Abstract
Solidification/stabilization technology is commonly used in the rehabilitation of dredged sediment due to its cost-effectiveness. However, traditional solidification/stabilization technology relies on cement, which increases the risk of soil alkalization and leads to increased CO2 emissions during cement production. To address this issue, this study proposed an innovative approach by incorporating bentonite and citrus peel powder as additives in the solidifying agent, with the aim of reducing cement usage in the dredged sediment solidification process. The research results showed that there is a significant interaction among cement, bentonite, and citrus peel powder. After response surface methodology (RSM) optimization, the optimal ratio of the cementitious mixture was determined to be 14.86 g/kg for cement, 5.85 g/kg for bentonite, and 9.31 g/kg for citrus peel powder. The unconfined compressive strength (UCS) of the solidified sediments reached 3144.84 kPa. The reaction products of the solidification materials, when mixed with sediment, facilitated adsorption, gelation, and network structure connection. Simultaneously, the leaching concentration of heavy metals was significantly decreased with five heavy metals (Zn, As, Cd, Hg, and Pb) leaching concentrations decreasing by more than 50%, which met the prescribed thresholds for green planting. This study demonstrated the ecological benefits of employing bentonite and citrus peel powder in the solidification process of dredged sediment, providing an effective solution for sediment solidification.
Graphical Abstract
The addition of citrus peel powder and bentonite to the traditional cement-based sediment significantly interacted with the development of UCS in the cement-based sediment. CAH, CSH, and LMP contributed significantly to the development of UCS in the solidified sediment. Moreover, when incorporated with bentonite and orange peel powder, the leaching concentration of heavy metals in the solidified sediment also met the requirements of green planting soil.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of heavy metals, organic pollutants, and nutrients in river sediment renders it an intrinsic source of pollution to the river, compromising the environmental quality if left untreated (Bao et al. 2016; Beljin et al. 2023). Currently, dredging is widely used as the primary method for sediment management in rivers. Nevertheless, the dredged sediment consistently exhibits high moisture content and low strength, making it unsuitable for direct resource utilization. Hence, it is essential to dispose the sediment produced during dredging.
Traditional sediment resource treatment methods include sediment washing, chemical extraction, and stabilization/solidification (Xu and Wu 2023). However, sediment washing carries the risk of heavy metal transfer, and the use of chemical additives negatively impacts the soil microorganisms and enzymes (Udovic and Lestan 2012), thereby compromising the ecological safety of the local soil. Among these methods, stabilization/solidification is the most commonly used due to its low cost, good treatment effect, and simple operation (Ma et al. 2018). The solidification process involves the addition of solidifying materials that react with the sediment to enhance its mechanical properties and stabilize heavy metals and organic matter. This transforms the sediment, which has a high moisture content and low strength, into solidified sediment with a certain strength that can be used in engineering (Pu et al. 2021). Cement and lime are the most used solidifying materials thus far. Cement solidifies sediment through the hydration process, producing high-strength calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH) (Gu et al. 2015; Lang et al. 2020). Lime is often used to neutralize acids in the sediment and create an alkaline environment since cement hydration requires alkalinity. However, the production of cement and lime consumes significant amounts of energy and generates excessive heat. According to literature sources, the cement industry alone accounted for nearly 5–7% of global anthropogenic CO2 emissions (Zhang et al. 2018). Dredged sediments often contain a substantial amount of organic matter and moisture, which obstruct the hydration of cement (Gussoni et al. 2004). Consequently, to effectively solidify dredged sediments, it is necessary to increase the cement dosage. Nevertheless, a notable drawback of using cement for sediment solidification is that the elevated alkalinity of Ca(OH)2 from the cement can infiltrate the aquatic environment (Lin et al. 2013), leading to significant adverse impacts on water quality and microorganisms (Maldonado-Alameda et al. 2021), as well as impeding the resource utilization of cement-based solidified sediments.
Bentonite is a type of layered silicate clay with a structure that consists of alternating positive and negative charges. Upon contact with bentonite, water molecules penetrate the gaps in its layered structure and interact with the ions present between the layers. These interactions cause water molecules to adhere to the surface of bentonite particles, resulting in strong water absorption and expansion properties (Dimirkou et al. 2002; Gokalp et al. 2011). Research findings indicated that when bentonite is mixed with cement, its water absorption and expansion properties allow it to absorb moisture from the cement slurry and fill the fine pores in the cement mixture. This, in turn, enhances the stability and strength of the sediment solidification system (Akguen 2010). The study by Kadhim et al. showed that the UCS of cement mixtures containing 15% bentonite increased by 21% compared to the control (Kadhim et al. 2022). Moreover, the surface of bentonite carries a predominantly negative charge, enabling it to adsorb positive charges or polar molecules (Doulia et al. 2009; Sdiri et al. 2014). Due to this adsorption capacity, bentonite exhibits high affinity for a wide range of substances like organic matter and heavy metal ions (Andini et al. 2006; Bao et al. 2016; Katsioti et al. 2008; Ouhadi et al. 2006). Hence, the combination of bentonite and cement in a solidification system can effectively adsorb pollutants in sediments, prevent their overflow and diffusion, thus safeguarding the surrounding environment.
