Abstract
Cadmium-contaminated soils are an urgent problem that needs to be solved in many countries and regions. In this study, a new heavy metal passivator, micro-nano nitrogen-doped biochar (Nm-NBC), was prepared by introducing nitrogen into biochar. Soybean was used as an experimental plant to compare the effects of corn straw biochar (CBC, not modified), ammonium chloride modified corn straw biochar (NBC), and micro-nano nitrogen-doped biochar (Nm-NBC) on the remediation of Cdcontaminated soil. The results showed that the biomass of soybean, pH, organic matter, and total nitrogen content of the Cd-contaminated soil significantly increased, and the available Cd in soil significantly reduced (P < 0.05) when CBC, NBC, and Nm-NBC were added. The effect was as follows: Nm-NBC > NBC > CBC; Nm-NBC had the best result. When 1% Nm-NBC added to the soil, the Cd content in beans reduced by 68.09%. BET, FTIR, XPS, and SEM were used to analyze the characteristics of Nm-NBC and its mechanisms in the remediation of Cd-contaminated soils. The results showed that Nm-NBC had larger specific surface area and abundant functional groups; -COOH and graphitic nitrogen in Nm-NBC can form Cd–O bond and Cd-π with Cd(II) in the soil. Therefore, Nm-NBC prepared by introducing nitrogen into biochar has a promising application in the remediation of Cd-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most toxic heavy metals, and Cd-contaminated soils are a major problem in India, Thailand, China, and other countries (Hamid et al. 2022). Cd in the soil has serious negative effects on microbial abundance, biogeochemical cycles, and enzymatic activities (Huang et al. 2021; Suhani et al. 2021), and Cd can enter the human body through the food chain, which will cause inflammation, autoimmune diseases, and organ failure (Popov et al. 2021). Therefore, the remediation of Cd-contaminated soils is an urgent problem to be solved.
For heavy metal contaminated soils, in situ remediation is a suitable technique through the use of amendments or fixatives in polluted soil, and the mobility and bioavailability of pollutant elements can be inhibited (Chen et al. 2021a, b; Bolan et al. 2014). As one of the passivators, biochar is widely used in the remediation of heavy metal-contaminated soil due to its large specific surface area and abundant functional groups. However, the effect of the original biochar on heavy metal-contaminated soil is not good, and it is necessary to improve the properties of origin biochar by using physical or chemical methods. Nitrogen (N) is one of the essential elements for crops. Nitrogen-rich biochar can also be used as a slow-release fertilizer to increase organic matter and total nitrogen content in the soil and improve crop yields (Chen et al. 2017; Li et al. 2022). In addition, previous studies have shown that nitrogen doping can promote the polarization of π electrons on the surface of biochar, generate more π electron-rich and π electron-deficient adsorption sites, and enhance the adsorption capacity of biochar for Cu(II) and Cd(II) (Yu et al. 2018a, b).
Corn straw is a common agricultural waste. According to statistics, the total annual output of corn straw in the world is 203 million tons, but the utilization of corn straw is only 2/3 (Ramanayaka et al. 2020). At present, he preparation of biochar from corn straw with pyrolysis technology can not only solve the problem of biomass resources waste, but also improve the utilization of biochar on the remediation of heavy metal contaminated soil and soil fertility (Zong et al. 2021). In this study, corn straw and soybean were used as research objects, and the characteristics of nitrogen-doped corn straw biochar and the mechanisms of Cd-contaminated soil remediation by micro-nano nitrogen-doped biochar were discussed.
Materials and methods
Preparation of biochar
Corn straw was purchased in Lianyungang (Jiangsu Province, China) and washed with deionized water, then dried in an oven (60 °C) for 24 h. Thirty grams of corn straw was added with 1.5 g·L−1 of ammonium chloride solution and soaked for 4 h and then placed in an oven (60 °C) for drying for 24 h. Corn straw and ammonium chloride–modified corn straw were placed into a muffle furnace for pyrolysis under the protection of N2 at 800 °C (2 h), and then corn straw biochar (CBC) and ammonium chloride–modified corn straw biochar (NBC) were obtained. Put a certain quality of NBC into a grinding bowl and ground for 5 min; the micro-nano nitrogen-doped corn straw biochar (NBC, < 0.074 mm) was screened out with a 200-mesh standard sieve.
