Abstract
The Kani Barazan and Yadegarlou wetlands in the southern part of Lake Urmia (Iran) have been substantially modified due to human activities and anthropogenic use. In recent years, freshwater-based eco-biological studies to recognize the quality of water resources have been greatly expanded. Microalgae and Cyanophyta are considered important bioindicators for the evaluation of water quality and wetland health worldwide. Herein, 22 microalgae and 5 Cyanophyta genera were identified in both wetlands, in which Cyanophyta has mainly caused blooms. Principal components analysis (PCA) was carried out based on links between the distribution of microalgae and Cyanophyta with physical and chemical parameters. The data showed that depth, turbidity, and the temperature had a significant influence on the microalga and Cyanophyta communities in both wetlands. Based on the biological properties, it seems that the Kani Barazan and Yadegarlou international wetlands experience meso-eutrophic conditions. The integration of the physical, chemical and biological parameters with the water quality index (WQI) revealed that both wetlands were polluted as a consequence of human activities. Moreover, a close relationship between WQI and the biological parameters was documented. Thus, we concluded that microalgae and Cyanophyta communities, their abundance patterns, and water quality changes could provide valuable data for the conservation of the Kani Barazan and Yadegarlou international wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growth and development of human societies are undoubtedly dependent on water resources, which are considered one of the most sensitive parts of the environment (Molnar 2010). Anthropogenic modification of waterways and human demands for freshwater have changed the condition of aquatic ecosystems worldwide. Therefore, the regulation of riverine ecosystems is the most extensive form of anthropogenic modification of natural flow regimes. Wetlands are considered to be the most known productive and valuable ecosystems playing an essential role in water resources management (Bassi et al. 2014). Moreover, wetlands can provide various economic, social, environmental, and cultural advantages in regional areas (Roebeling et al. 2016). Despite the implementation of regulatory protection programs, many wetlands have been degenerated by direct and indirect-related human activities (Junk et al. 2014). Thus, many wetlands need urgent restoration and conservation in large part because of the threatening of key functions and associated ecosystem services (Atazadeh et al. 2020).
Algal and Cyanophyta communities can respond daily to any physical, chemical, and biological characteristics in riverine ecosystems (Atazadeh et al. 2020). Indeed, algal periphyton communities are valuable indicators of ecological disturbance and provide important complementary evidence of ecosystem health and water quality over a range of temporal scales. Algae react directly to changes in nutrients, whereas invertebrates generally respond indirectly – mostly through the influence of water quality on habitat. For this reason, some approaches to understanding river conditions have been based on microalgae and Cyanophyta communities since they are sensitive to many physical, chemical, and biological changes.
The control of external and internal loading of nutrients, especially phosphorus and nitrogen contents, is a fundamental feature of water management efforts toward environmental sustainability and protection (Parris 2011). Rapid industrialization and overuse of chemical fertilizers and pesticides in agriculture have caused heavy pollution in aquatic ecosystems, leading to the deterioration of water quality and the elimination of aquatic biota (Kumar and Yaashikaa 2019). Inorganic chemicals, including some heavy metals such as Pb, Cd, and Cu, as well as inorganic ions such as NO3−, PO4−3, and SO4−2, have contributed to a higher portion of the contaminants in water resources than organic elements (Rahmanian et al. 2015). Nowadays, some physical and chemical parameters such as dissolved oxygen (DO), total dissolved solids (TDS), biochemical oxygen demand (BOD), total suspended solids (TSS), chemical oxygen demand (COD), pH, turbidity, and temperature are routinely utilized for the assessment of the water quality, verifying the impact of the distribution networks, and the efficiency of the treatment plants (Salari et al. 2018a). Furthermore, these parameters could be considered important indicators for the prediction of possible changes in the aquatic bio-ecosystems, the efficient management of water resources and also the determination of water quality (Cho et al. 2020).
Over the few past decades, water quality indices (WQIs) have been regarded as key tools for the surface water body classifications and the effective management of water resources. WQI is a mathematical indicator that integrates a large number of physicochemical characteristics data into a single number representing water quality levels. This indicator could also provide the required criteria for surface water classifications based on utilized parameters. The U.S. National Sanitation Foundation Water Quality Index (NSFWQI), Canadian Council of Ministers of the Environment WQI (CCMEWQI), British Columbia WQI (BCWQI), and Oregon WQI (OWQI) are the most common indices utilizing physical and chemical parameters for the evaluation of water body qualities (Burigato Costa et al. 2019; Şener et al. 2017). Recently, microalgae have been utilized as reliable bioindicators for the evaluation of water quality of wetlands and rivers, since the biodiversity and biodistribution of microalgal communities are highly sensitive to the physical, chemical and biological modifications that existed in the water resources (Atazadeh et al. 2007; Chun et al. 2018; Wu et al. 2017). More specifically, microbial community (microalgae and Cyanophyta) structure (MCS) could directly and sensitively respond to changes in conductivity, pH, organic pollutions, and nutrient availability. Thus, the evaluation of MCS could present the first indications of any alteration in wetlands. Moreover, both microalgae and Cyanophyta have a high species diversity, among which each strain is differentially sensitive to a wide range of physicochemical and environmental conditions. Accordingly, MCS could present a more reliable and precise indication of the nutrient status of wetlands compared to hydro-chemical features of the water. It should be noted that the existence and abundance of microalgae and Cyanophyta species in ecosystems could be determined by their interaction with physical and chemical factors (Agung et al. 2017). Additionally, the evaluation of the microbial communities can provide an insight into the state of the carbon cycle in different wetlands, upon which their response to climate changes could be determined (Mayali 2021).