Artificially synthesized polymer materials (e.g., PAC, PAM) are extensively utilized in cement products due to their ability to enhance the mechanical properties of solidified sediment. However, they have low biodegradability, and the intermediates generated during their degradation can have environmental implications (Okaiyeto et al. 2016). In contrast to synthetic polymer materials, biopolymer materials offer advantages, including biodegradability and the absence of toxic degradation products. Citrus peel powder is well-known for its high content of pectin, with pectin levels ranging from approximately 20 to 40% (Jeong et al. 2021). Pectin is an acidic polysaccharide that forms through covalent and hydrogen bond interactions. It contains various organic functional groups, including carboxyl groups, which enable it to bind with cations (Sengkhamparn et al. 2010). Research had revealed that incorporating pectin can markedly enhance the strength of solidified sediment (Eivazzadeh-Keihan et al. 2022; Lin et al. 2022). Mixing pectin with solidified sediment leads to the formation of a cross-linking structure between pectin and soil particles, resulting in enhanced compressive strength (Kavas et al. 2007). Simultaneously, pectin fills the gaps between solidified sediment particles, enhances soil compactness, and further improves its strength (Hazarika et al. 2018).
To reduce reliance on cement and alleviate its negative effects, this study examines the effects of employing bentonite with excellent water absorption properties and acidic citrus peel powder rich in pectin as additives on dredged sediments. The effectiveness of solidification was evaluated through testing the UCS, density, moisture content, and pH of the solidified sediment. Additionally, the leaching concentration of heavy metals from the solidified sediment was examined to assess the ecological benefits. The RSM was employed to optimize the solidifying material ratio and minimize cement consumption, using the UCS data obtained for various material ratios. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) methods were employed to examine solidified sediment specimens aged for 28 days. This analysis aims to assess the feasibility of using bentonite and citrus peel powder as solidifying agents by revealing the impact of different material ratios on the microscopic strength development of solidified sediment.
Materials and methods
Materials
The sediment utilized in this study was retrieved from the black and odorous section of a rural river in Nanjing, China. After undergoing a natural drying process for 2 days, the sediment underwent solidification/stabilization experiments. The moisture content, specific gravity, and pH of the sediment were measured to be 61.00 ± 1.24%, 1.78, and 8.07, respectively. The degree of heavy metal pollution in the raw sediments was low (Table S1), with the contents of four heavy metals (Pb, Cd, Cr, and Cu) being below the limit value for green planting soil (CJT340-2016), while other heavy metals (As, Hg, and Zn) have slightly exceeded their respective limit values. The solidification/stabilization materials employed in the study consisted of 425 ordinary Portland cement (supplied by Chongqing Senge Co., Ltd., with a specific gravity of 3.0), bentonite (supplied by Henan Zhong kai Material Factory, with a specific gravity of 2.2), and citrus peel powder (derived from Sichuan, with a specific gravity of 0.4). The chemical composition is shown in Table S2. The citrus peel powder was produced by grinding dried citrus peels at a temperature of 60 °C for 30 min. It possessed a particle size greater than 200 mesh and a pectin content of approximately 18.13%.
Preparation of solidified specimens and solidified sediment leachate specimens
Preparation of solidified specimens
A fixed mass of sediment was taken and mixed with the corresponding mass of solidifying agent using a stirrer. To prevent sticking and facilitate demolding, vegetable oil was evenly applied to the inside and bottom of the mold. The well-mixed specimen was then filled into a mold with an internal diameter of 45 mm and a height of 90 mm in 5 batches. This process ensured the compaction of the specimen and the removal of any air bubbles. Each time the mold was filled, it was immediately shaken up and down. After filling the mold, the surface of the specimen was leveled with a scraper and placed in a standard solidification room (China, LongHui, YH-40B) with a temperature of 20 ± 2 °C and humidity of 95% for 3 days before demolding. For the purpose of rural fieldwork compatibility, the demolded specimens were transferred to natural conditions and kept for up to 28 days.
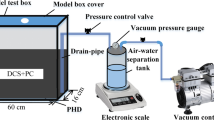
Preparation of solidified sediment leachate specimens
The solidified sediment was first air-dried and then crushed after the conservation period, with the crushed particles collected through a 115 mesh sieve. These sieved specimens were then added to distilled water in conical glass bottles at a mass ratio of 1:10. The conical glass bottles were placed on an oscillating device (China, Lichen, HY-4) with a fixed speed of 30 ± 2 r/min and a controlled temperature of 23 ± 2 ℃. After the shaking process, the filtrate was collected by passing it through a 0.45-μm filter membrane. The collected filtrate was immediately subjected to heavy metal concentration testing.