Characterization of biochar
Brunauer–Emmett–Teller (BET) surface area of corn straw biochar, nitrogen-modified corn straw biochar, and micro-nano nitrogen-doped corn straw biochar were performed using Tristar II 3020 (McMerritik Instruments, USA). The surface functional groups of biochar were determined by Fourier transform infrared spectrometer Nicolet 6700 (FTIR, Thermo Fisher Scientific, USA) in the wavelength range of 4000–550 cm−1. The surface chemistry of the micro-nano nitrogen-doped corn straw biochar before and after adsorption of Cd(II) was analyzed by X-ray photoelectron spectrometer ESCALAB 250Xi (XPS, MacMeratic Instruments, USA). Supra55 (SEM, Carl Zeiss AG, Germany) was used to observe the surface morphology of micro-nano nitrogen-doped biochar-adsorbed Cd(II). The pH of the biochar was measured by ST2100 (pH Meter, Ohaus, USA) (biochar: water = 1:20, w/v).
Pot experiment
Soybean (Yujiaolong Tezao 50, China) was used as an experimental plant to confirm the effects on the remediation of Cd-contaminated soil. The experimental pots (16 cm in diameter, 12 cm in height) were filled with 1 kg of dry soil, and 1% corn straw biochar (CBC), nitrogen-doped corn straw biochar (NBC), and micro-nano nitrogen-doped corn straw biochar (Nm-NBC) were added, respectively. All these treatments were divided into 5 groups: YW (uncontaminated soil), YW-Cd (Cd-contaminated soil), CBC, NBC, and Nm-NBC. Before sowing soybean seeds, the 70% of maximum water holding capacity of soil was maintained by deionized water. The soybean seeds (Tezao 50) with uniform size were selected, soaked in 75% alcohol for 1–2 min, soaked in 1% sodium hypochlorite for 4 min, and rinsed with sterile deionized water. After the seedlings were sprouted, 5 soybean seedlings with consistent growth were kept, and other soybean seedlings were removed. During the whole experiment, each pot was watered with 200 mL of deionized water every day to keep the soil moisture maintained at about 70% of the maximum water holding capacity in the field. All the treatment groups were sheltered by a canopy outside, maintained normal light, and changed the position of flowerpots every 3 days to ensure that the flowerpots of different treatment groups could obtain the same light level.
Physical and chemical properties of soil
The soil organic matter (SOM) was determined by potassium dichromate oxidation-oil bath heating. Total nitrogen in soil was determined by the Kjeldahl method, and the soil pH was measured using a pH meter.
Characterization of Cd species in the soil
The total Cd in the soil was determined with flame atomic absorption spectrometry after microwave digestion. The available Cd in the soil was extracted by DTPA (diethylenetriaminepentaacetic acid) and determined by flame atomic absorption spectrometry. The BCR (Bureau Community of Reference) sequential extraction was used in the chemical speciation analysis of Cd in the soil and divided into exchangeable fraction, oxidizable fraction, reducible fraction, and residual fraction (Shi et al. 2018).
Cd content in various organs of soybean
The mature soybeans were divided into roots, stems, leaves, and beans (including pods) and then dried at 80 °C to constant weight. Ground all parts of soybeans and pass them through a 60-mesh standard sieve. Weigh 0.25 g of the powder, transfer it to a PTFE digestion tube, and add 2 mL HF, 2 mL HNO3, and 5 mL H2O2 (30%). Put it into a microwave digestion system (Mars6, CEM, USA) for digestion. After the digestion completed, the liquid was filtered into a 25-mL colorimetric tube, diluted with deionized water to volume, and mixed, and then the Cd concentration in the solution was determined by flame atomic absorption spectrometry.
Statistical analysis
The experimental data were analyzed by Microsoft Excel 2010 and SPSS 26.0 software. The significant differences were further analyzed by one-way analysis of variance (P < 0.05).