Each chemical or biological parameter, per se, might present specific data about the status of aquatic ecosystems. Thus, the evaluation of water quality should be determined by both physical, chemical and biological properties (Gabriels et al. 2010). In detail, physical and chemical parameters might highlight the effects of stressors on the bio-ecosystems without elucidating how they could affect the biological communities.
Herein, we determined the water quality and ecological condition of the Kani Barazan and Yadegarlou international wetlands, located in the northwest of Iran, using several biological and physicochemical parameters. Both wetlands and their flora/fauna are affected by significant impacts of the agricultural activities in the region. At first, the MCS and function of the ecosystem were evaluated in the both Kani Barazan and Yadegarlou wetlands. Subsequently, some physical parameters (e.g., TSS and TDS) and chemical parameters (e.g., nitrate, orthophosphate, total nitrate, total phosphate, dissolved oxygen, and BOD) were considered to determine the quality of water samples using the NSFWQI model. Finally, using various biological parameters such as Chl-a, Chl-b, and AFDM, the direct correlation between microalgal communities and WQI was determined.
Materials and methods
Study area
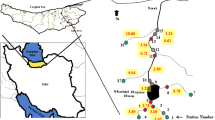
The Yadegarlou and Kani Barazan international wetlands are located in the southern part of Lake Urmia, NW Iran. The Yadegarluo wetland is almost 1530 ha area and situated at an average height of 1280 m asl and 22 km from Naghdeh city (West Azerbaijan province, Northwest Iran) in the geographical position of N 37° 1′ 32″ and E 45° 32′ 47″. The main water sources of this wetland are seasonal runoffs due to rainfall in the basin, seasonal flows of shallow rivers through permanent rivers, and groundwater leakages. The Kani Barazan wetland includes about 970 ha located at an average height of 1275 m asl and 30 km from Mahabad city (West Azerbaijan province, NW Iran) in the geographical position N 36° 59′ 34″ and E 45° 46′ 34″. The water source of this wetland is supplied by the Mahabad River (Fig. 1).
Water sampling, microalgae identification, and MCS monitoring
The water samples were collected monthly at 10 stations in the Kani Barazan and Yadegarlou wetlands from May to September 2019. Some geographical and climate limitations, such as the existence of high mountains associated with snowfall, blizzard, and freezing of wetlands have limited the water sampling during the cold seasons. The main criteria for the selection of sampling sites were anthropogenic effects, human activities, and climatic variability (e.g., inlet and outlet of the wetlands and polluted section of the wetlands). The geographical information of the sampling stations was collected using a GPS tracker. Microalgae and Cyanophyta communities have great diversity in which their structure and composition may vary depending on the nature of the substrate. Diatoms and soft algae were colonized on natural substrates such as cobbles, stones, mud, rock, woody debris, emergent, and submerged plants (Taylor et al. 2007).
Small boulders and pebbles were used for taking samples when cobbles were not available (Kelly et al. 1998). Microalgae and Cyanophyta were collected in three replicates from emergent and submerged surfaces including cobbles, pebbles or rocks, woody debris, aquatic plants, and mud within any of the established sampling sites. The microalgae and Cyanophyta were scraped from an area of 20–30 cm2 of a substrate using a soft toothbrush. The samples were washed in plastic trays with river water and the obtained algal suspensions were put into 250-mL collection bottles. The bio-distribution of identified microalgae was determined by a light microscopic study of five samples from each station. The separated samples were collected for the determination of biomass and the identification and counting of diatoms and soft algae. Five sample replicates were used to estimate the dry mass, AFDM, Chl-a, and Chl-b. An authentication key, algal flora, and some recent related references were carefully used for the correct identification of the isolated microalgae (Krammer 2000; Ling and Tyler 2001; Sonneman et al. 2000). All samples and slides were examined using a light microscope (Olympus CKX41). During microscopic analyses, the microalgae species were preserved by adding two drops of Lugol’s iodine (Wehr et al. 2015).
Physical and chemical properties of water samples
Some physical and chemical characteristics of the collected water samples such as temperature, pH, turbidity, and depth were measured in situ. To this end, samples for chemical analyses were stored at 4 °C in the dark and analyzed after 14 h from sampling. Analyses of nitrate, phosphate, TSS, TDS, DO, COD, BOD, cations, and anions were done based on the American Public Health Association protocol.
Atomic absorption spectrophotometry (AAS)
The acid-washed bottles were used to collect water samples to detect the concentrations of heavy metals in the wetlands. Samples were filtered using Whatman filter papers No. 42. The pH was adjusted to 2 using 10% nitric acid, and then samples were stored at 4 °C until the measurement by atomic absorption spectroscopy (WFX 130, Japan).
Evaluation of WQI
Several parameters included the concentration of heavy metals, nitrate, phosphate, sodium, bromine, as well as TSS, TDS, DO, BOD, pH, turbidity, conductivity, and electrical conductivity (EC) were considered for the computing of WQI. First, a unique weight (wi) was assigned to each parameter based on the minimum and maximum effects on the water quality where scale 1 and scale 5 represented the lowest and highest values, respectively (Table 1). Indeed, the highest weight equals 5, assigned to parameters that have critical health effects, and their presence above the critical concentration limits the usability of the resource for aquatic, domestic, and drinking purposes (Ramakrishnaiah et al. 2009). The relative weight (Wi) has been computed from the following equation:
where Wi is a relative weight, (wi) is the weight of each parameter, and (n) is the number of parameters. Then, a quality rating (qi) for each parameter was assigned by dividing its concentration to each water sample by its limit values provided by the World Health Organization (WHO), and the result was multiplied by 100:
where qi, Ci, and Si, respectively, presented the quality rating, the concentration of each chemical parameter in each water sample in mg/L, and the drinking water standard for each chemical parameter in mg/L based on the WHO guidelines. For calculating WQI, the SIi value was determined using the following equations:
where (SIi) is the subindex of the ith parameter; (qi) is the quality rating based on the concentration of the ith parameter (Rahmanian et al. 2015). The computed WQI values were classified into five categories (Rahmanian et al. 2015) including:
-
a).
