory response associated with diseases of old age initiates and propagates further pathologies (Fig. 1). Indeed, RA patients exhibit a reduced life expectancy of up to 18 years that has been attributed to an increased risk of cardiovascular events (Van Doornum et al. 2002). Metabolic syndrome is frequently observed in diverse inflammatory conditions, including psoriasis (Cohen et al. 2008), RA (Chung et al. 2008) and HIV (Sobieszczyk et al. 2008). Furthermore, muscle wasting (Morley et al. 2001) is substantially over-represented amongst RA (Beenakker et al. 2010), HIV (Wanke et al. 2000), cancer (Dewys et al. 1980) and COPD (Debigare et al. 2001) patients. The efficacy of therapies that target TNFα, IL-6 and IL-1β in treatment of a wide variety of inflammatory conditions (reviewed in Dinarello 2011) heavily implicates these cytokines as causative pathological agents. This is best characterised in the context of RA, in which anti-TNFα treatments are used not only for rapidly controlling joint damage but also reducing RA-associated insulin resistance (Gonzalez-Gay et al. 2010), osteoporosis (Seriolo et al. 2006) and CVD disease risks (Westlake et al. 2011). Additionally, NSAID use is associated with reduced cancer incidence and mortality (Rothwell et al. 2011; Ruder et al. 2011) and anti-IL-6 therapy may be an effective anti-cancer agent (Rossi et al. 2010). Nevertheless, though efficacious in acute myocardial infarction models, clinical trials of TNFα blockade in cardiac heart failure patients were either ineffective (Mann et al. 2004) or mediated deleterious effects (Chung et al. 2003). This may reflect cardioprotective effects of TNFα and IL-6 at low concentrations and durations of exposure (El Ani et al. 2007; Wang et al. 2007b). This highlights that although augmented levels of proinflammatory cytokines may be implicated in age-related immunopathology, any therapeutic approach must take into account their critical physiological roles.
Interventional approaches for reducing inflammation
A reduction in calorie intake (CR) appears to consistently decrease the biological rate of ageing in a variety of organisms, as well as protect against age-associated diseases. The anti-inflammatory effects of CR, rather than being simply a passive mechanism linked to the reduction in inflammatory stimuli, may also effect metabolic, hormonal and gene expression products that repress pathways of inflammation (Fontana 2009; Gonzalez et al. 2011). CR has been shown to enhance anti-inflammatory molecules (Swindell 2009) and inhibit proinflammatory mediators (Kim et al. 2002). However, CR has been shown to impact on immunity (Jolly 2004), improving IL-2 production and T-cell proliferation but impairing innate responses (Nayak et al. 2009). Furthermore, it has been suggested that there is an optimal window during adulthood where CR can delay T-cell senescence and improve immunity. Indeed, inappropriate initiation of CR may be harmful to the maintenance of T-cell function (Messaoudi et al. 2008).
Although CR has beneficial effects in humans (Heilbronn et al. 2006), such a diet is unlikely to be widely adopted and would pose a significant risk to the frail, critically ill or the elderly. This has led to the development of CR mimetic (CRM) compounds that provide some of the benefits of CR without a reduction in caloric intake (Ingram et al. 2004). The best studied CRM is resveratrol, a polyphenolic compound found in grapes, red wine, peanuts and some berries owing to its ability to activate SIRT1 and extend lifespan in cell cultures and animal models (reviewed in Baur and Sinclair 2006). SIRT1, a negative regulator of NF-κB, is a target of resveratrol and its supplementation has been shown to suppress the release of proinflammatory cytokines (Li et al. 2011b; Pearson et al. 2008). When given to healthy adults, their mononuclear cells showed a significant reduction in the generation of ROS and proinflammatory cytokines (Ghanim et al. 2010).
Major age-related health factors
Major risk factors for poor health in old age are also associated with alterations to systemic inflammatory markers, which underlie many of their health altering effects. Indeed, obesity, smoking and psychological stress are accompanied by an upregulation in circulating inflammatory parameters (reviewed by Gouin et al. 2008), also observed following the menopause (Abu-Taha et al. 2009) and may be associated with declining testosterone levels in ageing men (Maggio et al. 2006). Conversely, key behaviours associated with health benefit such as exercise (Carrel et al. 2009), weight loss amongst obese individuals (Forsythe et al. 2008) and smoking cessation (Bakhru and Erlinger 2005) are also coupled with reductions in systemic inflammatory indices. These circulating inflammatory molecules may propagate and exacerbate age-related inflammatory pathologies. Moreover, proinflammatory agents can induce insulin resistance (Wen et al. 2011), contributing towards a hyper-triglycaemic state, lipid accumulation in tissues with lipotoxic effects (reviewed in Sethi and Vidal-Puig 2007).