Results and discussion
Effects of different biochar on soil organic matter and total nitrogen content
Organic matter and soil total nitrogen content are important indicators of soil fertility, and biochar can improve soil organic matter and total nitrogen content (Riaz et al. 2017). From Fig. 1a, after 1% CBC, NBC, and Nm-NBC were added to the Cd-contaminated soil, the soil organic matter content in the soil increased from 28.43 to 31.80, 37.53, and 38.67 g kg−1, respectively. The pore structure of biochar itself can adsorb organic molecules, and it is easier for organic small molecules to polymerize into organic matter and increase the content of soil organic matter.
Effects of different biochar on soil physical and chemical properties: a organic matter; b the total nitrogen. YW-Cd, control group; CBC, corn straw biochar group; NBC, nitrogen-doped biochar group; Nm-NBC, micro-nano nitrogen-doped biochar group. Different letters indicate significant dsifferences (P < 0.05)
The variation of total nitrogen content in the soil was shown in Fig. 1b. When 1% CBC, NBC, and Nm-NBC were added to the Cd-contaminated soil, the total nitrogen content in soil increased from 1.58 to 1.69, 1.98, and 2.03 g kg−1, respectively. Xiu et al. (2021) reported that biochar can improve the nitrogen fixation capacity of soybean, thus increasing the total nitrogen content in soil. The nitrogen content of Nm-NBC and NBC itself is much higher than that of CBC, so it can more effectively improve the total nitrogen content of the soil.
Effects of different biochar on soil pH
The variation of soil pH will directly affect the soil clay, soil organic, inorganic colloids, and the adsorption capacity of Cd(II), thereby further affecting the migration and transformation of Cd in soil. Therefore, it is necessary to study the effects of different biochar on soil pH. From Fig. 2, after 1% CBC, NBC, and Nm-NBC were added to the Cd-contaminated soil, the soil pH increased by 0.10, 0.21, and 0.28 at 20 days, respectively. The increase in the pH value of these groups was due to the fact that the corn straw biochar itself was alkaline. After being added to the soil, its own alkaline substances were released into the soil and increased the pH value of the soil (Chen et al. 2021a, b). The change of pH value in Nm-NBC group was the largest that it was due to the smaller particle size of biochar, more evenly dispersed in the soil, and more alkaline substances released into the soil.
Effects of different biochar on Cd species in soil
The Cd accumulated by plants depends on the concentration of available Cd and exchangeable Cd in the soil, and it is usually related to soil pH and soil organic matter (Liu et al. 2018). As shown in Fig. 3a, after 1% CBC, NBC, and Nm-NBC were added to the Cd-contaminated soil, the soil pH increased, and the available Cd in the soil decreased by 0.76, 0.97, and 1.09 mg kg−1, respectively, after 50 days. On the 50th day, the pH values in the experimental groups were Nm-NBC > NBC > CBC > YW-Cd, respectively, which were opposite to the available Cd level in the soil. This was because the increase of soil pH in the experimental group affected the migration and transformation of available Cd in the soil, making it change to an inactive or fixed state.
Effects of different biochar on Cd species in soil: a available Cd in soil; b percentage of Cd species in soil. YW-Cd, control group; CBC, straw biochar group; NBC, nitrogen-doped biochar group; Nm-NBC, micro-nano nitrogen-doped biochar group. EXF, exchangeable fraction; RDF, reducible fraction; OXF, oxidizable fraction; RSF, residual fraction. Different letters indicate significant differences (P < 0.05)
As shown in Fig. 3b, the change of exchangeable Cd and reduced Cd in the experimental groups were the major compared with the control group. The percentage of exchangeable Cd content in experimental groups decreased by 5.2%, 10.8%, and 15.8%, and the reduced Cd content increased by 3.0%, 5.9%, and 12.8% compared with the control group, respectively. It can be concluded that the changes of Cd species in the experimental groups were mainly the transformation of exchangeable Cd and reduced Cd compared with the control group. The content of exchangeable Cd in soil was mainly related to soil pH value and soil organic matter. The pH value of the experimental groups was Nm-NBC > NBC > CBC, and the content of exchangeable Cd in the soil was Nm-NBC < NBC < CBC. This is because the increase of soil pH will promote the increase of negative charge on soil surface and enhance the adsorption of Cd. In addition, the increase of soil pH will lead to the hydroxide of Cd, which has a stronger affinity with the soil adsorption site than the free Cd ions. The increase of soil pH also leads to an increase of OH− in soil solution. Cd(II) can combine with CO32− and OH− to form insoluble Cd carbonate and Cd hydroxide precipitation, resulting in the decrease of exchangeable Cd content in soil. Therefore, the increase of pH in soil will reduce the content of exchangeable Cd and increase the content of reduced Cd.
