Abstract
The short-term association between ambient air pollution and hospital admissions for ischemic stroke is not fully understood. We examined the association between four regularly measured major ambient air pollutants, i.e., sulfur dioxide (SO2), nitrogen dioxide (NO2), photochemical oxidants (Ox), and particulate matter with aerodynamic diameters ≤ 2.5 μm (PM2.5), and hospital admissions for ischemic stroke by analyzing 3 years of nationwide claims data from 97 cities in Japan. We first estimated city-specific results by using generalized additive models with a quasi-Poisson regression, and we obtained the national average by combining city-specific results with the use of random-effect models. We identified a total of 335,248 hospital admissions for ischemic stroke during the 3-year period. Our analysis results demonstrated that interquartile range increases in the following four ambient air pollutants were significantly associated with hospital admissions for ischemic stroke on the same day: SO2 (1.05 ppb), 1.05% (95% CI: 0.59–1.50%); NO2 (6.40 ppb), 1.10% (95% CI: 0.61–1.59%); Ox (18.32 ppb), 1.43% (95% CI: 0.81–2.06%); and PM2.5 (7.86 μg/m3), 0.90% (95% CI: 0.35–1.45%). When the data were stratified by the hospital admittees’ medication use, we observed stronger associations with SO2, NO2, and PM2.5 among the patients who were taking antihypertensive drugs and weaker associations with SO2, NO2, and Ox among those taking antiplatelet drugs. Short-term exposure to ambient air pollution was associated with increased hospital admissions for ischemic stroke, and medication use and season may modify the association.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is a global public health concern, accounting for 5.5 million deaths in 2016 (Naghavi et al. 2017). In Japan, the age-adjusted mortality rate for stroke has decreased rapidly over the past decades but is still one of the leading causes of death (Ueshima 2007; WHO Global Health Observatory 2020). Epidemiological studies have suggested that ambient air pollution is associated with ischemic stroke (Verhoeven et al. 2021), which is the predominant stroke subtype. Several mechanisms are suspected to underlie this association, including inflammation, oxidative stress, lipid modification, and autonomic dysfunction (Lee et al. 2018). However, the existing epidemiological studies often used mortality as an outcome (Hong et al. 2002; Zanobetti and Schwartz 2009; Chen et al. 2017; Yorifuji et al. 2011; Michikawa et al. 2019; Shah et al. 2015; Niu et al. 2021). Hospital admission data are expected to be more sensitive and to capture the broader burden of air pollution, but most of the studies that used hospital admission data were based on a single city or region (Chan et al. 2006; Villeneuve et al. 2012; Collart et al. 2018; Montresor-López et al. 2016; Matsuo et al. 2016; Huang et al. 2017; Chen et al. 2019; Qi et al. 2020), which are susceptible to publication bias (Anderson et al. 2005). Several multi-city studies have been conducted, but most of these included a small number of cities (~ 10 cities) (Le Tertre 2002; Wellenius et al. 2005; Barnett et al. 2006; Larrieu et al. 2007; O’Donnell et al. 2011). The number of multi-city studies with large sample sizes is increasing (Tian et al. 2018; Gu et al. 2020; Stafoggia et al. 2020) but remains limited.
Moreover, the identification of subgroups that are especially susceptible to environmental pollutants is crucial for revealing underlying mechanisms and subgroups that should avoid exposure. Several studies suggested that patient characteristics may modify the association between ambient air pollution and ischemic stroke (O’Donnell et al. 2011; Villeneuve et al. 2012; Ho et al. 2018; Qi et al. 2020), but their results are unclear and based on small sample sizes. Large-scale studies with high statistical power are needed to clarify the association between air pollution and ischemic stroke.
We thus conducted the present study to examine the association between short-term exposure to ambient air pollutants and daily hospital admissions for ischemic stroke on a national scale in Japan. We used data from a nationwide claims database, which allowed us to include 97 cities across Japan. By using a large sample size, we examined modifications by patient characteristics that often require high statistical power.
Materials and methods
Study area and period
This study included 97 largest cities in Japan (Suppl. Fig. S1). All of the cities had a total population ≥ 200,000, and the cities included 50.4% of the national population as of the 2015 national census (Statistics Bureau, Ministry of Internal Affairs and Communications 2021). The study period was from 1 April 2016 to 31 March 2019 in all of the included cities.
Hospital admission data
Japan achieved universal health insurance coverage in 1961, and most people now receive medical treatments under the system. Almost all of the health insurance claims issued under the system are collected and stored in a national claims database called the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), which the Ministry of Health, Labour and Welfare of Japan (MHLW) manages. The MHLW provides researchers anonymized data from the database on request, and several studies have been conducted using the data from the database (Ishimaru et al. 2018; Izumi et al. 2018; Okumura et al. 2019).