Seasonal influenza
An influenza epidemic occurs each winter, infecting up to one fifth of individuals and causing substantial levels of morbidity and mortality amongst aged populations (Centres for Disease Control and Prevention 2010). Current UK health policy targets over 65-year-olds for influenza vaccination and cohort studies demonstrate a 47% reduction in all-cause mortality amongst old vaccinated individuals compared with their non-vaccinated counterparts (Wang et al. 2007a). However, this figure provokes controversy, as amongst aged individuals this vaccine has a low efficacy (Goodwin et al. 2006) and influenza is estimated to account for only 5% of excess deaths during winter (Simonsen et al. 2005). It is recognised that these cohort studies reporting large decreases in all-cause mortality following influenza vaccination are subject to a number of biases (reviewed in Baxter et al. 2010) and the finding of reduced mortality amongst vaccinated individuals outside of the influenza season is considered evidence of the strength of these biases (Campitelli et al. 2010). Nevertheless, we propose the systematic inflammatory response induced by influenza infection be considered and its role in propagating many age-related inflammatory pathologies, particularly CVDs. Vaccinating against influenza is associated with decreased incidence of myocardial infarction (Siscovick et al. 2000) and stroke (Lavallee et al. 2002). Moreover, in mice, influenza propagates the inflammatory response, the progression and thrombosis of atherosclerotic plaques (Naghavi et al. 2003). Influenza vaccination has been shown to be beneficial in reducing major cardiovascular events amongst acute coronary syndrome patients (Phrommintikul et al. 2011). Furthermore, many other disparate acute and chronic pathogens, such as Clostridium pneumoniae (Vainas et al. 2009), HIV (Ho and Hsue 2009), CMV (Simanek et al. 2011) and gum disease-causing bacteria (Kebschull et al. 2010), carry an elevated risk of CVD, suggesting that, rather than the nature of the infectious agent, it is the ensuing host-derived inflammatory response that drives this increased CVD risk. Thus, influenza infection may accelerate atherogenesis, resulting in death months after infection.
CMV
CMV is being increasingly implicated in age-related immune decline. Recent epidemiological data reveal that CMV infection is independently associated with premature mortality (Simanek et al. 2011) and is a component of the IRP (Wikby et al. 2002). CMV induces production of a variety of proinflammatory agents (Qiu et al. 2008), which although seemingly counterintuitive, is advantageous to the virus; inflammatory molecules induce CMV reactivation enabling spreading to other hosts through secretions associated with the inflammatory response (Freeman 2009). Thus, it is tempting to speculate that at least part of the deleterious effects of CMV may be related to its upregulation of proinflammatory agents. CMV seropositive subjects who had high baseline CRP levels were at a substantially increased risk of all-cause mortality compared to individuals that exhibited low CRP levels (Simanek et al. 2011). Moreover, individuals genetically enriched for longevity were more resistant to characteristic CMV-related immune alterations and exhibited significantly lower CRP levels (Derhovanessian et al. 2010). Thus, proinflammatory molecules and CMV may be engaged in a deleterious cycle with inflammation inducing CMV reactivation which in turn exacerbates the inflammatory response. Epidemiological studies associate CMV infection with multiple chronic inflammatory disorders, whereby active CMV replication is specifically detected at sites of inflammation (reviewed in Freeman 2009). Therefore, the age-related upregulation of proinflammatory molecules may mediate some of its effects through exacerbation of the frequency and consequences of CMV reactivation.
Concluding remarks
This article describes how ageing is accompanied by an upregulation of proinflammatory molecules that is heavily implicated in deleterious age-related alterations to T-cell immunity and in the pathophysiology of many common and severe diseases of old age. Major age-related risk factors for poor health, including infections with influenza and CMV, are also associated with increased levels of circulating inflammatory parameters, which may mediate some of their substantial deleterious health effects. These augmented systemic inflammatory molecules initiate and aggravate further age-related pathologies, drive CMV reactivation and induce T-cell senescence, which in turn further exacerbate inflammaging in multiple positive feedback loops. We propose that this may constitute a major force driving the ageing process.