Additives proportioning design and optimal design experiment
The specific additive ratios used in the single-factor experiment and heavy metal leaching test are listed in Table S3. In order to preliminarily determine the range of adding cement, bentonite, and citrus peel powder, three groups of single-factor experiments were conducted to study the effects of different additive ratios on moisture content, pH, and UCS. Through manipulation of a single variable, while maintaining the other two additive proportions at constant values, experiments were conducted within a range of 5 to 30 g/kg for the varying single variable. The purpose of setting up the leaching test group was to evaluate the effect of the addition amounts of three additives on the leaching of heavy metals in the solidified sediment.
After determining the optimal range of the three material addition amounts, the Box-Behnken experimental design method within the RSM was utilized to optimize the proportions of cement, bentonite, and citrus peel powder. The experimental results were fitted and optimized through multiple linear regression using Design Expert 11 software, with reference to the results of single-factor experiments. Subsequently, the best experimental results were evaluated through validation experiments. According to Box-Behnken, the RSM employed a 3-factor, 3-level experimental design consisting of 17 experiments (Table 1).
Measurement specimens and characterization methods
To examine the solidification effect of bentonite and citrus peel powder on cement-solidified sediment, the UCS of the solidified sediment was measured. The UCS and strain of the specimens were determined using Eqs. (1) and (2), respectively.
where \(p\) is the UCS (kPa); \(f\) is the load applied (N); and \(A\) is the area under stress (m2).
where \(\varepsilon\) is the strain (%); \(\Delta s\) is the compression (cm); and \(s\) is the length of the solidified sediment (cm).
The load-bearing capacity and compression behavior of the solidified sediment were determined using a universal testing machine (Japan, Shimadzu, AG–X plus), known for its high accuracy and ease of control. The test involved applying a vertical load at a constant displacement rate of 1 mm/min until failure occurred. The methods employed to analyze the pH, moisture content, and density of the solidified sediment specimens, as well as the concentration of heavy metals in the solidified sediment leachate, are detailed in Table S4.
Microstructural analysis of the selected specimens was performed using SEM (America, FEI, Inspect F50) and XRD (Germany, Bruker, D8-Discover). To prepare the specimens for SEM testing, they were freeze-dried with liquid nitrogen and subsequently subjected to vacuum sublimation for 48 h. Dried specimen pieces up to 7 mm in size were then prepared and covered with a layer of gold before being loaded into the SEM for imaging. XRD testing involved using granular specimen powder sieved through a 75-µm sieve. The specimens were scanned in steps of 0.02°, ranging from 5 to 90°, at a scan rate of 8°/min. The XRD results were analyzed using MDI Jade 6.5 materials analysis software. Statistical analysis was performed using SPSS 19.0.
Results and discussion
The effect of additives on the solidification of dredged sediment
Single-factor experiments were to explore the effect of additives on the solidification of dredged sediment. Figure 1 illustrates the effects of adding cement, bentonite, and citrus peel powder on the solidification effectiveness of sediment samples at 28-day curing period. The bentonite addition significantly affected the UCS of the solidified sediment. The UCS gradually decreased as the dosage of bentonite increases, starting from 5 g/kg. And when the bentonite addition was 5 g/kg, the strength of the solidified sediment reached the highest UCS of 2082.66 Kpa. Excessive addition of bentonite should be avoided as excessive bentonite could absorb water and disperse particles within the materials. Citrus peel powder addition also significantly influenced the UCS of the solidified sediment. With the addition of 5 g/kg of citrus peel powder, the UCS of the solidified sediment was 1549.85 kPa. Subsequently, adding 10 g/kg of citrus peel powder increased the UCS to 2061.71 kPa, but further additions resulted in a sharp decrease in UCS. The UCS values of the 28-day cured sediment samples rose from 1534.71 to 2002.55 kPa with the increase in cement addition from 5 to 30 g/kg. The moisture content in all solidified sediment samples reduced to less than 3% at 28-day cure time. Among the three solidifying materials, due to the presence of acidic substances in citrus peel powder, and the lack of alkaline substances in both bentonite and citrus peel powder themselves, with the addition of citrus peel powder, the alkalinity of the solidified sediment decreased, ensuring its ecological safety. However, excessive citrus peel powder could also have a negative impact on hydration reactions. Thus, based on the results of single-factor experiment, the preliminary ranges for the addition of bentonite and citrus peel powder were determined to be 0–10 g/kg and 5–15 g/kg, respectively. The cement content typically employed to solidify the sediment exceeds 5% (Malviya and Chaudhary 2006). To reduce cement consumption, it was advisable to keep the total amount of solidifying additives below 5%. Thus, it was recommended to limit the cement addition to the range of 5–15 g/kg.
