Abstract
In this study, a simple, one-pot, and eco-friendly biosynthesis of silver nanoparticles (AgNPs) was accomplished with the use of aqueous leaves extract of Cestrum nocturnum L.(AECN). Different techniques like ultraviolet–visible (UV–Vis) spectrophotometry, Fourier transform infrared (FTIR), X-ray diffraction (XRD), transmission electron microscopy (TEM), and scanning area electron diffraction were used to investigate the optical, operational, and physical properties of the green synthesized AECN-AgNPs.The AECN-AgNPs were further used for the detection of Hg2+ by UV–Vis and electrochemical methods. The disintegration of the AECN-AgNPs solution caused the formation of an Ag-Hg amalgam, which caused discoloration of the solution. Sensing performance for a variety of metals such as Na+, K+, Mg2+, Ca2+, Ni2+, Cu 2+, Fe3+, Zn2+, Co2+, Cd2+, Pb2+, As3+, and Mn2+ at 10-mM concentrations was measured in order to determine the selectivity of the sensor towards the Hg2+. For the electrochemical determination of 2 + Hg2+ , AECN-AgNPs were immobilized on a glassy carbon (GC) electrode, and the resulting modified electrode (GC/AECN-AgNPs) was characterized by cyclic voltammetry. This phenomenon is advantageously used for the sensitive determination of trace level Hg2+. GC/AECN-AgNPs demonstrated a linear calibration range of 100 nM to 10 μM and a limit of detection of 21 nM for Hg2+ determination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercuric ion (Hg2+) is considered the most stable toxic metal ion which pollutes the environment and affects human health (Toh et al. 2015; Tang et al. 2014; Zhou et al. 2013). Mercury is a heavy metal pollutant emitted by fossil fuel emissions (coal-burning thermal power plants), battery cells, mercury-based rituals, and hazardous material combustion (Park and Zheng 2012). It is reported that the Hg2+ level in the human body is gradually increasing through the food chain, causing serious and permanent injuries to the nervous system and brain (Zheng et al. 2003; Wei et al. 2013; Agraz et al. 1995). Its toxicity affects human health and causes severe damage to the kidney, heart, and lungs, as well as reducing the immune system (Azevedo et al. 2012; Rice et al. 2014; Chang et al. 1992) of human beings. The development of a sensitive, simple, and reliable technique is required for the routine detection of Hg2+ (Toh et al. 2015; Lou et al. 2011; Ding et al. 2016). Presently, a number of methods are available for Hg2+ detection, which include atomic absorption spectroscopy, atomic emission spectrometry, X-ray fluorescence spectrometry, colorimetric assay, and gas chromatography (Rey-Raap and Gallardo, 2012; Ioannidou et al. 2005; Aranda et al. 2013; Lin et al. 2016; Fang et al. 2016; Wu et al. 2012; Zaib et al. 2015). These techniques generally have high selectivity and sensitivity. However, sophisticated instruments, extensive sample pretreatments, and/or more samples are required, limiting their wide range of applications (Toh et al., 2015). Electrochemical methods are promising alternatives for sensitive Hg2 + determination in aqueous samples due to their low cost, high sensitivity, and short experimental time (Toh et al. 2015; Wei et al. 2013). The development of a well-organized method for the detection of Hg2+ is a matter of concern. Many different types of sensors have been developed, including those that use color, electrochemical methods, and fluorescence recognition (Cinti et al. 2016; Some et al. 2016; Wang and Bonakdar 1988). As a result, there is significant emphasis on the development of an effective sensor for the detection of mercury in the environment. For the determination of Hg2 + in the environment, several advanced instruments were used, including the inductively coupled plasma mass spectrometer (ICP-MS) (Cinti et al. 2016), the inductively coupled plasma optical emission spectrometer (ICP-OES) (Some et al. 2016), the cold vapor atomic absorption spectrometer (CVAAS) (Wang and Bonakdar, 1988), and the stripping voltammeter (Afkhami et al. 2012). The above instruments are expensive and time-consuming and necessarily involve a skilled operator for their handling. For the rapid detection of Hg2+, a few technologies based on spectrophotometric and probes of specific metals have been developed (Cinti et al. 2016; Some et al. 2016; Wang and Bonakdar 1988; Afkhami et al. 2012; Suaarez et al. 2000).
Electrochemical methods have been tested for Hg2+ determination using various materials/electrodes such as graphene oxide-ionic liquid composites/gold nanoparticles (Zhou et al. 2013), silver nanoparticle modified glassy carbon electrode (Toh et al. 2015), screen-printed carbon (Cinti et al. 2016; Some et al. 2016), carbon paste (Wang and Bonakdar 1988; Afkhami et al. 2012), and titanium dioxide (Suaarez et al. 2000). In spite of the above methods and materials, a sensitive electrode/material is still required for cost-effective, selective, and real-time determination of trace-level Hg2+ at low over potentials. Nowadays, scientists are interested in developing Hg2+ detection techniques that are eco-friendly, involving biomolecules and natural materials. Medicinal plants contain several phytoconstituents which may be useful for this purpose.