References
Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz MJ (2009) Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183:1393–1402
Akbar AN, Henson SM (2011) Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol 11:289–295
Bagnara GP, Bonsi L, Strippoli P, Bonifazi F, Tonelli R, D’Addato S, Paganelli R, Scala E, Fagiolo U, Monti D, Cossarizza A, Bonafe M, Franceschi C (2000) Hemopoiesis in healthy old people and centenarians: well-maintained responsiveness of CD34+ cells to hemopoietic growth factors and remodeling of cytokine network. J Gerontol A Biol Sci Med Sci 55:B61–B66
Bakhru A, Erlinger TP (2005) Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med 2:e160
Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I (1996) Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Aging 25:224–230
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Baxter R, Lee J, Fireman B (2010) Evidence of bias in studies of influenza vaccine effectiveness in elderly patients. J Infect Dis 201:186–189
Beenakker KG, Ling CH, Meskers CG, de Craen AJ, Stijnen T, Westendorp RG, Maier AB (2010) Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev 9:431–436
Birkenkamp KU, Coffer PJ (2003) Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans 31:292–297
Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S (2009) Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest 119:1167–1177
Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B (2003a) Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 115:278–283
Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK (2003b) Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol 132:24–31
Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ (2005) Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum 52:2996–3003
Calado RT, Young NS (2009) Telomere diseases. N Engl J Med 361:2353–2365
Campitelli MA, Rosella LC, Stukel TA, Kwong JC (2010) Influenza vaccination and all-cause mortality in community-dwelling elderly in Ontario, Canada, a cohort study. Vaccine 29:240–246
Carrel AL, McVean JJ, Clark RR, Peterson SE, Eickhoff JC, Allen DB (2009) School-based exercise improves fitness, body composition, insulin sensitivity, and markers of inflammation in non-obese children. J Pediatr Endocrinol Metab 22:409–415
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Centres for Disease Control and Prevention. Seasonal influenza (flu). Available at: http:www.cdc.gov/flu/about/qa/disease.htm Accessed 15 April 2010. Ref Type: Internet Communication
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107:3133–3140
Chung HY, Sung B, Jung KJ, Zou Y, Yu BP (2006) The molecular inflammatory process in aging. Antioxid Redox Signal 8:572–581
Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, Raggi P, Sokka T, Pincus T, Stein CM (2008) Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis 196:756–763
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30
Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS (1997) The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci 52:M201–M208
Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J (2008) Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology 216:152–155
Debigare R, Cote CH, Maltais F (2001) Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med 164:1712–1717
Derhovanessian E, Maier AB, Beck R, Jahn G, Hahnel K, Slagboom PE, de Craen AJ, Westendorp RG, Pawelec G (2010) Hallmark features of immunosenescence are absent in familial longevity. J Immunol 185:4618–4624
Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69:491–497
Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN (2011) Reversible senescence in human CD4+CD45RA+. J Immunol 187:2093–2100
Dinarello CA (2011) Blocking interleukin-1beta in acute and chronic autoinflammatory diseases. J Intern Med 269:16–28
Dodeller F, Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H (2005) The p38 mitogen-activated protein kinase regulates effector functions of primary human CD4 T cells. Eur J Immunol 35:3631–3642
Dunne PJ, Belaramani L, Fletcher JM, Fernandez DM, Lawrenz M, Soares MV, Rustin MH, Lam EW, Salmon M, Akbar AN (2005) Quiescence and functional reprogramming of Epstein–Barr virus (EBV)-specific CD8+ T cells during persistent infection. Blood 106:558–565
El Ani D, Zimlichman R, Mashiach Y, Shainberg A (2007) Adenosine and TNF-alpha exert similar inotropic effect on heart cultures, suggesting a cardioprotective mechanism against hypoxia. Life Sci 81:803–813
Ershler WB (2007) A gripping reality: oxidative stress, inflammation, and the pathway to frailty. J Appl Physiol 103:3–5
Feldmann M, Maini RN (2003) Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 9:1245–1250
Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN (2005) Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 175:8218–8225
Fontana L (2009) Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol 44:41–45
Forsythe LK, Wallace JM, Livingstone MB (2008) Obesity and inflammation: the effects of weight loss. Nutr Res Rev 21:117–133
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105
Freeman RB Jr (2009) The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant 9:2453–2458
Geist LJ, Hopkins HA, Dai LY, He B, Monick MM, Hunninghake GW (1997) Cytomegalovirus modulates transcription factors necessary for the activation of the tumor necrosis factor-alpha promoter. Am J Respir Cell Mol Biol 16:31–37
Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P (2010) An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab 95:E1–E8
