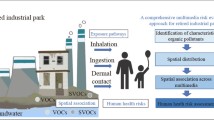
Abstract
Odor emission from the soil of pesticide-contaminated sites is a prominent environmental problem in China, but there are very few researches about the component and spatial distribution of odorous substances in the soil of contaminated sites. In this paper, to investigate the odor pollution condition of an organophosphorus pesticide production site in a city of South China, the odor pollutants in the soil and soil gas were analyzed and the key odor–contributing substances were identified. Besides, the correlation between the concentrations of odorous substances in soil and soil gas was analyzed, and the measured results were compared with the predicted results by the linear model and DED model. An off-line soil gas sampling device was designed to collect the gas emitted from soil because the groundwater level in the site was too shallow to build a soil gas well. The key odor substances were screened from the detection results of soil gas via odor activity value (OAV) analysis, which revealed that the key odorous substances included benzene, ethylbenzene, ammonia, toluene, m,p-xylene, methyl sulfide, dimethyl disulfide, and formaldehyde. Furthermore, the spatial distribution of the odor substances in the soil of the pesticide-contaminated site was closely related to the layout and geologic structure of the site. The odor pollutants in soil were mainly distributed near the phosmet production workshop and the drainage ditch network. As for the deep distribution, the odorous substances were mainly enriched in the silty clay or clay layer (5.6–11 m), followed by the sludge layer (1–3.6 m). Finally, the predicted model (linear model and DED model) analysis suggested that the linear model was more suitable for predicting the concentration of odorous substances in the soil gas with the detection data of soil in this pesticide-contaminated site.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are lots of report about the characteristics, distribution, and health risks of soil pollutants in different contaminated sites (Liu et al. 2015; Mcneel et al. 1997; Morra et al. 2011; Vijgen et al. 2018). However, they mainly focus on investigating toxic pollutants with health risks, while paying less attention to soil odor pollution (Tao et al. 2022; Wang et al. 2023). In fact, offensive odors pose a direct threat for human health and welfare, and the odor pollution of contaminated sites has raised more and more public attention (Yang et al. 2015). Organic solvents used in pesticide production, such as acetone, formaldehyde, xylene, benzene, toluene, and ethylbenzene, can cause odorous gas emission not only during the production process but also after the factory was closed. Presently, the research on odor pollution is mainly focused on the emission from production of factories, sewage treatment plants, and landfill leachate (Blanes-Vidal et al. 2009; Dincer et al. 2006; Jiao et al. 2023). It is reported that the main odorous substrates emitted during the pesticide production included hydrogen sulfide, methyl mercaptan, sulfur dioxide, methyl sulfide, and dimethyl sulfide (Jiao et al. 2023). However, there are few research literatures on soil odor pollution of pesticide-contaminated sites (Meng et al. 2022; Yan et al. 2022; Zhao et al. 2021).
Currently, there are two general approaches to odor measurement: olfactometric techniques for odor concentrations COD (OUE/m3) and analytical techniques for concentration measurements C (mg/m3) (Abraham et al. 2020; Sivret et al. 2016). Odor concentration of a gaseous sample is the number of dilutions with neutral gas, at which 50% of the panelists can still detect odor in the air sample. Olfactometry is not limited by the type and concentration range of odorants and can quickly reflect people’s intuitive perception of the odor. Although this method is simple and easy to operate, it has uncontrollable subjectivity by using human olfactory organs as identification carriers. Therefore, the stability of this method is doubtful (Zhao et al. 2015). Analytical techniques use the gas chromatography (GC) for detecting and analyzing the concentrations of substances in the gas, which is the most accurate and widely used chemical method. Analytical techniques are in principle more reliable than olfactometric techniques, but it cannot determine the perceivable odor intensity because there is great discrepancy among the odor detection thresholds (ODT) of different compounds (Davoli et al. 2003). To solve this problem, some studies proposed to identify the key odorants by calculating the odor activity value (OAV) of a single substance, the sum of OAVs for odorous mixtures (SOAV), and the odor contribution rate of each odorant (Sivret et al. 2016; Wu et al. 2017). OAV can better describe the properties of odor and has been widely used to evaluate the key odorants in gaseous mixtures cost-effectively (Wu et al. 2017). For example, Wu et al. measured the concentrations of individual compounds in the air of a waste disposal plant by analytical analysis and identified the key odor components by calculating the OAV values. The result found that a total of 28 odorants were detected and the H2S, trimethylamine, phenol, limonene, ethylbenzene, styrene, and NH3 were selected as key odorants for their OAV above 1 (Wu et al. 2015). The odor with complex compositions in the soil of pesticide-contaminated site was mainly derived from raw and materials, products, intermediate products, and degradation products in the production process. In order to economically and scientifically characterize odor of soil in the pesticide-contaminated site, the types and concentrations of odor mixtures in soil gas can be determined scientifically through combining analytical techniques and OAV.
Odor pollution in contaminated site can be determined by collecting soil gas and then analyzing the main components in the sample to determine the extent and scope of odor pollution. However, the depth of groundwater level in some area, such as the Pearl River Delta of South China, is generally shallow (about 1 ~ 2 m), which cannot meet the requirement of the standard for sampling of soil gas (ASTM D7758-17 2017). Meanwhile, the odorous substrates are often enriched in the clay of the saturated zone. Therefore, it is urgent to propose a new sampling method to detect odor pollution in the contaminated site such as with shallow groundwater. Alternatively, soil gas concentrations can be predicted using mathematical model based on the soil concentration (Johnson et al. 1993; Pavlovic et al. 2018). In general, the measured soil gas concentration accurately reflects the actual level of soil pollution. However, due to the unique advantages of simplicity and low cost, models are widely used in field studies. Models used for studying the concentration of volatile organic compounds in soil and soil gas include the linear adsorption model and the dual equilibrium desorption (DED) model (Chen et al. 1999, 2010; Johnson et al. 1993; Zhang et al. 2018). For example, Zhang et al. used the linear adsorption model and the DED model to estimate the concentrations of dichloromethane and benzene in soil gas at a hazardous material storage warehouse site in Handan, Hebei Province, North China (Zhang et al. 2018). Therefore, using soil gas–measured concentration or model-predicted concentration for odor pollution in shallow groundwater level site is worth studying.