Cestrum nocturnum L. belongs to the Solanaceae family. It is commonly known as a night blooming jasmine and is widely distributed all over tropical as well as subtropical areas of the world, including Australia, India, Southern China, and the USA. It is an important horticultural plant used for ornamental and medicinal purposes. Several studies have demonstrated that it has a great deal of pharmacological activity, including antibacterial (Al-Reza et al. 2009), hepatoprotective activity (Qadir et al. 2014), and anticoagulant activity (Tyagi et al. 2016). It also contains biologically active phytochemicals like phenols, glycosides, saponins, and flavonoids.
The objective of the current work was to synthesize photo-induced green silver nanoparticles from aqueous leaf extract of C. nocturnum and to immobilize them in glassy carbon electrode for the detection of the Hg2+ ion.
Materials and methods
Chemicals and reagents
During the experiment, salts of various cations (Pb(NO3)2, KCl, MgCl2, MnSO4, CaCl2, HgCl2, ZnCl2, CdCl2, CoCl2, NiCl2, CuCl2, As2O3, and FeCl3) were used. Experimental salt solutions (1 mM) were made by mixing salt with Milli-q water and adjusting the pH with either 1.0 N HClor 1.0 N NaOH. Before use, the glassware was immersed in chromic acid and rinsed thoroughly with double distilled water. All chemicals have been purchased from SRL Chemicals Pvt. Ltd., India. For the electrochemical experiments, triple distilled water was used.
Instrumentation
The optical, operational, and physical properties of the green synthesized AECN-AgNPs were studied using UV–Visible, FTIR, X-ray diffraction (XRD), transmission electron microscopy (TEM), and scanning area electron diffraction (SAED). All the electrochemical measurements were carried out using CH instruments electrochemical workstation (CHI-660C, USA). A three-electrode system having glassy carbon (GC) or AECN-AgNPs immobilized GC (GC/AECN-AgNPs) as the working electrode, Ag/AgCl (saturated with KCl) as the reference electrode, and platinum wire as the counter electrode was used for electrochemical measurements.
Preparation of leaf extract
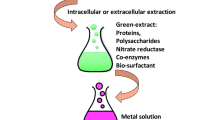
Fresh leaves of C. nocturnum were taken from the campus of Banaras Hindu University, Varanasi, India, and washed repeatedly with double distilled water to remove dirt particles and other adhering contaminants before being used to make the aqueous leaf extract. Thereafter, the leaves were allowed to air dry in the shade to completely remove moisture. The dried leaves were then grinded, and 25 g of leaf powder was added to 100 ml of double distilled water and boiled for 5 min at 60 °C. Aqueous filtrate of C. nocturnum was obtained by using Whatman filter paper No. 1. The prepared AECN was kept at 4 °C as a stock solution for future use.
Biosynthesis of AECN-AgNPs
For biosynthesis of AECN-AgNPs, 2 ml of AECN extract (1%) was added to 100 mL of AgNO3 (1 mM) solution. The reaction mixture was exposed to bright sunlight for 30 min. The reaction mixture had a neutral pH. In bright sunlight, the ambient temperature was 38 °C, with a solar intensity of 536 lx. The confirmation of synthesis of AECN-AgNPs was measured by using a UV–Vis spectrophotometer (Simadazu1800, Japan) between the ranges of 250 and 700 nm. All AECN-AgNPs biosynthesis procedures were carried out in direct sunlight in order to optimize the other factors individually. Factors like AECN inoculum dose (0.25% to 4.0%), duration of sunlight exposure (1–30 min), and AgNO3 concentration (0.5 to 5 mM) were optimized for proper synthesis of AECN-AgNPs. The biosynthesized AECN-AgNPs were isolated by centrifugation at 15,000 rpm for 15 min after the reaction was completed at these optimized reaction conditions. Further, AECN-AgNPs were re-dispersed in Milli-Q water to remove the organic substances and natural compounds. The procedure was executed multiple times, and the desired amount of AECN-AgNPs was obtained by drying.
Preparation of GC/AECN-AgNPs electrode
The GC electrode was cleaned to a mirror finish by hand polishing on a cloth pad with an aqueous neutral alumina slurry and then ultra-sonicated in water. The AECN-AgNPs suspension (10 μL, 1.0 mM) was drop casted on a dry and clean GC electrode and dried for 2 h. The resulting electrode, abbreviated as GC/AECN-AgNPs, was used for electrochemical studies and for the electrochemical determination of Hg2+.