We applied to the MHLW and obtained anonymized data from the NDB. The provided data consists of clinical information including anonymized personal identifiers, age groups, sex, date of admission, diagnosis codes linked with the 10th edition of the International Classification of Disease (ICD-10), codes for procedures and prescriptions, and the city of the hospital that issued the claim. To prevent the identification of individual patients, the MHLW provided only the city of the hospital and did not provide the names of the hospitals. The MHLW also provided only anonymized personal identifiers, called identifier (ID)1 (generated from the date of birth, sex, and the ID number of an insurer) and ID2 (generated from the date of birth, sex, and name) instead of individual numbers. We used the two anonymized IDs; if one or both IDs matched, we regarded the data as belonging to the same patient.
From the provided data, we identified individuals who were hospitalized with a primary diagnosis of ischemic stroke using ICD-10 code I63. We used the MHLW’s algorithm to decide the primary diagnoses (Ministry of Health, Labour and Welfare 2015; Okumura et al. 2019). As rehospitalization within a few days was regarded as a single episode of hospitalization and only the date for the first admission was available (Hayashida et al. 2021), we included only the first admission for such rehospitalization admissions. To further exclude recurrent or re-hospitalizations for ischemic stroke, we excluded the patients with outpatient or inpatient claims with an ischemic stroke disease code within 36 months before their current admission. We also excluded both patients without records of procedure codes for brain computed tomography (CT) or magnetic resonance imaging (MRI) after their hospital admission (in order to reduce diagnostic error) and the patients aged < 20 old because the number of these patients was too few.
Supplementary Figure S2 is the patient selection flow chart. For each patient, we examined the medication use of antihypertensive drugs, oral hypoglycemic drugs or insulin, lipid-modifying drugs, antiplatelet drugs, and anticoagulant drugs by using their pharmacy claims data in the 3 months prior to their admittance to the hospital (Fukai et al. 2020). Supplementary Table S1 shows the list of Anatomical Therapeutic Chemical codes for each medication.
We used the alternate definition of hospital admission for ischemic stroke as a sensitivity analysis. We limited the analyses to patients who were emergently hospitalized to a diagnosis procedure combination (DPC) hospital, which are top-ranked in Japan’s medical system, with DPC claims (Ando et al. 2018). We used the diagnosis codes recorded in the greatest-resource condition, trigger-for-hospitalization condition, or main condition, and we included patients with ischemic stroke (ICD-10 codes: I63) in any of the three conditions (Ando et al. 2018). As in the main analysis, we further excluded patients with outpatient or inpatient claims with an ischemic stroke disease code within 36 months before their admission, patients without records of brain CT or MRI after their hospital admission, and patients aged < 20 years.
Ambient air pollution and meteorological data
We obtained the hourly ambient air measurements of sulfur dioxide (SO2), nitrogen dioxide (NO2), photochemical oxidants (Ox), and particulate matter with an aerodynamic diameter of ≤ 2.5 μm (PM2.5) for each of the 97 cities from Japan’s National Institute for Environmental Studies. As there are no routine measurements for ozone (O3) in Japan, we used Ox as a proxy for O3 as in earlier studies (Bae et al. 2015; Vicedo-Cabrera et al. 2020; Ito et al. 2021). All of the included cities had more than one monitoring station for each included ambient air pollutant. These monitoring stations were located at sites that reflect the ambient exposure of the residents (Ministry of the Environment 2010). We calculated the daily mean concentrations for SO2, NO2, and PM2.5 and the daily maximum 8-h mean concentrations for Ox from the hourly measurements. We conducted the calculations only if > 18 hourly measurements were available; otherwise, the data were considered missing. We averaged across monitors for the cities with two or more monitoring stations. Data on ambient SO2, NO2, Ox, and PM2.5 were missing on 0.35%, 0.49%, 0.20%, and 0.84% of the days. We excluded these days from the analysis.
We also obtained daily mean ambient temperature and relative humidity data from the Japan Meteorological Agency (JMA). For cities without measurements for temperature (n = 25) or relative humidity (n = 46), we used the data measured at the nearest monitor in the same prefecture. Data on ambient temperature and relative humidity were missing on 0.02% and 0.12% of the days. We also excluded these days from the analyses.
Statistical analyses
We used a two-stage approach to examine the associations between ambient air pollution and hospital admissions for ischemic stroke. In the first stage, we built city-specific models using generalized additive models with quasi-Poisson regression for the hospital admissions. In the second stage, we used a random-effects meta-analysis with the restricted maximum likelihood estimation method to obtain the national average estimates from the first stage. We used the I2 statistics to examine heterogeneity between cities.
We included the following covariates based on previous studies: (1) a natural cubic smooth function of calendar time with seven degrees of freedom (df) per year for long-term trends (Dominici et al. 2006; Tian et al. 2018); (2) indicator variables for the days of the week and public holidays (Tian et al. 2018; Gu et al. 2020), and (3) a natural spline function of the 3-day moving average temperature (six df) and relative humidity (three df) to adjust for weather conditions (Dominici et al. 2006; Chen et al. 2017).
The final model is below:
where E(HAt) is the expected number of hospital admissions for ischemic stroke on day t; α is the intercept; Xt is the air pollutant concentrations on day t; f is a function defining the exposure–response relationship; ns(.) is the natural cubic spline function; time is the calendar time; DOWt is a categorical variable for the day of the week on day t; holidayt is an indicator variable of public holidays on day t; temperaturet is the 3-day moving average temperature on day t; relative humidityt is the 3-day moving average relative humidity on day t.