In this study, organophosphorus pesticide (phosmet, dimethoate, butachlor, and trichlorfon) production site with shallow groundwater in the Pearl River Delta of South China was selected as a typical contamination site to analyze the distribution of odorous contaminants in soil. The objectives of this study were to (a) propose a set of experimental device to collect odor gas from soil; (b) screen the odorous substances in the soil and identify the key odorants responsible for the odor emitted from soil gas; (c) research the extent of odorous contamination, their spatial distribution, and the influencing factors; and (d) explore the applicability of two adsorption/desorption models in predicting the concentration of odor pollutants in soil gas. The results will provide valuable information to understand the pollution status of odorous contaminants in pesticide-contaminated site and provide reference for the investigation and restoration of the odor-contaminated soil.
Materials and methods
Site characterization
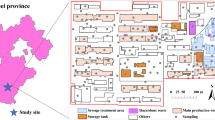
The typical pesticide factory site is located in Guangzhou, Guangdong Province, South China, where the depth of groundwater level is shallow (about 0.4 ~ 2.3 m). This pesticide factory was built in 1958 and produced various pesticides such as phosmet, dimethoate, butachlor, and trichlorfon. The plant was shut down in 1996, and the site was repurposed as a sports field and ground parking lot. The area of production workshops for dimethoate, butachlor, and trichlorfon were remediated, and buildings have been built. Therefore, the area of this study is concentrated in the phosmet workshop area and other ancillary facilities, covering 23,264 m2.
Previous investigation showed that the stratigraphy of the site can be generalized as backfill soil (1 ~ 3 m thickness), sludge soil (0 ~ 3.6 m thickness), silty clay or clay (1.6 ~ 6.6 m thickness), sandy (2.6 ~ 4.9 m thickness), and mudstone (2.00 ~ 3.80 m thickness). The overall distribution is shown in Fig. 1. The depth of groundwater level in this site is shallow (0.4 ~ 2.3 m), and the pollution distribution is in relatively deep soil. In some areas, the deep soil had a strong pungent odor, indicating that soil odor pollutants mainly existed in the saturated soil layer.
Sample collection and analysis
Odor screening sample collection
The possible pollutants of pesticide-contaminated site are extremely complex, involving not only various raw materials, solvents, intermediates, and products but also a large number of by-products, degradation products, and so on. Therefore, in order to screen the main odor pollutants in the soil, soil and soil gas pre-sampling were carried out for qualitative analysis. To the collect the heavily contaminated soil samples, the drilled soil of each sampling point was first quickly tested by a photoionization detector (PID, with measurement range 0–2000 ppm) during the soil sampling process, and the soil samples with higher PID (PGM-7340, RAE Systems, USA) readings and obvious stench were collected. Then, the collected samples were subjected to GC/MS or GC (QP2020NX, Shimadzu, Japan) scan analysis to determine the detectable pollutants in the soil and soil gas. A total of 10 soil samples and 5 soil gas samples were collected in this stage (Fig. 2). In view of the low sensitivity of amines in GC, parallel soil gas samples were taken for ion chromatography (IC, Aquion, Dionex, USA) analysis to determination of ammonia, methylamine, dimethylamine, and trimethylamine in soil gas.
Odor investigation sample collection
Soil sampling
Given the complexity of soil pollution, soil sampling is divided into two stages: preliminary sampling and supplementary sampling. The preliminary sampling stage identifies the general spatial distribution of odor pollution, while the supplementary sampling further determines the extent and boundary of odor pollution. In the preliminary sampling stage, the professional judgment method was used to arrange soil sampling points, mainly in potentially contaminated areas, including the phosmet workshop, the chloroacetic acid workshop, the fine workshop, the calcium plastic machine repair workshop, the sewage collection tank, and the drainage ditches. Among them, the phosmet production workshop, chloroacetic acid workshop, and fine workshop are used to the produce of pesticide phosmet, chloroacetic acid, and the phosphite, respectively. The calcium plastic workshop was built for storing raw materials before 1983, and after 1983, it was employed for the production of packaging boxes for pesticides. Those workshop locations at the production site are shown in Fig. 2 (blue font). Consideration is also given to pre-sampling soil pollution–heavy areas. Therefore, in this stage, soil samples of 26 soil points (S1–S26 in Fig. 2) were collected with a SH-30 model rig at different depths of the boreholes based on the distribution of soil layers. The final sampling depth was 9–15 m, and a total of 250 samples were collected.
On the basis of odor substance pollution analysis of preliminary investigation results, supplementary sampling was conducted around the points which exceeded the soil screening value recommended by the Ministry of Ecology and Environment of the People’s Republic of China (MEEPRC). Then, 16 additional sampling boreholes (X1–X16 in Fig. 2) were added in this study, and a total of 162 samples were collected. All soil samples were collected and preserved according to GB/T 36197–2018 (PRC 2018).