Mechanism of solidification and characterization of destruction
XRD analysis
As shown in Fig. 2, XRD analysis was performed on sediment specimens that underwent 28-day curing stabilization tests in order to determine the composition of specific minerals. These minerals included quartz (Si), CSH, CAH, high-sulfur-type calcium sulfur aluminate hydrate (AFt), calcium hydroxide (CH), high methoxyl pectin (HMP), and low methoxyl pectin (LMP). The purpose of these analyses was to evaluate the reactions that occurred during the mixing process of cement, bentonite, and citrus peel powder in the sediment.
As depicted in Fig. 2(a), the content of CAH and CSH increased with the cement addition due to the hydration reaction of silicate cement. This reaction generated CSH, CH, CAH, and AFt when added to the sediment (Pu et al. 2019). The hydration products mixed and filled the gaps between the sediment particles, collectively improving the strength of the solidified sediment (Wang et al. 2019a). The lower relative peak strength of CH resulted from its reaction with Al2O3 and SiO2 in the sediment, which leaded to the production of CAH and CSH, respectively (Gu et al. 2015). Equations (3) and (4) depict the chemical reactions involved.
Moreover, the relative peak strength of LMP was found to increase proportionally with the addition of cement due to the enhanced alkalinity of the solidified sediment. This alkalinity promoted the de-esterification process of HMP, resulting in the production of LMP. In the system, LMP readily reacted with Ca2+, Mg2+, and Al3+ to form structures resembling eggshells or globular colloids. Additionally, the inclusion of LMP enhanced the rate of cement hydration, resulting in a significant increase in the formation of CAH and CSH (Hazarika et al. 2018). These structures enhanced the overall strength of the solidified sediment (Guo et al. 2021; Sedan et al. 2007).
Figure 2 (b) illustrates the XRD patterns of solidified sediment specimens with varying additions of bentonite. The results showed that the peak strength of CSH initially increased, but then decreased with increasing the addition of bentonite. At low additions, SiO2 in bentonite reacted with the cement hydration product known as CH, leading to the production of CSH (Gu et al. 2015). However, the excessive water absorption and swelling of bentonite impeded the cement hydration reaction, and its loose internal structure resulted in a decrease in the strength of the solidified sediment (Boutammine et al. 2020; Katsioti et al. 2008). This finding aligned with the experimental conclusion regarding the influence of bentonite dosing on the UCS of solidified sediment.
Figure 2 (c) displays the XRD patterns of solidified sediment specimens with varying amounts of citrus peel powder. The relative peak strengths of CSH and CAH decreased due to the consumption of Ca(OH)2, a result of the acidic pectin in citrus peel powder. This consumption inhibited the reactions described by Eqs. (3) and (4), leading to a decrease in the formation of CSH and CAH. Simultaneously, the relative peak strength of HMP increased. However, the relative peak strength of LMP initially increased and then decreased. This occurred due to the partial de-esterification of HMP under the influence of CH, resulting in the generation of LMP. With the further addition of citrus peel powder, the acidic pectin consumed alkalinity, leading to a decrease in the production of LMP. However, it was also beneficial for reducing the overall alkalinity of the solidified sediment and improving soil alkalization issues.
SEM analysis
The solidified sediment specimens used in the curing stabilization material dosing test were kept for 28 days and were analyzed using SEM to observe their micro-morphology. Figure 3(a) and (c) demonstrate that the quantity of cement hydration products in the solidified sediment increases with the amount of cement dosing. These hydration products were primarily composed of fibrous, networked, needle, and rod-shaped CSH, with a small amount of caliche present. When zoomed in at a magnification of 20,000 times, it was evident that the pore space in the solidified sediment gradually decreases with an increase in cement addition. Moreover, the agglomerated and globular LMP-cemented particles (identified by the red circle) also showed an increasing trend. As the cement addition increased, the cement hydration products and LMP-cemented soil particles filled the pores of the solidified sediment, promoting its strength development. This microscopic observation explained why the UCS of the solidified sediment increases as the cement content in the curing agent increases.
SEM images of solidified sediment. Cement 10, a 4000 times. b 20000 times. Cement 30, c 4000 times. d 20000 times. Bentonite 10, e 4000 times. f 20000 times. Bentonite 30, (g) 4000 times. h 20000 times. Citrus peel powder 10, i 4000 times. j 20,000 times. Citrus peel powder 30, k 4000 times. l 20,000 times
The laminar and plate-like bentonite crystals (marked by arrow 1) in the solidified sediment increased with an increase in bentonite addition, as shown in Fig. 3 (e) and (g). Consequently, the CSH decreased. In Fig. 3(f), it was evident that the highly swollen lamellar and plate-like bentonite consistently filled the gaps between soil particles, resulting in a dense structure with smaller pores and higher strength. Figure 3 (f) and (h) reveal that an increase in bentonite addition causes over-expansion of bentonite, resulting in pore expansion, loosening of the solidified sediment structure, and a decrease in its strength. This microscopic explanation elucidated the impact of bentonite on the UCS of the solidified sediment.