Effects of different biochar on soybean biomass and Cd content in soybean organs
Biochar has lots of advantages, and it can promote the photosynthesis of soybeans; the absorption of nitrogen, phosphorus, and potassium; the growth of plants; and the yield of crops. Soybean is an important global source of protein and edible oil, but it is a sensitive to Cd pollution. If expose to excessive Cd, it will inhibit the growth of soybean, and Cd in the seeds will be far higher than the security value (Zhan et al. 2019). The risk of Cd accumulation in soybeans has got a great concern, so soybeans were selected to carry out the experiment.
From Fig. 4a, there is a comparison between the YW group (non-Cd-contaminated) and the YW-Cd group, which indicates that the growth of soybeans and the effect of the biomass of soybeans had been inhibited by Cd pollution. This is because Cd is a powerful enzyme inhibitor. The accumulation of Cd in plants leads to mitochondrial degeneration, abnormal mitosis, and chromosomal abnormality; affects cell cycle activity and catalytic and metabolic processes; and changes in gene and protein expression by blocking some essential enzymes (Kapoor et al. 2021). After adding 1% CBC, NBC, and Nm-NBC in the YW-Cd group, the biomass of soybean root had no significant change, but the aboveground biomass of all soybeans increased. Compared with the YW-Cd group, the aboveground biomass of soybeans in the CBC group, NBC group, and Nm-NBC group had an increase of 6.71%, 7.04%, and 11.88%, respectively, which indicated that the addition of biochar and nitrogen-doped biochar is beneficial to the growth of soybean. Nitrogen-doped biochar is beneficial to fungi and bacteria that are beneficial to the nitrogen accumulation of root (Yu et al. 2018a, b). The plant height, stem diameter, and leaf area of corn grown in micro-nano nitrogen-doped biochar group were significantly increased, compared with the other experimental groups (Yang et al. 2020).
Effects of different biochar on soybean: a soybean biomass; b Cd content in various organs of soybean. YW, no Cd-contaminated group; YW-Cd, control group; CBC, straw biochar group; NBC, nitrogen-doped biochar group; Nm-NBC, micro-nano nitrogen-doped biochar group. Different letters indicate significant differences (P < 0.05)
From Fig. 4b, after 1% CBC, NBC, and Nm-NBC were added to the Cd-contaminated soil, there was only a significantly different between the Cd content in the roots of the Nm-NBC group and YW-Cd group, and the Cd content in roots of the YW-Cd group decreased from 1.10 to 0.78 mg kg−1. The Cd content in the stem of the YW-Cd, CBC, NBC, and Nm-NBC groups was significantly different from the control group. Among them, Nm-NBC biochar had the best results, which could reduce the Cd content in the stem of the YW-Cd group from 1.09 to 0.24 mg kg−1. Comparing the Cd content in soybean leaves between the experimental group and the control group, there were significant differences between Cd content in the leaves of the YW-Cd group and the CBC, NBC, and Nm-NBC groups, and the Cd content in the leaves of the Nm-NBC group was the lowest (0.03 mg kg−1). The Cd content in soybean seeds was significantly different among the YW-Cd, CBC, NBC, and Nm-NBC groups. Nm-NBC biochar also had the best results, which could reduce the Cd content of soybean seeds in the YW-Cd group from 0.79 to 0.26 mg kg−1.
When biochar was added to Cd-contaminated soil, the soil pH increased, and Cd can be attached to the surface adsorption site of biochar through physical adsorption, ion exchange, and surface complexation and prevented the transfer of Cd from soil to soybean. Because of its larger specific surface area, richer functional groups, and higher content of micropores, Nm-NBC has a better passivation effect on Cd in the soil and Cd transfer into soybeans.