Sensing of multi metal ion
The AECN-AgNPs solution was being used as a key solution for various metal ion detection. A fixed concentration (200 μL) of various metal ion solutions (Na+, K+, Mn2+,Mg2+, Ca2+, Ni2+, Cu 2+, Zn2+, Fe3+, Co2+, As3+,Cd2+, Pb2+, and Hg2+) was added to an AECN-AgNPs (5 ml) solution, and the absorption spectra was recorded using a UV–Vis spectrophotometer. The highest sensing ability of AECN-AgNPs and the decolorization of AECN-AgNPs solution were measured in the presence of Hg2+. Therefore, various concentrations (10 nM to 100 μM) of Hg2+ were applied for the determination of the linear sensing range by using AECN-AgNPs solution.
Effect of pH on the detection of Hg2+
To evaluate the Hg2+ detecting ability of AECN-AgNPs at different pH (4–10), the 100 μM of HgCl2 was spiked in double distilled water. After that, the pH of the HgCl2 solution was maintained from pH 4 to pH 10 through NaOH (1 N) and HCl (1 N). When 1 ml of HgCl2 solution of each pH was added to 1 ml dark brown solution of AECN-AgNPs, a distinct color change of the solution was observed. The spectra were recorded by the UV–Vis spectrophotometer at different time intervals (0 to 10 min).
Characterization of synthesized AECN-AgNPs
TEM (TECNAI 20 G2) performed at a voltage of 100–200 kV was used for the analysis of the shape and size of the synthesized AgNPs. A thin film of green synthesized AECN-AgNPs sample was prepared by dropping a small amount on copper grid, and extra sample was removed by using blotting paper and kept it for drying at room temperature for 15–30 min. FTIR for the AECN-AgNPs powder and extract of the C. nocturnum was used to identify the functional groups in a (Bruker) spectrophotometer IR affinity-1 by employing the KBr pellet technique and registering amplitude waves ranging from 400 to 4000 cm−1. The crystallinity of the biosynthesized AECN-AgNPs was demonstrated by X-ray diffraction (Rigaku Miniflex II). The circular scattering rings observed in the selected area electron diffraction represent the crystalline nature of the biosynthesized AgNPs.
Colorimetric detection of Hg2+ in real water samples
For the sensing of Hg2+ions in real water samples, the water samples such as drinking water, tap water, and river water were collected and filtered via the Whatman filter paper. These water samples were treated with 200 μl of three different concentrations of Hg2+ (50, 100, and 200 nM), and the colorimetric changes in the presence of AECN-AgNPs (200 μl) solution were examined using a UV–Vis spectrophotometer.
Statistical analysis
All of the tests have been done three times. The data collected throughout the study was analyzed and represented as mean ± SD.
Results and discussion
Photo-induced biosynthesis of AECN-AgNPs
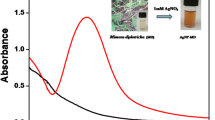
AECN (yellow color) was mixed with AgNO3 solution (colorless) and kept for 30 min under direct sunlight. The color of the reaction mixture changed from yellow to brown color (Fig. 1); this change in color indicates the biosynthesis of AECN-AgNPs. UV–Vis spectrophotometer result showed a sharp band in between 400 and 500 nm, while alone, AECN and AgNO3 solution did not show any SPR band (Fig. 1). The sharp SPR band indicates the biosynthesis of the AECN-AgNPs. The above result emphasizes the importance of sunlight in AECN-AgNPs biosynthesis. In order to maximize the efficiency of AECN-AgNPs biosynthesis, different factors like duration of light exposure, inoculum concentration, silver nitrate concentration, and pH of reaction mixtures were optimized.
Effect of light exposure on biosynthesis of AECN-AgNPs
The control samples containing AgNO3 or AECN alone did not show any change in color. Thus, the biosynthesis of AECN-AgNPs requires both AgNO3 and AECN. In the previous report (Kumar et al. 2017), the presence of light in the biosynthesis of AgNPs was required. The experiments were performed to optimize the duration of light exposure for the biosynthesis of AECN-AgNPs by exposing the reaction mixture to direct sunlight for various periods of time (1–30 min). It was observed that when reaction mixtures were subjected to direct sunlight, they changed color instantly within just a few seconds. UV spectra of the reaction mixture after each time period were recorded. Results revealed that SPR band intensity was increased with an increased duration of light exposure (Kumar et al. 2016a, b). At 30 min of sunlight exposure, the SPR band was very sharp. Furthermore, there was no change in SPR intensity (Fig. S1). It confirmed that the 30-min sunlight exposures to the reaction mixture were sufficient for AECN-AgNPs biosynthesis.