The surface morphology of solidified sediment specimens from the citrus peel powder group at 4000 × magnification is shown in Fig. 3(i) and (k). With increasing addition of citrus peel powder, the CSH content gradually decreased. This could be attributed to the acidic pectin, which inhibited the cement’s hydration reaction and reduced the production of CSH, consistent with the XRD test results. Figure 3 (j) and (l) reveal that when smaller amounts of citrus peel powder were mixed, clustered and spherical citrus peel pectin colloids could be observed, resulting in a denser structure and higher strength of the solidified sediment. However, with an excessive amount of citrus peel powder, the presence of colloids in the solidified sediment decreased, leading to increased cracks, looseness, and porosity, ultimately resulting in decreased strength.
Based on the SEM results of bentonite and citrus peel powder, it could be observed that the addition of 10 g/kg was more effective in sediment solidification than 30 g/kg. This also confirmed the rationality of the range of addition of bentonite and citrus peel powder in the single-factor experiment.
Destruction characterization
Typical damage patterns of consolidated soils during UCS testing are shown in Fig. 4. The crack unfolding patterns could be classified into three main types: (a) plastic flow shear damage, (b) brittle shear damage, and (c) brittle tensile crack damage. During the UCS testing of solidified sediment, it was observed that when the strength was low, the soil exhibits plastic damage and larger destructive strained. Conversely, when the strength was high, the soil shows brittle damage and smaller destructive strained. Additionally, the destructive strain was found to be inversely related to the strength of the solidified sediment.
The stress–strain curves of the specimens, which were cured with different material mixing ratios of curing agents, were tested at the 7th and 28th days (see Fig. 5). The stress corresponding to the point of maximum stress on the stress–strain curve represented the UCS of the solidified sediment. The stress of the solidified sediment specimen for the one-factor test ranged from 0 to 2400 kPa, with a destructive strain between 1.5 and 6%. The peak stress of the solidified sediment increased while the destructive strain decreased with an increase in curing time. Similarly, the peak stress of the solidified sediment increased while the destructive strain decreased with an increase in cement addition. Conversely, the peak stress of the solidified sediment decreased and the destructive strain increased with an increase in bentonite addition. Furthermore, the peak stress of the solidified sediment increased while the destructive strain decreased with an increase in citrus peel powder. Interestingly, the peak stress of the solidified sediment initially increased, then decreased, as the citrus peel powder was further increased, while the destructive strain initially decreased and then increased. These findings aligned with the analysis of microstructure conducted via XRD and SEM.
Ecological safety of solidified sediment
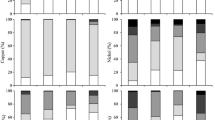
The ecological safety of the solidified sediments was further checked to ensure that the leaching concentrations of the seven heavy metals after solidification were all below the standard limits. Heavy metals leaching concentrations for raw and solidified sediments were presented in Table 2 and compared to that in green planting soil (CJT340-2016). Since the sediment and distilled water were mixed at a ratio of 1:10 in this research, the soil heavy metal content (mg/kg) in the standard was converted into the leaching concentration of heavy metals in the sediment (mg/L). The results indicated that the cured sediment exhibits reduced leaching concentrations of all heavy metals compared to the untreated sediment. This could be attributed to the adsorption of heavy metals by reaction products of cement, bentonite, and citrus peel powder, such as CSH and LMP. Additionally, heavy metals could react with cement components to form insoluble precipitates, thereby reducing their release (Guo et al. 2017).
With the increasing addition of cement, the leaching concentrations of Cu, Zn, As, and Pb in the solidified sediment specimens gradually decreased. This trend suggested that higher cement addition promotes an increase in system alkalinity, thereby facilitating the solidification and stabilization of heavy metals. The concentrations of Zn and As showed a more pronounced decrease, suggesting that cement has better solidification and stabilization capabilities for these metals. On the other hand, the leaching concentrations of Cu, Zn, and As in the solidified sediment specimens first decreased and then increased with increasing bentonite addition. Initially, the higher bentonite content allowed for greater adsorption of Cu, Zn, and As ions. However, the excessive increase in bentonite addition leaded to excessive expansion of the solidified sediment, resulting in the loosening of binding between some Cu, Zn, and As ions and the soil particles and an increase in leaching concentration. For both Cu and Zn, their leaching concentrations exhibited a nonlinear relationship with the addition of citrus peel powder. Specifically, as the addition of citrus peel powder increased, the leaching concentrations of Cu and Zn initially decreased and then increased. This occurrence could be attributed to the higher adsorption of Cu and Zn ions by pectin at lower additions of citrus peel powder (Wang et al. 2019b). However, with excessive citrus peel powder, a large amount of acidic substances hindered the hydration reaction, thereby reduced the hydration products of cement.