≤ 50 excellent water (acceptable for all organisms)
-
b).
50–100 good water (acceptable for all organisms)
-
c).
100–200 poor water or slightly polluted water (acceptable except for very sensitive species)
-
d).
200–300 very poor water or polluted water (only for very resistant organisms)
-
e).
≥ 300 unsuitable for drinking or heavily polluted water (unacceptable)
Biomass content
Samples were scratched and dried for 24 h at 60 °C and then weighed to determine the dry mass. For AFDM measurement, the samples were dried for 24 h at 60 °C, and subsequently the microalgal periphyton was scraped off, weighed, and combusted at 550 °C in a furnace for 5 h, and reweighed. Finally, AFDM was estimated based on the difference between the mass before and after incineration that represented as mg/cm−2 of the original substratum.
Photosynthetic pigments content
The photosynthetic pigment contents such as Chl-a, Chl-b, and total chlorophyll were measured based on the Dere (1998) procedure. In detail, 0.5 g of fresh samples were mixed with 10 ml of acetone (% 99) using a mortar and pestle on ice. The samples were filtered using a Whatman filter paper No. 42, and the absorbance of the supernatants was assessed at 662 and 645 nm. The content of the pigments was determined as:
Statistical analysis
The following statistical methods were used for the determination of transform data, and the application of techniques included Pearson’s correlation coefficient, principal components analysis (PCA), correspondence analysis (CA), and detrended correspondence analysis (DCA).
Principal component analysis (PCA) is a statistical method that illustrates the covariance of the variables. It allows for the identification of the principal directions (gradients) of the variations in the data to find the patterns in ecological datasets. Furthermore, PCA (indirect analyses) is an unconstrained linear response model used to explore relationships between species and environmental variables. After transformation, the data was used to generate a PCA ordination for the algae community patterns and environmental conditions.
Detrended correspondence analysis (DCA) was applied to species data that exhibited an arch when analyzed using the computationally simple CA. This often occurred where the axis 1 gradient was > 2. In addition, the interpretation of the DCA is easier than other techniques because the main axes are aligned to the biological gradients. In the present study, the DCA was performed on the microalgae and Cyanophyta dataset to determine species dispersal and the length of the species gradient.
The SPSS software was also used to calculate Pearson’s correlation matrix and standard deviations in the dataset. Further, the CANOCO software for Windows 4.5 was used to assess microalgae assemblage patterns under different flow regimes using PCA, CA, and DCA (Ter Braak and Smilauer 2002).
Results and discussion
The analyses of biological parameters
Biological parameters are fundamental and time-efficient criteria for the determination of water quality, and they may reflect the effects of external and internal pollutants. Thus, the MCS, algal blooms, and microalgae biomass were evaluated and discussed as fundamental biological parameters.
Assessment of MCS
There has been limited research on the use of microalgae and Cyanophyta for the assessment of wetland conditions in Iran. Based on morphological characteristics, 27 genera (22 microalgae and 5 Cyanophyta), mainly belonging to the Chlorophyta, Bacillariophyta, and Cyanophyta, were identified in the Kani Barazan and Yadegarlou wetlands (Fig. 2 and Tables 2 and 3). In addition, relative volume and the type of microbial communities were evaluated across the studied sites. The relative abundance of the algae and Cyanophyta communities was calculated by placing 1 mL of each sample into a Sedgewick-Rafter counting chamber. Cells were counted using an Olympus microscope at 100–1000 × magnification.
In the Yadegarlou wetland, the abundance of the green microalgae, especially Ankistrodesmus, Chlamydomonas, Chlorella, and Dunaliella, and some Cyanophyta such as Microcystis and Oscillatoria were higher than the other identified genera. Also, the populations of some Cyanophyta including Anabaena sp., Microcystis sp., and Merismopedia sp., were higher in the Kani Barazan wetland compared to the other identified microorganisms. Similar to the Yadegarlou wetland, the abundance of the Dunaliella species was remarkably high in the Kani Barazan wetland (Tables 2 and 3).
The DCA can display the reasons for connections between sites and dispersal of microalgae and Cyanophyta communities. Regarding the DCA data of the Yadegarlou wetland, the first axis presented a 21.7% cumulative percentage variance of the species-station relationship, while the second axis explained 11.2% (Fig. 3a). Concerning the Kani Barazan wetland, the first canonical axis explained 28.4% cumulative percentage variance of species-station relation, and the second axis described 15.1% (Fig. 3b). The DCA results indicate that there is a strong unimodal response of species to environmental factors. In other words, the DCA test suggests that PCA would be an appropriate means for exploring the relations between the species and environmental data sets. Exploration of the data using the computationally simple, unconstrained ordination technique of CA indicated that there were two strong gradients in the data sets. The DCA was used when gradient length was > 2 and to eliminate the arch effects for appropriate interpretation. The results of the DCA showed visualization plots due to an arch effect observed in CA plots where DCA was applied to remove the arch effect.
It should be noted that biological monitoring programs, especially microalgal-based ones, are reliable platforms for determining the biodiversity and the assembly of living organisms to assess the environmental changes and anthropogenic impacts (Elias and Bremec 1994). In addition, microalgae and Cyanophyta could be regarded as a powerful ecological toolkit to evaluate water quality in rivers and wetlands (Atazadeh et al. 2020, 2007).