Effect of inoculum (AECN) dose on biosynthesis of AECN-AgNPs
To optimize the suitable concentration of the inoculums for efficient biosynthesis of nanoparticles, different concentrations (0.25% to 4%) of AECN were added to the reaction mixture, while other factors were kept constant. After 30 min of exposure to direct sunlight, the color of the reaction mixture changed from yellow to brown (Fig. S2) (Kumar et al. 2016a, b). Results revealed that 0.25% and 0.5% of AECN inoculum doses in the reaction mixtures produced a single, broader, and less intense SPR band. This confirmed that there was no proper synthesis of AgNO3. Further, on increasing the AECN inoculum dose from 1.0 to 4.0%, the intensity and clarity of the SPR band enhanced after 30 min of light exposure, indicating good biosynthesis of AECN-AgNPs in higher proportions (Fig. S2). UV spectra of the reaction mixture supplemented with 4.0% AECN inoculum showed maximum absorbance at 452 nm. Thus, AECN-AgNPs synthesis was optimized at 4.0% AECN inoculum (Kumar et al. 2017). AECN-AgNPs were also synthesized at high AECN dosages, but clumping of AECN-AgNPs takes place. The sharpening and expansion of the SPR band occurred due to the clump formation of AECN-AgNPs (Kumar et al. 2016a, b).
Effect of AgNO3 concentration on biosynthesis of AECN-AgNPs
To decide the most effective concentration of AgNO3 for the biosynthesis of AECN-AgNPs, different AgNO3 concentrations (0.5 mM to 5 mM) were added to the reaction mixture supplemented with 4% AECN and exposed to sunlight for 30 min. The SPR bands of AECN-AgNPs with various AgNO3 concentrations (0.5 mM, 1.0 mM, 2.0 mM, 3.0 mM, 4.0 mM, 5.0 mM) are shown in Figure S3. Based on the above findings, the color of the reaction mixture darkened progressively as the concentration of AgNO3 (Kumar et al. 2016a, b) was increased after 30 min direct sunlight exposure (Fig. S3). As a result, 5 mM AgNO3 was best for AECN-AgNPs biosynthesis.
Effect of pH of reaction mixture
The pH is another important parameter that influences the biosynthesis of silver nanoparticles. The pH of the reaction mixture also affects the size as well as the shape of biosynthesized AgNPs. It has the ability to alter the charges of bio molecules, which might be affecting its stabilizing and capping properties. The peak absorption wavelength shifts from 427 to 439 nm as the pH increases from 4 to 10 (Fig. S4). The above results indicate that pH 6 is the most suitable for the synthesis of AECN-AgNPs. It was observed that when AECN was added to different pH solutions, the color of the reaction mixture changed very fast from yellow to dark brown color with increasing pH. Peak shifting at various pH levels suggests about size as well as shape of the green silver nanoparticles, which was regulated by the pH of the reaction mixture (Kumar et al. 2016a, b).
Characterization of biosynthesized AECN-AgNPs
HR TEM analysis
The shape, size, and structure of silver nanoparticles were investigated by using the HRTEM technique (Kumar et al. 2016a, b). HRTEM images of the AECN-AgNPs (Fig. 2A–B) clearly indicate that synthesized nanoparticles are highly stable and are spherical in shape with a smooth surface without agglomeration. The particle size distribution of AECN-AgNPs was observed between 2 and 20 nm with an average particle size of 10 nm (Fig. 2C). HR TEM images of selected area electron diffraction patterns (SAED) of AECN-AgNPs confirmed the crystalline characteristics of the silver nanoparticle (Fig. 2D).
XRD
The diffraction peaks were visible in the XRD pattern of biosynthesized AECN-AgNPs at 32.17°, 44.47°, 64.34°, and 76.99°which were related to Bragg reflections (111), (200), (220), and (311) respectively (JCPDS file no. 00–004-0783). The crystalline planes of metallic silver’s face-centered cubic (fcc) crystal structure are represented by these Bragg reflections (Fig. 3).
FTIR spectral analysis
FTIR analysis of AECN showed peaks at 3361, 3017, 2930, 1635, 1541, 1428, 1292, 1078, 868, 842.9, and 790.8 cm−1. Peaks 1382, 1246, 1075, 839.1, 824.8, and 777.3 cm−1 were found in the AECN-AgNPs (Fig. S4). The -OH and C-H groups were reduced and sifted at 3356 cm−1 and 3011 cm−1 in the FTIR spectra of AECN-AgNPs. The peak shifting and intensity reduction represent the extract’s -OH and C-H groups involved in AgNPs formation. Methylene C-H stretch, alkenyl C = C stretch, aliphatic nitro compound, and organic sulphate groups were also involved in AECN-AgNPs formation. FTIR spectra of AECN-AgNPs showed weaker peaks than AECN. As shown above in Figure S5, AECN-AgNPs lacked all the AECN peaks. It means that the carboxyl, hydroxyl, peroxide, nitro, and organic sulfate groups found in AECN phytochemicals play a role in silver nanoparticle reduction and stabilization (Coates 2006).
