Abstract
It is a common practice to improve the water environment of rivers and lakes in China by the enhancement and releasing (EAR) of silver carp (Hypophthalmichthys molitrix) and bighead carp (Hypophthalmichthys nobilis) for biomanipulation. However, the quantity of bighead carp and its effect on water quality and plankton community have been the focus of debate among ecologists. Herein, in order to more accurately simulate the environmental conditions of lakes, we selected earthen ponds with large areas adjacent to Lake Qiandao from May to August in 2016 to study the responses of water quality condition and plankton community to a gradient of bighead carp stocked alone. Experimental groups with different densities of carp stocked were set as follows: 12.1 (LF), 23.5 (MF), and 32.5 g/m3 (HF), and a control group with no fish (NF). Results showed that total phosphorus (TP) in the fish-containing groups considerably decreased, and the lowest chlorophyll-a concentration (chl-a) was detected in the MF group. The biomass accumulation of the crustacean zooplankton was suppressed after carp was introduced, but the diversity, richness, and evenness of the crustacean zooplankton were weakly affected, except in the HF group. Phytoplankton biomass especially that of cyanobacteria was grazed rapidly by fish in the MF and HF groups and biodiversity indices were considerably increased in the fish-containing groups, especially in the late stages of the experiment. At a fish stocking density of 23.5–38.8 g/m3, the highest efficiency in controlling cyanobacteria and promoting water condition was achieved, and the impact on zooplankton diversity was weak. Our results indicated that bighead carp can be included in the EAR of lakes and reservoirs, but the optimal density of bighead carp stocking should be carefully considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomanipulation, originally proposed by Shapiro et al. (1975), is a method for effectively controlling the amount of nuisance algae in lakes or reservoirs by reducing the predation pressure of planktivores on zooplankton and increasing zooplankton biomass. This method involves the use of piscivorous fish in controlling planktivorous fish or other fish-killing measures. It has been considered one of the most effective approaches for improving water quality in many eutrophic lakes and reservoirs (Gulati et al. 1990; ter Heerdt and Hootsmans 2007; McQueen 2010) and has now become a routine technique for managing lakes in Europe, Australia, and North America (Benndorf 1990; Mehner et al. 2004; Sierp et al. 2009; Jeppesen et al. 2012; Gophen and Snovsky 2015). However, the effectiveness of using traditional biomanipulation in controlling nuisance algae is often questioned because of the inefficient grazing of zooplankton on cyanobacteria or difficulty in sustaining high zooplankton biomass for long periods in complex lake ecosystems (Zhang et al. 2008; McQueen 2010; Jeppesen et al. 2012).
A similar but different approach is nontraditional biomanipulation, which was suggested by Xie and Liu (2001); this approach involves the use of filter-feeding planktivorous carps (i.e., bighead carp, Hypophthalmichthys nobilis, and the silver carp, Hypophthalmichthys molitrix) in directly controlling algal overgrowth. Nontraditional biomanipulation has attracted considerable interest and has been widely tested for its feasibility in algal control in China (Wang et al. 2009; Xiao et al. 2010; Yi et al. 2016a; Liu and Zhang 2016; Chen et al. 2020) and other countries (Zubcov et al. 2021; Mohammad and Haque, 2021). In fact, long before the concept of nontraditional biomanipulation had been proposed, the effects of using two species of carps on plankton community and water quality have been extensively studied worldwide (Leventer and Teltsch 1990; Starling 1993; Domaizon and Dévaux 1999; Post and McQueen 1987); meanwhile, silver and bighead carps have been used as core elements in aquatic environment protection-oriented lake fishery practices in many reservoirs and lakes, such as Lake Qiandao in China (Liu 2005). However, studies on the use of two filter-feeding carps in controlling algal overgrowth presented conflicting results (Zeng et al. 2010; Yi et al. 2016b), which have created many controversies over the feasibility of nontraditional biomanipulation. After carefully comparing and analyzing different studies, Liu and Zhang (2016) found that the seemingly different results were actually not contradictory to one another as previously believed. The different results might be attributed to differences in experimental designs or protocols. They found that the results showing the successful control of algae with carps are mostly from studies conducted in experimental systems with natural bottoms (Tan et al. 1995; Wang et al. 2009; Zhou et al. 2011), such as the natural lakes, ponds, and enclosures without artificial bottoms (i.e., with the natural bottoms of water bodies), whereas results showing failure in controlling algae with carps were from studies conducted in concrete or glass tanks or enclosures with artificial bottoms. Nevertheless, this finding has not convinced some researchers, who performed statistical analysis on many lakes either in the middle and lower reaches of the Yangtze River or in the Pearl River Delta region and found that high chlorophyll-a (chl-a) concentration in areas with high carp biomass and thus reached an opposite conclusion (Wang et al. 2008; Lin et al. 2020). Other researchers found that planktivorous carps reduce zooplankton biomass and even increase phytoplankton biomass, presenting results predicted by studies on traditional biomanipulation (Yi et al. 2016a, Shen et al. 2021). The effectiveness of stocking with bighead carps in lakes and reservoirs has been questioned because bighead carps predominantly feed on zooplankton (Cremer and Smitherman 1980; Burke et al. 1986; Dong and Li 1994; Li et al. 2018a) and silver carp mainly feed on phytoplankton (Xie 2003), although bighead carps are effective biological control agents for algal blooms (Cremer and Smitherman 1980; Xie 2001) and played an important role in the trophic dynamics of aquatic ecosystems during top-down and bottom-up processes (Carpenter et al. 1992; Schindler et al. 2001; Jeppesen et al. 2010). The view that bighead carps are ineffective algal control agents is supported by the fact that the mesh size of the gill raker of a bighead carp (with a relatively wide spacing of less than 20 μm) is considerably larger than that of a silver carp (with filtering particles of less than 12 μm (Opuszynski and Shireman 1993; Vörös 2000). However, evidence of the successful use of bighead carps in manipulation has been presented as well (Xie 1999, 2001; Ke et al. 2007; Chen et al. 2011; Jayasinghe et al. 2015; Nistor et al. 2018).
Bighead carps have been stocked in lakes and reservoirs for long times because of their high economic value and this method have yielded practical results (which often mean good growth; FAO 2018). Along with the silver carp, the bighead carp has become a dominant fish species in lakes and reservoirs in China and has thus exerted considerable impact on lake ecosystems; thus, its effects on plankton should be assessed. Moreover, experiments using bighead carp alone in investigating their effects on aquatic environments and plankton communities are necessary because whether or not bighead carps can be used in controlling nuisance algae is unclear. Some of the questions considered in the present study were as follows: (1) What are the real effects of bighead carp on plankton structures and nutrients in experimental water bodies; (2) can bighead carp yield a positive effect on water quality or phytoplankton; and what is the optimum stocking density for bighead carp? Previous studies that investigated the effects of using two species of carps on phytoplankton in lakes are often conducted in small enclosures (e.g., enclosures with sizes of approximately 10–50 m2) (Zhao et al. 2013, 2016; Li et al. 2018b). In such small systems, the walls of enclosures are conducive to the growth of epiphytes (Dickman 1968; Martin et al. 1992), which exert a huge impact on nutrient level and phytoplankton. Furthermore, environmental conditions in small experimental systems (e.g., enclosures) readily fluctuate and different (in comparison with the conditions in large experiment systems) from those in real scenarios (lakes or reservoirs) and might thus lead to large errors. Hence, in this study, we adopted large earthen ponds for the control study. We hope that by using larger experimental systems, we can eventually obtain the results closer to those obtained in actual situations in which bighead carp are stocked in lakes and reservoirs.
