Abstract
Diazinon is a widely used pesticide that can be effectively degraded in aqueous solutions via photocatalytic oxidation. This quantitative systematic review was conducted to shed light on the various aspects of photocatalytic diazinon removal based on evidence. A systematic search was performed in Scopus, PubMed, Web of Science, Embase, and Ovid databases with keywords including diazinon, photocatalysis, and their equivalents. The search was limited to original articles in English published between January 1, 2010, and March 25, 2021. The results were expressed by descriptive statistics including mean, SD, median, and percentiles, among others. The initial electronic and manual search retrieved 777 articles, among which 41 studies comprising 49 trials were qualified for data synthesis. The reported diazinon degradation rate ranged from 2 to 100%, with a mean ± SD of 59.17 ± 28.03%. Besides, ZnO/UV, WO3/UV, TiO2/UV, and TiO2/Vis, in sequence, were the most widely used processes with the highest efficacies. Solution pH in the range of 5–8, catalyst dose below 600 mg/L, diazinon initial concentration below 40 mg/L, and contact time of 20–140 min could be the optimum conditions. Diazinon degradation obeyed the first-order kinetic model with kobs between 0.0042 and 1.86 min−1 and consumed energy of 38.93–350.36 kWh/m3. Diazoxon and IMP were the most detected by-products of diazinon degradation although bioassay data were scarce. Based on the results, photocatalytic processes are very efficient in removing diazinon from aqueous solutions although more elaborate studies are needed to assess the mineralization rate and effluent toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photocatalytic oxidation processes are drawing much attention for the removal of toxic and refractory pollutants. These processes benefit from catalysts of metal oxides (ZnO, TiO2, WO3, CeO2, Fe2O3), metal sulfides (CdS, PbS, CuS), metal–organic frameworks (MOFs), polymer-based, and so on, in either pristine or modified forms with nano- or micro-sizes to accelerate photo-driven reactions (Choi et al. 2017; Zhang et al. 2020, Samy, Ibrahim et al. 2021). A photocatalytic process is based on the photo-stimulation of semiconductors using ultraviolet or visible light. By absorbing the light with energy enough to exceed the bandgap energy of the semiconductor catalyst, the catalyst generates a positive hole (hVB+) in its valence band due to electron detachment (Rezaei and Mohseni 2017; Hosseini and Mohebbi 2018). The reaction of the photo-created holes (hVB+) with H2O or OH− produces •OH radicals that strongly oxidize organic pollutants. Besides, the detached electron, which is transferred to the conduction band (eCB−), participates in the reduction of O2 to •O2−, which is another agent capable of oxidizing organic species (Cadiau et al. 2020, Liu, Guo et al. 2020). Reactions driving the photocatalytic degradation of an organic pollutant are as follows (Yap et al. 2019):
As mentioned, the photocatalyst and the light source are two main role players in the photocatalytic reactions to initiate. Simple photocatalysts such as TiO2, ZnO, and WO3 are desirably used for this purpose, as they are usually associated with low-cost and labor-saving preparation (Biglari et al. 2017, Hadei, Mesdaghinia et al. 2021). However, many researchers are intended to incorporate some compounds into the crystalline structure of the photocatalysts to overcome some problems with simple photocatalysts or enjoy photocatalysts with new features. Preventing from the quick recombination of electrons and holes (Kumara et al. 2021), narrowing the bandgap and hence expanding the light spectrum for photocatalytic activation (Gong, Wu et al. 2021), increasing the separation properties of the photocatalyst from the bulk solution (Wang, Gao et al. 2021), and promoting the photostability of the photocatalyst (Goulart, Santos et al. 2021) are some reasons for modifying simple photocatalysts. Therefore, binary photocatalysts such as MoS2/ZnS (Joy et al. 2017), tertiary photocatalysts such as TiO2-Au-CdS (Zhao et al. 2016), and doped photocatalysts such as Fe-TiO2 (Borji et al. 2014; Tabasideh et al. 2017), Ag-TiO2 (Parastar et al. 2013), and Er-ZnO (Chemingui et al. 2021) have been developed.
The light source provides the energy required for the excitation of the photocatalyst in photocatalysis reactions. The light needs to provide valence band electrons with ample energy to move to the conduction band. The UV light at the wavelength of 254–365 nm is most widely used for photo-activating the catalysts (Dong et al. 2005; Daiqi and Xiaoshan 2010), as it emits highly energetic photons capable of exciting a wide variety of semiconductors with various bandgaps including TiO2 (band gap energy 3.2 eV), ZnO (3.2 eV), ZnS (3.7 eV), WO3 (2.8 eV), and V2O5 (2.7 eV). A large portion of the operating costs of a photocatalytic system is devoted to the energy required by the light source. In recent decades, a significant contribution has been made to invent visible light-harvesting catalysts so they can be activated with less energy cost under visible light which is also safer than UV. Some of them include Pt-doped TiO2 (Nakaoka et al. 2010), N-doped TiO2 (Salarian et al. 2016), WO3-doped ZnO (Maleki, Moradi et al. 2020), and Cd3OSO4 (Mohammadzadeh Kakhki and Ahsani 2020), which can also be activated in solar systems.
Like any other technology, there are some pros and cons for photocatalytic oxidation processes. Concerning advantages, they run under ambient temperature and pressure; employ some photo-stable, low-cost, readily available, and effective catalysts; and are capable of complete oxidation of refractory organics without the generation of unwanted by-products including sludge (Gunasekar et al. 2013; Favier et al. 2019, Li, Ivanenko et al. 2019, Noor, Sajjad et al. 2020). Among the disadvantages, we can point to the need for some high-cost light sources, e.g., UV and xenon lamps, the occurrence of some competing side-reactions, the fast recombination of electron–hole in some photocatalysts, the need for downstream separation of the catalyst, and the loss of photo-activity of the catalyst in successive use which necessitates the continuous addition of the fresh catalyst (Yousaf, Nadeem et al., Katzenberg et al. 2020). Despite the limitations, the photocatalytic oxidation of persistent organic pollutants has shown to be promising in the detoxification of effluents containing pesticides and pharmaceutical residues, providing a cleaner environment.
Diazinon with the formula of C12H21N2O3PS is a commonly used insecticide relying on phosphorus as one of its main constituents. This organophosphorus insecticide is classified by the World Health Organization (WHO) as “probably carcinogenic to humans” (class II) chemicals used to control pests mostly in agricultural fields (Kalantary et al. 2014; Fritschi et al. 2015). Although its usage is widely restricted in some regions such as the USA and the European Union, it is still one of the popular insecticides around the world (Khun et al. 2021). Diazinon has been tracked in water resources including freshwater, seawater, and even wastewater treatment plant effluents and some foods (Hassan, Rahaman et al. 2019). It is toxic to aquatic organisms at a concentration of 350 ng/L (Kalantary et al. 2014). Although there are limited data on the carcinogenic effects of diazinon on humans, there is some evidence about the adverse effects of diazinon on the liver and kidneys (Fritschi et al. 2015, Hassan, Rahaman et al. 2019). Due to its relative water solubility, non-polarity, moderate mobility, and persistence in soil, diazinon makes a great deal of concern for surface and groundwater contamination (Brady et al. 2006; Saraji et al. 2018). Therefore, its removal from aqueous solutions before discharging to the environment is of utmost importance.
Based on a literature review, there are tens of studies showing that photocatalytic oxidation is capable of complete or nearly complete removal of diazinon. These studies have tried relatively fixed operational parameters but in wide ranges. For instance, contact time has changed from 3 to 360 min and diazinon concentration from 1 to 500 mg/L. Besides, studies have utilized different catalysts to examine their efficacy for photo-activation and diazinon removal. Therefore, a comprehensive study may be helpful to summarize the data of previous studies and make firm conclusions, with the hope that results would help better understand the status quo and design more effective studies in the future. As a result, this quantitative systematic review was conducted to explore the operational parameters, their levels, and effects on selected outcomes in the photocatalytic removal of diazinon using various catalysts and light sources.
Materials and methods
Study protocol and search strategy
The recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green 2008) were followed for the conduct of this study. The protocol of the study was submitted in the International Prospective Register of Systematic Reviews (ID = CRD42020166279). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher, Liberati et al. 2009) was used to organize the current report.