AECN-AgNPs-based detection of Hg2+
The biosynthesized AECN-AgNPs were evaluated as spectrophotometric sensor for Hg2+ recognition under optimum conditions. The potential of AECN-AgNPs to detect metal ions was investigated separately for Na+, K+, Mg2+, Ca2+, Ni2+, Cu 2+, Fe3+, Zn2+, Co2+, Cd2+, Pb2+, As3+, Mn2+, and Hg2+ at a fixed volume of 200 μl of metal ion solution mixed with 5 ml of AECN-AgNPs solution. The intensity of the SPR band changed slightly when different metals (Na+, K+, Mg2+, Ca2+, Ni2+, Cu 2+, Fe3+, Zn2+, Co2+, Cd2+, Pb2+, As3+, Mn2+, and Hg2+) were added to the AECN-AgNPs solution, whereas the color of the solution stayed unchanged (Fig S6A). While Hg2+ has been added, however, the intensity of the SPR band shifted significantly, and the color of the solution changed from brown to colorless. Based on calorimetry data, it was clear that Hg2+ shows the highest sensitivity factor as compared to other metals (Fig. S6B). It has been shown that AECN-AgNPs can identify Hg2+ with high sensitivity and specificity (Kumar et al. 2017; Chen et al. 2013). Adding Hg2+ metal ions to the AECN-AgNPs solution revealed that the absorbance intensity changed as the concentration of Hg+ increased (Fig. S7A). Figure S7B shows the linear relationship between the change in absorption spectra and different concentrations of Hg2+.
Effect of pH on the detection of Hg2+
AECN-AgNPs detect Hg2+metal ions in a wide range of diverse physiological pH of water systems. Because of the high stability of AECN-AgNPs over a wide pH range, we were able to investigate its Hg2+ sensing capability at various pH levels. AECN-AgNPs show different sensing efficacy for pH 4 to pH 10 (Fig. S8–14), but high sensing of Hg2+ ions took place at pH 6(λ max 248 nm) at 1 min (Fig. S10), while pH 4, 5, 7, 8, 9, and 10 also detect Hg2+ but take more time in comparison to pH 6. Based on our findings, it is clear that at pH 6, high sensing of Hg2+ ions took place (Fig. S10 & Table S1).
Electrochemical characterization of the modified electrode
Cyclic voltammetry (CV) response of the GC/AECN-AgNPs electrode was recorded in 0.1 M KCl solution at a scan rate of 20 mVs−1(Fig. 4A). GC/AECN-AgNPs shows oxidation and reduction signals at + 0.12 and -0.54 V, respectively, which may be attributed to the oxidation of Ag0 to Ag+ and the reduction of Ag+ to Ag0, respectively (Goulart et al. 2018; Sonkar and Ganesan 2015; Sonkar et al. 2016; Tang et al. 2009; Bard and Faulkner, 1980). To get a stable oxidation peak, the GC/AECN-AgNPs electrode was initially subjected to 10 continuous CV scans, and after getting a constant oxidation response, the electrode was used for further electrochemical experiments. The CV response of GC/AECN-AgNPs was recorded with different scan rates (10 to 200 mVs−1) and is shown in Fig. 4B. On increasing the scan rate, the peak current was also increased linearly (inset of Fig. 4B). It indicates a surface confined process as expected for the ACEN-AgNPs immobilized electrodes (Toh et al. 2015; Bard and Faulkner 1980).
Electrochemical determination of Hg2+ ions
In the absence of Hg2+ ions, the linear sweep voltammetry (LSV) of GC electrode (Fig. 5A-a) does not exhibit any significant redox signals. However, GC/AECN-AgNPs (Fig. 5A–b) represents an oxidation signal due to the oxidation of AgNPs at 1.10 V in the absence of Hg2+ ions (vide supra). In the presence of Hg2+ ions, GC/AECN-AgNPs (Fig. 5A-c) displays high oxidation peak current (36.1 μA) at 0.18 V. This current response is much higher than that observed at abare GC electrode (0.17 V, 8.1 μA) under similar conditions (Fig. 5A-d).