Material and methods
Experimental design
The experiment was conducted in eight earthen ponds with the depth of 2 m adjacent to a lake shore in the southwest portion of Lake Qiandao (118°38′58.70′′ E, 29°26′1.57′′ N) (Fig. 1). The experiment lasted for 102 days from May 6 to August 16, 2016. Before the experiment, we removed the sediment (approximately 5 cm) to minimize initial interference from variations during treatment, and the earthen ponds were filled with lake water from Lake Qiandao at a depth of 1.5 m. We ensured that the water remained stagnant for more than 2 weeks for the sedimentation of inorganic suspended solids, which may affect initial conditions in all treatments. Bighead carps were obtained from a nearby fish hatchery and acclimatized in a net cage in Lake Qiandao 12 days before the start of the experiment. We captured fish by using a basic seining net and minimized the handling of mortality by a short time before the carps were transferred to the earthen ponds. On May 6, six fish-containing ponds were selected randomly, as presented in Fig. 1. The average standing biomass of carp in Lake Qiandao was around 28.30 g/m2 (Li, 2016). However, earthen pond conditions could not fully represent the actual condition of the lake. Therefore, the three gradients set in the experimental groups included the carp density of the lake. The detailed information of the three gradients and earthen pond areas was shown as follows: LF (12.1 g/m3, 2980–3056 m2), MF (23.5 g/m3, 3012–3056 m2), HF (32.5 g/m3, 2998–3067 m2), and a control group with no fish (NF, 3000–3100 m2), with two replicates each (Fig. 1). The mean individual weight (± S.E.) and body length (± S.E.) of the carp stocked were 85.38 ± 1.44 g and 16.0 ± 0.1 cm, respectively.
Sampling and measurement
The earthen ponds were sampled on May 6 before the experiment for the assessment of pretreatment conditions, and the samples were taken at 10-day intervals until 16 August. Integrated water samples were collected using a 5 L modified Patalas bottle sampler from the surface (20 cm below the surface) and from the bottom layers (approximately 20 cm above the sediment to minimize disturbance) in each treatment on each sampling occasion. Water temperature (WT), transparency (WSD), dissolved oxygen (DO), pH, total nitrogen (TN), total phosphorus (TP), nitrate-N (NO3-N), nitrite-N (NO2-N), ammonia-N (NH3-N), chemical oxygen demand (CODMn), chl-a, and plankton communities were investigated for the comparison of the ability of fish to interfere with the characteristics of water environments.
The WSD value was measured with a 20 cm diameter Secchi’s disk, and DO, WT, and pH were determined with a YSI Professional Plus Water Quality Monitor (YSI Inc., Yellow Springs, Ohio, USA). Chl-a was tested using a new submersible probe (FluoroProbe, bbe-Moldaenke), which is widely used in limnology study (Garrido et al. 2019). Chl-a and physical factors were determined on the spot. In the laboratory, 500 mL of integrated water was collected for the testing of chemical parameters, including TN, TP, NO3-N, NO2-N, NH3-N, and CODMn, with spectrophotography methods according to SEPA (2002). All water samples were analyzed within 24 h.
Pretreated samples (50 mL) of crustacean zooplankton, including Cladocera and Copepoda, were collected by filtering 10 L of integrated water samples through a 64 μm mesh plankton net, which collected from the surface (20 cm below the surface) and from the bottom layers (approximately 20 cm above the sediment to minimize disturbance), and immediately preserved with a 5% final concentration of formaldehyde in glass bottles (Jin and Tu 1990). Entire samples of zooplankton were used in identifying species under a dissecting microscope with an ocular micrometer. Copepoda was identified according to Sheng (1979), and Copepoda volumes (copepodid and adult) were estimated using the geometric figures of their approximate shapes (Lawrence et al. 1987). Copepoda nauplii were counted without further taxonomic distinction. Cladocera was identified according to the method of Chiang and Du (1979), and biomass was estimated according to the method of Huang and Hu (1986). Rotifera was counted directly from the phytoplankton samples at 100× magnification (at least 100 individuals of the most abundant taxa), and enumerating sorted counting was performed according to the methods of Wang (1961) and Ruttner-Kolisko (1977). We calculated the body volume according to the geometric shapes provided by McCauley (1984). The dry weight of each Rotifera species was obtained by assuming a specific gravity of 1 and a dry weight/wet weight ratio of 0.1 (McCauley 1984).
A 1 L portion of water sampled was preserved with 1% of acidified Lugol’s iodine solution and concentrated to 50 mL after 48 h of sedimentation (Jin and Tu 1990). After mixing, 0.1 mL of concentrated samples was counted directly with a 0.1 mL counting chamber and a microscope at 400× magnifications. Phytoplankton taxonomy was identified at the species level (whenever possible) and enumerated using a compound microscope according to the method of Hu and Wei (2006). For phytoplankton biomass (wet weight) measurement, algal cells were considered to have equivalent geometric shapes (Hillebrand et al. 1999) and uniform specific gravity. Each subsample was counted, and the average of the three counts was used for statistical analysis (Zhang and He 1991). Measurements were made for 10–30 individuals per species in each sample.
Data analysis
The effects of fish biomass level on the water quality condition and chl-a were analyzed using one-way repeated-measures ANOVA performed in SPSS statistics 19.0 for windows (Statistics Product and Service Solutions, IBM Inc., USA) excluding first sampling (D1). Because fish were stocked after first sampling, data from D1 were excluded from the analysis. For low replication and statistical power, we choose a probability level of α < 0.10 to reduce the chance of making the type II error of failing to reject a false null hypothesis. We employed the Presence/Absence (P/A) methods to evaluate the effects of TN, TP, and plankton communities on fish stocks (Compte et al. 2012). The P/A values were calculated as follows: log10 (XPresence/XAbsence), where XPresence and XAbsence are the values of fish presence and fish absence groups for all sampling dates, respectively. If the P/A values were positive, it means the indicators in fish presence groups were higher than those in the fish absence groups, whereas a negative log ratio values indicated the opposite. Values close to 0 implied that the characteristics were unaffected by fish stocks.
Plankton diversity in all treatments was assessed using the Shannon-Wiener diversity index (Shannon 1948), Margalef’s richness index (Legendre and Legendre 1998), and Pielou evenness index (Pielou 1966). The dominant species of plankton in different treatments were ascertained using the McNaughton dominance index (McNaughton 1967).
The calculation formula of the dominance index (Y) is
The calculation formula of Shannon-Wiener diversity index (H’) is
The calculation formula of Margalef’s richness index (R) is
The calculation formula of Pielou evenness index (J) is
where ni represents the number of ith species, N represents the total amount of all species, S is the number of the species observed, and fi represents the appearance frequency of certain species at all sampling sites. Taxa with a Yi ≥ 0.02 were selected as dominant species (Deng et al. 2016).
Canonical correspondence analysis (CCA) and correlation coefficients were used in verifying the relationship between plankton species and environmental variables. CCA was performed using the CANOCO program (version 5.0). The model achieved was tested using Monte-Carlo permutation (ter Braak 1994). Environmental factors and dominant plankton species were transformed using log10 (x + 1; except pH) for increasing the importance of small values and achieving a normalized distribution. Environmental variables were selected according to significant statistical level in the forward selection (p < 0.05). The figures were devised with Origin 2019b (Origin Lab Corporation, USA).
Results
Effects of fish on environmental parameters
The WT varied from 24.5 to 33.8 °C (no difference was detected among the groups). Initially, all groups had similar WSD values, which ranged from 70.0 to 92.5 cm. Significant differences of WSD were observed among the groups (except NF vs. LF, Fig. 2, Table 1) after the fish stocking. No significant difference of DO was detected (Fig. 2, Table 1). The pH values in the MF group showed markedly lower than other groups (Fig. 2, Table 1). The concentration of nutrients and chl-a are shown in Fig. 3. Differences in NO3-N, NO2-N, and NH3-N were not observed among all groups. CODMn in the MF group was lower than that in LF and HF groups (Table 1). The TP value in the MF group showed lowest concentration compared with other groups, and almost showed notably difference among all the groups (except LF vs. HF, Table 1). Chl-a was significantly lower in the MF group than that in the NF, LF and HF groups.