Soil gas sampling
As a typical area with shallow groundwater level, the odor pollutants in the study area mainly were distributed in the saturated soil layer, which cannot meet the requirements for standard soil gas sampling (ASTM D7663-12 2018). As the odor pollution mainly spreads into the air through volatilization, a soil volatilization gas sampling device was invented in this study to collect soil gas from the saturated soil, and then, the concentration of odor substances in the soil gas was detected to characterize the odor pollution in the soil. The soil gas collection method used in this study was the static chamber method, which involves directly drilling to collect a certain amount of odor-contaminated saturated soil into a static chamber. The odor pollutants in the soil will then volatilize into the gas in the chamber. Three outlets are provided on the chamber, each of which is connected to a pump. The pumps are used to control the flow rate of the three outlets. The outlets are connected to absorbent solutions, adsorption tubes, and gas cylinder, for the measurement of ammonia odor, VOC odor, and sulfur odor substances, respectively. The schematic diagram of the sampling device is shown in Fig. 3. Considering the vertical distribution of soil gas, samples of different depths can be collected at each borehole according to the actual situation. For soil with large readings of PID or pungent odor, 1 kg was collected in the static chamber for soil gas sampling, and two parallel samples were collected for each sample. A total of 14 soil gas samples were collected in this study based on the odor distribution on site.
Analysis methods
The concentration of the odorous substances in soil samples was measured by Environmental Protection Agency (EPA) methods 8260C (EPA 2006) or HJ605 (PRC 2011). The volatile organic components in soil gas samples were analyzed using GC according to the HJ 583–2010 (PRC 2010). Methyl sulfide and dimethyl disulfide in soil gas samples were determined by GC according to GB/T 14678–1993 (PRC 1993). Formaldehyde in soil gas samples was tested by spectrophotometric method according to GB/T 15516–1995 (PRC 1995). Ammonia in soil gas samples was measured using ion chromatography according to HJ 1076–2019 (PRC 2019). All testing followed the requirements of the China Inspection Body and Laboratory Mandatory Approval (CMA) (Li et al. 2023). The quality control and assurance of testing were guaranteed with certified reference material, duplicates, and method blanks.
Key odor identifying methods
It is necessary to identify the key odorous substances with a significant contribution to the odor from the gas mixture for the treatment and restoration of pesticide-contaminated sites. Studies showed that the substances that produce strong odors are not necessarily those with the highest concentration, but with the highest odor activity value (OAV) (Blazy et al. 2015). The greater the OAV, the greater the contribution of the substance to the odor. The OAV can be calculated using Eq. (1), and the odor contribution (Pi) of a single substance gives the relative importance of the olfactory perception (Chai et al. 2022). Odorous substances with OAV equal to or above one are identified as substances with a significant contribution to the odor, referred to as the key odorous substances (Wu et al. 2015).
where OAVi is the odor activity value of a single odorous substance, Ci is the concentration of the odorous substance i (mg/m3), OTi is the olfactory threshold value of the odorous substance i (mg/m3), SOAV is the total odor activity value of all odorous substances, and Pi is the odor contribution rate of the odorous substance i (%).
Adsorption isotherms and model development
Linear adsorption model
The linear adsorption model assumes that VOCs in soil are linearly and reversibly adsorbed. Organic matter is in a three-phase dynamic equilibrium in soil, and its model equation is obtained by normalizing the linear adsorption of organic matter onto soil particles (Eq. (4)) and Henry’s law (Eq. (5)) (Karickhoff et al. 1979; Park 1999). If an NAPL phase is not present at the site, the mass of pollutants in the soil matrix gas, solid, and liquid phases satisfies the relationship in Eq. (6). The soil gas calculation formula can be obtained by combining the above equation, as shown in Eq. (7).
where qe and Ce represent the solid phase concentration and liquid phase concentration of soil particles, respectively, when organic matter is adsorbed in equilibrium; Cs is the concentration of odorous substances in soil (mg/kg); Csg is the concentration of odorous substances in soil gas (mg/L); ρb is the dry bulk density of soil (g/cm3); Koc is the organic carbon–water partition coefficient (L/kg); foc is the organic carbon content in soil, dimensionless, and can be calculated by soil organic matter content (fom), foc = fom/1.72 (Sabljić 1989); θws represents the volumetric air content, dimensionless; and θas represents the volumetric water content, dimensionless.
DED model
A large number of studies showed that the adsorption of pollutants in soil is not always linear (Weber et al. 2001; Weber and Huang 1996). The DED model assumes that adsorption consists of two parts. The first part is reversible adsorption, which usually occurs at high concentrations of pollutants, and in this part, the amount of adsorption is linearly related to the concentration of pollutants in the water phase. The second part is irreversible adsorption, which usually occurs at low concentrations of pollutants, and in this part, the amount of adsorption is represented as a Langmuir type in relation to the concentration of pollutants in the water phase(Kan et al. 1998). Assuming that adsorption is the linear sum of the first and second parts, the soil adsorption in the DED model can be calculated as follows:
where C is the concentration of pollutants in the water phase (mg/L); \({K}_{\mathrm{oc}}^{\mathrm{first}}\) and \({K}_{\mathrm{oc}}^{\mathrm{second}}\) are the organic carbon–water partition coefficients for the first and second parts (L/kg), where the value of \({K}_{\mathrm{oc}}^{\mathrm{first}}\) equals Koc and \({K}_{\mathrm{oc}}^{\mathrm{second}}\) can be calculated using Eq. (11) (Chen et al. 2010); Kow is the partition coefficient of n-octanol/water (L/L); Csat is the solubility of the pollutant (mg/L); f represents the fraction of the second compartment that is saturated upon exposure, 0 ≤ f ≤ 1, and literature suggests taking f = 1 (Chen et al. 1999, 2010); and \({q}_{\mathrm{max}}^{\mathrm{second}}\) is the maximum adsorption capacity of the second part (mg/kg), calculated by Eq. (12). Combined with Eqs. (8) ~ (10), the total adsorption of VOCs by soil solid particles under the DED model can be obtained, and the relationship formula between Cs and Csg is finally obtained. For convenience of representation, a coefficient A and functions F(Cs) and G(Cs) of Cs are first established, as shown in Eqs. (13) ~ (15). And the expression for calculating soil gas Csg from soil concentration Cs under the DED model is derived as Eq. (16).