We used single-pollutant models that contained one air pollutant at a time as the main models. We examined the association with the air pollutant concentrations of the same day (lag 0) throughout this study based on earlier studies (Shah et al. 2015; Tian et al. 2018). We also examined the air pollutant concentrations of the prior day (lag 1) and 2 days ago (lag 2) to explore temporal associations. The function f for the main models was defined in the following equation:
where xp,t−l is a concentration of an air pollutant, p = SO2, NO2, Ox, or PM2.5, on day t, with lagged day l = 0, 1, 2; and βp is the parameter to estimate.
We further performed multi-pollutant model analyses to examine the stability of the estimated associations and to differentiate the role of each air pollutant by including air pollutants that were significant in the single-pollutant model. The function f for multi-pollutant models was defined in the following equation:
We additionally estimated the exposure–response curve to get the overall shape of the associations for each pollutant (Samoli et al. 2005; Chen et al. 2012). From an explanatory graphical analysis, we used cubic spline functions with knots at 0.6 and 1.5 ppb for SO2, 7.0 and 14.0 ppb for NO2, 30.0 and 50.0 ppb for Ox, and 7.5 and 15.0 μg/m3 for PM2.5. These values are almost the 25th and 75th percent quantiles of the distributions for many of the included cities. We estimated five regression coefficients of the cubic spline functions and the corresponding variance–covariance matrix in each city and obtained the national average by random-effect models. We restricted this analysis to days with concentrations above the 0.5th and below the 99.5th quantile because extreme values could overly impact the whole exposure–response curves. The function f for the exposure–response curve was defined in the following equation (Samoli et al. 2005):
where xp,i is the place of the ith knot for the air pollutant, p; and ()+ is a notation representing a constant function of 0 for negative inputs and an identity function for non-negative inputs.
We performed stratification analyses by age group (20–64, 65–74, and ≥ 75 years), sex, medication use (i.e., antihypertensive drugs, oral hypoglycemic drugs or insulin, lipid-modifying drugs, antiplatelet drugs, and anticoagulant drugs), region (East and West Japan), and season (warm and cool season). We divided the cities into those in East Japan and West Japan based on the definition by the JMA (The Japan Meteorological Agency 2021) with the modification of incorporating North Japan into East Japan. We excluded the city of Naha from the stratification analysis by region, as it is located on a remote island (Suppl. Fig. S1). We defined the warm season as April–September and the cold season as October–March. The statistical significance of the differences in the estimates between groups was tested by calculating the 95% confidence interval (CI) as follows:
where \({\widehat{Q}}_{1}\) and \({\widehat{Q}}_{2}\) are effect estimates for each group, and \({\widehat{SE}}_{1}\) and \({\widehat{SE}}_{2}\) are their respective standard errors (Zeka et al. 2006). We performed the following sensitivity analyses: (1) using alternative df values for calendar time (5–9 per year), temperature (3–7), and relative humidity (2–6), and (2) using the alternate definition for hospital admissions for ischemic stroke.
We performed cross-sectional dependency tests to examine the cross-sectional interdependence between included cities. We used Pesaran’s CD test (Pesaran 2021) defined by the following equation:
where N is the number of cities; T is the number of days during the study period; and \({\widehat{\rho }}_{ij}\) is the correlation among response residuals between city i and city j. We present the estimated effects and their 95% CIs as the percent change (PC) per one interquartile range (IQR) range increase in air pollutants. We used the mean of the IQRs of the 97 cities: 1.05 ppb for SO2, 6.40 ppb for NO2, 18.32 ppb for Ox, and 7.86 μg/m3 for PM2.5. Probability (p)-values < 0.05 were considered significant. We used R (ver. 4.1.1) for the statistical analyses and used the “metafor” package to conduct the meta-analysis (Viechtbauer 2010). We also used the “mixmeta” package to estimate the exposure–response curves (Sera et al. 2019).
Results
Descriptive analysis
A total of 335,248 hospital admissions in the 97 Japanese cities during the period from 1 April 2016 to 31 March 2019 were analyzed. Table 1 summarizes the demographic characteristics of the included patients, and Table 2 provides the summary statistics of ambient air pollutants and meteorological conditions in the 97 Japanese cities during the study period. The mean of the annual means of ambient air pollutants in each city was 1.6 ppb for SO2, 10.25 ppb for NO2, 41.98 ppb for Ox, and 11.65 µg/m3 for PM2.5. Supplementary Table S2 lists Pearson’s correlation coefficients between ambient air pollutants and meteorological conditions. The concentrations of ambient air pollutants were positively correlated, except for the correlation between NO2 and Ox.
Regression analysis
The national average estimates of the associations between ambient air pollutant levels and hospital admissions for ischemic stroke are given in Table 3. We observed small between-city heterogeneity for all four of the ambient air pollutants and lag structure combinations, where the I2 statistic ranged from 0.0 to 18.55%. The analysis results demonstrated that IQR increases in the concentrations of SO2, NO2, Ox, and PM2.5 on the same day were associated with 1.05% (95% CI: 0.59–1.50%), 1.10% (95% CI: 0.61–1.59%), 1.43% (95% CI: 0.81–2.06%), and 0.90% (95% CI: 0.35–1.45%) increases in hospital admissions for ischemic stroke, respectively.