A LSV response of GC (a, d) and GC/AECN-AgNPs (b, c) in absence (a, b) and presence (c, d) of 10 μM Hg2+ in 0.1 M KCl at a scan rate of 20 mVs−1. B LSV response at GC/AECN-AgNPs with different additions of Hg2+ from 100 nM to 40 μM. Inset represents the zoomed view of the oxidation signals. C Plot representing the variation of oxidation current for Hg2+ with respect to its concentration. D Calibration plot for Hg2+ determination from 100 nM to 10 μM
It is known that the Hg2+ ions in the presence of an excess of Cl− ions undergo to form HgCl42− which is further converted to Hg2Cl2 (Wei et al. 2013). Accordingly, the oxidation signal observed at the GC/AECN- AgNPs electrode in the presence of Hg2+ may be due to the oxidation of Hg+ to Hg2+ ions (Wei et al. 2013). This indicates that the Hg+ is electro catalytically oxidized to form Hg2+ at the GC/AECN-AgNPs electrode and this phenomenon can be used for the sensitive determination of Hg2+ originally present in a sample. The LSV oxidation peak current continuously increases with an increase in the concentration of added Hg2+ (Fig. 5B). The oxidation current reaches to a saturation level at high concentrations (> 20 μM) of Hg2+ probably due to the saturation of AECN-AgNPs catalytic sites (Tang et al. 2009; Bard and Faulkner, 1980)(Fig. 5C). The linear calibration range for the determination of Hg2+ at the GC/AECN-AgNPs electrode is found to be 100 nM to 10 μM (Fig. 5D). The limit of detection (3σ/m, where σ = standard deviation, m = slope of the calibration plot) is found to be 21 nM. These studies demonstrate the trace level determination of Hg2+ using the prepared AECN-AgNPs. In the electrochemical experiment, Hg2+ was determined using AgNPs. The following section discusses a proposed mechanism for the electrochemical determination of Hg2+ at the AgNPs modified electrode (Bhattacharjee and Chakraborty 2014; Suherman et al. 2017; Rastogi et al. 2014). The proposed reaction between Hg2+ and AgNPs may occur in the following steps:
Practical application
The proposed sensing activity for the detection of Hg+2at a nanomolar level may be applied to different samples. Here, it has been utilized in the detection of the Hg2+ level of four water samples (drinking water, tap water, pond water, and river water). A blank test was performed by the addition of a certain volume of water sample spiked with NaCl aqueous solution, and no change in spectra was found. A recovery experiment using different concentrations of Hg2+, including the maximum permitted level (200 nM), was also performed. A good recovery (more than 96%) and RSD (relative standard deviation) less than 5% were obtained as shown in Table S2.
The final outcome reveals that the composition of the drinking water and tap does not significantly interfere with Hg+ detection in comparison of the pond and river water sample. There may be a potential application of our proposed spectrophotometric method for the detection of Hg+ toxicity in real water samples.
Conclusions
The AECN-AgNPs were successfully synthesized via a green method using C. nocturnum aqueous leaf extract. The synthesized AECN-AgNPs were characterized by UV–Vis spectroscopy, TEM, XRD, and FTIR. The average particle size of AECN-AgNPs was 10 nm, and the shape of the nanoparticles was spherical. The biosynthesized AECN-AgNPs also showed the ability to specifically sense harmful Hg2+ ions in a wide pH range at different time intervals. AECN-AgNPs were immobilized on the GC electrode, and the modified electrode, GC/AECN-AgNPs, demonstrates high electro catalytic activity for the oxidation of Hg+ which can be used for the determination of Hg2+ originally present in a sample. The linear calibration range and detection limits are 100 nM to 10 μM and 21 nM, respectively. The proposed electrode, GC/AECN-AgNPs, could be a promising electrochemical sensor for the determination of trace level of Hg2+ in biological, pharmaceutical, food, and water samples. The proposed colorimetric assay holds great potential for the safety control of drinking water and the determination of Hg+2concentrations in various other water samples. Conclusively, AECN-AgNPs is an effective material for the detection of Hg2+using electrochemical and absorption methods.
Data availability
Data used in this study are freely available to the public from the described sources in the method section.