Mean values (hollow square symbol “white square”) for WSD (cm), DO (mg/L), and pH (−) for each sample. Abnormal values are marked using the solid rhombus symbol “black diamond”. Different letters indicate significant differences in all treatments at p < 0.1, as determined by the post-hoc test (LSD) of the variance analysis (ANOVA). Treatments were treated as fixed factors
Mean values (hollow square symbol “white square”) for NH3-N (mg/L), NO2-N (mg/L), NO3-N (mg/L), CODMn (mg/L), TN (mg/L), TP (mg/L), and Chl-a (μg/L) in the experiment period (except first sampling). Abnormal values are marked using the solid rhombus symbol “black diamond”. Different letters indicate significant differences in all treatments at p < 0.1, as determined by the post-hoc test (LSD) of the variance analysis (ANOVA). Treatments were treated as fixed factors
The ratios of TN and TP values (P/A values) between the fish presence and absence groups are shown in Fig. 4. The two curves showed downward trends as sampling time increased, and both parameters varied by sampling time. All groups had similar patterns of fluctuation. The P/A values of TN and TP, respectively, became negative after the fourth sampling (D4, June 8) and the third sampling point (D3, May 27). The REG2 curve of TP showed the lowest level.
Effects of fish on zooplankton community
In this study, the composition of zooplankton and its biomass (including Cladocera, Copepoda, and Rotifera) were identified and calculated for each treatment. A total of 97 zooplankton species were recorded in all treatments over the entire experimental period, including 30 Cladocera species, 36 Copepoda species, and 31 Rotifera species. The NF group showed the highest number of species in all treatments, containing 77 species (20 Cladocera species, 32 Copepoda species, and 25 Rotifera species), whereas the LF group had the least number of zooplankton, containing 59 species (14 Cladocera species, 25 Copepoda species, and 20 Rotifera species). In addition, 64 species of zooplankton (18 Cladocera, 25 Copepoda, and 21 Rotifera species) were found in the MF group, and 70 zooplankton species were found in the HF group (19 Cladocera, 28 Copepoda, and 23 Rotifera species). The detailed composition of zooplankton in each experimental group was shown in Electronic Supplementary Material 1. All experimental groups had the same dominated species, Phyllodiaptomus tunguidus. NF group was mainly dominated by Cladocera and Copepoda, including Moina rectirostris, P. tunguidus, Neodiaptomus schmackeri, etc., whereas LF group was mainly Rotifera, such as Ploesoma hudsoni, Polyarthra trigla. MF and HF groups had similar distributions, including M. rectirostris, P. tunguidus, P. trigla, etc.
Figure 5 shows the changes in the biomass of zooplankton in each experimental group at different sampling times. The biomass of crustacean zooplankton was much higher in the NF group than in the fish-containing groups (Fig. 5, p = 0.012). The biomass of Cladocera, Copepoda, and Rotifera in all treatments was similar at the start of the experiment. The status was broken after bighead carp was stocked. The fish absence groups showed higher Cladocera and Copepoda biomass than those in LF (p = 0.044), MF (p = 0.021), and HF groups (p = 0.010). Cladocera biomass showed a rapidly decreasing trend in all treatments. Meanwhile, Copepoda biomass remained high in the fish absence groups, and Copepoda biomass growth was inhibited in the fish presence groups. The Rotifera biomass in the fish absence groups was lower than that in the fish presence groups at the early stage of the experiment (before D6, p = 0.104), but an opposite result was obtained at the late stage (p = 0.041). Over the entire period, crustacean zooplankton contributed to the majority of biomass in the NF group, consisting of approximately 65.5% of wet weight of zooplankton. The proportions of Rotifera biomass in the LF and HF groups were 74.6% and 73.8%, respectively, and the proportion of crustaceans in the MF group was 54.8%.
The Shannon-Wiener diversity index, Pielou evenness index, and Margalef’s richness index of the zooplankton community were calculated over the entire experimental period (Fig. 6). The indices of the fish absence groups were higher than those of the presence groups. And the zooplankton community was more significantly inhibited by the introduction of bighead carp, and the diversity, evenness, and richness showed a more obvious downward trend compared with the fish absence groups.
To investigate differences in each treatment, we calculated the natural logarithmic ratios of the three zooplankton catalogs and compared the results from the fish presence and the absence groups (Fig. 7). Cladocera ratios in all the fish presence groups were negative at all sampling times (except in MF at D1 and HF at D9). Copepoda ratios in the fish presence groups were also negative (except in MF and HF at D1). Rotifera ratios in the fish presence groups present were positive from D2 to D6, when the early stages of the experiment were performed, and a negative proportion was observed from D7 to the end of the experiment. Different distributions of dominated species were found among treatments, as shown in Table 2. The NF group had six species, which were mainly from the Cladocera and Copepoda. The LF group included five predominant species, mainly from Rotifera. Macrozooplankton biomass decreased, and a similar phenomenon was observed in the HF group. The MF group was involved in five dominant species, mainly Cladocera and Rotifera.
P/A values (log10 (XPresence/XAbsence)) for CLA (Cladocera), COP (Copepoda), and ROT (Rotifera) for all samplings in each treatment, where REG1, REG2, and REG3 are shown for the ratios of the values between LF, MF, HF, and NF, respectively. A blue line with a blue dot means P/A value < 0 (left to the dashed line) and a red line with a red dot means P/A value > 0 (right to the dashed line)
Fish effects on phytoplankton community
Eight types of phytoplankton, including Cyanophyta, Chlorophyta, Bacillariophyta, Cryptophyta, Pyrroptata, Euglenophyta, Xanthophyta, and Chrysophyta, were observed. A total of 148 phytoplankton species were recorded in all treatments during the entire sampling period, and 118 species of phytoplankton belonging to 62 genera were found in the fish absence groups, 124 species belonged to 63 genera in the LF group, 110 species belonged to 62 genera in the MF group, and 117 species belonged to 65 genera in the HF group. The detailed composition of phytoplankton in each experimental group was shown in Electronic Supplementary Material 2. The most frequent phytoplankton taxa in all treatments were Cyanophyta, Chlorophyta, and Bacillariophyta (Fig. 8). The biomass of these three types of algae accounted for the largest proportion of phytoplankton. The NF group had 98.23%, LF had 94.98%, MF had 82.17%, and HF had 90.02%. Changes in phytoplankton biomass in the NF and LF groups showed different trends compared with the MF and HF groups (Fig. 9). The different stocking densities of bighead carp had different control pressures on the main population of phytoplankton (Fig. 10). The MF and HF groups had different inhibitory effects on blue-green algae (except at D2), and the control effects increased gradually with sampling time. The LF group presented positive values at the former stage and negative values at the late stage. The green algal ratios in all the fish presence groups showed no apparent effects before D4 and then had a positive increase during the rest of the experiment. Overall, the P/A values of diatoms were positive in all the fish presence groups.
P/A values (log10 (XPresence/XAbsence)) for CYA (Canophyta), CHL (Chlorophyta), and BAC (Bacillariophyta) for all samples in each treatment, where REG1, REG2, and REG3 are shown for the ratios of the values between LF, MF, HF, and NF, respectively. A blue line with a blue dot means P/A value < 0 (left to the dashed line) and a red line with a red dot means P/A value > 0 (right to the dashed line)
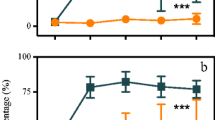
Bighead carp stocking not only affected the biomass of the main phytoplankton population but also the community structure (Fig. 6). In the fish absence groups, phytoplankton diversity, evenness, and richness were lower than those in the fish presence groups, especially after D5. The compositions of the dominant phytoplankton species varied among the treatments (Table 3). The NF group consisted mainly of the phytoplankton of cyanobacteria, including Microcystis incerta (Y = 0.128), Anabaena circinalis (Y = 0.066), and Anabaena smithii (Y = 0.161). As the density of stocked fish increased, the composition of the dominant species in fish presence groups gradually changed to that of Chlorophyta species.