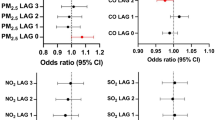
Table 4 shows the estimates from the multi-pollutant model, which included SO2, NO2, Ox, and PM2.5. We observed that the associations of NO2 and Ox remained significant. Figure 1 depicts the exposure–response curves between air pollutant concentrations and hospital admissions for ischemic stroke, indicating generally positive concentration–response curves. We observed an apparent elevation in relative risks even at relatively low air pollutant concentrations in the exposure–response curves of SO2, NO2, and PM2.5.
The exposure–response curve for concentrations of sulfur dioxide, nitrogen dioxide, photochemical oxidants, and particulate matter with an aerodynamic diameter of ≤ 2.5 μm associated with hospital admissions for ischemic stroke in 97 cities in Japan, April 2016–March 2019. The x-axis is the concentrations of air pollutants on the same day of admissions for ischemic stroke. The y-axis is the log relative risk. The solid line represents the mean estimates, and the dotted line shows their 95% confidence intervals
Stratification and sensitivity analysis
Figure 2 illustrates the estimated effects modified by age group, sex, medication use, region, and season. We observed significantly stronger associations with SO2, NO2, and PM2.5 among the patients taking antihypertensive drugs but significantly weaker associations with SO2, NO2, and Ox among the patients taking antiplatelet drugs. We observed stronger associations with Ox during the warm season. The estimates for alternative df values are provided in Supplementary Table S3, and the demographic characteristics of the patients by the alternate definition of hospital admissions for ischemic stroke are summarized in Supplementary Table S4. Supplementary Table S5 shows the estimates for the alternate definition. We obtained similar estimates as in the main analysis after changing the df values and when using the alternate definition for hospital admissions for ischemic stroke. Supplementary Table S6 provides the results of the cross-sectional dependency tests. We observed significant cross-sectional dependences in all of the tested models.
Discussion
In this time-series study, we examined the associations between the concentrations of four ambient air pollutants and the hospital admissions for ischemic stroke in 97 cities in Japan. We observed that increases in the same-day concentrations of ambient SO2, NO2, and PM2.5 were positively associated with hospital admissions for ischemic stroke, and the data for NO2 and Ox remained significant in the multi-pollutant model. The stratification analysis revealed that the associations with SO2, NO2, and PM2.5 were stronger among the patients taking antihypertensive drugs, and weaker associations with SO2, NO2, and Ox were present among the patients taking antiplatelet drugs. The association between the hospital admissions and Ox was stronger in the warm season.
Although several large-scale studies have examined the association between exposure to ambient air pollutants and hospital admissions for ischemic stroke, their results have been inconsistent. A study of Medicare beneficiaries aged ≥ 65 years in nine US cities indicated that an IQR increase in the ambient particulate matter with aerodynamic diameter ≤ 10 μm (PM10) (22.96 μg/m3), CO (0.71 ppm), NO2 (11.93 ppb), and SO2 (6.96 ppb) concentrations on the same day were associated with 1.03% (95% CI: 0.04–2.04%), 2.83% (95% CI: 1.23–4.46%), 2.94% (95% CI: 1.78–4.12%), and 1.35% (95% CI: 0.43–2.29%) increases in hospital admissions for ischemic stroke, respectively (Wellenius et al. 2005). A study of 172 Chinese cities revealed that a 10-μg/m3 increase in ambient PM2.5, SO2, NO2, and O3, and a 1-mg/m3 increase in ambient CO in the same day were associated with 0.39% (95% CI: 0.20–0.48%), 1.37% (95% CI: 1.05–1.70%), 1.82% (95% CI: 1.455–2.19%), 0.01% (95% CI: − 0.14–0.16%), and 3.24% (95% CI: 2.055–4.43%) increases in hospital admissions for ischemic stroke, respectively (Tian et al. 2018). A nationwide study from Taiwan reported that an IQR increase in PM2.5 (22.26 μg/m3) concentrations in the 2 days prior to admission was associated with an adjusted odds ratio of 1.055 (95% CI: 1.014–1.097) in hospital admissions for ischemic stroke (Yang et al. 2021).
On the other hand, several studies did not observe significant positive associations between ambient air pollutants and hospital admissions for ischemic stroke. A study of eight urban cities in France reported that an increase of 10 μg/m3 in ambient PM10, NO2, and O3 concentrations was associated with 0.2% (95% CI: − 1.6–1.9%), − 0.2% (95% CI: − 1.1–0.7%), and − 0.4% (95% CI: − 1.2–0.3%) changes in hospital admissions for stroke, respectively (Larrieu et al. 2007). An investigation of eight cities in Canada reported that an increase of 10 μg/m3 in ambient PM2.5 was associated with a − 0.7% (95% CI: − 6.3–5.1%) change in hospital admissions for stroke (O’Donnell et al. 2011). Another examination of eight cities across Europe reported that PM10 and black smoke concentrations were not associated with hospital admission for smoke (Le Tertre 2002). A study of seven cities in New Zealand and Australia reported that ambient NO2, CO, PM2.5, PM10, and O3 concentrations were not associated with hospital admission for stroke (Barnett et al. 2006). The inconsistency of the results of the above-cited studies may be partly explained by differences such as ambient air pollutants levels, meteorological conditions, population characteristics, sample sizes, and study designs (Verhoeven et al. 2021).