Result and discussion
Key odorous substances in soil gas
During the odor pollutant screening sample collection, 10 soil samples and 5 soil gas samples were collected for qualitative analysis. A total of 119 kinds of VOCs and 139 kinds of semi-volatile organic compounds (SVOCs) were detected in the soil samples by GC/MS scanning. These organic substances were compared with the list of odorous substances proposed by the Japan Odor Environment Association in 1988 (Nagata 1988), and the detected odorous substances in the soil included benzene, toluene, o-xylene, m,p-xylene, ethylbenzene, carbon disulfide, dimethyl disulfide, and methyl sulfide. Meanwhile, the results of the GC/MS analysis of the soil gas samples showed that the odorous substances included benzene, toluene, o-xylene, m,p-xylene, methyl sulfide, dimethyl disulfide, formaldehyde, carbon disulfide, and ammonia. In addition, IC analysis showed that the soil gas contained ammonia. Therefore, the soil gas of the pesticide-contaminated site in this study contained 10 odorous substances including benzene, toluene, o-xylene, m,p-xylene, methyl sulfide, dimethyl disulfide, formaldehyde, carbon disulfide, and ammonia.
To further investigate the sources of odor in the soil and the contribution of odorous substances, 14 soil gas samples were collected for quantitative analysis. The OAVi of each substance were calculated using the average concentration of odorous substances in the soil gas samples and their odor threshold. The results are shown in Table 1. The average concentrations of benzene, ethylbenzene, ammonia, toluene, m,p-xylene, methyl sulfide, dimethyl disulfide, formaldehyde, o-xylene, and carbon disulfide in the soil gas were 306.16, 2.52, 3.44, 3.60, 3.80, 0.03, 0.02, 1.19, 1.09, and 0.25 mg/m3, respectively. The corresponding OAVi values were 32.54, 28.01, 14.93, 9.01, 8.83, 3.71, 2.06, 1.77, 0.83, and 0.43. Therefore, the key odorous substances with OAVi greater than one were benzene, ethylbenzene, ammonia, toluene, m,p-xylene, methyl sulfide, dimethyl disulfide, and formaldehyde. The cumulative contribution of benzene, ethylbenzene, ammonia, toluene, and m,p-xylene accounted for more than 90% of the total odor contribution. Most odorous contaminants came from raw materials or intermediate products in the pesticide production process, such as benzene, ethylbenzene, ammonia, toluene, m,p-xylene, and formaldehyde. Other contaminants came from by-products or degradation products, such as methyl sulfide and dimethyl disulfide. The types and sources of key odorous substances in this study are highly consistent with the Yan et al.’s research which explored the odorous substances in the soil. The results showed that the raw and auxiliary materials used in the production process are the main source of odorous substances in the soil, and the key odor substances were dimethyl sulfide, dimethyl disulfide, and toluene, trichloroethylene of organochlorine and organophosphorus pesticide-contaminated site (Yan et al. 2022). For the remediation the odorous substances detected in the soil, sulfur-containing and nitrogen-containing compounds usually have reducibility and are easily oxidized, which can be treated through chemical oxidation technologies. The BTEX (benzene, toluene, ethylbenzene, and m,p-xylene) is usually relatively stable and requires the use of Fenton oxidation technology. The ammonia, formaldehyde, and carbon disulfide with highly volatile, room temperature desorption technology can be used for treatment.
The concentration contribution PCi of the odorous chemical in each soil gas sample and the odor contribution Pi of each chemical are shown in Fig. 4. It is demonstrated that the trend of odor activity value contribution is very different from that of concentration contribution. Most of the odorous substances in soil gas are benzene, and the concentration of benzene is very high in all 12 samples, accounting for more than 70% of the pollutants in soil gas. However, due to the high odor threshold of benzene of 9.41 mg/m3, its contribution to odor was only 5 to 60%, with an average contribution rate of 32.54%. For sulfur compounds (methyl sulfide and dimethyl disulfide) which contain typical odor-causing groups (sulfur-containing groups) (Jiao et al. 2023), the maximum concentration contribution of methyl sulfide (Cmax = 0.049 mg/m3) was 2.17% but with an maximum contribution rate of 37.26%, and the maximum concentration contribution of dimethyl disulfide (Cmax = 0.033 mg/m3) was 2.36% with an maximum contribution rate of 25.99%. Although their concentrations in soil gas were relatively low, their contribution to odor cannot be ignored. Thus, it is accurate and convenient to employ OAV to evaluate the contribution of each odorous substances.
Spatial distribution of odorous substances in soil
To reveal the horizontal distribution characteristics of the key odorous substances in the soil, Surfer 14 with kriging interpolation method was used to draw the spatial distribution of the key odor species (benzene, toluene, xylene, m,p-dimethylbenzene, methyl sulfide, dimethyl disulfide, formaldehyde) (Fig. 5) (Martínez-González et al. 2022). In view of that ammonia in the soil generally exists in the form of NH3 and NH4+, under certain conditions, NH4+ can be converted into NH3 volatilization. It is difficult to detect the concentration of ammonia in soil accurately, so the spatial distribution of ammonia in soil is not included here. The statistical results of the key odorous substance content data are shown in Table 2. The maximum content values of benzene, toluene, xylene, m,p-dimethylbenzene, methyl sulfide, dimethyl disulfide, and formaldehyde in the soil were 3018.73, 163.65, 248.71, 121.81, 51.30, 88.70, and 46.18 mg/kg, respectively. The standard deviations were 460.24, 25.01, 37.86, 19.23, 9.19, 13.86, and 8.09 mg/kg, respectively. It can be seen that the content of odorous substances in different sampling points varied greatly, which showed that the distribution of pollutants in this site is extremely uneven.