To find studies that used photocatalytic processes for removing diazinon from aqueous solutions, a systematic search was performed in Scopus, PubMed, Web of Science, Embase, and Ovid databases. The search strategy contained the MeSH and non-MeSH terms including diazinon or equivalents (dimpylate or neocidol) and photocatalysis, photocatalytic, or photocatalyst in titles, abstracts, or keywords. Full search strategy in Scopus, for instance, was as follows: photocatalysis OR “photo catalysis” OR photocatalytic OR “photo catalytic” OR photocatalyst OR “photo catalyst” AND diazinon OR dimpylate OR neocidol. To avoid missing relevant papers possibly absent in the electronic search, the reference lists of the eligible studies were also checked out manually.
Eligibility criteria and study selection
Original articles in the English language investigating the photocatalytic removal of diazinon from aqueous solutions reporting on the diazinon degradation rate were included in the study. To obtain the most recent achievements, the search was limited to papers published between January 1, 2010, and March 25, 2021. Studies using auxiliary oxidants or purging gases, diazinon mixture with another pollutant, or those with non-extractable data were excluded from the study. The papers were reviewed and screened by two authors (FBA and MD) for the eligibility criteria, and any discrepancies in the study selection were discussed and resolved by the third author (A. h. M).
Quality assessment
The quality assessment of the selected studies was done by a researcher-made scale. This was done to avoid the risk of bias due to unqualified studies. The scale was constructed for the methodological assessment of interventional photochemical studies. The comments of five experts were sought to validate the scale. As shown in Supplementary File Table S1, the scale included 10 items with yes/no answers on various methodological aspects. Studies with at least five yeses were used for data extraction.
Data extraction
Two researchers (FBA and MD) independently read the selected papers and extracted the following data: authors’ name, article title, journal name, publication year, origin country, light source, catalysts, operational parameters, and their levels, degradation rate, mineralization rate, energy consumption, and reusability data. To affirm the accuracy of data, the extracted data were scrutinized, and any disagreement was discussed to reach a consensus. If there was some ambiguity in expressing data in a paper, we contacted the authors via email.
Data analysis
Quantitative data on operational variables and outcome measures were analyzed and presented with descriptive statistics including mean, standard deviation (SD), mode, minimum, maximum, the first percentile (Q1), median, the third percentile (Q3), the 90th percentile, the 95th percentile, and the 99th percentile. The statistics were computed separately for various photocatalytic processes grouped based on the types of the base photocatalyst and light source (UV/Vis). The relationships between operational variables and diazinon removal rates were examined using histograms drawn for removal rates above the 75th percentile (Q3) in each category. ImageJ software was used to extract data from graphs and OriginPro 2015 for drawing the graphs.
Results
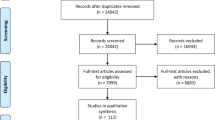
Figure 1 illustrates the PRISMA 2009 flow diagram of the study selection process. In summary, the electronic search retrieved 765 articles (453 in Scopus, 292 in Web of Science, 4 in PubMed, 10 in Embase, and 6 in Ovid). Besides, 12 studies were found in the reference list of relevant articles. Of 777 studies, 71 were examined in full-text. However, 30 studies were removed because of various reasons (Fig. 1). Finally, 41 articles were subject to quality assessment. Based on the quality assessment, the scores ranged from 5 to 10, indicating all the studies were qualified for data synthesis.
Table 1 summarizes the results of 41 relevant studies (comprising 49 trials) published since 2010 to investigate the effectiveness of photocatalytic processes for diazinon removal from aqueous solutions. There were 29 articles from Iran, four from Egypt, three from Indonesia, one from Thailand, one from Bangladesh, one from Japan, one from Vietnam, and one from China. Of 49 trials, UV lamps/LED were the sources of light in 30 (61.22%) trials, visible light in 15 (30.61%) trials, and sunlight in four (8.16%) trials. Thus, about 61% of the trials were conducted in the UV light spectrum and 39% in the visible light spectrum. A wide variety of catalysts were used for the photocatalytic removal of diazinon. We categorized them based on the main component exhibiting the photocatalytic property: TiO2-based (n = 22 studies; 53.66%), ZnO-based (n = 10; 24.39%), WO3-based (n = 2; 4.88%), metal–organic framework (MOF)-based (n = 2; 4.88%), VO4-based (n = 1; 2.44%), chalcogenide metal-based (n = 1; 2.44%), MgO-based (n = 1; 2.44%), hydroxyapatite-based (n = 1; 2.44%), and polymer-based (n = 1; 2.44%). Only 14 (34.15%) studies used pure compounds as photocatalysts, while the majority of them (n = 27; 66%) used a modification method for manipulating the photocatalytic materials. The reasons for modifications included expanding the photocatalytic activity into the visible light spectrum, increasing the capability of the process in removing diazinon, and facilitating the separation of photocatalyst residue among others. Initial solution pH, initial diazinon concentration, catalyst dose, and contact time were the most widely used operational variables whose effects were studies on the outcomes including diazinon degradation rate, mineralization rate, and energy consumption. We found that 21/49 (42.8%) trials claimed maximum diazinon removal rates of above 90% and 8/13 (61.5%) trials reported maximum mineralization rates of above 75%. Concerning the reusability of the photocatalyst, a vast majority of the studies reported no significant change in the efficacy of the photocatalyst for diazinon removal after 2 to 7 cycles of use.
Efficacy of photocatalytic removal of diazinon
The removal efficiency was the most intended outcome for researchers to seek in studies of photocatalytic removal of diazinon (n = 2427 data). Hassan and Elhadidy et al. (2017) proposed the following reactions for the degradation of diazinon in the presence of TiO2:
Figure 2A shows a box plot summarizing the efficacy of various types of photocatalytic processes for diazinon abatement. As can be seen, overall, the mean ± SD of the degradation rate was 59.17 ± 28.03%, with a median and IQR of 60% and 48.73%, respectively. Besides, 25% of the removal percentages were above 84.73% (Q3 value), and 10% of them were above 96% (90th percentile). Based on the process, the highest mean removal efficiency was obtained for MgO/UV (94.4 ± 3.08%), followed by MOF/UV (84.3 ± 15.9%), and the least removal efficiency was attained for ZnO/visible light (14.9 ± 8.6%). However, these results cannot be conclusive, as they were obtained from limited studies and/or trials, as shown in Supplementary File Table S2, and could be the subject of bias. Among the most widely used processes (those used in at least two studies), the highest mean removal rate belonged to ZnO/UV with 62.6 ± 26.01%, followed by TiO2/visible light with 59.8 ± 23.7% and TiO2/UV with 54.8 ± 26.9%. However, these three processes were adequate to bear maximum removal rates of 100%, most probably depending on operational variables. Figure 2B shows the histogram of the diazinon degradation rate above the third percentile, which is 84.73%, for various photocatalytic processes. This histogram depicts that ZnO/UV, WO3/UV, TiO2/UV, and TiO2/Vis, in sequence, are probably the most efficient photocatalytic processes for diazinon removal because they are more likely to generate degradation rates of above 84.73%.
A Box plot of variations of degradation rate in experimental studies of photocatalytic removal of diazinon, showing minimum, Q1, median, Q3, maximum, and mean (●) values; B histogram of degradation rates above the third percentile (84.73%) based on various photocatalytic processes, showing ZnO/UV, WO3/UV, TiO2/UV, and TIO2/Vis as the best processes, in sequence, applied for diazinon abatement
The mineralization rate is usually determined by measuring total organic carbon (TOC) in the treated sample. A 100% mineralization rate indicates no organic carbon content in the treated effluent. The mineralization reaction of diazinon in the presence of oxygen as the oxidizing agent is proposed as follows (Nakaoka et al. 2010):
In photocatalytic processes, the complete oxidation of diazinon is achieved, if any, at very long irradiation durations, which seems economically undesirable. We found only two processes for which the mineralization rates were reported (n = 124 data) (Supplementary File Table S2). The mean mineralization rate was 47.5 ± 27.5% for TiO2/UV and 60.6 ± 28.2% for TiO2/Vis. Pure TiO2 is a semiconductor with a wide bandgap of 3.2 eV, which is only activated with the energy of photons from UV light (Drygała 2021). However, modified TiO2 may have a narrower bandgap, with the possibility of being activated with lower energies in the spectrum of visible light. This may also have led to its higher ability to mineralize diazinon. However, one should note that heterogeneities between subgroups in this study, e.g., concerning initial diazinon concentration, may explain some differences in the obtained results.