Our present findings regarding the exposure–response curve for SO2, NO2, and PM2.5 showed seeming elevations in the relative risks even at relatively low concentrations of these pollutants. Similar to these findings, earlier studies described exposure–response curves with elevations in the relative risks even at low concentrations of pollutants (Huang et al. 2017; Gu et al. 2020; Stafoggia et al. 2020). These results suggested that reducing these ambient air pollutants below these concentrations may still benefit public health.
Although several studies have examined whether patient characteristics may modify the association between ambient air pollution and ischemic stroke, the results have been unclear. For example, a study from Canada examined possible modifications of the association between PM2.5 and ischemic stroke by a history of diabetes, atrial fibrillation, hypertension, stroke or transient ischemic attack, smoking, and sex (O’Donnell et al. 2011). They reported that the association was significantly stronger among patients with a history of diabetes, whereas no significant differences in other characteristics were identified. Another study from Canada examined effect modification by a history of heart disease, stroke, hypertensive medication, diabetes medication, antiplatelet medication, and anticoagulant medication on the association between NO2 and ischemic stroke in the warm season; none of these factors exerted a significant effect (Villeneuve et al. 2012). A study from China reported that patients with hyperlipidemia showed stronger associations between PM10 and O3 and ischemic stroke in the warm season, but the heterogeneity test did not show significant differences between patients with and without hyperlipidemia (Qi et al. 2020).
Our stratification analyses showed that the associations between hospital admissions for stroke and the values of SO2, NO2, and PM2.5 were about ≥ 3 times greater among the patients taking antihypertensive drugs. In line with this finding, several research groups have reported a modification effect of hypertension on the association with heart rate variability, suggesting that hypertension may modify the association between ambient air pollution and cardiovascular diseases through cardiac autonomic dysfunction (Holguín et al. 2003; Liao 2004; Park et al. 2005, 2010). This hypothesis may explain the observed modification effect among patients taking antihypertensive drugs in the present study. In addition, as hypertension and air pollution cause similar physiological changes associated with cardiovascular diseases, e.g., systemic inflammation and oxidative stress (Briones and Touyz 2010; Lee et al. 2018), this similarity may explain our present results.
The stratification analysis also showed that the associations with SO2, NO2, and Ox were almost null among the patients taking antiplatelet drugs, in contrast to the patients not taking these medications. Exposure to air pollutants was reported to lead to platelet activation (Lucking et al. 2008; Jacobs et al. 2010), which is the central mechanism in ischemic stroke pathophysiology (Fisher et al. 1982; Joseph et al. 1989). One explanation for the present study’s results may be that antiplatelet drugs may have reduced these patients’ platelet activation by exposure to ambient air pollution and prevented ischemic stroke. However, as the patients taking antiplatelet drugs were likely to have more comorbidities and spend less time outdoors, the results may be attributable in part to the differences in exposure to ambient air pollution rather than physiological mechanisms. Further studies with more detailed individual-level data are needed to clarify.
There are several study limitations to address. First, as we used data from a claims database, misclassifications of the outcomes could have occurred due to diagnostic or coding errors. These errors are likely to be independent of air pollution concentrations and may have biased our estimates toward the null. However, we obtained similar estimates using the alternate definition for hospital admissions for ischemic stroke, suggesting the robustness of our estimates. Second, we used the average of all monitoring stations in each city instead of personal exposure to ambient air pollutants. This exposure assessment may have caused an exposure measurement error, which may have biased our estimates toward the null (Zeger et al. 2000).
Third, as we included only the four ambient air pollutants, unmeasured air pollutants may mediate the actual associations from the same source. For example, NO2 serves as a proxy for other unmeasured traffic-related pollutants, including noise, road dust, and ultrafine particles (Seaton 2003; Ito et al. 2007). Alternatively, combined effects of air pollutants such as the oxidant capacity rather than individual pollutants may be responsible for the observed association (Williams et al. 2014). We also did not consider the components of the PM2.5, due to a lack of data. Fourth, as we used the medication status to characterize patient characteristics, we could not discriminate whether the observed effect modification was from the treated disease, the medication itself, and/or the characteristics of the patients taking medications. Fifth, we observed significant cross-sectional dependency implying spillover effects of air pollution and/or unmeasured factors, such as infectious disease outbreaks or other hazardous air pollutants. Future studies should consider these factors. Lastly, this study included only relatively populated cities in order to minimize model convergence issues, and thus the generalizability of the results is limited.