Characterizations of biochar
The ability of biochar to immobilize heavy metals in soil is closely related to the specific surface area, micropore structure, functional groups, and pH of biochar (Lv et al. 2021). BET, X-ray photoelectron spectroscopy, scanning electron microscopy, and energy electron spectroscopy-area scanning were used to analyze the specific surface area, microporous structure, and functional groups and indicated the mechanism of Nm-NBC immobilizing Cd in soil.
BET
BET can measure the total area of a unit mass of a substance according to the pore size of the substance’s surface to accommodate the gas. The BET results of Nm-NBC and CBC showed that the specific surface area of Nm-NBC was 336.17 m2 g−1, which was 78 times that of CBC (4.27 m2 g−1). By comparing the pore size distribution of NBC and Nm-NBC, it was found that the pore size distribution of Nm-NBC was more uniform, most of which were concentrated at about 1.8776 nm, and the numbers of micropores were more than that of NBC.
FTIR
The infrared spectra (400–4000 cm−1) of CBC and NBC were shown in Fig. 5. The band range 650–850 cm−1 is related to the aromatic structure. The signal at 1081 cm−1 was attributed to the C-O and C–C groups (Yin et al. 2019). The peak at 1576 cm−1 was assigned to the C = N group (Zhang et al. 2022). The peaks at 3481 cm−1 were assigned to the -N–H and -OH groups’ bending vibrations (Sridhara Chary et al. 2008).
Corn straw contains more natural pores, which can absorb some ammonia nitrogen in water during impregnation. The electronegativity of biochar and the number of functional groups will change when nitrogen element is doped into the biochar (Kasera et al. 2021). Therefore, compared with CBC, the infrared spectrum peaks of NBC are gentler. However, at 3481 cm−1, NBC has stretching vibrations of -N–H and -OH, which indicates that the biochar prepared by doping corn straw with ammonium chloride contains a certain number of amino or hydroxyl functional groups. Xi et al. (2015) also proved that lone pair electrons provided by N atoms in C-N functional groups can combine with Cd(II) to produce a complexation reaction, increasing the adsorption capacity of biochar for Cd(II).
XPS
XPS (X-ray photoelectron spectroscopy) can be used for qualitative analysis of elemental composition, ion valence state, and molecular structure of biochar surface. The X-ray photoelectron spectra of C1s before and after adsorption of Cd(II) on Nm-NBC are shown in Fig. 6a. The C1s spectra of Nm-NBC before Cd(II) adsorption had a peak at 284.15 eV, 285.48 eV, and 286.77 eV, respectively, and the corresponding functional groups are C/C-N, -C–OH/C-(O)-C, and O-C = O/C≡N (Zhang et al. 2015, 2019; Zhu et al. 2019). The C1s spectra of adsorbed Cd(II) on Nm-NBC had a peak at 284.26 eV and 285.88 eV, respectively, and the corresponding functional groups are C/C-N (Wang et al. 2020) and -C–OH/C-(O)-C (Kasera et al. 2021).
The X-ray photoelectron spectra of O1s before and after adsorption of Cd(II) on Nm-NBC are shown in Fig. 6b. The O1s spectra of Nm-NBC before Cd(II) adsorption had a peak at 532.50 eV and 534.67 eV, respectively, and the corresponding functional groups are C = O and –COOH (Kasera et al. 2021; Wang et al. 2020; Zhou et al. 2007); the O1s spectrum of Nm-NBC after adsorption of Cd(II) had a peak at 531.83 eV, and the corresponding functional group is C = O. X-ray photoelectron spectra of N1s before and after nm-NBC adsorption of Cd2+ are shown in Fig. 6c. N1s before Nm-NBC adsorption of Cd(II) had a peak at 398.64 eV, 400.12 eV, and 401.36 eV, respectively, and the corresponding functional groups are pyridine nitrogen, pyrrole nitrogen, and graphite nitrogen (Kubier et al. 2019); the spectra N1s of Nm-NBC adsorbed Cd(II) had a peak at 398.11 eV and 400.36 eV, respectively, and the corresponding functional groups are pyridine nitrogen and pyrrole nitrogen (Kasera et al. 2021). The X-ray photoelectron spectrum of Cd3d after adsorption of Cd(II) on Nm-NBC was shown in Fig. 5d. The spectrum of Cd3d before adsorption of Cd(II) on Nm-NBC had a peak at 405.98 eV and 412.55 eV, respectively, corresponding to Cd3d5/2 and Cd3d3/2 (Liang et al. 2017).