Operational parameters
Solution pH
Initial solution pH is an influential factor that affects the performance of the photocatalytic degradation of diazinon. Figure 3A summarizes the variations of solution pH based on the photocatalytic process applied in various studies for assessing the effect of pH on diazinon removal. As can be seen, pH varied in a relatively constant range in different photocatalytic processes. As a whole, the pH varied from 2 to 12, with a mean and SD of 6.86 and 2.69 and a median and IQR of 7 and 4, respectively. However, 99% of the solution pH values were adjusted below 11 (Supplementary File Table S3). Concerning diazinon removal efficiency, the optimal pH was obtained in the neural condition in most studies (Hossaini et al. 2014, 2017; Moussavi et al. 2014; Jonidi-Jafari et al. 2015; Shirzad-Siboni et al. 2015; Salarian et al. 2016; Baneshi et al. 2017; Jonidi-Jafari et al. 2017; Shirzad-Siboni et al. 2017, Ahmadifard, Heydari et al. 2019, Ghodsi, Esrafili et al. 2020, Maleki, Moradi et al. 2020), an acidic condition in some cases (Nakaoka et al. 2010, Yang et al. 2010, Gar Alalm et al. 2015, Jafari et al. 2016, Mohagheghian et al. 2016, Zangeneh et al. 2018, Phuong, Chu et al. 2019, Rahimi-Nasrabadi, Ghaderi et al. 2019, Khaghani and Zare 2020), and alkaline condition in a few studies (Fadaei and Dehghani 2012, Molla, Furukawa et al. 2019). This could also be seen in Fig. 3B, which depicts the histogram of pH for diazinon removal efficiencies above the third percentile (74%) (n = 139). As seen, in about 69% (n = 96/139) of the cases, a removal of > 74% occurred in a pH range of 5–8, while pHs between 8 and 12 accounted for around 19% of the high removal percentages.
A Box plot of variations of solution pH in experimental studies of photocatalytic removal of diazinon, showing minimum, Q1, median, Q3, maximum, and mean (●) values; B histogram of applied solution pH in photocatalytic experiments (n = 139) giving a diazinon degradation rate above the third percentile (> 74%), showing pH 5.0–8.0 as the optimum pH
The variation in removal efficiency in different pH values is mostly related to pKa of diazinon, pHzpc of the used catalyst, and other operational conditions. As the pKa of diazinon is 2.6, the functional groups on the surface of diazinon are negatively and positively charged above and below pH 2.6, respectively. On the other hand, the pHzpc of the catalyst plays an important role in the status of the surface charge of the catalyst. It is expected that at pHs below pHzpc, the surface of the catalyst is negatively charged and vice versa. Therefore, considering the pka of diazinon and the pHzpc of the catalyst, it is believed that the best removal efficiency happens between the pHzpc of the catalyst and pka of diazinon (Gar Alalm et al. 2015, Jafari et al. 2016, Hossaini et al. 2017, Ahmadifard, Heydari et al. 2019, Khoiriah et al. 2020a, b). This improvement occurs because of the increase in electrostatic attraction between diazinon molecules and active sites of the catalyst due to a rise in attractive forces within this pH range (Gar Alalm et al. 2015, Ghodsi, Esrafili et al. 2020). This accelerates the adsorption of diazinon on the catalyst surface, which is necessary for the direct oxidation of diazinon. As much as electron–hole pairs at the surface of the catalyst recombine in a shorter time, this mechanism will be more essential for the degradation of diazinon (Jafari et al. 2016, Ahmadifard, Heydari et al. 2019). On the other hand, it is shown that by increasing pH from neural to alkaline values, a decreasing trend is observed in diazinon removal percentages (Hossaini et al. 2014, Jonidi-Jafari et al. 2015, Jafari et al. 2016, Mohagheghian et al. 2016, Baneshi et al. 2017, Hossaini et al. 2017, Jonidi-Jafari et al. 2017, Ghodsi, Esrafili et al. 2020, Khoiriah et al. 2020a, b). In some cases, this may be attributed to the homogeneity of charges on the catalyst surface and diazinon molecules (both with negative charges) above the pHzpc of the catalyst. As a result, a repulsive force is generated, and the removal efficiency of diazinon decreases (Gar Alalm et al. 2015; Jonidi-Jafari et al. 2015, Ghodsi, Esrafili et al. 2020).
Mohagheghian et al. investigated the removal efficiency of diazinon using WO3/UV. It was shown that by increasing pH from 3 to 11, the diazinon removal decreased from 99.88 to 19.8%. They believed that this decreasing trend could be related to the pHzpc of WO3 that is negative in alkaline conditions, so the adsorption of diazinon on the catalyst surface was prevented (Mohagheghian et al. 2016). Nakaoka et al. investigated the photodegradation of diazinon by platinized TiO2 at pH values 2–11. They reported that by increasing pH up to 7, there was a growing trend in removal efficiency. However, the trend sharply dropped after neural pH up to pH 11. Therefore, they postulated that OH− ions increased by increasing pH, so enhancing the production of •OH free radicals through the interaction between OH− ions and photo-generated holes. Therefore, the competition between diazinon and hydroxyl radicals for the surface sites of the catalyst causes a decrease in the removal efficiency in alkaline pH values (Nakaoka et al. 2010).
Catalyst dose
The photocatalyst is one of the most determining constituents of photocatalytic processes. What can make a photocatalyst preferable to another includes parameters such as suitable distribution of particle sizes, high surface areas, high chemical stability, and ability to absorb sunlight/UV. There are different methods and materials used for photocatalyst preparation. Semiconductors that can act as photocatalysts can be made in single, composite, modified, or doped forms (Moussavi et al. 2014, Hossaini et al. 2017, Ahmadifard, Heydari et al. 2019, Ayoubi-Feiz et al. 2019, Ghodsi, Esrafili et al. 2020, Joubani et al. 2020). One of the most widely used photocatalysts for diazinon removal is TiO2. It is widely used either in the single, doped, or binary forms (Nakaoka et al. 2010; Hossaini et al. 2014; Gar Alalm et al. 2015; Jonidi-Jafari et al. 2015; Jafari et al. 2016; Baneshi et al. 2017; Hassan et al. 2017; Mirmasoomi et al. 2017; Usman et al. 2017; Ayoubi-Feiz et al. 2018, Sheydaei, Karimi et al. 2019). Another commonly used photocatalyst for diazinon removal is ZnO (Fadaei and Dehghani 2012, Moussavi et al. 2014, Shirzad-Siboni et al. 2015, Hossaini et al. 2017, Jonidi-Jafari et al. 2017, Shirzad-Siboni et al. 2017, Rezaei, Dehghanifard et al. 2019, Maleki, Moradi et al. 2020, Mohammadia et al. 2020). Moreover, some nanostructured composite photocatalysts have been recently used for diazinon removal (Yang et al. 2010, Mohagheghian, Ayagh et al. 2017, Jansanthea et al. 2018, Zangeneh et al. 2018, Rahimi-Nasrabadi, Ghaderi et al. 2019, Ghodsi, Esrafili et al. 2020).