Conclusion and policy recommendation
We observed significant associations between exposure to ambient air pollutants and hospital admissions for ischemic stroke in 97 cities in Japan. Our results also indicate that medication use and the season might modify these associations. However, as there are several study limitations due to our secondary use of claims data, our findings should be confirmed by studies with more detailed and precise data, such as registry-based data.
Our results suggest the following policy recommendation: the air quality standards in Japan should be reconsidered. In 2018, the ratios of monitoring stations that complied with the air quality standards were almost 100% for SO2 and NO2, 93.5% for PM2.5, and almost 0% for Ox (Ito et al. 2021). These results implied that most of the areas were considered at the “safe levels” for SO2, NO2, and PM2.5. However, we observed significant positive associations between these air pollutants and hospital admissions for ischemic stroke, suggesting that the current air quality standards might not be adequate from a public health perspective. Moreover, the exposure–response curve for SO2, NO2, and PM2.5 showed seeming elevations in the relative risks even at relatively low concentrations of these pollutants, implying that further reductions in the levels of these air pollutants might still be beneficial.
Data Availability
The dataset analyzed in this study is not publicly available due to data protection requirements from Japan’s MHLW.
References
Anderson HR, Atkinson RW, Peacock JL et al (2005) Ambient particulate matter and health effects: publication bias in studies of short-term associations. Epidemiology 16:155–163. https://doi.org/10.1097/01.ede.0000152528.22746.0f
Ando T, Ooba N, Mochizuki M et al (2018) Positive predictive value of ICD-10 codes for acute myocardial infarction in Japan: a validation study at a single center. BMC Health Serv Res 18:895. https://doi.org/10.1186/s12913-018-3727-0
Bae S, Lim Y-H, Kashima S et al (2015) Non-linear concentration-response relationships between ambient ozone and daily mortality. PLoS ONE 10:e0129423. https://doi.org/10.1371/journal.pone.0129423
Barnett AG, Williams GM, Schwartz J et al (2006) The effects of air pollution on hospitalizations for cardiovascular diseasein elderly people in Australian and New Zealand cities. Environ Health Perspect 114:1018–1023. https://doi.org/10.1289/ehp.8674
Briones AM, Touyz RM (2010) Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 12:135–142. https://doi.org/10.1007/s11906-010-0100-z
Chan C-C, Chuang K-J, Chien L-C et al (2006) Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei. Taiwan Eur Heart J 27:1238–1244. https://doi.org/10.1093/eurheartj/ehi835
Chen C, Wang X, Lv C et al (2019) The effect of air pollution on hospitalization of individuals with respiratory and cardiovascular diseases in Jinan. China Medicine (baltimore) 98:e15634. https://doi.org/10.1097/MD.0000000000015634
Chen R, Kan H, Chen B et al (2012) Association of particulate air pollution with daily mortality: the China Air Pollution and Health Effects Study. Am J Epidemiol 175:1173–1181. https://doi.org/10.1093/aje/kwr425
Chen R, Yin P, Meng X et al (2017) Fine particulate air pollution and daily mortality. a nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med 196:73–81. https://doi.org/10.1164/rccm.201609-1862OC
Collart P, Dubourg D, Levêque A et al (2018) Short-term effects of nitrogen dioxide on hospital admissions for cardiovascular disease in Wallonia, Belgium. Int J Cardiol 255:231–236. https://doi.org/10.1016/j.ijcard.2017.12.058
Dominici F, Peng RD, Bell ML et al (2006) Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295:1127–1134. https://doi.org/10.1001/jama.295.10.1127
Fisher M, Levine PH, Fullerton AL et al (1982) Marker proteins of platelet activation in patients with cerebrovascular disease. Arch Neurol 39:692–695. https://doi.org/10.1001/archneur.1982.00510230018006
Fukai K, Nagata T, Mori K, et al (2020) Validation of self‐reported medication use for hypertension, diabetes, and dyslipidemia among employees of large‐sized companies in Japan. J Occup Health 62. https://doi.org/10.1002/1348-9585.12138
Gu J, Shi Y, Chen N et al (2020) Ambient fine particulate matter and hospital admissions for ischemic and hemorrhagic strokes and transient ischemic attack in 248 Chinese cities. Sci Total Environ 715:136896. https://doi.org/10.1016/j.scitotenv.2020.136896
Hayashida K, Murakami G, Matsuda S, Fushimi K (2021) History and profile of Diagnosis Procedure Combination (DPC): development of a real data collection system for acute inpatient care in Japan. J Epidemiol 31:1–11. https://doi.org/10.2188/jea.JE20200288
Ho AFW, Zheng H, De Silva DA et al (2018) The relationship between ambient air pollution and acute ischemic stroke: a time-stratified case-crossover study in a city-state with seasonal exposure to the Southeast Asian haze problem. Ann Emerg Med 72:591–601. https://doi.org/10.1016/j.annemergmed.2018.06.037