Monitoring of harmful blooms by Cyanophyta
Our preliminary data, based on the sampling method, revealed that Cyanophyta was predominantly present in the surficial depth of the water samples, while the Bacillariophyta were mainly detected in the deeper part of the Yadegarlou and Kani Barazan wetlands. This issue could be utilized to confirm the occurrence of Cyanophyta blooms and their intensity in the Yadegarlou and Kani Barazan international wetlands. Based on our findings, blooms of harmful Cyanophyta such as Anabaena sp., Oscillatoria sp., and Microcystis sp. have occurred in the Yadegarlou and Kani Barazan wetlands (Tables 2 and 3).
Notably, the increase of external nutrient inputs such as nitrates and phosphates provided by human activities, discharge of human wastewater and agricultural water drains, and climate changes are the most important reasons for Cyanophyta blooms observed in the Yadegarlou and Kani Barazan wetlands. Apart from the external agents, the internal release of pollutants from the nutrients embedded into the sediments is another reason for the occurrence of Cyanophyta blooms. They are mainly observed under hypoxic circumstances at sediment–water interfaces (Lyu et al. 2020). It is believed that dredging and a recently emerged approach known as modified local soil (MLS) technology could be successfully applied to remove the internal pollutants from sediments (Pan et al. 2019). Based on our preliminary inquiries, dredging is being performed in the Yadegarlou and Kani Barazan wetlands that resulted in the worsening of Cyanophyta blooms that occurred by internal and external pollutant loadings.
In addition to toxicity, the Cyanophyta blooms might affect the smell and taste of water resources which could be dangerous and undesirable for human and aquatic biota usage (Zhao et al. 2015). Unfortunately, the Cyanophyta blooms occur in most parts of the world, especially South Korean coastal waters, Indian and Pacific oceans, and the water resources of Japan, China, Turkey, and Iran (Balkis et al. 2016; Gholami et al. 2019; Sakamoto et al. 2020; Tester et al. 2020). In this sense, the water resource managers and freshwater ecologists currently have used the ecological response models for the sustainable management of Cyanophyta blooms (Li et al. 2021).
Determination of chlorophyll concentrations and biomass
The concentration of Chl-a and Chl-b was different in the studied sites (Fig. 4). In the Yadegarlou wetland, site number 4 showed the highest concentration of Chl-a and Chl-b, while site number 3 presented the lowest. Notably, the concentration of Chl-a and Chl-b was different in the Kani Barazan wetland, where sites number 3 and 5 represented the lowest and highest concentrations, respectively (Fig. 4a and b). Furthermore, our results revealed that sites number 4 and 5 from the Yadegarlou and Kani Barazan wetlands represented the highest values of dry mass and AFDM, but site number 3 showed the lowest in both wetlands (Fig. 4c and d). A direct correlation between biomass content analyses and DCA data was observed, in which stations with high biomass contents showed high levels of microalgae and Cyanophyta biodiversity. It is reported that the initial microalgae and Cyanophyta production and biomass are increased by providing a higher concentration of nutrients, light availability, and an optimized temperature. Supplying these factors during dry seasons might result in a higher value of primary productivity in the water resources (Sutherland et al. 2015). Additionally, biomass parameters could represent some data about the importance of a taxonomic unit within a community or the influence of a community on an ecosystem in which their implications could be used for trophic-level interactions and energy flows (Steinman et al. 2017).
Photosynthetic pigment, dry mass, and AFDM contents of total identified microalgae and Cyanophyta. panels a and b represented Chl-a and Chl-b contents in the Yadegarlou and Kani Barazan wetlands, respectively. Panels c and d presented dry mass and AFDM contents in the Yadegarlou and Kani Barazan wetlands, respectively (data indicate means and SD)
Physical and chemical parameters of wetland water
The water quality management policy for surface waters mainly relies on a wide variety of physical and chemical parameters. To this end, the measured values of the chemical and physical parameters collected from 10 sites are presented in Tables 4 and 5. It is believed that the appropriate concentration of nitrate and phosphate in water resources has a significant effect on the growth of microalgae (Lapointe 2019). Our results showed the same homogeneity in the concentration of nitrogen and phosphorus in most sites, confirming a high concentration of the nutrients in both Yadegarlou and Kani Barazan wetlands compared to the WHO guidelines. The enrichment of the aquatic ecosystems with nitrogen and phosphorus can cause NH3 emission and nitrification that create an undesirable disturbance in organism balances and water quality (Martínez-Espinosa et al. 2021). Thus, eutrophication is mainly regarded as a continuous environmental threat derived directly or indirectly from human activities. Of note, the control of nutrient levels and eutrophication in the aquatic ecosystems is considered one of the primary objectives of environmental policies (Hering et al. 2015).
Moreover, our data show that the carbonate system (i.e., carbonate, bicarbonate, carbonate) could play a major role in the increase of buffering capacity in both wetlands (Tables 4 and 5). The mean values of DO were recorded as 3.37 and 2.99 mg/L, respectively, in the Yadegarlou and Kani Barazan wetlands and were lower than the WHO standards. As an important physicochemical-related indicator, DO is essential for the survival of many aquatic organisms (Atazadeh et al. 2020). Possibly, the decreased exchange of oxygen between the atmosphere and water in the wetlands has occurred due to water stagnation in these lentic systems.