Canonical correspondence analysis of dominant species of plankton and environmental factors
Multivariate statistical approaches were used in assessing the relationship of dominant zooplankton or phytoplankton species with environmental variables in different fish stocks. The results of the DCA analysis indicated a good dispersion of plankton species on axes with high gradient length (> 3), which is suitable for unimodal methods, such as CCA (Šmilauer and Lepš 2014). CCA was performed to explore the relationship between environmental factors (predictor variables) and phytoplankton or zooplankton species (response variable) in the experimental ponds (Fig. 11). The dominant species of phytoplankton were apparently affected by the environmental factors (Fig. 11a). A total of 54.3% of cumulative variance in species was explained by the first two CCA axes, and over 90% of correlation was found between environmental factors and phytoplankton assemblies. The application of forward selection using the Monte-Carlo test confirmed that the first two axes were highly significant (p = 0.002; Table 4). Multiple environmental variables (e.g., TP, DO, pH, TN, WT, and fish biomass) played significant roles in driving the succession of species (Table 5). Cyanophyta showed a significant suppression by fish, and positive correlations with pH, DO, and nutrient level. Responses of dominant zooplankton species to environmental variables have been observed in Fig. 11b. The detailed results of the coordinate were shown in Table 4 and Table 5. A total of 40.8% of cumulative variance in species was explained by the first two CCA axes, and over 89.6% of correlation was found between environmental variables and zooplankton assemblies. Environmental variables (chl-a, TP, pH, WT, fish biomass, and WSD) exerted considerable impacting on zooplankton community (Table 5). Fish biomass showed the inhibition of crustacean zooplankton, and an opposite result was obtained in Rotifera. Copepoda showed a positive relationship with chl-a.
Ordination diagram of canonical correlation analysis (CCA) shows dominant species of phytoplankton (a) and zooplankton (b) and environmental characteristics (arrows) in all treatments. Abbreviated names are as follows: TN total nitrogen, TP total phosphorus, DO dissolved oxygen, pH, WT water temperature, Fish fish biomass in various groups, Chl-a chlorophyll-a, CLA1 B. longirostris, CLA2 L. rectirostris, COP1 P. tunguidus, COP2 N. schmackeri, COP3 T. hyalinus, COP4 Nauplii (Copepoda), ROT1 P. hudsoni, ROT2 P. trigla, ROT3 T. rousseleti, CYA1 M. incerta, CYA2 A. circinalis, CYA3 A. smithii, CYA4 M. punctata, CHL1 S. quadricauda, CHL2 S. carinatus, CHL3 S. bijugatus, CHL4 S. bicaudatus, CHL5 C. microporum, CHL6 C. vulgaris, CHL7 W. linearis, BAC1 S. acus, BAC2 M. sulacata
Discussions
Trophic cascade of bighead carp on plankton community structure and water environment
Phosphorous transferred to the pelagic zone via the feeding of fish is an important flux in lakes (Carpenter et al. 1992). Filter-feeding fish plays an important role in “phosphorus pumps” in many lakes and accounted for 70% of the phosphorus excreted from pelagic fish in filter-feeding fish-dominated lakes (Carpenter et al. 1992; Sterner and George 2000). Studies on the nutrient budget mediated by filter-feeding fish revealed their regulatory effects on nutrients in experimental water bodies. Chen et al. (1991) showed that the amounts of nutrients released by filter-feeding fish in Lake Donghu were lower than the optimal algal production requirements (Chen et al. 1991), suggesting that the excretion of nutrients by fish in eutrophic environments is not critical to algal growth (Lu et al. 2002). Research on the ecological stoichiometry of filter-feeding fish and their driven nutrient recycling in Lake Qiandao showed similar results. The nitrogen and phosphorus released by the excretion of filter-feeding fish only accounted for 0.237% and 0.788% of algal primary production needs, respectively (Li 2010). Therefore, the nutrients released by filter-feeding fish were insignificant compared with those required by algae in Lake Qiandao (Li 2010). Given that the N/P ratio of bighead carp is much higher than that of other food organisms, excess nitrogen is discharged, and phosphorus is fixed, and thus homeostasis of its element composition is sustained (Li 2010). At the end of the current experiment, the weights of bighead carp in the LF, MF, and HF groups were 1.78, 1.55, and 1.58 times those of their respective initial weights. Li (2010) concluded that the contents of N and P in bighead carp were 9.99 ± 0.07% and 3.70 ± 0.09%, respectively. According to these proportions, the effective preservation of N and P of the current treatments was calculated as follows: LF, N 4211.34–4317.78 g, P 1559.75–1599.18 g; MF, N 5835.86–5920.30 g, P 2161.43–2192.70 g; HF, N 8726.66–8929.49 g, and P 3232.10–3307.22 g. TP in each treatment group was significantly lower than that in the control group, and the effect intensity gradually increased with experimental time, mainly showing a downward trend in the P/A curve (Fig. 4). The inhibition intensity of TN fluctuated greatly with sampling time. Therefore, the bighead carp played a key role in the phosphorus sink in the experimental pond. Meanwhile, bighead carp improved the WSD and maintained the stability of the pH value. As mentioned in the introduction, experiments were carried out using earthen ponds rather than enclosures or concrete ponds (without natural bottom) will directly determine the final results of the experiment.
Some studies directly or indirectly emphasized that bighead carp is selective in phytoplankton feeding and shows no interest in cyanobacteria (Brooks and Dodson 1965; Cremer and Smitherman 1980). The results of other studies concluded that the sparser gill rakers of bighead carp automatically filter out the flow of small algae and therefore opportunistically “select” relatively large particles, such as zooplankton, compared with those of silver carp (Opuszynski and Shireman 1993; Xie and Liu 2001). Some researchers found intact and undamaged algae in the excreted feces of bighead carp, which were cytoactive (Xie 2003). Even these algae showed high growth rates and photosynthetic activity (Wang et al. 2014), which provide evidence of the indigestible use of algae by bighead carp. Liu and Zhang (2016) had systematically explained these research results. However, many studies confirmed that bigheaded carp is efficient in controlling total phytoplankton biomass when phytoplankton is dominated by either net phytoplankton or cyanobacteria concentrated in dense blooms or floating mats (Laws and Weisburd 1990; Radke and Kahl 2002; Collins and Wahl 2017). In the current study, no cyanobacterial blooms were observed in the treatment groups. In the control groups, blooms suddenly occurred after the sixth sampling (D6, June 30), and a WSD of 0.40 m was obtained in the worst condition at the last sampling time. The community composition and biomass of Cyanophyta, Chlorophyta, and Bacillariophyta occupied the main proportions of phytoplankton in all the experimental groups (Opuszynski and Shireman 1993; Tang et al. 2002; Yi et al. 2016a). The LF group had the most phytoplankton species, whereas the MF group had the least, suggesting that there was a weak correlation between the number of phytoplankton species and bighead carp stocks. Strong predation intensity on phytoplankton biomass (in particular, cyanobacteria taxa) was cascaded by bighead carp from the P/A value of phytoplankton and CCA analysis (Fig. 11a), and MF and HF groups displayed greater cyanobacteria reduction rates than that the LF group, and the dominant species transferred to green algae and diatoms. The phytoplankton biomass of the LF group reached its peak at the fourth sampling time (the cyanobacteria biomass accounted for 90.92% of the total biomass) but decreased rapidly thereafter, further indicating that bighead carp stocking had an inhibitory effect on cyanobacteria. The H′, R, and J indices of phytoplankton in the control group were the lowest among the experimental groups possibly because of the blooms that occurred. Therefore, bighead carp can play roles in controlling algae biomass and improving phytoplankton diversity and has the best effect in the MF and HF groups.