The results of the leaching tests shows that the solidified sediment meets the technical requirements of class 1 soil according to China’s planting soil for green standard (CJT340-2016).
Optimization of additives ratio by response surface method
Because the UCS directly determined the pathway of sediment resource utilization, the response surface was analyzed with the UCS of the solidified sediment as the response value. Design-Expert 11 software was utilized for a quadratic multiple regression analysis of the experimental data in Table S5. Consequently, the quadratic polynomial equation for the UCS was obtained as follows:
where Y represents the predicted value of UCS, while A, B, and C correspond to the respective addition of cement, bentonite, and citrus peel powder.
The coefficients of the factors and the analysis of variance utilizing the F-distribution method are presented in Table 3. The F value of 57.67 indicates that the model is significant. The F value of the lack of fit is 2.25, and the p value is 0.2246, which means that the lack of fit is not significant. As indicated in Table 3, the model terms A, B, C, A2, B2, and C2 exhibit significance (p < 0.05), suggesting the reliability of the data (Abdulhameed et al. 2021; Jawad et al. 2020). The correlation coefficient R2 = 0.9696 of the adjusted regression equation signifies a sound fit of the model with actual values, enabling the prediction of UCS. Figure 6 displays a comparison between the measured and predicted values of the 28-day UCS, revealing a linear distribution of the measured and predicted values. In Fig. 7, the external studentized residuals for each trial fall within the range of ± 4.81963, without any outliers and with a center close to 0, confirming the accuracy of the model’s predictions (Danmaliki et al. 2017). Analyzing the F values of each individual-factor model terms A, B, and C in Table 3 enables the determination of the order of their effects on Y (UCS), which is judged to be A (cement addition) > B (bentonite addition) > C (citrus peel powder addition).
The 3D response surface in Fig. 8(a) shows the relationship between UCS and the dosage of both cement and bentonite. The cement content had a greater influence on UCS compared to bentonite. The contour plot in Fig. 8(b) exhibited a saddle shape, which signified a noteworthy interaction between the addition of cement and bentonite. At a bentonite dosage of 6 g/kg, the continual addition of cement substantially improved the UCS of the solidified sediment. However, surpassing a bentonite dosage of 6 g/kg resulted in a diminishing promotional effect of cement on the UCS. This could be attributed to the repulsive force acting within the excessive bentonite, resulting in particle dispersion and weak bonds between particles and sediment, devoid of the robust bonds generated by cement hydration products (Estabragh et al. 2022). Figure 8 (c) illustrates the relationship between UCS and additions of cement and citrus peel powder. The influence of cement addition on UCS was more significant than the addition of citrus peel powder. The contour plot in Fig. 8(d) exhibits a saddle shape, indicating a significant interaction between the addition of cement and citrus peel powder. When the addition of citrus peel powder was close to 9 g/kg, cement could maximize the improvement of UCS in solidified sediment. However, once it exceeded 9 g/kg, citrus peel powder would negatively affect UCS. Hydration reactions could result in a pH increase above 10 (Galan et al. 2021). However, Fig. 1(b) illustrates a final pH of only 6.74 for sample citrus peel powder 30. Consequently, the excessive amount of acidic pectin in the citrus peel powder significantly depleted alkalinity (Wang et al. 2023). This depletion was detrimental to the progression of hydration reactions and, as a result, negatively affected the UCS. Figure 8 (e) demonstrates the impact of bentonite and citrus peel powder addition on the UCS of solidified sediment, with both having a threshold for improving UCS, with bentonite having a greater impact. The contour plot in Fig. 8(f) is similar to a circle, indicating that the interaction between bentonite and citrus peel powder is not significant. This is because excess pectin from citrus peel powder reduces alkalinity and prevents the silica in the bentonite from reacting with the hydration products of the cement, and excess bentonite leads to loss of UCS in the setting sediment due to excessive swelling.
Based on a combination of Fig. 8(a), (c), (e), it could be observed that cement exerted the strongest impact on the UCS compared to the effects of bentonite and citrus peel powder. This finding aligns with the outcomes of the significance analysis performed on the regression model. The additive ratios were optimized based on an analysis of the independent and interaction effects of the individual factors in order to determine the most favorable combination. At the maximum response value, the recommended additions for each material were the following: cement 14.86 g/kg, citrus peel powder 9.31 g/kg, and bentonite 5.85 g/kg, in a ratio of 2.54:1.59:1. The predicted UCS of the solidified sediment was 3181.40 kPa. By using these specific additive ratios in three parallel tests, the average compressive strength of the solidified sediment was measured to be 3144.84 kPa. This result was 57% higher than the UCS of sample “cement 30” (2002.55 kPa), indicating that citrus peel powder and bentonite have advantages in improving the UCS of solidified sediments compared to cement-based S/S method.