Previous research has indicated that phytoplankton biomass and production in most lentic systems are controlled by nitrate and phosphate supply, leading to an increase in system turbidity and intense Cyanophyta blooms (Yang et al. 2020). Hence, Cyanophyta blooms might cause further intensification of anoxia and eutrophication in these ecosystems. Moreover, the mean value of COD, required for the oxidation of chemical wastes was higher than the tolerance limit in both wetlands. Furthermore, the mean value of BOD, a primary water quality factor, was relatively high in the Yadegarlou and Kani Barazan wetlands. It should be noted that the introduction of sullage, sewage, and other different pollutants from urban and/or industrial areas could increase the BOD of water resources. It subsequently resulted in intense depletion of DO and a strong decline in the number of aquatic organisms (Kumar and Yaashikaa 2019). We identified that the observed blooms in the Yadegarlou and Kani Barazan wetlands are effectively related to the inappropriate levels of COD, BOD, and DO that could promote the rapid growth of Cyanophyta. In addition, the pH of water samples has a relatively alkaline status (i.e., ~ 8) causing rapid growth of Cyanophyta and could also contribute to the occurrence of the blooms in the Yadegarlou and Kani Barazan wetlands (Tables 4 and 5).
Further data confirmed that the content of three heavy metals in the water samples were higher than the quantification limits: Cu (Kani Barazan = 0.214 mg/L; Yadegarlou = 0.268 mg/L), Pb (Kani Barazan = 0.050 mg/L; Yadegalou = 0.042 mg/L), and Cd (KaniBarazan = 0.0048 mg/L; Yadegarlou = 0.0034 mg/L). Thus, the concentration of heavy metals in the studied water bodies needs further attention for the protection of both wetlands in large part because of their toxic effects on the aquatic biota utilized as human food. It seems that the occurrence of blooms in the studied wetlands came from the heavy metals tolerance potential of the microalgae and Cyanophyta enabling them to grow in highly polluted conditions. As a result, the presence of heavy metals could intensify the blooms in the water resources. Further results also confirmed the high capacity of EC values of the water samples, reflecting the enrichment of water in cations and anions. The concentration of major ions in the wetlands and their relative relationships are considered appropriate indicators to explore the lithological origin of the minerals (Atazadeh et al. 2020). The water of both wetlands showed a high concentration of ions, especially a very high sodium content. This condition caused a higher increase in the TDS value that might be derived from human activities (Tables 4 and 5). Indeed, TDS included the ionized and non-ionized components that affected the water conductivity. Based on the relationship between TDS and EC, high TDS levels indicate low-quality water (Salari et al. 2018b). The evaluation of the TSS (as an essential parameter for the exploration of the pollution in the water resources) revealed that the mean value of this factor is intensely high over the WHO standards in the Yadegarlou and Kani Barazan wetlands. TSS is one of the main factors that strongly deteriorates the quality of water resources and leads to a significant degradation in aquatic ecosystems (Bilotta and Brazier 2008).
Assessment of the water quality using WQI
The WQI was calculated through the NSFWQI method and is strongly recommended for reliable determination of water quality in the Yadegarlou and Kani Barazan wetlands. The weight values were determined for each water quality parameter according to their relative importance in the water quality for aquatic biota and drinking purposes (Table 1). In the studied wetlands, water samples were not suitable for aquatic biota and drinking usages in most sites because of the high levels of nitrate and phosphate. Moreover, our data showed that the Pb and Cd levels in the Yadegarlou and Kani Barazan wetlands exceeded the normal levels. The accumulation of trace metals in waters was due to the presence of natural or anthropogenic sources that affected human health and aquatic life (Ugulu et al. 2019). In this sense, the highest weight was assigned to some parameters such as nitrate, phosphate, and heavy metals (Table 1). Heavy metals appeared to be more important due to their different toxic effects on various aquatic species (Bibi et al. 2007).
Our results also confirmed the incoherence of turbidity, BOD, and DO parameters with world standards for aquatics in both studied wetlands. The turbidity value highly depended on the levels of total solids, plankton density, suspended materials, and various human activities (Chen et al. 2016; Shi et al. 2017). Moreover, the high level of BOD occurred because of sewage entry and the killing of aquatic biota that leads to the consumption of aquatic oxygen by microorganisms (Yin et al. 2017). Our data showed that the turbidity, BOD, and DO parameters were assigned a weight of 4 that shows their importance in water quality. Further results showed that the pH and EC values were slightly higher than the levels reported by the WHO. Thus, we assigned pH and EC a weight of 3, reflecting their importance in water quality (Table 1). The pH of water samples is dependent on the geological nature of the source, the presence of dissolved solids, and phytoplankton activity (Boyd 2020). The EC level (as an indirect consequence of the concentration of dissolved salts) could rise through the effects of natural atmospheric factors, the presence of certain sedimentary rocks, or a human source such as industry or wastewater output (Meena and Bhargava 2012). Therefore, EC might impose a significant effect on the acceptability of water for human and aquatic usage. Furthermore, TSS and TDS parameters were assigned a weight of 2, demonstrating their importance in water quality. The minimum weighting of 1 was assigned to Br and Na because of their lower importance in water quality (Table 1). WQI values are 184.579 and 545.411 for Yadegarlou and between 160.9 and 482.123 for Kani Barazan wetlands (Table 6).
Based on the data represented as water categories, our water samples could be categorized as follow: (1) “slightly polluted,” (2) “polluted,” and (3) “heavily polluted” (Table 6). In this sense, stations 1, 2, and 5 in Yadegarlou and stations 1 and 2 in Kani Barazan wetlands are classified as polluted. Station 3 in both wetlands is considered slightly polluted, while station 4 in Yadegarlou and stations 4 and 5 in Kani Barazan wetland are described as heavily polluted (Table 6). Consequently, the WQI data confirmed that the studied wetlands provided low-quality water not only for drinking but also for aquatic biota. It should be noted that many wetlands worldwide have lost their hydrological and ecological functions because of increasing anthropogenic pressures (Lee et al. 2006). These phenomena are more disastrous for urban wetlands that have been exposed to land use policy and increased economic activities (Mei et al. 2018).