Our study found that intense predation by bighead carp reduced the biomass of zooplankton, shifting community structure toward smaller individuals (such as Rotifera and Copepoda nauplii; (Collins and Wahl 2018). The ability of fish to detect and capture a particular prey depends on the conspicuousness and escape behavior of that prey (Lazzaro 1987; Opuszynski and Shireman 1993). Cladocera is more vulnerable than Copepoda and is easier to be captured by filter-feeding planktivores (Szlauer 1965; Drenner et al. 1978; Drenner et al. 1982; Gophen et al. 1983), but the bighead carp had a strong effect on Cladocera and Copepoda biomass possibly because of the lack of Cladocera in the treatment groups (Yi et al. 2016a). The standing crops of crustaceans and Rotifera showed different changing trends (Fig. 5, Fig. 7) in the different treatment groups with different predation pressures, which was consistent with previous studies (Cremer and Smitherman 1980; Burke et al. 1986; Opuszynski and Shireman 1993; Yi et al. 2016a). In the early stages of the experiment, Rotifera biomass in the treatment groups was higher than that in the control group (Fig. 5), which was consistent with Rosińska et al. (2019). However, when cyanobacterial blooms appeared in the control group, Rotifera biomass increased rapidly. Cyanobacterial blooms severely restrict the growth of macrozooplankton and thus improve the competitiveness of smaller individuals (Lampert 1987; Fulton III and Paerl 1988). In the late stages of the experiment, the crustacean and phytoplankton standing crop of the treatment groups were at low levels. In the current experiment, earthen ponds were used for the control study. The result showed that bighead carp had a faster filter-feeding rate in these earthen ponds than in natural water bodies. Given that large ponds were used, the biomass of small zooplankton, particularly Rotifera, showed a decreasing trend. Filter-feeding fish stocking can lead to phytoplankton miniaturization (Ma et al. 2010), which is conducive to zooplankton feeding. In the ecosystems of natural lakes and reservoirs, filter-feeding fish feed on large phytoplankton, and thus nutrients are effectively used by micro- and nanophytoplankton, which in turn serves a food source for zooplankton. However, although filter-feeding fish may feed on zooplankton, silver and bighead carps and zooplankton have distinct ecological niches in the ecosystems of large or medium natural lakes and reservoirs, which enable zooplankton to thrive in waters without filter-feeding fish (Liu and Zhang 2016). The effect of filter-feeding fish on zooplankton is mainly reflected in the density and biomass of zooplankton rather than in the compositions of their species (Liu 1997; Li et al. 2018a). The findings from our experiment on the HF group showed that the number of zooplankton species was not markedly reduced compared with that in the control group. The H′, R, and J′ indices were slightly suppressed in the LF and MF groups compared with those in the controls, and strong inhibition was observed in the HF group. These findings are consistent with those in previous studies (Liu 1997; Mei 2010). Therefore, filter-feeding fish and zooplankton can achieve cooperation in algal control at a reasonable stocking density in natural water bodies.
Optimal density for the management of water environment using bighead carp alone
A study on Lake Taihu suggested that silver and bighead carps in mixed or separate cultures at high densities can be useful in controlling the occurrence of cyanobacterial blooms and the effective density of carp-controlled blooms is between 46 and 50 g/m3 and is based on the current state of a lake (Xie 2003). Ke et al. (2007) predicted that a higher stocking density (more than 40 g/m3) increases the controlling effect on microcystis biomass. Zhang et al. (2008) reviewed many field studies performed on scales from microcosmic to full lakes and concluded that filter-feeding carps reared at 30–70 g/m3 are effective in preventing the rapid growth of algae. A modified minimal model of planktivory by Attayde et al. (2010) presented a unimodal relationship between the biomass of omnivorous filter-feeding fish and phytoplankton biomass, and when the dry weight of the fish was 20 g/m3 (approximately 50 g/m3 wet weight), the plankton community had a stable state. A similar relationship was reported by Liu (2010), who concluded that algal control is effective when the biomass of filter-feeding fish reaches or exceeds the threshold.
Threshold density varies among researchers when the optimal algal density that can be effectively controlled by filter-feeding fish is considered. The interference of the external environment is an important factor that determines the direction of experimental results during in situ experiments. The experiments mentioned above have natural bottoms, and the experimental water body size was controlled. Season, duration, fish stocking density (whether carps stocked achieve threshold density), and the type of nutrition research water or nutritional status affect optimal threshold density and even directly lead to the failure of an experiment. A “Hopf bifurcation” tipping point and alternative stable states in which filter-feeding fish obviously can affect phytoplankton biomass decreasing and without having a significant impact on zooplankton diversity and stability (Attayde et al. 2010).
In this study, although the MF and HF groups can effectively control phytoplankton biomass (especially cyanobacteria), the protection of zooplankton diversity, richness, and evenness, the stocking density of the MF group exerted a higher effect than the HF group, and nutrient reduction (especially TP) was also more pronounced in the MF group. At the beginning of the experiment, the stocking density of the MF group was 23.5 g/m3, and at the end of the experiment, the existing density of bighead carp was 38.8 g/m3. Thus, bighead carp can be included in the EAR of lakes and reservoirs for the control of algal biomass and improvement of the status of water nutrients. However, for the protection of aquatic biodiversity, the amount of bighead carp stocking should be carefully considered.
Conclusion
In the current study, we carried out the experiment in eight earthen ponds, which had larger areas with nature bottoms and were similar to nature water body types, to research the effects of bighead carp stocking alone on eutrophic water columns. The results showed that (1) fish presence groups showed an improvement effect on water quality condition, acting as reductions in TN and TP, promotion in WSD, maintenance of the pH values at a low and stable state; (2) the phytoplankton biomass (especially cyanobacteria) were suppression markedly after carps stocked and different pressures were observed among the treatments, and the MF and HF groups tended to have better controls; (3) the bighead carp showed a clear preference for macro-crustacean zooplankton, especially in the HF group; and (4) the compositions and community structures of plankton showed different changing trends with the H′, R, J′ indices of phytoplankton in the fish presence groups and increased compared with those in the control groups, and meanwhile decreasing trends were observed in zooplankton in the HF groups, and no significant decrease in the LF and MF groups was observed. At a fish stocking density of 23.5–38.8 g/m3, after comprehensively comparing the effects of three stocking densities set in this experiment on the water environment indicators and on plankton, the highest efficiency in controlling cyanobacteria and promoting water condition was achieved, and the impact on zooplankton diversity was weak. Our results indicated that bighead carp can be included in the EAR of lakes and reservoirs, but the optimal density of bighead carp stocking should be carefully considered.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Attayde JL, van Nes EH, Araujo AIL, Corso G, Scheffer M (2010) Omnivory by planktivores stabilizes plankton dynamics, but may either promote or reduce algal biomass. Ecosystems 13:410–420. https://doi.org/10.1007/s10021-010-9327-4