References
Afkhami A, Madrakian T, Sabounchei SJ, Rezaei M, Samiee S, Pourshahbaz M (2012) Construction of a modified carbon paste electrode for the highly selective simultaneous electrochemical determination of trace amounts of mercury(II) and cadmium(II). Sens Actuators B Chem. https://doi.org/10.1016/j.snb.2011.10.073
Agraz R, Sevilla MT, Hernandez L (1995) Voltammetric quantification and speciation of mercury compounds. J Electroanal Chem. https://doi.org/10.1016/0022-0728(95)03955-G
Al-Reza SM, Rahman A, Kang SC (2009) Chemical composition and inhibitory effect of essential oil and organic extracts of Cestrum nocturnum L. on food-borne pathogens. Int J Food Sci Technol. https://doi.org/10.1111/j.1365-2621.2009.01939.x
Aranda PR, Colombo L, Perino E, de Vito IE, Raba J (2013) Solid-phase preconcentration and determination of mercury(II) using activated carbon in drinking water by X-ray fluorescence spectrometry. X-Ray Spectrom. https://doi.org/10.1002/xrs.2440
Azevedo BF, Furieri LB, Peçanha FMI, Wiggers GA et al (2012) Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol. https://doi.org/10.1155/2012/949048
Bard AJ, Faulkner LR (1980) Electrochemical methods fundamentals and applications, 2nd edn. Wiley, New York. https://doi.org/10.1021/ed060pa25
Bhattacharjee Y, Chakraborty A (2014) Label-free cysteamine-capped silver nanoparticle-based colorimetric assay for Hg (II) detection in water with subnanomolar exactitude. ACS Sustain Chem Eng. https://doi.org/10.1021/sc500339n
Chang LW (1992) The concept of direct and indirect neurotoxicity and the concept of toxic metal/essential element interactions as a common biomechanism underlying metal toxicity. In: Isaacson RL, Jensen KF (eds) The vulnerable brain and environmental risks. Springer, Boston, pp 61–82. https://doi.org/10.1021/ac00153a011
Chen L, Fu X, Lu W, Chen L (2013) Highly sensitive and selective colorimetric sensing of Hg2+ based on the morphology transition of silver nanoprisms. ACS Appl Mater Interfaces. https://doi.org/10.1021/am3020857
Cinti S, Santella F, Moscone D, Arduini F (2016) Hg2+ detection using a disposable and miniaturized screen-printed electrode modified with nanocomposite carbon black and gold nanoparticles. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-6118-2
Coates J (2006) Interpretation of infrared spectra a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, Chichester. https://doi.org/10.1002/9780470027318.a5606
Ding Y, Wang S, Li J, Chen L (2016) Nanomaterial-based optical sensors for mercury ions. Trends Anal Chem. https://doi.org/10.1016/j.trac.2016.05.015
Fang Y, Pan Y, Li P, Xue M, Pei F, Yang W, Ma N, Hu Q (2016) Simultaneous determination of arsenic and mercury species in rice by ion-pairing reversed phase chromatography with inductively coupled plasma mass spectrometry. Food Chem. https://doi.org/10.1016/j.foodchem.2016.07.003
Goulart LA, Gonçalves R, Correa AA, Pereira EC, Mascaro LH (2018) Synergic effect of silver nanoparticles and carbon nanotubes on the simultaneous voltammetric determination of hydroquinone, catechol, bisphenol A and phenol. Microchim Acta. https://doi.org/10.1007/s00604-017-2540-5
Ioannidou MD, Zachariadis GA, Anthemidis AN, Stratis JA (2005) Direct determination of toxic trace metals in honey and sugars using inductively coupled plasma atomic emission spectrometry. Talanta. https://doi.org/10.1016/j.talanta.2004.05.018
Kumar V, Bano D, Mohan S, Singh DK, Hasan SH (2016a) Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater Lett. https://doi.org/10.1016/j.matlet.2016.05.097
Kumar V, Singh DK, Mohan S, Hasan SH (2016b) Photo-induced biosynthesis of silver nanoparticles using aqueous extract of Erigeron bonariensis and its catalytic activity against Acridine Orange. J Photochem Photobiol B Biol. https://doi.org/10.1016/j.jphotobiol.2015.12.011
Kumar V, Singh DK, Mohan S, Bano D, Gundampati RK, Hasan SH (2017) Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion. J Photochem Photobiol B Biol. https://doi.org/10.1016/J.JPHOTOBIOL.2017.01.022
Lin Y, Yang Y, Li Y, Yang L, Hou X, Feng X, Zheng C (2016) Ultrasensitive speciation analysis of mercury in rice by headspace solid phase microextraction using porous carbons and gas chromatography-dielectric barrier discharge optical emission spectrometry. Environ Sci Technol. https://doi.org/10.1021/acs.est.5b04328
Lou T, Chen Z, Wang Y, Chen L (2011) Blue-to-red colorimetric sensing strategy for Hg2+ and Ag+ via redox-regulated surface chemistry of gold nanoparticles. ACS Appl Mater. https://doi.org/10.1021/am200130e
Park JD, Zheng W (2012) Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. https://doi.org/10.3961/jpmph.2012.45.6.344
Qadir MI, al Murad MS, Ali M, Saleem M, Farooqi AA (2014) Hepatoprotective effect of leaves of aqueous ethanol extract of Cestrum nocturnum against paracetamol-induced hepatotoxicity. Bangladesh J Pharmacol. https://doi.org/10.3329/bjp.v9i2.17770