As shown in Fig. 5, the key odorous substances were mainly present near the phosmet production workshop and the drainage ditch. Comparatively, the odorous substance content was low or even not detected in the area surrounding the calcium plastic machine repair workshop, the phosphate warehouse, and the phosphate ore yard. Overall, the raw and auxiliary materials and their degradation products in the production process are the main sources of odorous substances in the soil, and the horizontal distribution of the pollution has a significant correlation with the production process and layout of the pesticide-contaminated site. For example, formaldehyde is the main raw material in the production process of phosmet. The BTEX (benzene, toluene, ethylbenzene, and xylene) are the main solvent in the production process of pesticide such as dimethoate, butachlor, and trichlorfon. Moreover, the dimethyl sulfide and dimethyl disulfide in soil are mainly distributed in the phosmet production workshop and its downstream drainage ditch. The workshop has been used to produce products such as phosphorus pentasulfide that are the main raw materials in the production process. The phosphorus pentasulfide could enter the soil environment through leakage and then gradually convert into dimethyl sulfide and dimethyl disulfide through chemical and biological degradation (Zhang et al. 2013).
In addition, the soil odor pollution in the south is more severe than that in the north, which is mainly due to the impact of adjacent land plots. The surroundings of the site include the following: to the west of the site are small streams and residential areas; to the east of the site are the temporary warehouse, garbage dump, etc.; to the north side of the site are the ore raw material warehouse, phosphorus warehouse, phosphorus trichloride workshop, bucket washing yard, etc.; and to the south side of the site are the previously used workshops for methylamine, dimethoate, sulfide, and butachlor which have now been converted into residential buildings. Therefore, the southern side of the site is severely affected and polluted by the adjacent former methylamine workshop, dimethoate workshop, sulfide workshop, parka workshop, and butachlor workshop on the south side of the site.
Figure 6 illustrates the vertical distributions of odorous substances in the soil profiles of boreholes S10, S18, S26, X6, X7, and X10, which were located in the area surrounding the phosmet production workshop and the drainage ditch. During site sampling, the odor of the soil in these holes was very obvious. In particular, S10 with a strong odor showed very high benzene concentration levels, among which the highest concentration (304.73 mg/kg) was at the depth of 1.8–2.4 m. As shown in Fig. 1S, the relationship between benzene concentration and depth at borehole S10 shows two concentration peaks at depths of 1.8–2.4 m and 7.0–8.0 m, with concentrations of 304.73 mg/kg and 294.99 mg/kg, respectively. Consistent with the pollution characteristics of other boreholes, the odorous substances were generally concentrated in the sludge layer (1–3.6 m) and the silty clay or clay layer (5.6–11 m) of the site. The clay layer is the main aquiclude with a relatively compact soil structure and measured permeability coefficient between 2.88 × 10−7 and 9.79 × 10−6 cm/s (Table S2). This layer can block the vertical migration of odorous substances through soil capillary force and adsorption, resulting in the accumulation of pollutants. The sludge layer has a high organic matter content and a lower permeability coefficient, ranging from 2.18 × 10−8 to 8.13 × 10−6 cm/s (Table S2), which also inhibited the migration and diffusion of pollutants to some extent. In contrast, due to the loose structure of the sandy layer, odorous substances can diffuse and migrate in it. The vertical distribution generally showed a trend of increasing first and then decreasing. This result is consistent with the existing view (Liu et al. 2015; Yan et al. 2022). For example, Liu et al. explored the DDT vertical migration in pesticide-producing sites, and the result showed that DDT migrated more slowly due to poor permeability in silty clay or clay (Liu et al. 2015).
Over the decades, the odorous substances have penetrated from the surface layer to deep layer of soil, and the maximum contamination depth of soil reached 13 m. The depth of groundwater level in this site is shallow (0.40–2.31 m). When there is no anthropogenic disturbance, the odorous substances in contaminated saturated soil could threaten human health through vapor intrusion (McHugh et al. 2017). Meanwhile, due to the low content of vapor intrusion, the concentration of odor may not be enough to reach the human olfactory threshold. When the contaminated soil is excavated, a large amount of odorous substances will evaporate into the air and can produce strong pungent odors. Therefore, it is necessary to detect concentration of the key odorants in saturated soil layers, to provide reference for the effective control of odorous substances in the remediation process of pesticide-contaminated site. Especially for highly toxic BTEX (benzene, toluene, ethylbenzene, and xylene) or sulfides compounds, targeted protective measures could be taken to prevent soil gas poisoning accidents.
Relationship of odorous substances in soil and in soil gas
According to the soil gas detection results, most of the odorous substances in the site were benzene (more than 70% in soil gas). Therefore, benzene was selected as the representative contaminant to analyze the accuracy of the linear model and the DED model in predicting the concentration of odorous substances in soil gas, and ethylbenzene was selected is a comparative study. The data pairs of the detected soil accounted for approximately 55.20% of the total and were mainly concentrated around the phosmet production workshop and the drainage ditch, and then, the data pairs with soil concentration below the detection limit were removed. It has been reported that different soil matrices have escape behaviors that differ substantially; the soil matrix type affects the fit (Zhang et al. 2019). Considering that the odorous substances were generally concentrated in the sludge layer and the silty clay or clay layer, so in this study, the soil samples corresponding to soil gas are basically clay or sludge soil (Table S1). Figure 7a shows the correlation between the concentration of benzene in soil gas (Csg) and the concentration of benzene in soil (Cs), and the correlation coefficient R2 can reach 0.93. Figure S2 shows the correlation of ethylbenzene in soil gas and soil, and the correlation coefficient Re2 can reach 0.92. Overall, whether it is benzene or ethylbenzene, as the concentration of benzene Cs increases, the measured Csg also increases.