Holguín F, Téllez-Rojo MM, Hernández M et al (2003) Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology 14:521–527. https://doi.org/10.1097/01.ede.0000081999.15060.ae
Hong Y-C, Lee J-T, Kim H et al (2002) Effects of air pollutants on acute stroke mortality. Environ Health Perspect 110:187–191. https://doi.org/10.1289/ehp.02110187
Huang F, Luo Y, Tan P et al (2017) Gaseous air pollution and the risk for stroke admissions: a case-crossover study in Beijing, China. Int J Environ Res Public Health 14:E189. https://doi.org/10.3390/ijerph14020189
Ishimaru M, Matsui H, Ono S et al (2018) Preoperative oral care and effect on postoperative complications after major cancer surgery: preoperative oral care and postoperative complications after major cancer surgery. Br J Surg 105:1688–1696. https://doi.org/10.1002/bjs.10915
Ito A, Wakamatsu S, Morikawa T, Kobayashi S (2021) 30 years of air quality trends in Japan. Atmosphere 12:1072. https://doi.org/10.3390/atmos12081072
Ito K, Thurston GD, Silverman RA (2007) Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol 17:S45–S60. https://doi.org/10.1038/sj.jes.7500627
Izumi K, Morimoto K, Hasegawa N, et al (2018) Epidemiology of adults and children treated for nontuberculous mycobacterial pulmonary disease in Japan. Ann Am Thorac Soc AnnalsATS.201806–366OC. https://doi.org/10.1513/AnnalsATS.201806-366OC
Jacobs L, Emmerechts J, Mathieu C et al (2010) Air pollution–related prothrombotic changes in persons with diabetes. Environ Health Perspect 118:191–196. https://doi.org/10.1289/ehp.0900942
Joseph R, D’Andrea G, Oster SB, Welch KM (1989) Whole blood platelet function in acute ischemic stroke. Importance of dense body secretion and effects of antithrombotic agents. Stroke 20:38–44. https://doi.org/10.1161/01.STR.20.1.38
Larrieu S, Jusot J-F, Blanchard M et al (2007) Short term effects of air pollution on hospitalizations for cardiovascular diseases in eight French cities: the PSAS program. Sci Total Environ 387:105–112. https://doi.org/10.1016/j.scitotenv.2007.07.025
Le Tertre A (2002) Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health 56:773–779. https://doi.org/10.1136/jech.56.10.773
Lee KK, Miller MR, Shah ASV (2018) Air pollution and stroke. J. Stroke 20:2–11. https://doi.org/10.5853/jos.2017.02894
Liao D (2004) Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol 159:768–777. https://doi.org/10.1093/aje/kwh109
Lucking AJ, Lundback M, Mills NL et al (2008) Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J 29:3043–3051. https://doi.org/10.1093/eurheartj/ehn464
Matsuo R, Michikawa T, Ueda K et al (2016) Short-term exposure to fine particulate matter and risk of ischemic stroke. Stroke 47:3032–3034. https://doi.org/10.1161/STROKEAHA.116.015303
Michikawa T, Ueda K, Takami A et al (2019) Japanese Nationwide Study on the association between short-term exposure to particulate matter and mortality. J Epidemiol 29:471–477. https://doi.org/10.2188/jea.JE20180122
Ministry of Health, Labour and Welfare (2015) Methods for identifying one principle diagnose. https://www.mhlw.go.jp/content/12400000/000681207.xls. Accessed 1 Feb 2022
Ministry of the Environment (2010) Continuous monitoring manual for atmosphere environment, 6th edition. http://www.env.go.jp/air/osen/manual_6th/index.html. Accessed 1 Feb 2022
Montresor-López JA, Yanosky JD, Mittleman MA et al (2016) Short-term exposure to ambient ozone and stroke hospital admission: a case-crossover analysis. J Expo Sci Environ Epidemiol 26:162–166. https://doi.org/10.1038/jes.2015.48
Naghavi M, Abajobir AA, Abbafati C et al (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 390:1151–1210. https://doi.org/10.1016/S0140-6736(17)32152-9
Niu Z, Liu F, Yu H et al (2021) Association between exposure to ambient air pollution and hospital admission, incidence, and mortality of stroke: an updated systematic review and meta-analysis of more than 23 million participants. Environ Health Prev Med 26:15. https://doi.org/10.1186/s12199-021-00937-1
O’Donnell MJ, Fang J, Mittleman MA et al (2011) Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology 22:422–431. https://doi.org/10.1097/EDE.0b013e3182126580
Okumura Y, Sugiyama N, Noda T, Tachimori H (2019) Psychiatric admissions and length of stay during fiscal years 2014 and 2015 in Japan: a retrospective cohort study using a nationwide claims database. J Epidemiol 29:288–294. https://doi.org/10.2188/jea.JE20180096
Park SK, Auchincloss AH, O’Neill MS et al (2010) Particulate air pollution, metabolic syndrome, and heart rate variability: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 118:1406–1411. https://doi.org/10.1289/ehp.0901778
Park SK, O’Neill MS, Vokonas PS et al (2005) Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect 113:304–309. https://doi.org/10.1289/ehp.7447
Pesaran MH (2021) General diagnostic tests for cross-sectional dependence in panels. Empir Econ 60:13–50. https://doi.org/10.1007/s00181-020-01875-7