Interactions between physicochemical and biological parameters
Regarding the PCA of the Yadegarlou wetland, the first axis explained 40.0% of cumulative percentage variance of microalgae species-environment relation, while the second axis described 12.6% (Fig. 5a). The highest separation of species was associated with temperature, depth, HCO3−, and turbidity. The number of harmful microalgae species was enhanced with the increasing concentration of heavy metals. Nutrients can apparently play a significant role in the distribution patterns of most species since Oedogonium was highly associated with nitrate and phosphate contents. Besides, Navicula, Chroococus, and Cosmarium were significantly associated with turbidity, depth, and temperature. In contrast, Denticula and Cyclotella had a significant relationship with pH, BOD, COD, and TSS. Concerning the Kani Barazan wetland, the first axis explained 24.7% cumulative percentage variance of species-environment relation, while the second axis represented 16.5% (Fig. 5b). The PCA analysis showed that nitrate content, depth, CO3, turbidity, and pH had a remarkable influence on the MCS in the Kani Barazan wetland. Moreover, we found that cations and anions had a low effect on the MCS, although Navicula and Cocconoeis, Spirulina, and Melosira showed a direct connection with cations and ions. In contrast, Anabaena, Chroococus, and Dunaliella had a significant relationship with BOD and COD parameters. To sum up, depth, turbidity, and temperature had substantial influences on the MCS in the Kani Barazan and Yadegarlou wetlands.
Principal components analysis (PCA). Panels a and b respectively described the PCA ordination of potential microalgae and Cyanophyta species and physicochemical factors in the Yadegarlou and Kani Barazan wetlands. Percentages in parenthesis are the cumulative percentage variance of species—physicochemical factors relation explained by the axis. Depth, turbidity, and temperature presented significant impacts on the microalgae and Cyanophyta communities in both wetlands
Correlation between WQI and biological parameters
WQI is generally evaluated using physical and chemical characteristics. Biological features obtained from MCSs related research could be included in the assessment of WQI (Pham 2017). Similar to macrophytes, zooplankton, microalgae as well as Cyanophyta could react to disturbances in environments derived from natural or anthropogenic origins. Therefore, these microorganisms and their distribution could be considered a bioindicator for the assessment of WQI (Lyu et al. 2020). Based on our findings, a direct relationship exists between WQI and DCA analysis, and thus a trend of increased abundance indices of microalgae and Cyanophyta was observed by the increment of WQI (Fig. 3a and b) (Table 6). In this context, a comprehensive comparison was achieved between WQI and biological parameters (i.e., Chl-a, Chl-b, dry mass, and AFDM) by linear regression analysis (Fig. 6). As a result, the obtained data displayed a high level of correlation and consistency among the parameters in the Yadegarlou and Kani Barazan wetlands. Previous reports on the performance of wetlands have mainly focused on the hydrologic characteristics, improvement in ecological features, or ecosystem services provided by wetland restoration (Mao et al. 2019). In contrast, the biological data could represent a more reliable assessment for long-term ecological changes in the quality of water systems (Hellawell 2012). In the past, water quality monitoring programs have focused on water chemistry criteria. Nowadays, they are more focused not only on water chemistry but also on biological characteristics. Indeed, this is essential to demonstrate the effect of pollutants on biota, which in turn reflects the impact of contaminants on water quality. Water in aquatic ecosystems can be classified, based on biology, hydrology, and quality, into different ecological categories of conditions such as bad, poor, moderate, good, or excellent. Of note, short generation times and rapid response to environmental changes offer the possibility to study the transgenerational effects of pollutant exposure. Being a model of choice for the surveys of the long-term effects of pollutant exposure at the population level has led to MCS being regarded as a more reliable, inexpensive, and fast biomarker for the evaluation of the quality of the water resources (Atazadeh et al. 2021).
Relationship between WQI and biological parameters. Schemes a and b represent the correlation of WQI and chl-a in the Yadegarlou and Kani Barazan wetlands. Schemes c and d describe the correlation of WQI and dry mass in the Yadegarlou and Kani Barazan wetlands. Schemes e and f explain the correlation of WQI and AFDM in the Yadegarlou and Kani Barazan wetlands
Conclusion
The relationship between biological and chemical conditions is an important issue in establishing a framework for water management. Thus, we have tried to assemble the impact of both attributes on the primary productivity, HABs, and WQI of the wetlands. The evaluation of WQI by physicochemical traits and biological metrics revealed that the water quality of both wetlands could be classified as polluted to heavily polluted. According to the PCA analysis, the Yadegarlou and Kani Barazan wetlands represented biological and limnological differences in the physicochemical parameters. The PCA analysis showed that microalgae and Cyanophyta abundance and their distribution patterns could be used as more powerful indicators for the evaluation of water quality of the wetlands compared to the physicochemical parameters. Water characteristics and also microalgal activities could be influenced by the nutrient composition of wetlands induced by external and internal pollutants. Thus, our data provide a more holistic view of the water quality of both wetlands due to the inclusion of microalgal metrics in the physicochemical attributes. Importantly, the Yadegarlou and Kani Barazan international wetlands deserve further attention for understanding anthropogenic activities toward wetland management and bringing more benefits to humans and the environment.
Data availability
The data for the assessment included in the study is given as supplementary material.