Figure 7b shows the logarithmic relationship between Csg and Cs. The black dots represent the measured concentration of benzene in soil gas in this study, the blue dots represent the concentration predicted by the linear model, and the red dots represent the concentration predicted by the DED model. The dashed red line represents the soil gas odor threshold of benzene. Figure 7b clearly shows that only two of the 14 odorous soil gas samples did not exceed the benzene odor threshold (9.41 mg/m3), and the maximum concentration was 2570.0 mg/m3, 273.1 times higher than the odor threshold. The soil gas concentrations predicted by the linear model are higher than the measured values. In contrast, the results of the DED model are generally lower than the measured results, which agrees with existing research results (Smith et al. 1990). It is worth noting that the results of laboratory simulation researches indicated that the DED model can better predict the concentration of VOCs in soil gas than the linear model (Kan et al. 1997, 1998, 2002). Reasons for this could be that the soil characteristics (such as soil moisture content, organic matter content, and volumetric air content) may influence the application of the DED model to the field (Zhang et al. 2019). For example, Zhang et al. collected 128 sets of soil and soil gas samples from 5 contaminated sites and then found that linear model results are closer to the measured soil gas concentrations, and the DED model may underestimate risks (Zhang et al. 2019). Therefore, the applicability of linear model and DED model differs under laboratory and field conditions, and the linear model was more suitable to predict the concentration of soil gas in contaminated site.
Limitation and application
Previous researches have pointed out that soil moisture greatly affects the vapor exposure concentration of VOCs in the environment (Chiou and Shoup 1985). On the one hand, soil moisture greatly affects the partitioning of VOCs in the soil environment; on the other hand, soil moisture affects the VOC migration (Zhang et al. 2022). According to the study of Zhang et al., the experimental results indicate that the vapor exposure concentration decreases gradually to 0 when soil relative saturation is above 0.8 (Zhang et al. 2022). Therefore, in the absence of anthropogenic disturbance, the presence of groundwater in the saturated soil results in very low vapor exposure concentrations of odorous substances in the soil to the gaseous environment (McHugh et al. 2017). Once the saturated soil is exposed to the air due to excavation, the soil moisture is significantly reduced and the VOCs through the pathways of vapor phase diffusion and advection into the air. Then, the vapor concentrations of odorous substances increase sharply, producing unpleasant gases that pose a threat to human health. Therefore, it is very meaningful to use the saturated soil volatile gas sampling device proposed in this study to simulate the diffusion and advection of odor pollutants into the air concentration of saturated soil under excavation exposure environmental conditions. However, due to the small volume of the sampling device and the limited amount of soil samples collected, the detection concentration of soil gas samples collected by this device is not equivalent to the concentration of odor pollutants in the air when the soil excavation of the site is exposed to the air. Certainly, the concentration of odor pollutants in the air caused by soil excavation is also a subject worth further longitudinal study.
This study suggests that the measured concentration for odorous compounds volatilized from soil can be used to estimate the potential odor impact of the saturated layer soil in the pesticide-contaminated site. Moreover, models could be used to evaluate the odor concentrations in soil gas from those in soil. The two approaches of odor investigation, chemical analysis of saturated soil gas or prediction by the model, should be used combined in practical work. According to this paper and existing literature, the models have a good fit under strict conditions in laboratories (Chen et al. 2010; Kan et al. 1998; Zhang et al. 2019), but with a poor fit to the experimental data in field conditions. Overall, it is best to use chemical analysis of saturated soil gas to measure polluted concentration for odorous compounds.
Considering the pollutants that each model is suitable for simulating may be different, the performance results of the ethylbenzene with the linear and DED model are shown in Figure S2. By comparing the performance of two models for different odorants, the linear model results are generally closer to the measured soil gas concentrations than the DED model. Moreover, the results of the linear model are mostly greater than the measured results, but the results of the DED model are lower than the measured results. Due to the sensitivity and complexity of odor pollution, the DED model with lower prediction results is not recommended for predicting odor pollution concentration of soil gas in pesticide-contaminated sites which are often heavily polluted. In addition, before quantitatively evaluating the odor pollution, qualitative analysis of the composition of pollutants in soil gas should be conducted first to determine the possible pollution area and depth. Researches showed that contamination depth of soil has a significant correlation with the production duration, soil permeability, and properties of contaminants. The pesticide production sites that had been in production for > 30 years have a depth of contamination of > 10 m, while those produced for < 20 years have a depth of contamination of < 8 m (Li et al. 2023). Therefore, the saturated soil layer of the pesticide production site that has been production for a long time is highly likely to have odor pollution, where the soil gas sampling or prediction of odor pollution concentration in gas by the linear model should be conducted.
Conclusions
In this study, odor gas samples from a typical pesticide-contaminated site with shallow groundwater were collected by designed sampling device and measured by GC and IC. The results showed that the odorous substances included benzene, toluene, xylene, methylbenzene, ethylbenzene, methylene sulfide, dimethyl disulfide, formaldehyde, carbon disulfide, and ammonia. Evaluation of the OAV revealed that benzene (32.54%), toluene (28.01%), o-xylene (14.93%), m,p-xylene (9.01%), ethylbenzene (8.83%), carbon disulfide(3.71%), dimethyl disulfide(2.06%), and methyl sulfide (1.77%) contributed the most of the odor at the pesticide-contaminated site. Finally, soil gas concentrations predicted by the linear model and the DED model were compared with measured values, and the result showed that the prediction result of linear model was more accurate. Moreover, the DED model with low prediction result is not recommended to predict the concentration of soil gas for sensitive pesticide-contaminated sites.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author.