Conclusion
This research proposed an environmentally friendly solidification method to reduce the cement consumption and improve the ecological safety of the sediment after solidification. The interactive effects of citrus peel powder, bentonite, and cement on the solidified sediment were investigated using single-factor experiments and RSM. The optimal addition amounts for each solidification additive were as follows: 14.86 g/kg of cement, 5.85 g/kg of bentonite, and 9.31 g/kg of citrus peel powder. The strength of the solidified sediment could reach up to 3144.84 kPa. XRD and SEM analysis revealed that HMP in citrus peel powder reacts with cement hydration product CH, resulting in the formation of LMP. The resulting gelatinous pectin particles connected the solidified sediment, thereby enhancing its strength. SiO2 in bentonite reacted with cement hydration product CH, leading to the formation of CSH. Additionally, the plate-like and layered structure of bentonite filled the gaps in the solidified sediment, thereby enhancing its strength. Furthermore, by investigating the leaching concentrations of heavy metals, it was discovered that the pectin in citrus peel powder and bentonite exhibit adsorption properties, resulting in decreased leaching concentrations of heavy metals compared to the original sediment. As solidification additives, citrus peel powder and bentonite not only had significant advantages in improving the strength of sediment and reducing alkalinity, but also met the standards for green planting of solidified sediment. This study provided an effective method for the safe resource utilization of dredged sediment.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Abdulhameed AS, Hum N, Rangabhashiyam S et al (2021) Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. J Environ Chem Eng 9:105530
Akguen H (2010) Geotechnical characterization and performance assessment of bentonite/sand mixtures for underground waste repository sealing. Appl Clay Sci 49:394–399
Andini S, Cioffi R, Montagnaro F et al (2006) Simultaneous adsorption of chlorophenol and heavy metal ions on organophilic bentonite. Appl Clay Sci 31:126–133
Bao JP, Wang L, Xiao M (2016) Changes in speciation and leaching behaviors of heavy metals in dredged sediment solidified/stabilized with various materials. Environ Sci Pollut Res 23:8294–8301
Beljin J, Arsenov D, Slijepcevic N et al (2023) Recycling of polluted dredged sediment—building new materials for plant growing. Waste Manag 166:13–24
Boutammine H, Salem Z, Khodja M (2020) Petroleum drill cuttings treatment using stabilization/solidification and biological process combination. Soil Sediment Contam 29:1–15
Danmaliki GI, Saleh TA, Shamsuddeen AA (2017) Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon. Chem Eng J 313:993–1003
Dimirkou A, Ioannou A, Doula M (2002) Preparation, characterization and sorption properties for phosphates of hematite, bentonite and bentonite-hematite systems. Adv Coll Interface Sci 97:37–61
Doulia D, Leodopoulos C, Gimouhopoulos K et al (2009) Adsorption of humic acid on acid-activated Greek bentonite. J Colloid Interface Sci 340:131–141
Eivazzadeh-Keihan R, Noruzi EB, Aliabadi HAM et al (2022) Recent advances on biomedical applications of pectin-containing biomaterials. Int J Biol Macromol 217:1–18
Estabragh AR, Amini M, Javadi AA et al (2022) Remediation of a clay soil contaminated with phenanthrene by using mixture of bentonite and cement. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.14055
Galan I, Muller B, Briendl LG et al (2021) Continuous optical in-situ pH monitoring during early hydration of cementitious materials. Cem Concr Res 150:106584
Gokalp Z, Basaran M, Uzun O (2011) Compaction and swelling characteristics of sand-bentonite and pumice-bentonite mixtures. Clay Miner 46:449–459
Gu K, Jin F, Al-Tabbaa A et al (2015) Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng Geol 195:53–62
Guo B, Liu B, Yang J et al (2017) The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: a review. J Environ Manag 193:410–422
Guo C, Li XF, Gong T et al (2021) Gelation of Nicandra physalodes (Linn.) Gaertn. polysaccharide induced by calcium hydroxide: A novel potential pectin source. Food Hydrocoll 118:106756
Gussoni M, Greco F, Bonazzi F et al (2004) H-1 NMR spin-spin relaxation and imaging in porous systems: an application to the morphological study of white Portland cement during hydration in the presence of organics. Magn Reson Imaging 22:877–889
Hazarika A, Hazarika I, Gogoi M et al (2018) Use of a plant based polymeric material as a low cost chemical admixture in cement mortar and concrete preparations. J Build Eng 15:194–202
Jawad AH, Mohammed IA, Abdulhameed AS (2020) Tuning of fly ash loading into chitosan-ethylene glycol diglycidyl ether composite for enhanced removal of reactive red 120 dye: optimization using the box-behnken design. J Polym Environ 28:2720–2733
Jeong D, Park H, Jang BK et al (2021) Recent advances in the biological valorization of citrus peel waste into fuels and chemicals. Biores Technol 323:124603