References
Agung BM, Piranti AS, Carmudi C (2017) Relationship between water quality and abundance of Cyanophyta in Penjalin Reservoir. UNEJ e-Proceeding [S.l.], p. 52–56. https://jurnal.unej.ac.id/index.php/prosiding/article/view/4134. Accessed 15 Apr 2021
Atazadeh E, Barton A, Shirinpour M, Zarghami M, Rajabifard A (2020) River management and environmental water allocation in regulated ecosystems of arid and semi-arid regions–a review. Fundam Appl Limnol 193(4):327–345
Atazadeh E, Gell P, Mills K, Barton A, Newall P (2021) Community structure and ecological responses to hydrological changes in benthic algal assemblages in a regulated river: application of algal metrics and multivariate techniques in river management. Environ Sci Pollut Res Int 28:39805–39825
Atazadeh I, Sharifi M, Kelly M (2007) Evaluation of the Trophic Diatom Index for assessing water quality in River Gharasou, Western Iran. Hydrobiologia 589:165–173
Balkis N, Balci M, Giannakourou A, Venetsanopoulou A, Mudie P (2016) Dinoflagellate resting cysts in recent marine sediments from the Gulf of Gemlik (Marmara Sea, Turkey) and seasonal harmful algal blooms. Phycologia 55:187–209
Bassi N, Kumar MD, Sharma A, Pardha-Saradhi P (2014) Status of wetlands in India: a review of extent, ecosystem benefits, threats and management strategies. J Hydrol Reg Stud 2:1–19
Bibi MH, Ahmed F, Ishiga H (2007) Assessment of metal concentrations in lake sediments of southwest Japan based on sediment quality guidelines. Environ Geol 52:625–639
Bilotta GS, Brazier RE (2008) Understanding the influence of suspended solids on water quality and aquatic biota. Water Res 42:2849–2861
Boyd CE (2020): Microorganisms and water quality, Water Quality. Springer 233–267
Burigato Costa C, da Silva ML, Almeida AK, Leite IR, de Almeida IK (2019) Applicability of water quality models around the world-a review. Environ Sci Pollut 26:36141–36162
Chen X, Shen Z, Yang Y (2016) Response of the turbidity maximum zone in the Yangtze River Estuary due to human activities during the dry season. Environ Sci Pollut 23:18466–18481
Cho KH, Pachepsky Y, Ligaray M, Kwon Y, Kim KH (2020) Data assimilation in surface water quality modeling: a review. Water Res 186:116307
Chun SJ, Cui Y, Ahn CY, Oh HM (2018) Improving water quality using settleable microalga Ettlia sp. and the bacterial community in freshwater recirculating aquaculture system of Danio rerio. Water Res 135:112–121
Elias R, Bremec C (1994) Biomonitoring of water quality using benthic communities in Blanca Bay (Argentina). Sci Total Environ 158:45–49
Gabriels W, Lock K, De Pauw N, Goethals PL (2010) Multimetric Macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnologica 40:199–207
Gholami Z, Mortazavi MS, Karbassi A (2019) Environmental risk assessment of harmful algal blooms case study: Persian Gulf and Oman Sea located at Hormozgan Province, Iran. Hum Ecol Risk Assess 25:271–296
Hellawell JM (2012) Biological indicators of freshwater pollution and environmental management. Springer Science & Business Media, pp. 546. https://doi.org/10.1007/978-94-009-4315-5
Hering D et al (2015) Managing aquatic ecosystems and water resources under multiple stress–an introduction to the MARS project. Sci Total Environ 503–504:10–21
Junk WJ, Piedade MTF, Lourival R, Wittmann F, Kandus P, Lacerda LD, Bozelli RL, Esteves FdA, Nunes da Cunha C, Maltchik L (2014) Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquat Conserv 24:5–22
Kelly M, Cazaubon A, Coring E, Dell’Uomo A, Ector L, Goldsmith B, Guasch H, Hürlimann J, Jarlman A, Kawecka B (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10:215–224
Krammer K (2000): Diatoms of Europe, Volume 1: The genus Pinnularia. Gantner Verlag, 703
Kumar PS, Yaashikaa P (2019) Agriculture pollution, Advanced Treatment Techniques for Industrial Wastewater. IGI Global, pp. 134–154
Lapointe BE (2019) Chasing Nutrients and algal blooms in Gulf and Caribbean Waters: a personal story. Gulf Caribb Res 30:xvi–xxx
Lee S, Dunn R, Young R, Connolly R, Dale P, Dehayr R, Lemckert C, McKinnon S, Powell B, Teasdale P (2006) Impact of urbanization on coastal wetland structure and function. Austral Ecol 31:149–163
Li K, Xu T, Xi J, Jia H, Gao Z, Sun Z, Yin D, Leng L (2021) Multi-factor analysis of algal blooms in gate-controlled urban water bodies by data mining. Sci Total Environ 753:141821
Ling H, Tyler PA (2001) Australian Freshwater Algae (exclusive of diatoms). Acta Bot Hung 43:222–223
Lyu T, Song L, Chen Q, Pan G (2020) Lake and River Restoration: Method, Evaluation and Management. Water 12:977
Mao X, Wei X, Jin X, Tao Y, Zhang Z, Wang W (2019) Monitoring urban wetlands restoration in Qinghai Plateau: integrated performance from ecological characters, ecological processes to ecosystem services. Ecol Indic 101:623–631
Martínez-Espinosa C, Sauvage S, Al Bitar A, Green PA, Vörösmarty CJ, Sánchez-Pérez JM (2021) Denitrification in wetlands: a review towards a quantification at global scale. Sci Total Environ 754:142398
Mayali X (2021) Microscale carbon cycling between bacteria and algae under the sun. Microbes: The Foundation Stone of the Biosphere: 205–210
Meena BS, Bhargava N (2012) Physico-chemical characteristics of groundwater of some villages of Dag block in Jhalawar district of Rajasthan state (India). Rasayan J Chem 5:438–444
Mei Y, Sohngen B, Babb T (2018) Valuing urban wetland quality with hedonic price model. Ecol Indic 84:535–545
Molnar JJ (2010) Climate change and societal response: livelihoods, communities, and the environment. Rural Sociol 75:1–16