The examined studies utilized catalysts at different doses ranging from 1 to 6,000 mg/L. Based on Fig. 4A, the mean ± SD in the whole experiment was 578.7 ± 765.7 mg/L, with a median and IQR of 250 and 700 mg/L, respectively. Besides, 95% of the experiments were conducted in the presence of a 2000 mg/L catalyst dose (Supplementary File Table S4). Concerning the effect of the catalyst dose, the results showed that the removal efficiency of diazinon is enhanced by increasing the catalyst dose. This can be due to enhancing the available surface area, which provides more surfaces for diazinon molecules to be adsorbed. As a result, by enhancing available active sites, the chance of electron–hole pair formation increases, so more reactive radicals will be produced in the solution. However, the extra use of the photocatalyst can grow the turbidity of the solution, which, in turn, can inhibit the light penetration or enhance light scattering. Furthermore, the increasing dose can cause agglomeration of particles and consequently decrease the available active sites of the photocatalyst (Yang et al. 2010, Jonidi-Jafari et al. 2015, Shirzad-Siboni et al. 2015, Jafari et al. 2016, Salarian et al. 2016, Shirzad-Siboni et al. 2017, Zangeneh et al. 2018, Molla, Furukawa et al. 2019, Phuong, Chu et al. 2019, Toolabi et al. 2019, Ghodsi, Esrafili et al. 2020, Khoiriah et al. 2020a, b). In research conducted by Salarian and Hami et al. (2016), nitrogen-doped TiO2 nanosheets were produced through the hydrothermal method and used for diazinon removal under solar radiation. It was observed that when the amount of N-TiO2 increased up to 2.7 g/L, the degradation of diazinon was accelerated, and after that, a decreasing trend appeared. They stated that the reason for the enhanced degradation of diazinon could be due to the highly available active sites on TiO2, increased absorption of light photons on active sites, and high penetration of light into the solution. However, they inferred that the subsequent falling trend in removal efficiency was because of the enhanced turbidity of the solution induced by overdosing the catalyst and consequently light scattering. Figure 4B also provides information in favor of these explanations. The figure shows the histogram of the catalyst dose for diazinon removal efficiencies above the third percentile of 78% (n = 116). Notably, the majority of the high removal rates (86/116; 74.13%) were obtained approximately at doses below 600 mg/L, and the minority of them could be efficient at doses above this value. This confirms that (1) increasing catalyst dose can lead to increased removal only in a certain range and (2) photocatalytic processes are very efficient even at low catalyst doses although this largely depends on the type of the catalyst and its structural and chemical quality to be efficiently activated at the presence of light.
A Box plot of variations of catalyst dose in experimental studies of photocatalytic removal of diazinon, showing minimum, Q1, median, Q3, maximum, and mean (●) values; B histogram of applied catalyst dose in photocatalytic experiments (n = 116) giving a diazinon degradation rate above the third percentile (> 78%), showing doses < 600 mg/L as the optimum catalyst dose
Initial diazinon concentration
In the examined studies, the range of initial concentration of diazinon varied widely from 1 to 500 mg/L, with a mean ± SD of 32.4 ± 35.84 mg/L and median and IQR of 20 and 30 mg/L, respectively (Fig. 5A). However, 99% of the initial concentrations adjusted were equal to or below 100 mg/L (Supplementary File Table S5). Although these high concentrations are very less likely to occur in natural waters or even industrial wastewaters, in experimental studies, it is necessary to change the levels of diazinon concentration in an adequately wide range. Thus, one can elucidate the efficacy of the treatment in response to varying concentrations of the pollutant. Besides, wastewaters usually contain a mixture of various organic pollutants, summing up to a few tens of milligram per litter concentrations, which may compete for •OH that act unspecific to the target pollutants. One way for simulating these high concentrations is to add to the diazinon concentration in the experiments. Taking a glance at Supplementary File Table S5, it is seen that the mode concentration of diazinon was 10 mg/L, implying that researchers tended to keep a balance between real and experimental conditions.
A Box plot of variations of diazinon initial concentration (mg/L) in experimental studies of photocatalytic removal of diazinon, showing minimum, Q1, median, Q3, maximum, and mean (●) values; B histogram of applied diazinon initial concentration in photocatalytic experiments (n = 128) giving a diazinon degradation rate above the third percentile > 85.5%, showing diazinon initial concentration < 40 mg/L as optimum
Analyzing the results of different studies, an increase in the initial concentration of diazinon led to a decrease in removal efficiency. Besides, Fig. 5B shows the distribution of initial concentrations for removal efficiencies above the third percentile of 85.5% (n = 128). As can be seen, almost all of the high removal percentages (124/128; 96.87%) were observed at the presence of low concentrations of diazinon (≤ 40 mg/L), which is also indicative of the inverse relationship between diazinon removal and its initial concentration. This can be related to the more diazinon molecules adsorbed on the catalyst surface at higher concentrations; thus, the active sites on the catalyst and consequently the production of reactive radicals would be limited. Moreover, the high density of diazinon molecules in the solution can inhibit light photons to reach the catalyst surface. Maleki and Moradi et al. (2020) investigated the effects of tungsten oxide-doped zinc oxide nanoparticles immobilized on glass substrates as a catalyst on photocatalytic removal of diazinon. The results showed that after 60 min, the removal efficiency reduced from 88.6 to 44.4% by raising the concentration of diazinon from 10 to 200 mg/L (Maleki, Moradi et al. 2020). Jafari and Moussavi et al. (2016) conducted the study of the degradation of diazinon using UVC and UVC/TiO2 processes. In this study, they investigated the effect of three different initial concentrations of diazinon on the photocatalytic process (5, 10, and 20 mg/L) at a constant dose of TiO2 (100 mg/L) and pH = 7. The results showed that the highest removal rate (especially at initial contact times) was obtained at the initial concentration of 5 mg/L, and after 40 min, the complete degradation occurred (Jafari et al. 2016). Fadaei and Dehghani (2012) investigated the removal of two organophosphorus pesticides (diazinon and malathion) using zinc oxide as the catalyst. To determine the effect of the initial concentration of diazinon on the photocatalytic process, the initial concentrations of 100, 300, and 500 µg/L were tested. They reported that by increasing initial concentration from 100 to 500 µg/L, the degradation rate dropped from 69.88 to 55.2%. They attributed this reduced degradation rate to the (1) formation and adsorption of degradation by-products on the catalyst surface, which ended in occupying active sites on the catalyst surface, (2) decreased rate of light absorption due to increased adsorption of diazinon molecules on the catalyst surface, (3) enhancement of solution turbidity through the increase of diazinon concentration which inhibited light photons to penetrate the solution, (4) absorption of light photons in the path length by diazinon molecules rather than the catalyst, which could reduce catalytic efficiency, and (5) adsorption of diazinon molecules, instead of OH− ions, on the catalyst surface, thereby reducing the generation of •OH radicals and the degradation rate, as well (Fadaei and Dehghani 2012).
Contact time
In the reviewed studies, the contact time varied between 3 and 360 min (Fig. 6A). The mean ± SD in overall experiments was 67.4 ± 47.04 min, with a median and IQR of 60 and 60 min, respectively. However, 90% of the experiments were conducted at contact times below 120 min (Supplementary File Table S6), indicating the photocatalytic process acts relatively fast for diazinon removal. Figure 6B provides more information about the relationship of contact time in the photocatalytic process with the diazinon removal rate. As can be seen, removal efficiencies above the third percentile of 85% (n = 64) were more likely to obtain at contact times from 20 to 140 min (61/64; 95.3%). However, this finding could be biased due to the effect of other operational parameters, especially diazinon initial concentration.
A Box plot of variations of contact time (min) in experimental studies of photocatalytic removal of diazinon, showing minimum, Q1, median, Q3, maximum, and mean (●) values; B histogram of applied contact time in photocatalytic experiments (n = 64) giving a diazinon degradation rate above the third percentile (> 85%), showing contact time of 20–140 min as optimum
As known, when the contact time increases, more electrons will be excited at the catalyst surface, so increasing the chance of reactive radical production. This increase in contact time can enhance the possibility of collision between diazinon molecules and reactive radicals, leading to improved degradation and efficiency (Toolabi et al. 2019). In some cases, this increase in removal rate by increasing the contact time stops in a certain period, and after that, there is a constant trend or slight increase in the decomposition rate (Baneshi et al. 2017; Toolabi et al. 2019, Ghodsi, Esrafili et al. 2020). In a study conducted by Baneshi and Rezaei et al. (2017) on the photocatalytic degradation of diazinon by Fe-doped TiO2, the effect of contact time was investigated from 5 to 90 min. The results showed an increasing trend in diazinon removal up to 60 min (98.57%), and after that, the removal rate was negligible; so 60 min contact time was assigned as optimum in the study (Baneshi et al. 2017). Molla and Furukawa et al. (2019) investigated the effect of irradiation time on diazinon removal using nanosized-photocatalyst TiO2 in water under sunlight irradiation. The results show a sharp increase in removal efficiency up to 10 min. However, the complete decomposition occurred at 60 min (Molla, Furukawa et al. 2019). Hossaini and Moussavi et al. (2017) conducted a study on diazinon removal through CNS-ZnO/LED photocatalytic process. It was observed that by increasing contact time to 100 min, diazinon decomposition increased to 53.8%. However, after 100 min up to 200 min, this increase became slow and degradation reached 54.9%. Therefore, in this study, the optimum contact time for diazinon removal was determined as 100 min (Hossaini et al. 2017).