Benndorf J (1990) Conditions for effective biomanipulation; conclusions derived from whole-lake experiments in Europe. Hydrobiologia 200-201:187–203. https://doi.org/10.1007/BF02530339
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28–35. https://doi.org/10.1126/science.150.3692.28
Burke JS, Bayne DR, Rea H (1986) Impact of silver and bighead carps on plankton communities of channel catfish ponds. Aquaculture 55:59–68. https://doi.org/10.1016/0044-8486(86)90056-6
Carpenter SR, Kraft CE, Wright R, He X, Soranno PA, Hodgson JR (1992) Resilience and resistance of a lake phosphorus cycle before and after food web manipulation. The American Naturalist 140:781–798. https://doi.org/10.1086/285440
Chen G, Wu ZH, Gu BH, Liu DY, Li X, Wang Y (2011) Isotopic niche overlap of two planktivorous fish in southern China. Limnology 12:151–155. https://doi.org/10.1007/s10201-010-0332-2
Chen SL, Liu XF, Hua L (1991) The role of silver carp and bighead in the cycling of nitrogen and phosphorus in the East Lake ecosystem. Acta Hydrobiologica Sinica 15:8–26 (in chinese)
Chen ZQ, Zhao D, Li ML, Tu WG, Luo XM, Liu X (2020) A field study on the effects of combined biomanipulation on the water quality of a eutrophic lake. Environmental Pollution 265, Part A, 115091. https://doi.org/10.1016/j.envpol.2020.115091
Chiang SC, Du NS (1979) Fauna Sinica, Crustacea, Freshwater Cladocera. Science Press, Academia Sinica, Beijing, China (in chinese)
Collins SF, Wahl DH (2017) Invasive planktivores as mediators of organic matter exchanges within and across ecosystems. Oecologia 184:521–530. https://doi.org/10.1007/s00442-017-3872-x
Collins SF, Wahl DH (2018) Size-specific effects of bighead carp predation across the zooplankton size spectra. Freshwater Biology 63:700–708. https://doi.org/10.1111/fwb.13109
Compte J, Gascón S, Quintana XD, Boix D (2012) The effects of small fish presence on a species-poor community dominated by omnivores: example of a size-based trophic cascade. Journal of Experimental Marine Biology and Ecology 418-419:1–11. https://doi.org/10.1016/j.jembe.2012.03.004
Cremer MC, Smitherman RO (1980) Food habits and growth of silver and bighead carp in cages and ponds. Aquaculture 20:57–64. https://doi.org/10.1016/0044-8486(80)90061-7
Deng JM, Qin BQ, Sarvala J, Salmaso N, Zhu GW, Ventelä AM, Zhang YL, Gao G, Nurminen L, Kirkkala T, Tarvainen M, Vuorio K (2016) Phytoplankton assemblages respond differently to climate warming and eutrophication: a case study from Pyhäjärvi and Taihu. Journal of Great Lakes Research 42:386–396. https://doi.org/10.1016/j.jglr.2015.12.008
Dickman M (1968) The effect of grazing by tadpoles on the structure of a periphyton community. Ecology 49:1188–1190. https://doi.org/10.2307/1934511
Domaizon I, Dévaux J (1999) Impact of moderate silver carp biomass gradient on zooplankton communities in a eutrophic reservoir. Consequences for the use of silver carp in biomanipulation. Comptes Rendus de l'Académie des Sciences-Series III - Sciences de la Vie 322:621–628. https://doi.org/10.1016/S0764-4469(00)88532-7
Dong SL, Li DS (1994) Comparative studies on the feeding selectivity of silver carp Hypophthalmichthys molitrix and bighead carp Aristichthys nobilis. Journal of Fish Biology 44:621–626. https://doi.org/10.1111/j.1095-8649.1994.tb01238.x
Drenner RW, Strickler JR, O'Brien WJ (1978) Capture probability: the role of zooplankter escape in the selective feeding of planktivorous fish. Journal of the Fisheries Research Board of Canada 35:1370–1373. https://doi.org/10.1139/f78-215
Drenner RW, deNoyelles F, Kettle JD (1982) Selective impact of filter-feeding gizzard shad on zooplankton community structure. Limnology and Oceanography 27:965–968. https://doi.org/10.4319/lo.1982.27.5.0965
FAO (2018) The state of world fisheries and aquaculture 2018. Food and Agriculture Organization of the United Nations, UN.
Fulton RS III, Paerl HW (1988) Effects of the blue-green alga Microcystis aeruginosa on zooplankton competitive relations. Oecologia 76:383–389. https://doi.org/10.1007/BF00377033
Garrido M, Cecchi P, Malet N, Bec B, Torre F, Pasqualini V (2019) Evaluation of FluoroProbe® performance for the phytoplankton-based assessment of the ecological status of Mediterranean coastal lagoons. Environmental Monitoring and Assessment 191:204. https://doi.org/10.1007/s10661-019-7349-8
Gophen M, Drenner RW, Vinyard GL (1983) Cichlid stocking and the decline of the Galilee Saint Peter's fish (Sarotherodon galilaeus) in Lake Kinneret, Israel. Canadian Journal of Fisheries and Aquatic Sciences 40:983–986. https://doi.org/10.1139/f83-124
Gophen M, Snovsky G (2015) Silver carp (Hypophthalmichthys molitrix, Val. 1844) stocking in Lake Kinneret (Israel). Open Journal of Ecology 5:343–351. https://doi.org/10.4236/oje.2015.58028
Gulati RD, Lammens EHRR, Meijer ML (1990) Biomanipulation, tool for water management; Proceedings of an international conference held in Amsterdam, The Netherlands, 8-11 August, 1989 (Developments in Hydrobiology). Kluwer Academic Publishers, Netherlands
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35:403–424. https://doi.org/10.1046/j.1529-8817.1999.3520403.x
Hu HJ, Wei YX (2006) The freshwater algae of China-Systematics, Taxonomy and Ecology. Science Press, Beijing, China (in chinese)
Huang XF, Hu CY (1986) Regression equations of the body weight to body length for common freshwater species of Cladocera. In: Chinese Crustacean Society (ed), Transaction of the Chinese Crustacean Society. No. 1. Science Press, Beijing, China (in chinese)
Jayasinghe UAD, García-Berthou E, Li ZJ, Li W, Zhang TL, Liu JS (2015) Co-occurring bighead and silver carps show similar food preference but different isotopic niche overlap in different lakes. Environmental Biology of Fishes 98:1185–1199. https://doi.org/10.1007/s10641-014-0351-7
Jeppesen E, Meerhoff M, Holmgren K, González-Bergonzoni I, Mello FT, Declerck SAJ, De Meester L, Søndergaard M, Lauridsen TL, Bjerring R, Conde-Porcuna JM, Mazzeo N, Iglesias C, Reizenstein M, Malmquist HJ, Liu ZW, Balayla D, Lazzaro X (2010) Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 646:73–90. https://doi.org/10.1007/s10750-010-0171-5
Jeppesen E, Søndergaard M, Laurids TL, Davidson TA, Liu ZW, Mazzeo N, Trochine C, Özkan K, Jensen HS, Trolle D, Starling F, Lazzaro X, Johansson LS, Bjerring R, Liboriussen L, Larsen SE, Landkildehus F, Egemose S, Meerhoff M (2012) Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Advances in Ecological Research 47:411–488. https://doi.org/10.1016/B978-0-12-398315-2.00006-5
Jin XC, Tu QY (1990) Survey standards of lake eutrophication, 2nd edn. China Environmental Press, Beijing, China (in chinese)
Ke ZX, Xie P, Guo LG, Liu YQ, Yang H (2007) In situ study on the control of toxic Microcystis blooms using phytoplanktivorous fish in the subtropical Lake Taihu of China: a large fish pen experiment. Aquaculture 265:127–138. https://doi.org/10.1016/j.aquaculture.2007.01.049
Lampert W (1987) Laboratory studies on zooplankton-cyanobacteria interactions. New Zealand Journal of Marine and Freshwater Research 21:483–490. https://doi.org/10.1080/00288330.1987.9516244
Lawrence SG, Malley DF, Findlay WJ, Maclver MA, Delbaere IL (1987) Method for estimating dry weight of freshwater planktonic Crustaceans from measures of length and shape. Canadian Journal of Fisheries and Aquatic Sciences 44. https://doi.org/10.1139/f87-301
Laws EA, Weisburd RSJ (1990) Use of silver carp to control algal biomass in aquaculture ponds. The Progressive Fish-Culturist 52:1–8. https://doi.org/10.1577/1548-8640(1990)0522.3.CO;2
Lazzaro X (1987) A review of planktivorous fishes: their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia 146:97–167. https://doi.org/10.1007/bf00008764
Legendre P, Legendre L (1998) Numerical ecology, volume 20, 2nd edn (Developments in Environmental Modelling), Elsevier.