References
Abraham MH, Acree WE, Cometto-Muñiz JE (2020) Descriptors for terpene esters from chromatographic and partition measurements: estimation of human odor detection thresholds. J Chromatogr A 1609:428–460
ASTM D7758-17 (2017) Standard practice for passive soil gas sampling in the vadose zone for source identification, spatial variability assessment, monitoring, and vapor intrusion evaluations. http://www.astm.org/cgi-bin/resolver.cgi?D7758-17
ASTM D7663-12 (2018) Standard practice for active soil gas sampling in the vadose zone for vapor intrusion evaluations. http://www.astm.org/cgi-bin/resolver.cgi?D7663-12(2018)e1; https://doi.org/10.1520/D7663-12R18E01
Blanes-Vidal V, Hansen MN, Adamsen A, Feilberg A, Petersen SO, Jensen BB (2009) Characterization of odor released during handling of swine slurry: part I. Relationship between odorants and perceived odor concentrations. Atmospheric Environment 43(18):2997–3005
Blazy V, de Guardia A, Benoist JC, Daumoin M, Guiziou F, Lemasle M, Wolbert D, Barrington S (2015) Correlation of chemical composition and odor concentration for emissions from pig slaughterhouse sludge composting and storage. Chem Eng J 276:398–409
Chai F, Li P, Li L, Qiu Z, Han Y, Yang K (2022) Dispersion, olfactory effect, and health risks of VOCs and odors in a rural domestic waste transfer station. Environ Res 209:112879
Chen W, Kan AT, Tomson MB, Vignona LC (1999) Adsorption-desorption behaviors of hydrophobic organic compounds in sediments of Lake Charles, Louisiana, USA. Environ Toxicol Chem 8(18):1610–1616
Chen W, Kan AT, Newell CJ, Moore EM, Tomson MB (2010) More realistic soil cleanup standards with dual-equilibrium desorption. Ground Water 40(2):153–164
Chiou CT, Shoup TD (1985) Soil sorption of organic vapors and effects of humidity on sorptive mechanism and capacity. Environ Sci Technol 19(12):1196–1200
Davoli E, Gangai ML, Morselli L, Tonelli D (2003) Characterisation of odorants emissions from landfills by SPME and GC/MS. Chemosphere 51(5):357–368
Dincer F, Odabasi M, Muezzinoglu A (2006) Chemical characterization of odorous gases at a landfill site by gas chromatography–mass spectrometry. J Chromatogr A 1122(1):222–229
Jiao R, Zhang J, Yang X, Zhu X, Gao W, Wang D (2023) Key odorant identification and odor treatment technology evaluation during the chemical synthesis process of typical pesticide. Process Saf Environ Prot 173:961–969
Johnson PC, Hertz MB, Byers DL (1993) Estimates for hydrocarbon vapor emissions resulting from service station remediations and buried gasoline-contaminated soils. Principles and Practices for Petroleum Contaminated Soils 14:465–496
Kan AT, Fu G, Hunter MA, Tomson MB (1997) Irreversible adsorption of naphthalene and tetrachlorobiphenyl to lula and surrogate sediments. Environ Sci Technol 31(8):2176–2185
Kan AT, Fu G, Hunter M, Chen W, Ward CH, Tomson MB (1998) Irreversible sorption of neutral hydrocarbons to sediments : experimental observations and model predictions. Environ Sci Technol 32(7):892–902
Kan AT, Fu G, Tomson MB (2002) adsorption/desorption hysteresis in organic pollutant and soivsedirnent interaction. Environ Sci Technol 28(5):859–867
Karickhoff SW, Brown DS, Scott TA (1979) Sorption of hydrophobic pollutants on natural sediments. Water Res 13(3):241–248
Li M, Chen Q, Yang L, Zhang Y, Jiang J, Deng S, Wan J, Fan T, Long T, Zhang S, Lin Y (2023) Contaminant characterization at pesticide production sites in the Yangtze River delta: residue, distribution, and environmental risk. Sci Total Environ 860:160156
Liu L, Bai L, Man C, Liang W, Li F, Meng X (2015) DDT vertical migration and formation of accumulation layer in pesticide-producing sites. Environ Sci Technol 49(15):9084–9091
Martínez-González A, Monzó-Cabrera J, Martínez-Sáez AJ, Lozano-Guerrero AJ (2022) Minimization of measuring points for the electric field exposure map generation in indoor environments by means of Kriging interpolation and selective sampling. Environ Res 212:113577
McHugh T, Loll P, Eklund B (2017) Recent advances in vapor intrusion site investigations. J Environ Manag 204(15):783–792
Mcneel PJ, Dibley V (1997). Case study comparisons of vapor inhalation risk estimates: ASTM RBCA model predictions vs site specific soil vapor data.
Meng J, Wang J, Li W, Zou K, Han M, Xing J, Zhai Z, Jing B, Xiao X (2022) Odorants in the organophosphorus pesticide contaminated soils: identification, prioritization, and quantification using GC-HRMS based on target and non-target screening. Int J Mass Spectrom 482:116930
Morra P, Leonardelli L and Spadoni G (2011). The volatilization of pollutants from soil and groundwater: its importance in assessing risk for human health for a real contaminated site.
Nagata Y (1988). Measurement of odor threshold value of odor substances by triangle odor bag method. 118–127, Meeting of the Japan Society of Air Pollution.