Kadhim MJ, Kamal HM, Hasan LM (2022) Hydro-mechanical properties of cement mortar using bentonite as partial cement replacement. Int J Nanoelectron Mater 15:241
Katsioti M, Katsiotis N, Rouni G et al (2008) The effect of bentonite/cement mortar for the stabilization/solidification of sewage sludge containing heavy metals. Cement Concr Compos 30:1013–1019
Kavas T, Olgun A, Erdogan Y et al (2007) The effect of pectin on the physicochemical and mechanical properties of cement containing boron. Build Environ 42:1803–1809
Lang L, Liu N, Chen B (2020) Strength development of solidified dredged sludge containing humic acid with cement, lime and nano-SiO2. Constr Build Mater 230:116971
Lin C, Zhu W, Han J (2013) Strength and leachability of solidified sewage sludge with different additives. J Mater Civ Eng 25:1594–1601
Lin D, Ma Y, Qin W et al (2022) The structure, properties and potential probiotic properties of starch-pectin blend: a review. Food Hydrocoll 129:107644
Ma Y, Liu Z, Xu Y et al (2018) Remediating potentially toxic metal and organic co-contamination of soil by combining in situ solidification/stabilization and chemical oxidation: efficacy, mechanism, and evaluation. Int J Environ Res Public Health 15:2295
Maldonado-Alameda A, Giro-Paloma J, Rodríguez-Romero A et al (2021) Environmental potential assessment of MSWI bottom ash-based alkali-activated binders. J Hazard Mater 416:125828
Malviya R, Chaudhary R (2006) Factors affecting hazardous waste solidification/stabilization: a review. J Hazard Mater 137:267–276
Okaiyeto K, Nwodo UU, Okoli SA et al (2016) Implications for public health demands alternatives to inorganic and synthetic flocculants: bioflocculants as important candidates. Microbiologyopen 5:177–211
Ouhadi V, Yong R, Sedighi M (2006) Desorption response and degradation of buffering capability of bentonite, subjected to heavy metal contaminants. Eng Geol 85:102–110
Pu SY, Zhu ZD, Wang HR et al (2019) Mechanical characteristics and water stability of silt solidified by incorporating lime, lime and cement mixture, and SEU-2 binder. Constr Build Mater 214:111–120
Pu HF, Mastoi AK, Chen XL et al (2021) An integrated method for the rapid dewatering and solidification/stabilization of dredged contaminated sediment with a high water content. Front Environ Sci Eng 15:64
Sdiri AT, Higashi T, Jamoussi F (2014) Adsorption of copper and zinc onto natural clay in single and binary systems. Int J Environ Sci Technol 11:1081–1092
Sedan D, Pagnoux C, Chotard T et al (2007) Effect of calcium rich and alkaline solutions on the chemical behaviour of hemp fibres. J Mater Sci 42:9336–9342
Sengkhamparn N, Sagis LMC, De Vries R et al (2010) Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench). Food Hydrocoll 24:35–41
Udovic M, Lestan D (2012) EDTA and HCl leaching of calcareous and acidic soils polluted with potentially toxic metals: remediation efficiency and soil impact. Chemosphere 88:718–724
Wang F, Kong X, Wang D et al (2019a) The effects of nano-C-S-H with different polymer stabilizers on early cement hydration. J Am Ceram Soc 102:5103–5116
Wang RS, Liang RH, Dai TT et al (2019b) Pectin-based adsorbents for heavy metal ions: a review. Trends Food Sci Technol 91:319–329
Wang SC, Nguyen T, Peng H et al (2023) Sodium removal from bauxite desilication product (sodalite) aided by chelating effects of inorganic and organic acids. J Environ Manag 338:117837
Xu QQ, Wu BR (2023) Recent progress on ex situ remediation technology and resource utilization for heavy metal contaminated sediment. Toxics 11:207
Zhang CY, Han R, Yu B et al (2018) Accounting process-related CO2 emissions from global cement production under shared socioeconomic pathways. J Clean Prod 184:451–465
Funding
This research was financially supported by National Key Research and Development Program of China (2019YFD1100205).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yu-Jia Deng: writing—original draft. Zhi-Xuan Yue: writing—review and editing. Zi-Jie Wang: review and editing. Qi Huang: conceptualization, investigation, figure editing. Prof. Xiao-Li Yang: writing—review and editing, resources, project administration, supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree to publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Bentonite and citrus peel powder were effective additives for sediment solidification
• The use of bentonite and citrus peel powder reduced the addition of cement
• The optimum ratio of cement, citrus peel powder, and bentonite was 2.54:1.59:1
• Pectin in citrus peel powder could promote UCS of sediment
• Sediment’s ecological security was strengthened after the solidification
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, YJ., Yue, ZX., Wang, ZJ. et al. Optimization and mechanism of the novel eco-friendly additives for solidification and stabilization of dredged sediment. Environ Sci Pollut Res 31, 25964–25977 (2024). https://doi.org/10.1007/s11356-024-32865-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32865-2