Leventer H, Teltsch B (1990) The contribution of silver carp (Hypophthalmichthys molitrix) to the biological control of Netofa reservoirs. Hydrobiologia 191:47–55. https://doi.org/10.1007/BF00026038
Li PP (2010) Ecological stoichiometry of silver and bighead carps and their driven nutrient recycling in Lake Qiandao. Shanghai Ocean University, Shanghai, China (in chinese)
Li YL (2016) Fish resource stock and its distribution based on hydroacoustic surveys in Qiandao Lake. Shanghai Ocean University, Shanghai, China (in chinese)
Li XM, Zhu YJ, Gong JL, Wang XG, Yang DG (2018a) Zooplankton community composition and environmental characteristic in block partition pond with high density of bighead carp (Aristichthys nobilis). Acta Hydrobiologica Sinica 42:400–405 (in chinese)
Li BX, Chen MH, Liao HP, Sun YY, Tang HJ (2018b) Combined effects of dietary phosphorus level and polyculture on fish production, water quality, and plankton composition, in intensive culture of Crucian carp. Isr J Aquacult Bamidgeh 70:1516. https://doi.org/10.46989/001c.20919
Lin QQ, Chen QH, Peng L, Xiao LJ, Lei LM, Jeppesen E (2020) Do bigheaded carp act as a phosphorus source for phytoplankton in (sub) tropical Chinese reservoirs? Water Research 180:115841. https://doi.org/10.1016/j.watres.2020.115841
Liu ES (2010) Analysis on biomanipulation, non-traditional biomanipulation and discussion of the coun-termeasures of biomanipulation application in waters. Journal of Lake Sciences 22:307–314 (in chinese)
Liu QG (2005) Aquatic environment protection oriented (AEPO) fishery in L. Qiandaohu and Its influences on the lake ecosystem, East China Normal University, Shanghai, China (in chinese)
Liu QG, Zhang Z (2016) Controlling the nuisance algae by silver and bighead carps in eutrophic lakes: disputes and consensus. Journal of Lake Sciences 28:463–475 (in chinese)
Liu ZW (1997) Predation and biodiversity in aquatic ecosystem. Transactions of oceanology and limnology 2:19–24. https://doi.org/10.9774/GLEAF.978-1-909493-38-4_2
Lu M, Xie P, Tang HJ, Shao ZJ, Xie LQ (2002) Experimental study of trophic cascade effect of silver carp (Hypophthalmichthys molitrixon) in a subtropical lake, Lake Donghu: on plankton community and underlying mechanisms of changes of crustacean community. Hydrobiologia 487:19–31. https://doi.org/10.1023/A:1022940716736
Ma H, Cui FY, Liu ZQ, Fan ZQ, He WJ, Yin PJ (2010) Effect of filter-feeding fish silver carp on phytoplankton species and size distribution in surface water: a field study in water works. Journal of Environmental Sciences 22:161–167. https://doi.org/10.1016/S1001-0742(09)60088-7
Martin TH, Crowder LB, Dumas CF, Burkholder JM (1992) Indirect effects of fish on macrophytes in Bays Mountain Lake: evidence for a littoral trophic cascade. Oecologia 89:476–481. https://doi.org/10.1007/BF00317152
McCauley E (1984) Chapter 7. The estimation of the abundance and biomass of zooplankton in samples. In: Downing JA, Rigler FH (eds) A manual on methods for the assessment of secondary productivity in fresh waters. Blackwell Scientific, Oxford
McNaughton SJ (1967) Relationships among functional properties of Californian grassland. Nature 216:168–169. https://doi.org/10.1038/216168b0
McQueen DJ (2010) Freshwater food web biomanipulation: a powerful tool for water quality improvement, but maintenance is required. Lakes and Reservoirs: Research and Management 3:83–94. https://doi.org/10.1111/j.1440-1770.1998.tb00035.x
Mehner T, Arlinghaus R, Berg S, Dörner H, Jacobsen L, Kasprzak P, Koschel R, Schulze T, Skov C, Wolter C, Wysujack K (2004) How to link biomanipulation and sustainable fisheries management: a step-by-step guideline for lakes of the European temperate zone. Fisheries Management and Ecology 11:261–275. https://doi.org/10.1111/j.1365-2400.2004.00401.x
Mei Z (2010) The influences of silver carp and bighead in bio-manipuation pen on the community structure of Crustacean. Shanghai Ocean University, Shanghai, China (in chinese)
Mohammad N, Haque MM (2021) Impact of water quality on fish production in several ponds of Dinajpur Municipality Area, Bangladesh. International Journal of Science and Innovative Research 2:0100042IJESIR
Nistor V, Savin C, Tenciu M, Mocanu EE, Jecu E (2018) Eșanu VO (2018) Avoidance of algae bloom, key factor in the sustainable development of eco-effective growth techniques of carp (Cyprinus carpio) with silver carp (Hypophthalmichthys molitrix) and bighead carp (Hypophthalmichthys nobilis). Research Journal of Agricultural Science 50:127–135
Opuszynski K, Shireman JV (1993) Food habits, feeding behaviour and impact of triploid bighead carp, Hypophthalmichthys nobilis, in experimental ponds. Journal of Fish Biology 42:517–530. https://doi.org/10.1111/j.1095-8649.1993.tb00356.x
Pielou EC (1966) The measurement of diversity in different types of biological collections. Journal of Theoretical Biology 13:131–144. https://doi.org/10.1016/0022-5193(66)90013-0
Post JR, McQueen DJ (1987) The impact of planktivorous flsh on the structure of a plankton community. Freshwater Biology 17:79–89. https://doi.org/10.1111/j.1365-2427.1987.tb01030.x
Radke RJ, Kahl U (2002) Effects of a filter-feeding fish [silver carp, Hypophthalmichthys molitrix (Val.)] on phyto- and zooplankton in a mesotrophic reservoir: results from an enclosure experiment. Freshwater Biology 47:2337–2344. https://doi.org/10.1046/j.1365-2427.2002.00993.x
Rosińska J, Romanowicz-Brzozowska W, Kozak A, Goldyn R (2019) Zooplankton changes during bottom-up and top-down control due to sustainable restoration in a shallow urban lake. Environmental Science and Pollution Research 26:19575–19587. https://doi.org/10.1007/s11356-019-05107-z
Ruttner-Kolisko A (1977) Suggestions for biomass calculation of plankton rotifers. Arch Hydrobiol Beih Ergebn Limnol 8:71–76
Schindler DE, Knapp RA, Leavitt PR (2001) Alteration of nutrient cycles and algal production resulting from fish introductions into mountain lakes. Ecosystems 4:308–321. https://doi.org/10.1007/s10021-001-0013-4
SEPA (2002) Analytical methods for water and wastewater monitor, 4th edn. Chinese Environment Science Press, Beijing, China (in chinese)
Shannon CE (1948) A mathematical theory of communication. Bell System Technical Journal 27:623–656. https://doi.org/10.1002/j.1538-7305.1948.tb00917.x
Shapiro J, Lamarra V, Lynch M (1975) Biomanipulation: an ecosystem approach to lake restoration, Limnological Research Center, University of Minnesota, America.