Kinetic studies
Zero-, first-, and second-order kinetic models have been utilized for assessing the kinetics of diazinon removal in heterogeneous photocatalytic systems. The linear forms of the equations for zero-, first-, and second-order kinetic models are shown in Eqs. (17) to (19):
In these equations, C0 is the initial diazinon concentration (mg/L), Ct is the diazinon concentration at time t (mg/L), t is the reaction time (min), k0 is the zero-order reaction constant (mg/L/min), kobs is the first-order reaction constant (1/min), and k2 is the second-order reaction constant (L/mg/min). Kinetic studies are usually conducted under fixed (optimum) conditions of initial diazinon concentration, solution pH, catalyst dose, etc. but the reaction time varies up to a few hours. Finally, the experimental data are fitted with the kinetic models to find the best fitting model. To do so, plots are drawn with Ct against t for the zero-order model, lnC0/Ct against t for the first-order model, and 1/Ct against t for the second-order model. The plot with the highest R2 value (closest to 1) shows the best describing model, and the intercept and the slope of the plot are utilized to calculate the rate constant.
Herein, we found 23 studies that investigated the kinetics of photocatalytic diazinon abatement. Of them, only six studies (Jonidi-Jafari et al. 2015, Jonidi-Jafari et al. 2017, Mohagheghian, Ayagh et al. 2017, Shirzad-Siboni et al. 2017, Ghodsi, Esrafili et al. 2020, Khoiriah et al. 2020a, 2020b) tried all the three kinetic states in order to prove the kinetics of the reaction, all of which showed that the first-order kinetic model best explains the experimental results. The other studies (n = 17 studies) only tried the first-order model presumably based on the assumption that diazinon degradation at low concentrations under photocatalytic conditions follows the first-order reaction laws (Jafari et al. 2016). Based on Table 1, the highest reaction rates were 1.86 min−1 (Fadaei and Dehghani 2012), 1.7 min−1 (Nakaoka et al. 2010), 0.127 min−1 (Samy, Ibrahim et al. 2021), 0.1234 min−−1 (Jonidi-Jafari et al. 2015), and 0.1233 min−1 (Mohagheghian et al. 2016), in sequence, which seems to be inversely related to the reaction time in the kinetic experiments (3 min, 30 min, 60 min, 120 min, and 120 min, respectively).
The Langmuir–Hinshelwood (L–H) model has been developed to describe the heterogeneous photocatalytic systems in which the substrate (herein diazinon) is adsorbed on the photocatalyst following a Langmuir isotherm, and the reaction takes place based on the rules of the first-order model (Ohtani 2011). Besides, the adoption equilibrium state should remain during photocatalytic degradation. In such a system, the rate of adsorption is always faster than the rate of degradation, and the photo-absorption process limits the rate of substrate abatement. The following equation is used to express the L–H model (Shirzad-Siboni et al. 2015):
where kc is the reaction rate constant of the surface-adsorbed substrate with electron–holes (mg/L/min) and K is the adsorption equilibrium constant (L/mg). To obtain the parameters, this equation can also be written as follows (Mohagheghian, Ayagh et al. 2017):
By plotting reciprocal kobs (obtained from the first-order reaction at different initial diazinon concentrations) against initial diazinon concentration, one can calculate K and kc values from the intercept and the slope of the plot, respectively. This was done by seven studies (Jonidi-Jafari et al. 2015, Shirzad-Siboni et al. 2015, Mohagheghian et al. 2016, Jonidi-Jafari et al. 2017, Mohagheghian, Ayagh et al. 2017, Shirzad-Siboni et al. 2017, Maleki, Moradi et al. 2020), which reported K values from 0.023 (Maleki, Moradi et al. 2020) to 0.176 L/mg (Shirzad-Siboni et al. 2017) and kc values ranging from 0.006 (Mohagheghian et al. 2016) to 0.926 mg/L/min (Shirzad-Siboni et al. 2015).
Energy consumption
Energy consumption is among various factors that can affect the selection of photocatalytic processes, as it affects the cost-effectiveness of the process. In the examined studies, the energy consumption was expressed as the number of kWh of electrical energy needed for one order of magnitude reduction (i.e., 90% removal) in diazinon concentration in 1 m3 of the solution, known as electrical energy per order (EEO). Therefore, it is directly related to the power of the light source and the duration of irradiation to reach a 90% removal rate and is applied only for artificial light sources. For this purpose, kobs from the first-order kinetic model can be utilized to compute the EEO values. Seven studies (Jonidi-Jafari et al. 2015, Shirzad-Siboni et al. 2015, Mohagheghian et al. 2016, Jonidi-Jafari et al. 2017, Mohagheghian, Ayagh et al. 2017, Shirzad-Siboni et al. 2017, Maleki, Moradi et al. 2020) used the following equation to calculate the EEO values as kWh/m3/order (Maleki, Moradi et al. 2020):
where P is the power of the light source (kW) and V is the solution volume (L). According to Table 1, the EEO was between 38.93 (Mohagheghian et al. 2016) and 350.36 kWh/m3 (Jonidi-Jafari et al. 2017). It seems that the lowest EEO values correspond to lower initial diazinon concentrations, as such solutions need less power and time to reach a 90% removal rate.
By-products
The formation of by-products is always one of the most challenging aspects of photocatalytic processes. Since photocatalytic processes are used to decompose toxic pollutants, it is expected that the effluent has less toxicity than the influent. However, it is not always true. Seven studies (Nakaoka et al. 2010, Jonidi-Jafari et al. 2017, Shirzad-Siboni et al. 2017, Ahmadifard, Heydari et al. 2019, Toolabi et al. 2019, Khaghani and Zare 2020, Samy, Ibrahim et al. 2021) investigated the formation of by-products of diazinon in photocatalytic degradation. The identified by-products are listed in Table S7.
The by-products produced at different reaction times may show the degradation pathway of the original compound. In photocatalytic processes, there are different pathways and consequently different intermediates for diazinon degradation depending on the experimental condition (Khaghani and Zare 2020). Diazoxon is mainly produced due to the desulfuration of diazinon via the substitution of oxygen by sulfur in the P = S bond through •OH radical attack or by direct diazinon oxidation. In some studies, diazoxon is reported as a more toxic compound than diazinon (Ahmadifard, Heydari et al. 2019, Toolabi et al. 2019). Another common intermediate is 2-isopropyl-6-methyl-4-pyrimidinol (IMP), which is suggested to be produced through the break of the pyrimidine ring (P-O) in diazinon, C-O bond in diazoxon, or even via the hydrolysis of diazinon. This compound is reported to be less toxic than primary diazinon (Čolović et al. 2010; Nakaoka et al. 2010, Ahmadifard, Heydari et al. 2019, Molla, Furukawa et al. 2019). The hydroxylation of the propyl group in diazinon is another phenomenon, which can cause further by-products such as hydroxydiazinon,2-hydroxydiazinon, 1-hydroxy isopropyl diazinon, and O,O-diethyl-O-(2-isopropyl-6-ethyl-5-hydrixypyrimidine-4-yl) (Nakaoka et al. 2010, Ahmadifard, Heydari et al. 2019, Molla, Furukawa et al. 2019, Khaghani and Zare 2020). These intermediates can turn into diazinon aldehyde and diazinon methyl ketone through further oxidation (Nakaoka et al. 2010). Besides, the oxidation of the C-O bond of diazinon can yield diethyl thiophosphate (DETP) or triethyl thiophosphate (TETP). The further degradation of these products can lead to triethyl phosphate or diethyl phosphonate production. These two products can also be produced through the hydrolysis of diazoxon. In the next step, triethyl phosphate or diethyl phosphonate can be converted into phosphoric acid and finally, carbon dioxide, phosphate, and water (Ahmadifard, Heydari et al. 2019, Molla, Furukawa et al. 2019, Samy, Ibrahim et al. 2021).