Above all, after Nm-NBC adsorbed Cd(II), some peaks of functional groups such as -COOH and graphitic nitrogen moved or disappeared. Combined with Fig. 6, it can be further concluded that -COOH in Nm-NBC is involved in the formation of Cd–O bonds, and the graphitic nitrogen in Nm-NBC also participated in the formation of Cd-π (Chen et al. 2018).
SEM
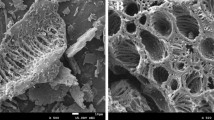
SEM (scanning electron microscope) can observe the surface morphology of biochar samples. EDS (energy dispersive spectroscopy) can analyze the elemental composition of biochar microregion. Therefore, the adsorption mechanism of Nm-NBC on Cd(II) was further analyzed by X-ray photoelectron spectroscopy, scanning electron microscope, and energy electron spectrum-surface scanning.
It can be seen from Fig. 7 that there are many irregular crystals on the SEM of Nm-NBC. Combined with the SEM mapping after biochar adsorbed Cd(II), it can be inferred that the Cd(II) and functional groups on the surface of Nm-NBC complexed and fixed on the surface of biochar after Nm-NBC was added to the Cd solution. It can also be seen from SEM mapping and energy spectrum of Nm-NBC after the Cd(II) adsorption by NBC that nitrogen element was also successfully doped into Nm-NBC.
Mechanisms of Cd-contaminated soil remediation by Nm-NBC
From Fig. 8, Nm-NBC, a new heavy metal passivator, has larger specific surface area, stronger adsorption capacity for Cd(II), and more amino or hydroxyl functional groups compared with CBC and NBC. The main mechanisms of Cd-contaminated soil remediated by Nm-NBC are that hydroxyl or carboxyl functional groups in Nm-NBC complexed with Cd(II) to form Cd–O bond. In Nm-NBC, pyridine nitrogen, pyrrole nitrogen, and graphite nitrogen complexed with Cd(II) to form Cd-π bond; CO32− in Nm-NBC precipitates with Cd(II) to form CdCO3. In addition, Cd(II) will be also exchanged with cations in Nm-NBC and attached to the surface of biochar.
Conclusion
The main purpose of this study was to investigate the effects of particle size and nitrogen doping biochar on the remediation of Cd-contaminated soil. The results showed that the biomass of soybeans increased when CBC, NBC, and Nm-NBC passivators were added to Cd-contaminated soil, and the biomass of soybean in the Nm-NBC group significantly increased by 11.88% (P < 0.05). The increase of soybean biomass was related to the changes in soil’s physical and chemical properties. It was found that adding biochar to the Cd-contaminated soil could increase the pH, organic matter, and total nitrogen content of soil, and the effect was in the order of Nm-NBC > NBC > CBC. In addition, the addition of passivator significantly reduced (P < 0.05) the content of available Cd in the soil and promoted the conversion of exchangeable Cd to reduced Cd in the soil, and the improvement effect was in the order of Nm-NBC > NBC > CBC; Nm-NBC had also the best result. The addition of 1% Nm-NBC to Cd-contaminated soil can effectively reduce the Cd content in soybean seeds by 68.09%.
In order to further study the mechanism of Cd-contaminated soil remediated by these passivators, these biochars were characterized and analyzed, and it was found that the specific surface area, micropore structure, and functional groups of these biochars were quite different. The results of BET showed that Nm-NBC had more micropores and larger specific surface area among them, and most of the pores were about 1.8776 nm in diameter; the specific surface area of Nm-NBC was 336.17 m2 g−1, and it was 78.73 times as much as that of CBC (4.27 m2 g−1). The XPS results of before and after adsorption Cd(II) by Nm-NBC showed that functional groups O-C = O/C≡N, -COOH, pyridine nitrogen, and pyrrolic nitrogen in Nm-NBC participated in the formation of Cd–O bonds; the functional group graphitic nitrogen participates in the formation of Cd-π bonds. SEM mapping also confirmed the successful doping of N element into Nm-NBC and the presence of complexes with Cd(II) on the Nm-NBC surface. Therefore, Nm-NBC prepared by introducing nitrogen element into biochar has a promising application in the remediation of Cd-contaminated soil.