Shen RJ, Gu XH, Chen HH, Mao ZG, Zeng QF, Jeppesen E (2021) Silver carp (Hypophthalmichthys molitrix) stocking promotes phytoplankton growth by suppression of zooplankton rather than through nutrient recycling: an outdoor mesocosm study. Freshwater Biology 66:1074–1088. https://doi.org/10.1111/fwb.13700
Sheng JR (1979) Fauna Sinica, Crustacea, Freshwater Copepoda. Science Press, Academia Sinica, Beijing, China (in chinese)
Sierp MT, Qin JG, Recknagel F (2009) Biomanipulation: a review of biological control measures in eutrophic waters and the potential for Murray Cod Maccullochella peelii peelii to promote water quality in temperate Australia. Reviews in Fish Biology and Fisheries 19:143–165. https://doi.org/10.1007/s11160-008-9094-x
Šmilauer P, Lepš J (2014) Multivariate analysis of ecological data using CANOCO 5. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9781139627061
Starling FLRM (1993) Control of eutrophication by silver carp (Hypophthalmichthys molitrix) in the tropical Paranoa Reservoir (Brasilia, Brazil): a mesocosm experiment. Hydrobiologia 257:143–152. https://doi.org/10.1007/BF00765007
Sterner RW, George NB (2000) Carbon, nitrogen, and phosphorus stoichiometry of cyprinid fishes. Ecology 81:127–140. https://doi.org/10.2307/177139
Szlauer L (1965) The refuge ability of plankton animals before plankton eating animals. Pol Arch Hydrobiol 13:89–95
Tan YJ, Li JL, Kang CX (1995) Studies on the effect of phosphorus fertilization and the role of siver carp and bighead carp in the control of eutrophication in enclosures. In: Zhu XB , Shi ZF (eds), Studies on the ecology of pond fish culture in China. Shanghai Scientific and Technical Publishers, Shanghai, China (in chinese)
Tang HJ, Xie P, Lu M, Xie LQ, Wang J (2002) Studies on the effects of silver carp (Hypophthalmichthys molitrix) on the phytoplankton in a shallow hypereutrophic lake through an enclosure experiment. Intl Rev Hydrobiol 87:107–119. https://doi.org/10.1002/1522-2632(200201)87:1<107::AID-IROH107>3.0.CO;2-J
ter Braak CJF (1994) Canonical community ordination. Part I: Basic theory and linear methods. Écoscience 1:127–140. https://doi.org/10.1080/11956860.1994.11682237
ter Heerdt G, Hootsmans M (2007) Why biomanipulation can be effective in peaty lakes. Hydrobiologia 584:305–316. https://doi.org/10.1007/s10750-007-0594-9
Vörös L (2000) Effective control of nuisance cyanobacterial blooms by biomanipulation. Egyptian Journal of Phycology 1, 267-277. 10.21608/egyjs.2000.113265
Wang HJ, Liang XM, Jiang PH, Wang J, Wu SK, Wang HZ (2008) TN : TP ratio and planktivorous fish do not affect nutrient-chlorophyll relationships in shallow lakes. Freshwater Biology 53:935–944. https://doi.org/10.1111/j.1365-2427.2007.01950.x
Wang JJ (1961) Fauna of freshwater Rotifera in China. Science Press, Beijing, China (in chinese)
Wang S, Wang QS, Zhang LB, Wang JX (2009) Large enclosures experimental study on algal control by silver carp and bighead. China Environmental Science 29:1190–1195 (in chinese)
Wang YP, Gu XH, Zeng QF, Gu XK, Mao ZG (2014) Growth and photosynthetic activity of Microcystis colonies after gut passage through silver carp and bighead carp. Acta Ecologica Sinica 34:1707–1715 (in chinese)
Xiao LJ, Ouyang H, Li HM, Chen MR, Lin QQ, Han BP (2010) Enclosure study on phytoplankton response to stocking of silver carp (Hypophthalmichthys molitrix) in a eutrophic tropical reservoir in South China. Intl Rev Hydrobiol 95:428–439. https://doi.org/10.1002/iroh.201011249
Xie P (1999) Gut contents of silver carp, Hypophthalmichthys molitrix, and the disruption of a centric diatom, Cyclotella, on passage through the esophagus and intestine. Aquaculture 180:295–305. https://doi.org/10.1016/S0044-8486(99)00205-7
Xie P (2001) Gut contents of bighead carp (Aristichthys nobilis) and the processing and digestion of algal cells in the alimentary canal. Aquaculture 195:149–161. https://doi.org/10.1016/S0044-8486(00)00549-4
Xie P, Liu JK (2001) Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: a synthesis of decades of research and application in a subtropical hypereutrophic lake. The Scientific World Journal 1:337–356. https://doi.org/10.1100/tsw.2001.67
Xie P (2003) Silver carp and bighead, and their use in the control of algal blooms. Science Press, Beijing, China (in chinese)
Yi CL, Guo LG, Ni LY, Luo CQ (2016a) Silver carp exhibited an enhanced ability of biomanipulation to control cyanobacteria bloom compared to bighead carp in hypereutrophic Lake Taihu mesocosms. Ecological Engineering 89:7–13. https://doi.org/10.1016/j.ecoleng.2016.01.022
Yi CL, Guo LG, Ni LY, Yin CJ, Wan J, Yuan CB (2016b) Biomanipulation in mesocosms using silver carp in two Chinese lakes with distinct trophic states. Aquaculture 452:233–238. https://doi.org/10.1016/j.aquaculture.2015.11.002
Zeng QF, Gu XH, Mao ZG, Zhou LH, Gao HM (2010) Ecological effect of the excretion from silver carp and bighead carp in algal bloom control: a review. Chinese Journal of Ecology 29:1806–1811 (in chinese)
Zhang JM, He ZH (1991) Handbook of inland waters fishery natural resources inquires. China Agriculture Press, Beijing, China (in chinese)
Zhang X, Xie P, Huang XP (2008) A review of nontraditional biomanipulation. The Scientific World Journal 8:1184–1196. https://doi.org/10.1100/tsw.2008.144
Zhao SY, Sun YP, Lin QQ, Han BP (2013) Effects of silver carp (Hypophthalmichthys molitrix) and nutrients on the plankton community of a deep, tropical reservoir: an enclosure experiment. Freshwater Biology 58:100–113. https://doi.org/10.1111/fwb.12042
Zhao SY, Sun YP, Han BP (2016) Top-down effects of bighead carp (Aristichthys nobilis) and Leptodora richardi (Haplopoda, Leptodoridae) in a subtropical reservoir during the winter-spring transition: a mesocosm experiment. Hydrobiologia 765:43–54. https://doi.org/10.1007/s10750-015-2398-7
Zhou XY, Zhang GF, Liu QG, Yan LL, Li JL (2011) Effects of Hyriopsis cumingii and Aristichthys nobilis on the enclosures phytoplankton community of Hypophthalmichthys molitrix pond. Journal of Fisheries of China 35:729–737
Zubcov E, Andreev N, Ungureanu L, Miron LD, Bagrin N, Zubcov N, Bulat D, Bileţchi L, Vulpe V (2021) Maintaining of good water quality—a prerequisite for healthy farmed fish. Sustainable use and protection of animal world in the context of climate change. 2021, 23-28. https://doi.org/10.53937/icz10.2021.03
Acknowledgments
The author acknowledges JY Pan, LP Ren, LS Chen, and GX He for their contributions during the field survey to this study.
Funding
This work was supported by the [National Key Research and Development Program and Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-Year Plan Period] (Grant numbers [2019YFD0900605] and [2015BAD13B00]).
Author information
Authors and Affiliations
Contributions
Experiment design, material preparation, and data analysis were performed by Z Zhang and QG Liu. Plankton identifications were done by YX Shi and JW Zhang. The first draft of the manuscript was written by Z Zhang, and QG Liu commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declare that they agree with the publication of this paper in this journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
11356_2022_19923_MOESM1_ESM.docx
Electronic Supplementary Material 1: Species composition of zooplankton in each experimental group. Electronic Supplementary Material 2: Species composition of phytoplankton in each experimental group (DOCX 53 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Shi, Y., Zhang, J. et al. Experimental observation on the effects of bighead carp (Hypophthalmichthys nobilis) on the plankton and water quality in ponds. Environ Sci Pollut Res 29, 56658–56675 (2022). https://doi.org/10.1007/s11356-022-19923-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19923-3