Hossaini and Moussavi et al. (2017) bioassayed the toxicity of by-products of diazinon degradation by Vibrio fischeri. Their results showed that the photoluminance of bacteria reduced by 55.3% and 8.9% in the influent and the sample treated by the CNS-ZnO/LED process, respectively, indicating the degradation of diazinon to less toxic compounds (Hossaini et al. 2017). Khaghani and Zare (2020) surveyed the toxicity of malathion and diazinon in a UV/nano-Zn process. They detected diethyl phosphate (DEP), diethyl thiophosphate (DETP), 2-isopropyl-6-methyl-4-pyrimidinol (IMP), hydroxydiazinon, O-analog diazinon (diazoxon), and some other compounds as the by-products of diazinon degradation. Moreover, the results showed that the by-products produced through diazinon decomposition were more in number and toxicity than those produced after malathion degradation. In this study, the EC50 values were 2.24 for Nitrobacter and 2.82 mg/L for Nitrosomonas, indicating that Nitrobacter was more sensitive than Nitrosomonas when exposed to photocatalytic by-products (Khaghani and Zare 2020).
One of the limitations of this study was the inclusion of studies conducted merely on diazinon, which limits the generalizability to other pesticides and refractory pollutants. Besides, there was high heterogeneity between studies concerning variables affecting the photocatalytic removal of diazinon, which may have been a source of bias. Despite limitations, this study provided data on the most important operational variables affecting photocatalysis reactions, as well as degradation rates, kinetics, required energy, and by-products in various photocatalytic processes, which may be used in setting up laboratory or even large-scale treatment systems. Future systematic reviews may provide information on non-operational variables and fixed conditions, e.g., reactor volume, mixing intensity, and light source power, as well as effluent toxicity, in the photocatalytic removal of diazinon or other persistent pollutants.
Conclusion
This quantitative systematic review could retrieve 41 studies assessing the photocatalytic removal of diazinon from aqueous solutions in 49 trials. About 63% of the trials were conducted in the UV light spectrum and 37% in the visible light spectrum. Besides, 14 studies utilized pure compounds as photocatalysts, while 27 of them used a modified catalyst. TiO2-based materials were the most frequent catalysts (n = 22 studies), followed by ZnO-based catalysts (n = 10 studies). All of the studies reported data on the diazinon degradation rate, which ranged from 2 to 100%, with a mean ± SD of 59.17 ± 28.03%. Based on the process, MgO/UV and MOF/UV were the most efficient processes for diazinon removal, and ZnO/Vis was the least efficient one. However, among the most widely used processes with the highest efficacies were ZnO/UV, WO3/UV, TiO2/UV, and TiO2/Vis, in sequence. Solution pH in the range of 5–8, catalyst dose below 600 mg/L, diazinon initial concentration below 40 mg/L, and contact time between 20 and 140 min could be the optimum conditions for diazinon degradation using photocatalytic oxidation. The degradation of diazinon obeys the first-order kinetic model with kobs between 0.0042 and 1.86 min−1, which depends on reaction time. The consumed energy ranged from 38.93 to 350.36 kWh/m3, which varied as a function of initial diazinon concentration. Diazoxon and IMP are the most detected by-products of diazinon degradation, although bioassay data are scarce. Based on the results, photocatalytic processes are very efficient for removing diazinon from aqueous solutions, although more elaborate studies are needed to assess the mineralization rate and toxicity of the effluent.
Data availability
All data used in this article are available for everyone on reasonable request.
References
Ahmadifard, T., R. Heydari, M. J. Tarrahi and G. S. Khorramabadi (2019). "Photocatalytic degradation of diazinon in aqueous solutions using immobilized MgO nanoparticles on concrete." Int. J. Chem. React. Eng. 17(9).
MG Alalm A Tawfik SJJMES Ookawara 2015 Combined Solar Advanced Oxidation and PAC Adsorption for Removal of Pesticides from Industrial Wastewater 6 800 809
Ayoubi-Feiz B, Mashhadizadeh MH, Sheydaei M (2018) Preparation of reusable nano N-TiO2/graphene/titanium grid sheet for electrosorption-assisted visible light photoelectrocatalytic degradation of a pesticide: effect of parameters and neural network modeling. J Electroanal Chem 823:713–722
Ayoubi-Feiz B, Mashhadizadeh MH, Sheydaei M (2019) Degradation of diazinon by new hybrid nanocomposites N-TiO2/graphene/Au and N-TiO2/graphene/Ag using visible light photo-electro catalysis and photo-electro catalytic ozonation: optimization and comparative study by Taguchi method. Sep Purif Technol 211:704–714
Baneshi MM, Rezaei S, Sadat A, Mousavizadeh A, Barafrashtehpour M, Hekmatmanesh H (2017) Investigation of photocatalytic degradation of diazinon using titanium dioxide (TiO2) nanoparticles doped with iron in the presence of ultraviolet rays from the aqueous solution. Biosci Biotechnol Res Commun 10(1):60–67
Biglari H, Afsharnia M, Alipour V, Khosravi R, Sharafi K, Mahvi AH (2017) A review and investigation of the effect of nanophotocatalytic ozonation process for phenolic compound removal from real effluent of pulp and paper industry. Environ Sci Pollut Res 24(4):4105–4116
Borji SH, Nasseri S, Mahvi AH, Nabizadeh R, Javadi AH (2014) Investigation of photocatalytic degradation of phenol by Fe (III)-doped TiO2 and TiO2 nanoparticles. J Environ Health Sci Eng 12(1):1–10
Brady J, Wallender W, Werner I, Fard BM, Zalom F, Oliver M, Wilson B, Mata M, Henderson J, Deanovic L (2006) Pesticide runoff from orchard floors in Davis, California, USA: a comparative analysis of diazinon and esfenvalerate. Agric Ecosyst Environ 115(1–4):56–68
Cadiau A, Kolobov N, Srinivasan S, Goesten MG, Haspel H, Bavykina AV, Tchalala MR, Maity P, Goryachev A, Poryvaev AS (2020) A titanium metal–organic framework with visible-light-responsive photocatalytic activity. Angew Chem 132(32):13570–13574
Chemingui H, Mzali JC, Missaoui T, Konyar M, Smiri M, Yatmaz HC, Hafiane A (2021) Characteristics of Er-doped zinc oxide layer: application in synthetic dye solution color removal. Desalin Water Treat 209:402–413
Choi S, Sang B-I, Hong J, Yoon KJ, Son J-W, Lee J-H, Kim B-K, Kim H (2017) Catalytic behavior of metal catalysts in high-temperature RWGS reaction: In-situ FT-IR experiments and first-principles calculations. Sci Rep 7(1):1–10
Čolović M, Krstić D, Petrović S, Leskovac A, Joksić G, Savić J, Franko M, Trebše P, Vasić V (2010) Toxic effects of diazinon and its photodegradation products. Toxicol Lett 193(1):9–18
Daiqi, Y. Y. H. B. Y. and C. Xiaoshan (2010). "Characterization and photocatalytic performance of CdS-TiO2/MWCNTs [J]." Chin. J. Environ. Eng. 2.
Dong Y-C, Bai Z-P, Zhong L-W, Wang Z-S, Zhu T (2005) Purification of indoor ammonia with nano-TiO2 loaded on fabric. China Environ Sci 25:26
Drygała, A. (2021). Influence of TiO2 film thickness on photovoltaic properties of dye-sensitized solar cells. IOP Conference Series: Earth and Environmental Science, IOP Publishing.
Fadaei AM, Dehghani MH (2012) Photocatalytic oxidation of organophosphorus pesticides using zinc oxide. Res J Chem Environ 16(3):104–109
Favier L, Rusu L, Simion AI, Hlihor RM, Pacala ML, Augustyniak A (2019) Efficient degradation of clofibric acid by heterogeneous photocatalytic oxidation process. Environ Eng Manag J 18(8):1683–1692
Fritschi L, McLaughlin J, Sergi C, Calaf G, Le Curieux F, Forastiere F, Kromhout H, Egeghy P, Jahnke G, Jameson C (2015) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Red 114(2):70134–70138
Gar Alalm M, Tawfik A, Ookawara S (2015) Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: operational conditions, kinetics, and costs. J Water Process Eng 8:55–63
Ghodsi, S., A. Esrafili, R. R. Kalantary, M. Gholami and H. R. Sobhi (2020). "Synthesis and evaluation of the performance of g-C3N4/Fe3O4/Ag photocatalyst for the efficient removal of diazinon: Kinetic studies." J. Photochem. Photobiol. A: Chem. 389.