Giunta S (2008) Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm-aging, from robustness to frailty. Inflamm Res 57:558–563
Gomez CR, Acuna-Castillo C, Nishimura S, Perez V, Escobar A, Salazar-Onfray F, Sabaj V, Torres C, Walter R, Sierra F (2006) Serum from aged F344 rats conditions the activation of young macrophages. Mech Ageing Dev 127:257–263
Gonzalez O, Tobia C, Ebersole J, Novak M (2011) Caloric restriction and chronic inflammatory diseases. Oral Dis 18:16–31
Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J (2010) Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci 1193:153–159
Goodwin K, Viboud C, Simonsen L (2006) Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159–1169
Gorczynski RM, Terzioglu E (2008) Aging and the immune system. Int Urol Nephrol 40:1117–1125
Goronzy JJ, Shao L, Weyand CM (2010) Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am 36:297–310
Gouin JP, Hantsoo L, Kiecolt-Glaser JK (2008) Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation 15:251–259
Grivennikov SI, Karin M (2011) Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis 70(Suppl 1):i104–i108
Gupta S, Bi R, Kim C, Chiplunkar S, Yel L, Gollapudi S (2005) Role of NF-kappaB signaling pathway in increased tumor necrosis factor-alpha-induced apoptosis of lymphocytes in aged humans. Cell Death Differ 12:177–183
Gupta S, Su H, Bi R, Gollapudi S (2006) Differential sensitivity of naive and memory subsets of human CD8+ T cells to TNF-alpha-induced apoptosis. J Clin Immunol 26:193–203
Haynes L, Eaton SM (2005) The effect of age on the cognate function of CD4+ T cells. Immunol Rev 205:220–228
Haynes L, Eaton SM, Swain SL (2000) The defects in effector generation associated with aging can be reversed by addition of IL-2 but not other related gamma(c)-receptor binding cytokines. Vaccine 18:1649–1653
Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E (2006) Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295:1539–1548
Ho JE, Hsue PY (2009) Cardiovascular manifestations of HIV infection. Heart 95:1193–1202
Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS (2004) Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci 1019:412–423
Jolly CA (2004) Dietary restriction and immune function. J Nutr 134:1853–1856
Kebschull M, Demmer RT, Papapanou PN (2010) “Gum bug, leave my heart alone!”—epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res 89:879–902
Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY (2002) Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev 123:1589–1595
Kim DH, Kim JY, Yu BP, Chung HY (2008) The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology 9:33–47
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321
Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T (2011) Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev 10:370–378
Lavallee P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P (2002) Association between influenza vaccination and reduced risk of brain infarction. Stroke 33:513–518
Lencel P, Magne D (2011) Inflammaging: the driving force in osteoporosis? Med Hypotheses 76:317–321
Li C, Beavis P, Palfreeman AC, Amjadi P, Kennedy A, Brennan FM (2011a) Activation of p38 mitogen-activated protein kinase is critical step for acquisition of effector function in cytokine-activated T cells, but acts as a negative regulator in T cells activated through the T-cell receptor. Immunology 132:104–110
Li H, Yan Z, Zhu J, Yang J, He J (2011b) Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology 60:252–258
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2:725–734
Libby P, Okamoto Y, Rocha VZ, Folco E (2010) Inflammation in atherosclerosis: transition from theory to practice. Circ J 74:213–220
Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C (2005) Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing 2:8
Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L (2006) Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 91:345–347
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T (2004) Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109:1594–1602
Merritt C, Enslen H, Diehl N, Conze D, Davis RJ, Rincon M (2000) Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8(+) but not CD4(+) T cells. Mol Cell Biol 20:936–946
Messaoudi I, Fischer M, Warner J, Park B, Mattison J, Ingram DK, Totonchy T, Mori M, Nikolich-Zugich J (2008) Optimal window of caloric restriction onset limits its beneficial impact on T-cell senescence in primates. Aging Cell 7:908–919
Monteiro R, Azevedo I (2010) Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm doi:10.1155/2010/289645
Morales I, Farias G, Maccioni RB (2010) Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation 17:202–204
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS (2001) Sarcopenia. J Lab Clin Med 137:231–243
Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W (2003) Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 107:762–768
Nayak BN, Friel JK, Rempel CB, Jones PJ (2009) Energy-restricted diets result in higher numbers of CD4+, CD8+, immunoglobulins (A, M, and G), and CD45RA cells in spleen and CD4+, immunoglobulin A, and CD45RA cells in colonic lamina propria of rats. Nutr Res 29:487–493
Parish ST, Wu JE, Effros RB (2009) Modulation of T lymphocyte replicative senescence via TNF-{alpha} inhibition: role of caspase-3. J Immunol 182:4237–4243
Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A (2009) Cytomegalovirus and human immunosenescence. Rev Med Virol 19:47–56
Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R (2008) Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8:157–168
Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A (2011) Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 32: 1730–1735
Qiu H, Straat K, Rahbar A, Wan M, Soderberg-Naucler C, Haeggstrom JZ (2008) Human CMV infection induces 5-lipoxygenase expression and leukotriene B4 production in vascular smooth muscle cells. J Exp Med 205:19–24
Reed JR, Vukmanovic-Stejic M, Fletcher JM, Soares MV, Cook JE, Orteu CH, Jackson SE, Birch KE, Foster GR, Salmon M, Beverley PC, Rustin MH, Akbar AN (2004) Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med 199:1433–1443
Rincon M, Pedraza-Alva G (2003) JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol Rev 192:131–142
Riou C, Yassine-Diab B, Van Grevenynghe J, Somogyi R, Greller LD, Gagnon D, Gimmig S, Wilkinson P, Shi Y, Cameron MJ, Campos-Gonzalez R, Balderas RS, Kelvin D, Sekaly RP, Haddad EK (2007) Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med 204:79–91
Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B (2010) A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer 103:1154–1162
Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377:31–41
Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ (2011) Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol 106:1340–1350
Schafer PH, Wang L, Wadsworth SA, Davis JE, Siekierka JJ (1999) T cell activation signals up-regulate p38 mitogen-activated protein kinase activity and induce TNF-alpha production in a manner distinct from LPS activation of monocytes. J Immunol 162:659–668
Seriolo B, Paolino S, Sulli A, Ferretti V, Cutolo M (2006) Bone metabolism changes during anti-TNF-alpha therapy in patients with active rheumatoid arthritis. Ann N Y Acad Sci 1069:420–427
Sethi JK, Vidal-Puig AJ (2007) Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 48:1253–1262
Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE (2011) Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6:e16103
Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA (2005) Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 165:265–272
Siscovick DS, Raghunathan TE, Lin D, Weinmann S, Arbogast P, Lemaitre RN, Psaty BM, Alexander R, Cobb LA (2000) Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol 152:674–677
Sobieszczyk, ME, Hoover, DR, Anastos, K, Mulligan, K, Tan, T, Shi, Q, Gao, W, Hyman, C, Cohen, MH, Cole, SR, Plankey, MW, Levine, AM, Justman, J (2008) Prevalence and predictors of metabolic syndrome among HIV-infected and HIV-uninfected women in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 48:272–280
Swindell, WR (2009) Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics. 10:585.
Vainas T, Sayed S, Bruggeman CA, Stassen FR (2009) Exploring the role of Chlamydia pneumoniae in cardiovascular disease: a narrative review. Drugs Today 45(Suppl B):165–172
van de Berg PJ, van Stijn A, ten Berge IJ, van Lier RA (2008) A fingerprint left by cytomegalovirus infection in the human T cell compartment. J Clin Virol 41:213–217
van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, Henson SM, ten Berge IJ, Akbar AN, van Lier RA (2010) Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol 184:3417–3423
Van Doornum S, McColl G, Wicks IP (2002) Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum 46:862–873
Wang CS, Wang ST, Lai CT, Lin LJ, Chou P (2007a) Impact of influenza vaccination on major cause-specific mortality. Vaccine 25:1196–1203
Wang Y, Yin B, Liu S, Xue S (2007b) Cardioprotective effect by tumor necrosis factor-alpha and interleukin-6 through late preconditioning in unstable angina patients. Arch Med Res 38:80–85
Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL (2000) Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis 31:803–805
Weinberger B, Lazuardi L, Weiskirchner I, Keller M, Neuner C, Fischer KH, Neuman B, Wurzner R, Grubeck-Loebenstein B (2007) Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol 68:86–90
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP (2011) Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12:408–415
Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ (2011) Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology 50:518–531
Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F (2002) Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol 37:445–453
Williams JG, Jurkovich GJ, Hahnel GB, Maier RV (1992) Macrophage priming by interferon gamma: a selective process with potentially harmful effects. J Leukoc Biol 52:579–584
Zhang X, Sun S, Hwang I, Tough DF, Sprent J (1998) Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591–599
Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J (2002) Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells. J Exp Med 195:283–293
Acknowledgements
The authors wish to acknowledge support from the Medical Research Council (RM), and the British Biotechnology and Biological Sciences Research Council (ANA, SMH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authorship
R.M. surveyed the literature and wrote the paper. S.M.H. and A.A. reviewed the manuscript.
About this article
Cite this article
Macaulay, R., Akbar, A.N. & Henson, S.M. The role of the T cell in age-related inflammation. AGE 35, 563–572 (2013). https://doi.org/10.1007/s11357-012-9381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-012-9381-2