Park HS (1999) A method for assessing soil vapor intrusion from petroleum release sites: multi-phase/multi-fraction partitioning. Global Nest Int J 1(3):195–204
Pavlovic DM, Glavac A, Gluhak M, Runje M (2018) Sorption of albendazole in sediments and soils: isotherms and kinetics. Chemosphere Environ Toxicol Risk Ass 193:635–644
Ministry of Ecology and Environment, PRC (1993) Air quality-determination of sulfuretted hydrogen,methyl sulfhydryl,dimethyl sulfide and dimethyl disulfide -Gas chromatographyd (GB/T 14678–93). China Environmental Publishing Group
Ministry of Ecology and Environment, PRC (1995) Air quality-determination of formaldehyde-acetylacetone spectrophotometric method (GB/T 15516–1995). China Environmental Publishing Group
Ministry of Ecology and Environment, PRC (2010) Ambient air-Determination of benzene and its analogies using sorbent adsorption thermal desorption and gas chromatography (HJ 583–2010). China Environmental Publishing Group
Ministry of Ecology and Environment, PRC (2011) Soil and sediment-determination of volatile organic compounds purge and trap gas chromatography/mass spectrometry method (HJ 605–2011). China Environmental Publishing Group
Ministry of Ecology and Environment, PRC (2018) Soil quality-guidance on sampling techniques (GB/T 36197–2018). China Environmental Publishing Group
Ministry of Ecology and Environment, PRC (2019) Ambient air—determination of ammonia, methylamine, dimethylamine and trimethylamine—ion chromatography (HJ 1076–2019). China Environmental Publishing Group
Sabljić A (1989) Quantitative modeling of soil sorption for xenobiotic chemicals. Environ Health Perspect 83:179–190
Sivret EC, Wang B, Parcsi G, Stuetz RM (2016) Prioritisation of odorants emitted from sewers using odour activity values. Water Res 88:308–321
Smith JA, Chiou CT, Kammer JA, Kile DE (1990) Effect of soil moisture on the sorption of trichloroethene vapor to vadose-zone soil at Picatinny Arsenal. New Jersey Environmental Sci Technol 24(5):676–683
Tao Y, Liu J, Xu Y, Liu H, Yang G, He Y, Xu J, Lu Z (2022) Suspecting screening “known unknown” pesticides and transformation products in soil at pesticide manufacturing sites. Sci Total Environ 808:152074
USEPA (2006) Method 8260C: Test methods for evaluating solid waste, physical/chemical. Methods: method 8260B: volatile organic compounds by gas chromatography/mass spectrometry (GC/MS). https://www.epa.gov/sites/default/files/2018-06/documents/
Vijgen J, Weber R, Lichtensteiger W, Schlumpf M (2018) The legacy of pesticides and POPs stockpiles—a threat to health and the environment. Environ Sci Pollut Res 25(32):31793–31798
Wang M, Jiang D, Ding D, Deng S, Kong L, Wei J, Xia F, Li M, Long T (2023) Spatiotemporal characteristics and dynamic risk assessment of a multi-solvents abandoned pesticide-contaminated site with a long history, in China. J Environ Manage 336:117633
Weber WJ, Huang W (1996) A distributed reactivity model for sorption by soils and sediments. 4. Intraparticle heterogeneity and phase distribution relationships under nonequilibrium conditions. Environ Sci Technol 30(3):881–888
Weber WJ, Boeuf L, Young TM, Huang W (2001) Contaminant interactions with geosorbent organic matter: insights drawn from polymer sciences - ScienceDirect. Water Res 35(4):853–868
Wu C, Liu J, Yan L, Chen H, Shao H, Meng T (2015) Assessment of odor activity value coefficient and odor contribution based on binary interaction effects in waste disposal plant. Atmos Environ 103:231–237
Wu C, Liu J, Zhao P, Li W, Yan L, Piringer M, Schauberger G (2017) Evaluation of the chemical composition and correlation between the calculated and measured odour concentration of odorous gases from a landfill in Beijing, China. Atmos Environ 164:337–347
Yan M, Hongguang Z, Yi S, Fan Z, Yujun Y, Weiguang Z, Xiaoming D (2022) Screening and distribution characteristics of odorous substances in soil of typical pesticide-contaminated site. Res Environ Sci 35(6):8
Yang W, Weifang LI, Liu B (2015) Odour prediction model using odour activity value from pharmaceutical industry. Austrian Contribut Vet Epidemiol 8:51–60
Zhang H, Schuchardt F, Li G, Yang J, Yang Q (2013) Emission of volatile sulfur compounds during composting of municipal solid waste (MSW). Waste Manage 33(4):957–963
Zhang R, Zhong M, Jiang L, Zhang D, Zhnag R, Wu Y, Fu Q (2018) Health risk assessment of volatile organic compounds based on DED model. J Environ Sci Res 31(01):170–178
Zhang R, Jiang L, Zhong M, Han D, Zheng R, Fu Q, Zhou Y, Ma J (2019) Applicability of soil concentration for VOC-contaminated site assessments explored using field data from the Beijing-Tianjin-Hebei urban agglomeration. Environ Sci Technol 53(2):789–797
Zhang R, Zhong M, Jiang L, Fu Q, Wang S, Zhang W, Li X, Ma L (2022) Effect of vapour-solid interfacial adsorption on benzene multiphase partition and its implication to vapour exposure assessment of contaminated soil in arid area. J Environ Manage 315:115182
Zhao Y, Lu W, Wang H (2015) Volatile trace compounds released from municipal solid waste at the transfer stage: evaluation of environmental impacts and odour pollution. J Hazard Mater 300:695–701
Zhao X, Ma H, Lu J, Yin T, Gong X (2021) Characteristics and source apportionment of volatile organic compounds during the remediation of contaminated sites in Zhenjiang, China. Int J Environ Sci Technol 18(12):2271–2282
Funding
This work was financially supported by the National Environmental Protection Public Welfare Industry Targeted Research Fund (PM-zx703-202204–069).
Author information
Authors and Affiliations
Contributions
T. Y. designed and performed the experiment and wrote the manuscript. J. T., C. X., L. L., and C. H. helped in the pot experiments and analyzed the samples. Z. W. and J. C. provided advice on the design of the research. G. L. provided advice on the design of the research, analyzed the data, and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Kitae Baek
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, T., Wang, Z., Liu, G. et al. Contaminant characterization of odor in soil of typical pesticide-contaminated site with shallow groundwater. Environ Sci Pollut Res 30, 121182–121195 (2023). https://doi.org/10.1007/s11356-023-30639-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30639-w