Gong, B., P. Wu, J. Yang, X. Peng, H. Deng and G. Yin (2021). "Electrochemical and photocatalytic properties of Ru-doped TiO2 nanostructures for degradation of methyl orange dye." Int. J. Electrochem. Sci. 16(2).
Goulart, L. A., G. O. Santos, K. I. Eguiluz, G. R. Salazar-Banda, M. R. Lanza, C. Saez and M. A. Rodrigo (2021). "Towards a higher photostability of ZnO photo-electrocatalysts in the degradation of organics by using MMO substrates." Chemosphere 271: 129451.
Gunasekar V, Divya B, Brinda K, Vijaykrishnan J, Ponnusami V, Rajan K (2013) Enzyme mediated synthesis of Ag–TiO2 photocatalyst for visible light degradation of reactive dye from aqueous solution. J Solgel Sci Technol 68(1):60–66
Hadei, M., A. Mesdaghinia, R. Nabizadeh, A. H. Mahvi, S. Rabbani and K. Naddafi (2021). "A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO 2." Environ Sci Pollut Res: 1–17.
Hassan AF, Elhadidy H, Abdel-Mohsen AM (2017) Adsorption and photocatalytic detoxification of diazinon using iron and nanotitania modified activated carbons. J Taiwan Inst Chem Eng 75:299–306
Hassan, M., T. Rahaman, K. Ferdous, M. Parvez and M. Islam (2019). "Effect of dietary exposure to diazinon on different organs and hematological parameters of rabbit." J. Entomol. Zool. Stud.
Higgins, J. P. and S. Green (2008). "Cochrane handbook for systematic reviews of interventions."
Hossaini H, Moussavi G, Farrokhi M (2014) The investigation of the LED-activated FeFNS-TiO2 nanocatalyst for photocatalytic degradation and mineralization of organophosphate pesticides in water. Water Res 59:130–144
Hossaini H, Moussavi G, Farrokhi M (2017) Oxidation of diazinon in cns-ZnO/LED photocatalytic process: catalyst preparation, photocatalytic examination, and toxicity bioassay of oxidation by-products. Sep Purif Technol 174:320–330
Hosseini F, Mohebbi S (2018) Photocatalytic oxidation based on modified titanium dioxide with reduced graphene oxide and CdSe/CdS as nanohybrid materials. J Clust Sci 29(2):289–300
Jafari SJ, Moussavi G, Hossaini H (2016) Degradation and mineralization of diazinon pesticide in UVC and UVC/TiO2 process. Desalination Water Treat 57(8):3782–3790
Jansanthea P, Chomkitichai W, Ketwaraporn J, Pookmanee P, Phanichphant S (2018) Template-free hydrothermal synthesis of CeVO4 for photocatalytic degradation of insecticide diazinon. Digest J Nanomater Biostruct 13(4):969–975
Jonidi-Jafari A, Gholami M, Farzadkia M, Esrafili A, Shirzad-Siboni M (2017) Application of Ni-doped ZnO nanorods for degradation of diazinon: kinetics and by-products. Sep Sci Technol 52(15):2395–2406
Jonidi-Jafari A, Shirzad-Siboni M, Yang J-K, Naimi-Joubani M, Farrokhi M (2015) Photocatalytic degradation of diazinon with illuminated ZnO–TiO2 composite. J Taiwan Inst Chem Eng 50:100–107
Joubani MN, Zanjanchi M, Sohrabnezhad S (2020) The carboxylate magnetic–Zinc based metal-organic framework heterojunction: Fe3O4-COOH@ ZIF-8/Ag/Ag3PO4 for plasmon enhanced visible light Z-scheme photocatalysis. Adv Powder Technol 31(1):29–39
Joy M, Mohamed AP, Warrier K, Hareesh U (2017) Visible-light-driven photocatalytic properties of binary MoS2/ZnS heterostructured nanojunctions synthesized via one-step hydrothermal route. New J Chem 41(9):3432–3442
Kalantary RR, Shahamat YD, Farzadkia M, Esrafili A, Asgharnia H (2014) Heterogeneous photocatalytic degradation of diazinon in water using nano-TiO2: modeling and intermediates. Eur J Exp Biol 4(1):186–194
Katzenberg A, Raman A, Schnabel NL, Quispe AL, Silverman AI, Modestino MA (2020) Photocatalytic hydrogels for removal of organic contaminants from aqueous solution in continuous flow reactors. React Chem Eng 5(2):377–386
Khaghani R, Zare MR (2020) Toxicity of malathion and diazinon byproducts generated through the UV/nano-Zn process. Health Scope 9(1):7
Khoiriah K, Safni S, Syukri S, Gunlazuardi J (2020a) The operational parameters effect on photocatalytic degradation of diazinon using carbon and nitrogen modified TiO2. Rasayan J Chem 13(3):1919–1925
Khoiriah K, Wellia DV, Gunlazuardi J, Safni S (2020b) Photocatalytic degradation of commercial diazinon pesticide using C, N-codoped TiO2 as photocatalyst. Indones J Chem 20(3):587–596
Khun KK, Ash GJ, Stevens MM, Huwer RK, Wilson BA (2021) Compatibility of Metarhizium anisopliae and Beauveria bassiana with insecticides and fungicides used in macadamia production in Australia. Pest Manag Sci 77(2):709–718
Kumara KJ, Krishnamurthy G, Walmik P, Naik S, Rani RP, Naik N (2021) Synthesis of reduced graphene oxide decorated with Sn/Na doped TiO2 nanocomposite: a photocatalyst for Evans blue dye degradation. Emerg Mater 4(2):457–468
Li, Z., A. Ivanenko, X. Meng and Z. Zhang (2019). "Photocatalytic oxidation of methanol to formaldehyde on bismuth-based semiconductors." J. Hazard. Mater. 380: 120822.
Liu, C., W. Guo, J. Chen, J. Zou, Z. Wang and L. Wu (2020). "Ultrathin ZnTi-LDH nanosheets for photocatalytic aerobic oxidation of aniline based on coordination activation." Catal. Sci. Technol.
Maleki, A., F. Moradi, B. Shahmoradi, R. Rezaee and S. M. Lee (2020). "The photocatalytic removal of diazinon from aqueous solutions using tungsten oxide doped zinc oxide nanoparticles immobilized on glass substrate." J. Mol. Liq. 297.
Mirmasoomi SR, Ghazi MM, Galedari M (2017) Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep Purif Technol 175:418–427
Mohagheghian, A., K. Ayagh, K. Godini and M. Shirzad-Siboni (2017). "Using amino-functionalized Fe3O4-WO3 nanoparticles for diazinon removal from synthetic and real water samples in presence of UV irradiation." J. Adv. Oxid. Technol. 20(2).
Mohagheghian A, Karimi SA, Yang JK, Shirzad-Siboni M (2016) Photocatalytic degradation of diazinon by illuminated WO3 nanopowder. Desalin Water Treat 57(18):8262–8269
Mohammadia M, Malekib A, Zandib S, Mohammadib E, Ghahremanib E, Yangc J-K, Leed S-M (2020) Photocatalytic decomposition of aqueous diazinon using reduced graphene/ZnO nanocomposite doped with manganese. Desalin Water Treat 184:315–325
Mohammadzadeh Kakhki, R. and F. Ahsani (2020). "Development of a novel and high performance visible‐light‐induced Cd3OSO4 nanophotocatalyst for degradation of diazinon." Appl. Organomet. Chem. 34(9): e5770.
Moher, D., A. Liberati, J. Tetzlaff, D. G. Altman and P. Group (2009). "Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement." PLoS MED 6(7): e1000097.
Molla, M. A. I., M. Furukawa, I. Tateishi, H. Katsumata and S. Kaneco (2019). "Mineralization of Diazinon with nanosized-photocatalyst TiO2 in water under sunlight irradiation: optimization of degradation conditions and reaction pathway." Environ. Technol.