Qi X, Wang Z, Guo X et al (2020) Short-term effects of outdoor air pollution on acute ischaemic stroke occurrence: a case-crossover study in Tianjin, China. Occup Environ Med 77:862–867. https://doi.org/10.1136/oemed-2019-106301
Samoli E, Analitis A, Touloumi G et al (2005) Estimating the exposure–response relationships between particulate matter and mortality within the APHEA Multicity Project. Environ Health Perspect 113:88–95. https://doi.org/10.1289/ehp.7387
Seaton A (2003) Hypothesis: Ill health associated with low concentrations of nitrogen dioxide–an effect of ultrafine particles? Thorax 58:1012–1015. https://doi.org/10.1136/thorax.58.12.1012
Sera F, Armstrong B, Blangiardo M, Gasparrini A (2019) An extended mixed-effects framework for meta-analysis. Stat Med 38:5429–5444. https://doi.org/10.1002/sim.8362
Shah ASV, Lee KK, McAllister DA et al (2015) Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 350:h1295. https://doi.org/10.1136/bmj.h1295
Stafoggia M, Renzi M, Forastiere F, et al (2020) Short-term effects of particulate matter on cardiovascular morbidity in Italy: a national analysis. Eur J Prev Cardiol zwaa084. https://doi.org/10.1093/eurjpc/zwaa084
Statistics Bureau, Ministry of Internal Affairs and Communications (2021) Population Census. https://www.stat.go.jp/english/data/kokusei/index.html. Accessed 1 Feb 2022
The Japan Meteorological Agency (2021) General Information on Climate of Japan. https://www.data.jma.go.jp/gmd/cpd/longfcst/en/tourist.html. Accessed 1 Feb 2022
Tian Y, Liu H, Zhao Z et al (2018) Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLOS Med 15:e1002668. https://doi.org/10.1371/journal.pmed.1002668
Ueshima H (2007) Explanation for the Japanese Paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb 14:278–286. https://doi.org/10.5551/jat.E529
Verhoeven JI, Allach Y, Vaartjes ICH et al (2021) Ambient air pollution and the risk of ischaemic and haemorrhagic stroke. Lancet Planet Health 5:e542–e552. https://doi.org/10.1016/S2542-5196(21)00145-5
Vicedo-Cabrera AM, Sera F, Liu C, et al (2020) Short term association between ozone and mortality: global two stage time series study in 406 locations in 20 countries. BMJ m108. https://doi.org/10.1136/bmj.m108
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 36. https://doi.org/10.18637/jss.v036.i03
Villeneuve PJ, Johnson JYM, Pasichnyk D et al (2012) Short-term effects of ambient air pollution on stroke: who is most vulnerable? Sci Total Environ 430:193–201. https://doi.org/10.1016/j.scitotenv.2012.05.002
Wellenius GA, Schwartz J, Mittleman MA (2005) Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke 36:2549–2553. https://doi.org/10.1161/01.STR.0000189687.78760.47
WHO Global Health Observatory (2020) Global Health Estimates: life expectancy and leading causes of death and disability. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. Accessed 1 Feb 2020
Williams ML, Atkinson RW, Anderson HR, Kelly FJ (2014) Associations between daily mortality in London and combined oxidant capacity, ozone and nitrogen dioxide. Air Qual Atmosphere Health 7:407–414. https://doi.org/10.1007/s11869-014-0249-8
Yang C-P, Li C-Y, Huang W-J et al (2021) Short-, mid-, and long-term associations between PM2.5 and stroke incidence in Taiwan. J Occup Environ Med 63:742–751. https://doi.org/10.1097/JOM.0000000000002222
Yorifuji T, Kawachi I, Sakamoto T, Doi H (2011) Associations of outdoor air pollution with hemorrhagic stroke mortality. J Occup Environ Med 53:124–126. https://doi.org/10.1097/JOM.0b013e3182099175
Zanobetti A, Schwartz J (2009) The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect 117:898–903. https://doi.org/10.1289/ehp.0800108
Zeger SL, Thomas D, Dominici F et al (2000) Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect 108:419–426. https://doi.org/10.1289/ehp.00108419
Zeka A, Zanobetti A, Schwartz J (2006) Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol 163:849–859. https://doi.org/10.1093/aje/kwj116
Acknowledgements
We thank the Ministry of Health, Labour and Welfare of Japan for managing and providing the extracted data from the NDB.
Author information
Authors and Affiliations
Contributions
K. Hasegawa: conceptualization, methodology, software, formal analysis, investigation, resources, data curation, writing (original draft), visualization. T. Tsukahara: conceptualization, methodology, validation, writing (review and editing). T. Nomiyama: conceptualization, methodology, writing (review and editing), supervision, project administration.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Committee for Medical Ethics of Shinshu University School of Medicine (protocol code: 4550; date of approval: 13 November 2019).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hasegawa, K., Tsukahara, T. & Nomiyama, T. Short-term associations of ambient air pollution with hospital admissions for ischemic stroke in 97 Japanese cities. Environ Sci Pollut Res 29, 78821–78831 (2022). https://doi.org/10.1007/s11356-022-21206-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21206-w