Pan G, Miao X, Bi L, Zhang H, Wang L, Wang L, Wang Z, Chen J, Ali J, Pan M, Zhang J, Yue B, Lyu T (2019) Modified Local Soil (MLS) technology for harmful algal bloom control, sediment remediation, and ecological restoration. Water 11:1123
Parris K (2011) Impact of agriculture on water pollution in OECD countries: recent trends and future prospects. Int J Water Resour Dev 27:33–52
Pham L (2017) Comparison between Water Quality Index (WQI) and biological indices, based on planktonic diatom for water quality assessment in the Dong Nai River, Vietnam. Pollution 3:311–323
Rahmanian N, Ali SHB, Homayoonfard M, Ali N, Rehan M, Sadef Y, Nizami A (2015) Analysis of physiochemical parameters to evaluate the drinking water quality in the state of Perak, Malaysia. J Chem 2015
Ramakrishnaiah C, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. J Chem 6:523–530
Roebeling P, Abrantes N, Ribeiro S, Almeida P (2016) Estimating cultural benefits from surface water status improvements in freshwater wetland ecosystems. Sci Total Environ 545–546:219–226
Sakamoto S, Lim WA, Lu D, Dai X, Orlova T, Iwataki M (2020) Harmful algal blooms and associated fisheries damage in East Asia: current status and trends in China, Japan, Korea and Russia. Harmful Algae: 101787
Salari M, Salami Shahid E, Afzali SH, Ehteshami M, Conti GO, Derakhshan Z, Sheibani SN (2018a) Quality assessment and artificial neural networks modeling for characterization of chemical and physical parameters of potable water. Food Chem Toxicol 118:212–219
Salari M, Shahid ES, Afzali SH, Ehteshami M, Conti GO, Derakhshan Z, Sheibani SN (2018b) Quality assessment and artificial neural networks modeling for characterization of chemical and physical parameters of potable water. Food Chem Toxicol 118:212–219
Şener Ş, Şener E, Davraz A (2017) Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci Total Environ 584–585:131–144
Shi Z, Xu J, Huang X, Zhang X, Jiang Z, Ye F, Liang X (2017) Relationship between nutrients and plankton biomass in the turbidity maximum zone of the Pearl River Estuary. J Environ Sci 57:72–84
Sonneman JA, Sincock A, Fluin J, Reid M, Newall P, Tibby J, Gell P (2000) An illustrated guide to common stream diatom species from temperate Australia. Identification Guide 33:5–166
Steinman AD, Lamberti GA, Leavitt PR, Uzarski DG (2017): Biomass and pigments of benthic algae, Methods in Stream Ecology, Volume 1. Elsevier 223–241
Sutherland DL, Howard-Williams C, Turnbull MH, Broady PA, Craggs RJ (2015) Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Biores Technol 184:222–229
Taylor J, Harding W, Archibald C (2007) A methods manual for the collection, preparation and analysis of diatom samples. Water Research Commission (WRC): 11–23
Ter Braak CJ, Smilauer P 2002: CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5), www. canoco. com
Tester PA, Litaker RW, Berdalet E (2020) Climate change and harmful benthic microalgae. Harmful Algae 91:101655
Ugulu I, Khan ZI, Rehman S, Ahmad K, Munir M, Bashir H, Nawaz K (2019) Trace metal accumulation in Trigonella foenum-graecum irrigated with wastewater and human health risk of metal access through the consumption. Bull Environ Contam Toxicol 103:468–475
Wehr JD, Sheath RG, Kociolek JP (2015): Freshwater algae of North America: ecology and classification. Elsevier
Wu N, Dong X, Liu Y, Wang C, Baattrup-Pedersen A, Riis T (2017) Using river microalgae as indicators for freshwater biomonitoring: review of published research and future directions. Ecol Indic 81:124–131
Yang W, Zhao Y, Wang D, Wu H, Lin A, He L (2020) Using principal components analysis and IDW interpolation to determine spatial and temporal changes of surface water quality of Xin'anjiang River in Huangshan, China. Int. J. Environ. Res. Public Health 17
Yin H, Lu Y, Xu Z, Li H, Schwegler BR (2017) Characteristics of the overflow pollution of storm drains with inappropriate sewage entry. Environ Sci Pollut Res 24:4902–4915
Zhao H-j, Wang Y, Yang L-l, Yuan L-w, Peng D-c (2015) Relationship between phytoplankton and environmental factors in landscape water supplemented with reclaimed water. Ecol Indic 58:113–121
Acknowledgements
We wish to express our appreciation to the University of Tabriz and Research Center for Pharmaceutical Nanotechnology (RCPN) at the Tabriz University of Medical Sciences, Iran, for their financial and technical support. We would like to thank Prof. J. Beardall (Monash University, Australia) and Dr. R. Tarrahi (University of Tabriz, Iran) for editing the manuscript. We also would like to thank two anonymous reviewers for their comments and corrections which greatly improved the final version of the manuscript.
Funding
This project is part of a Ph.D. thesis financially supported by the University of Tabriz and RCPN at the Tabriz University of Medical Sciences (Grant ID: RCPN60572(.
Author information
Authors and Affiliations
Contributions
All authors conceived and planned the project. HG performed the experiments and analyzed the data. The manuscript was written and edited by HG, EA, and AM. EA finalized the latest version of the manuscript. All authors contributed to discuss the results.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Alexandros Stefanakis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goshtasbi, H., Atazadeh, E., Fathi, M. et al. Using physicochemical and biological parameters for the evaluation of water quality and environmental conditions in international wetlands on the southern part of Lake Urmia, Iran. Environ Sci Pollut Res 29, 18805–18819 (2022). https://doi.org/10.1007/s11356-021-17057-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17057-6