Data availability
All data generated or analyzed during this study are included in this article.
References
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils - to mobilize or to immobilize? J Hazard Mater 266:141–166
Chen G, Feng T, Li Z, Chen Z, Chen Y, Wang H, Xiang Y (2017) Influence of sulfur on the arsenic phytoremediation using Vallisneria natans (Lour.) Hara. Bull Environ Contam Toxicol 99:411–414
Chen G, Ran Y, Ma Y, Chen Z, Li Z, Chen Y (2021a) Influence of Rahnella aquatilis on arsenic accumulation by Vallisneria natans (Lour.) Hara for the phytoremediation of arsenic-contaminated water. Environ Sci Pollut R 28:44354–44360
Chen X, Lewis S, Heal KV, Lin Q, Sohi SP (2021b) Biochar engineering and ageing influence the spatiotemporal dynamics of soil pH in the charosphere. Geoderma 386:114919
Chen Z, Liu T, Tang J, Zheng Z, Wang H, Shao Q, Chen G, Li Z, Chen Y, Zhu J, Feng T (2018) Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ Sci Pollut R 25:11854–11866
Hamid Y, Liu L, Usman M, Tang L, Lin Q, Saqib Rashid M, Ulhassan Z, Hussain MI, Yang X (2022) Organic/inorganic amendments for the remediation of a red paddy soil artificially contaminated with different cadmium levels: leaching, speciation, and phytoavailability tests. J Environ Manag 303:114148
Huang L, Yang X, Xie Z, Li S, Liang X, Hu Z (2021) Residual effects of sulfur application prior to oilseed rape cultivation on cadmium accumulation in brown rice under an oilseed rape-rice rotation pot experiment. Ecotox Environ Safe 225:112765
Kapoor D, Singh S, Ramamurthy PC, Jan S, Bhardwaj S, Gill SS, Prasad R, Singh J (2021) Molecular consequences of cadmium toxicity and its regulatory networks in plants. Plant Gene 28:100342
Kasera N, Hall S, Kolar P (2021) Effect of surface modification by nitrogen-containing chemicals on morphology and surface characteristics of N-doped pine bark biochars. J Environ Chem Eng 9:105161
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388
Li J, Xia C, Cheng R, Lan J, Chen F, Li X, Li S, Chen J, Zeng T, Hou H (2022) Passivation of multiple heavy metals in lead-zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: mechanisms and microbial community evolution. Sci Total Environ 803:149866
Liang J, Li X, Yu Z, Zeng G, Luo Y, Jiang L, Yang Z, Qian Y, Wu H (2017) Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II). ACS Sustain Chem Eng 5:5049–5058
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194:495–503
Lv G, Yang T, Chen Y, Hou H, Liu X, Li J, Wei L, Li J (2021) Biochar-based fertilizer enhanced Cd immobilization and soil quality in soil-rice system. Ecol Eng 171:106396
Popov AA, Mirkov I, Tucovic D, Kulas J, Zeljkovic M, Popovic D, Ninkov M, Jankovic S, Kataranovski M (2021) Immunomodulation by heavy metals as a contributing factor to inflammatory diseases and autoimmune reactions: cadmium as an example. Immunol Lett 240:106–122
Ramanayaka S, Tsang DCW, Hou D, Ok YS, Vithanage M (2020) Green synthesis of graphitic nanobiochar for the removal of emerging contaminants in aqueous media. Sci Total Environ 706:135725
Riaz M, Roohi M, Arif MS, Hussain Q, Yasmeen T, Shahzad T, Shahzad SM, Muhammad HF, Arif M, Khalid M (2017) Corncob-derived biochar decelerates mineralization of native and added organic matter (AOM) in organic matter depleted alkaline soil. Geoderma 294:19–28
Shi Y, Gan L, Li X, He S, Sun C, Gao L (2018) Dynamics of metals in backfill of a phosphate mine of Guiyang, China using a three-step sequential extraction technique. Chemosphere 192:354–361
Sridhara Chary N, Kamala CT, Samuel Suman Raj D (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotox Environ Safe 69:513–524
Suhani I, Sahab S, Srivastava V, Singh RP (2021) Impact of cadmium pollution on food safety and human health. Current Opinion Toxicol 27:1–7