Moussavi G, Hossaini H, Jafari SJ, Farokhi M (2014) Comparing the efficacy of UVC, UVC/ZnO and VUV processes for oxidation of organophosphate pesticides in water. J Photochem Photobiol a: Chem 290:86–93
Nakaoka Y, Katsumata H, Kaneco S, Suzuki T, Ohta K (2010) Photocatalytic degradation of diazinon in aqueous solution by platinized TiO2. Desalin Water Treat 13(1–3):427–436
Noor, S., S. Sajjad, S. A. K. Leghari, I. Ahmad and T. Mahmood (2020). "Visible light efficient and photo stable nanostructure of GO/CuO/m-TiO2 ternary composite." Mater. Res. Express 6(12): 1250d1258.
Ohtani, B. (2011). Chapter 10 - photocatalysis by inorganic solid materials: revisiting its definition, concepts, and experimental procedures. Adv Inorg Chem. R. v. Eldik and G. Stochel, Academic Press. 63: 395–430.
Parastar S, Nasseri S, Borji SH, Fazlzadeh M, Mahvi AH, Javadi AH, Gholami M (2013) Application of Ag-doped TiO2 nanoparticle prepared by photodeposition method for nitrate photocatalytic removal from aqueous solutions. Desalin Water Treat 51(37–39):7137–7144
Phuong, N. M., N. C. Chu, D. V. Thuan, M. N. Ha, N. T. Hanh, H. D. T. Viet, N. T. M. Thu, P. V. Quan and N. T. T. Truc (2019). "Novel removal of diazinon pesticide by adsorption and photocatalytic degradation of visible light-driven Fe-TiO2 /Bent-Fe photocatalyst." J. Chem.
Rahimi-Nasrabadi, M., A. Ghaderi, H. R. Banafshe, M. Eghbali-Arani, M. Akbari, F. Ahmadi, S. Pourmasoud and A. Sobhani-Nasab (2019). "Preparation of Co2TiO4/CoTiO3/polyaniline ternary nano-hybrids for enhanced destruction of agriculture poison and organic dyes under visible-light irradiation." J. Mater. Sci.: Mater. Electron. 30(17): 15854–15868.
Rezaei R, Mohseni M (2017) Impact of pH on the kinetics of photocatalytic oxidation of 2,4-dichlorophenoxy acetic acid in a fluidized bed photocatalytic reactor. Appl Catal B 205:302–309
Rezaei, S. S., E. Dehghanifard, M. Noorisepehr, K. Ghadirinejad, B. Kakavandi and A. R. Esfahani (2019). "Efficient clean-up of waters contaminated with diazinon pesticide using photo-decomposition of peroxymonosulfate by ZnO decorated on a magnetic core/shell structure." Environ Manage. 250: 109472.
Salarian AA, Hami Z, Mirzaei N, Mohseni SM, Asadi A, Bahrami H, Vosoughi M, Alinejad A, Zare MR (2016) N-doped TiO2 nanosheets for photocatalytic degradation and mineralization of diazinon under simulated solar irradiation: optimization and modeling using a response surface methodology. J Mol Liq 220:183–191
Samy, M., M. G. Ibrahim, M. Fujii, K. E. Diab, M. ElKady and M. G. Alalm (2021). "CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor." Chem. Eng. J. 406: 127152.
Saraji M, Jafari MT, Amooshahi MM (2018) Sol–gel/nanoclay composite as a sorbent for microextraction in packed syringe combined with corona discharge ionization ion mobility spectrometry for the determination of diazinon in water samples. J Sep Sci 41(2):493–500
Sheydaei, M., M. Karimi and V. Vatanpour (2019). "Continuous flow photoelectrocatalysis/reverse osmosis hybrid reactor for degradation of a pesticide using nano N-TiO2/Ag/Ti electrode under visible light." J. Photochem. Photobiol. A: Chem. 384: 112068.
Shirzad-Siboni M, Jonidi-Jafari A, Farzadkia M, Esrafili A, Gholami M (2017) Enhancement of photocatalytic activity of Cu-doped ZnO nanorods for the degradation of an insecticide: kinetics and reaction pathways. Environ Manage 186:1–11
Shirzad-Siboni M, Khataee A, Vahid B, Joo SW (2015) Synthesis, characterization and immobilization of ZnO nanosheets on scallop shell for photocatalytic degradation of an insecticide. Sci Adv Mater 7(4):806–814
Tabasideh S, Maleki A, Shahmoradi B, Ghahremani E, McKay G (2017) Sonophotocatalytic degradation of diazinon in aqueous solution using iron-doped TiO2 nanoparticles. Sep Purif Technol 189:186–192
Toolabi A, Malakootian M, Ghaneian MT, Esrafili A, Ehrampoush MH, AskarShahi M, Tabatabaei M, Khatami M (2019) Optimizing the photocatalytic process of removing diazinon pesticide from aqueous solutions and effluent toxicity assessment via a response surface methodology approach. Rend Lincei Sci Fis Nat 30(1):155–165
Usman MR, Noviyanti AR, Eddy DR (2017) Photocatalytic degradation of diazinon using titanium oxide synthesized by alkaline solvent. Indones J Chem 17(1):22–29
Wang, S., H. Gao, L. Fang, Q. Hu, G. Sun, X. Chen, C. Yu, S. Tang, X. Yu and X. Zhao (2021). "Synthesis of novel CQDs/CeO2/SrFe12O19 magnetic separation photocatalysts and synergic adsorption-photocatalytic degradation effect for methylene blue dye removal." Adv. Chem. Eng. 6: 100089.
Yang ZP, Gong XY, Zhang CJ (2010) Recyclable Fe3O4/hydroxyapatite composite nanoparticles for photocatalytic applications. Chem Eng Sci 165(1):117–121
Yap H, Pang Y, Lim S, Abdullah A, Ong H, Wu C-H (2019) A comprehensive review on state-of-the-art photo-, sono-, and sonophotocatalytic treatments to degrade emerging contaminants. Int J Environ Sci Technol 16(1):601–628
Yousaf, F., F. Nadeem and A. El Zerey-Belaskri "Comparative analysis of conventional treatment methodologies and advanced processing techniques for reutilization of polluted ground water–a comprehensive review." Int. J. Chem. Biol. Sci.
Zangeneh H, Zinatizadeh AA, Feyzi M, Zinadini S, Bahnemann DW (2018) Photomineralization of recalcitrant wastewaters by a novel magnetically recyclable boron doped-TiO2-SiO2 cobalt ferrite nanocomposite as a visible-driven heterogeneous photocatalyst. J Environ Chem Eng 6(5):6370–6381
Zhang R, Wang J, Zhu X, Liu X, Liu H, Zhou Y, Dong S, La P, Yao J, Liu B (2020) Phase-separated Ce–Co–O catalysts for CO oxidation. Int J Hydrog Energy 45(23):12777–12786
Zhao H, Wu M, Liu J, Deng Z, Li Y, Su B-L (2016) Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl Catal B 184:182–190
Acknowledgements
The authors would like to thank the authors of relevant papers who responded to emails and provided us with additional information on their work.
Funding
The study was funded by the Institute for Environmental Research, Tehran University of Medical Sciences, Tehran, Iran (Grant #48166). The funder had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Amir-Hossein Mahvi: conceptualization, critical revision, and editing of the manuscript.
Fateme Barjasteh-Askari: initial search, data extraction, manuscript drafting, data analysis, and interpretation.
Mojtaba Davoudi: initial search, data extraction, manuscript drafting, data analysis, and interpretation.
Ramin Nabizadeh: conceptualization, critical revision, and editing of the manuscript.
Simin Naseri: critical revision and editing of the manuscript.
Aliasghar Najafpoor: data analysis, interpretation, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Research Ethics Committee of Faculty of Health, Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1400.196).
Consent to participate
Not applicable.
Consent for publication
All the authors read and approved the final version of the manuscript and consented to publish with this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Sami Rtimi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barjasteh-Askari, F., Nasseri, S., Nabizadeh, R. et al. Photocatalytic removal of diazinon from aqueous solutions: a quantitative systematic review. Environ Sci Pollut Res 29, 26113–26130 (2022). https://doi.org/10.1007/s11356-022-18743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18743-9