Rastogi PK, Ganesan V, Krishnamoorthi S (2014) A promising electrochemical sensing platform based on a silvernanoparticles decorated copolymer for sensitive nitrite determination. J Mater Chem A. https://doi.org/10.1039/C3TA13794E
Rey-Raap N, Gallardo A (2012) Determination of mercury distribution inside spent compact fluorescent lamps by atomic absorption spectrometry. Waste Manage. https://doi.org/10.1016/j.wasman.2011.12.001
Rice KM, Walker EM, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health. https://doi.org/10.3961/jpmph.2014.47.2.74
Some IT, Sakira AK, Mertens D, Ronkart SN, Kauffmann JM (2016) Determination of groundwater mercury (II) content using a disposable gold modified screen printed carbon electrode. Talanta. https://doi.org/10.1016/j.talanta.2016.02.033
Sonkar PK, Ganesan V (2015) Synthesis and characterization of silver nanoparticle-anchored amine-functionalized mesoporous silica for electrocatalytic determination of nitrite. J Solid State Electrochem. https://doi.org/10.1007/s10008-014-2725-3
Sonkar PK, Ganesan V, Gupta R, Yadav DK (2016) Simple route to anchor silver nanoparticles into thiol-functionalized mesoporous silica: synthesis, characterization and electrochemical applications. J Nanoparticle Res. https://doi.org/10.1007/s11051-016-3607-7
Suaarez MF, Mills A, Egdell RG, Compton RG (2000) Anodic stripping voltammetry with photochemical preconcentration at nanocrystalline TiO2 films: detection of Ag and Hg2+. Electroanalysis. https://doi.org/10.1002/(SICI)1521-4109(20000401)12:63.3.CO;2-9
Suherman AL, Ngamchuea K, Tanner EE, Sokolov SV, Holter J, Young NP, Compton RG (2017) Electrochemical detection of ultratrace (picomolar) levels of Hg2+ using a silver nanoparticle-modified glassy carbon electrode. Anal Chem. https://doi.org/10.1021/acs.analchem.7b01304
Tang Y, Allen BL, Kauffman DR, Star A (2009) Electrocatalytic activity of nitrogen-doped carbon nano tube cups. J Am Chem Soc. https://doi.org/10.1021/ja904595t
Tang S, Tong P, Lu W, Chen J, Yan Z, Zhang L (2014) A novel label-free electrochemical sensor for Hg2+ based on the catalytic formation of metal nanoparticle. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2014.02.074
Toh HS, Jurkschat K, Compton RG (2015) The influence of the capping agent on the oxidation of silver nanoparticles: nano-impacts versus stripping voltammetry. Chem Eur J 21:2998–3004. https://doi.org/10.1002/chem.201406278
Tyagi CK, Jhade D, Shah SK (2016) Evaluation of anticoagulant activity of aqueous extract of Cestrum nocturnum. Int J Phytomed. https://doi.org/10.5138/09750185.1969
Wang J, Bonakdar M (1988) Preconcentration and voltammetric measurement of mercury with a crown-ether modified-carbon-paste electrode. Talanta. https://doi.org/10.1016/0039-9140(88)80085-7
Wei Y, Yang R, Liu JH, Huang XJ (2013) Selective detection toward Hg (II) and Pb (II) using polypyrrole/carbonaceous nanospheres modified screen-printed electrode. Electrochim Acta. https://doi.org/10.1016/j.electacta.2013.05.004
Wu L, Long Z, Liu L, Zhou Q, Lee YI, Zheng C (2012) Microwave-enhanced cold vapor generation for speciation analysis of mercury by atomic fluorescence spectrometry. Talanta. https://doi.org/10.1016/j.talanta.2012.03.009
Zaib M, Athar MM, Saeed A, Farooq U (2015) Electrochemical determination of inorganic mercury and arsenic-a review. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2015.07.058
Zheng W, Aschner M, Ghersi-Egea JF (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. https://doi.org/10.1016/S0041-008X(03)00251-5
Zhou N, Li J, Chen H, Liao C, Chen Z, Chen L (2013) A functional graphene oxide-ionic liquid composites/gold nanoparticles sensing platform for ultrasensitive electrochemical detection of Hg2+. Analyst. https://doi.org/10.1039/C2AN36405K
Funding
This paper was supported in part of grant from UGC (F-1–17-1/2014–15/RGNF-2014–15-SC-UTT-58260) to New Delhi, Government of India, and is gratefully acknowledged. PKS acknowledges the University Grant Commission, New Delhi, India (UGC No.F.30–431/2018 (BSR), M-14–58), and Institute of Eminence (IoE) Seed Grant, Banaras Hindu University, India (Scheme No.6031), for the financial support. Author Kavindra Nath Tiwari also acknowledges to IoE, Banaras Hindu University, Varanasi, India, for the financial support for the research work (Scheme No.6031).
Author information
Authors and Affiliations
Contributions
P Kumar: optimization of AECN-AgNPs synthesis. KN Tiwari, SK Mishra: supervision, methodology. PK Sonkar and V Ganesan: electrochemical detection and validation of Hg2+ sensing. AK Singh, J Singh, JDixit: plant material collection and extract preparation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, P., Sonkar, P., Tiwari, K.N. et al. Sensing of mercury ion using light induced aqueous leaf extract mediated green synthesized silver nanoparticles of Cestrum nocturnum L. Environ Sci Pollut Res 29, 79995–80004 (2022). https://doi.org/10.1007/s11356-022-19357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19357-x