Wang T, Liu S, Mao W, Bai Y, Chiang K, Shah K, Paz-Ferreiro J (2020) Novel Bi2WO6 loaded N-biochar composites with enhanced photocatalytic degradation of rhodamine B and Cr(VI). J Hazard Mater 389:121827
Xi Y, Luo Y, Luo J, Luo X (2015) Removal of cadmium(II) from wastewater using novel cadmium ion-imprinted polymers. J Chem Eng Data 60:3253–3261
Xiu L, Zhang W, Wu D, Sun Y, Zhang H, Gu W, Wang Y, Meng J, Chen W (2021) Biochar can improve biological nitrogen fixation by altering the root growth strategy of soybean in Albic soil. Sci Total Environ 773:144564
Yang J, Chen M, Yang H, Xu N, Feng G, Li Z, Su C, Wang D (2020) Surface heterogeneity mediated transport of hydrochar nanoparticles in heterogeneous porous media. Environ Sci Pollut R 27:32842–32855
Yin W, Zhang W, Zhao C, Xu J (2019) Evaluation of removal efficiency of Ni(II) and 2,4-DCP using in situ nitrogen-doped biochar modified with aquatic animal waste. ACS Omega 4:19366–19374
Yu L, Yu M, Lu X, Tang C, Liu X, Brookes PC, Xu J (2018a) Combined application of biochar and nitrogen fertilizer benefits nitrogen retention in the rhizosphere of soybean by increasing microbial biomass but not altering microbial community structure. Sci Total Environ 640–641:1221–1230
Yu W, Lian F, Cui G, Liu Z (2018b) N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193:8–16
Zhan J, Twardowska I, Wang S, Wei S, Chen Y, Ljupco M (2019) Prospective sustainable production of safe food for growing population based on the soybean (Glycine max L. Merr.) crops under Cd soil contamination stress. J Clean Prod 212:22–36
Zhang F, Wang X, Yin D, Peng B, Tan C, Liu Y, Tan X, Wu S (2015) Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J Environ Manage 153:68–73
Zhang H, Wang T, Sui Z, Zhang Y, Sun B, Pan W (2019) Enhanced mercury removal by transplanting sulfur-containing functional groups to biochar through plasma. Fuel 253:703–712
Zhang J, Huang D, Shao J, Zhang X, Zhang S, Yang H, Chen H (2022) A new nitrogen-enriched biochar modified by ZIF-8 grafting and annealing for enhancing CO2 adsorption. Fuel Process Technol 231:107250
Zhou J, Sui Z, Zhu J, Li P, Chen D, Dai Y, Yuan W (2007) Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 45:785–796
Zhu L, Tong L, Zhao N, Li J, Lv Y (2019) Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 219:493–503
Zong Y, Xiao Q, Malik Z, Su Y, Wang Y, Lu S (2021) Crop straw-derived biochar alleviated cadmium and copper phytotoxicity by reducing bioavailability and accumulation in a field experiment of rice-rape-corn rotation system. Chemosphere 280:130830
Funding
This work was supported by the Scientific Research Foundation of Hunan Provincial Education Department (No. 21B0451), the Teaching Research and Reform Project of Hunan Province (HNJG-2022–0174), and the National Natural Science Foundation of China (No. 41501343).
Author information
Authors and Affiliations
Contributions
Guoliang Chen: conceptualization, methodology, writing-reviewing and editing. Yongqing Ma: experiments, formal analysis, data curation, writing-Original draft. Wenting Xu: experiments. Zhang Chen: writing-reviewing. Zhixian Li: writing-reviewing. Jianlin Zhou: writing-reviewing; Weijian Yu: writing-reviewing.
Corresponding author
Ethics declarations
Ethics approval
The submitted study has not been published elsewhere in any form or language (partially or in full).
Consent for publication
The authors give their consent for submission and publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, G., Ma, Y., Xu, W. et al. Remediation of cadmium-contaminated soil by micro-nano nitrogen-doped biochar and its mechanisms. Environ Sci Pollut Res 30, 48078–48087 (2023). https://doi.org/10.1007/s11356-023-25674-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25674-6