Abstract
Glyphosate-based herbicides (GBH) are the most widely used herbicide for treatment of crops in the world. The digestive tract is one of the first systems exposed to pesticides, and damage to this system can affect the general health of individuals. The aim of this study was to evaluate the effects of subchronic inhalation and oral exposure to GBH on the digestive tract in rats. Six groups of Wistar rats (male and female) were exposed to nebulization with three concentrations of GBH [3.71 × 10−3 grams of active ingredient per hectare (g.a.i./ha), 6.19 × 10−3 g.a.i./ha and 9.28 × 10−3 g.a.i./ha] administered orally or by inhalation for 75 days. Bone marrow cells, smears of the tongue and fragments of the tongue, oesophagus, stomach and intestine were collected for histopathological analysis. Congestion, inflammation, an increase in the number of mast cells and nucleoli-organizing regions were detected in the tongue in the groups exposed to GBH. Females had a higher number of mast cells in the tongue than males. Animals in the groups exposed to higher concentrations of GBH showed dysplasia in the oesophagus and small and large intestine regardless of sex. Gastric changes were not observed. Animals exposed to GBH showed increased micronucleus formation. Our data indicate that GBH causes oral allergies and dysplastic lesions in the oesophagus and small and large intestine and has genotoxic potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The herbicide glyphosate [N-(phosphonomethyl)glycine (C3H8NO5P)] has high efficiency in combating weeds, perennials, monocots and dicots and is one of the most commonly used herbicides in the world, corresponding to 60% of the use of herbicides of its class (Benbrook 2016; Conrad et al. 2017). Glyphosate is among the best-selling active ingredients for domestic and non-professional use (Conrad et al. 2017).
The results of a large study evaluating the occurrence of glyphosate in the environment in the USA indicated that glyphosate and aminomethylphosphonic acid (AMPA), its main metabolite, are widely present in the environment, and its residues are found both in agricultural and domestic areas (Battaglin et al. 2014). In other studies, it has been shown that glyphosate and AMPA residues are present in both glyphosate-resistant and non-resistant plants, especially in glyphosate-resistant soybeans. Therefore, residues of glyphosate and AMPA and their adjuvants can be found in foods and diets made from plants treated with GBH (Mertens et al. 2018). Glyphosate and AMPA residues are frequently detected in wheat, canola, barley, beans and many other crops (Vandenberg et al. 2017), in addition to processed foods (Myers et al. 2016). Glyphosate and AMPA not only contaminate soil and food but also affect aquatic systems, even groundwater (Sanchis et al. 2012), and can also be detected in surface water (Battaglin et al. 2014). Non-occupational human exposure is increasing, not only due to the widespread use of this herbicide in crops but also because the half-life of glyphosate in water and soil is longer than previously thought (Myers et al. 2016), favouring non-occupational contamination by the oral route. Glyphosate residues have been found in the urine of humans, especially in consumers of predominantly non-organic foods (Krüger et al. 2014)
The risks of damage caused by pesticides affect workers and directly affect consumers, who may suffer the consequences of daily consumption of food contaminated by pesticides (EPA 1993). Glyphosate residues can be detected in food, drinking water and human blood and urine (Qiu et al. 2020). Therefore, the European Food Safety Authority (EFSA) has determined that the acceptable daily intake of glyphosate is 0.5 mg/kg/day and that the limit for drinking water is 0.1 μg glyphosate/L (EFSA 2015).
Glyphosate is the most commonly used herbicide worldwide, in part due to the assumption of the general population that it affects only plants but not animals (Chłopecka et al. 2014; Myers et al. 2016). Although studies carried out by industry, and the initial studies carried out by the US Environmental Protection Agency, suggested that glyphosate is slightly toxic to non-target species (such as birds, fish and aquatic invertebrates), and recent studies of glyphosate have highlighted the harmful toxic effects and raised concerns regarding the safety of glyphosate for other species (Battaglin et al. 2014). Glyphosate causes various morphological, physiological and biochemical changes in cells, including mammalian cells (Chłopecka et al. 2014), such as oxidative stress, and consequently damages organs and promotes of the onset of cancer (Landrigan and Belpoggi 2018).
The glyphosate active ingredient is not pure but instead is combined with other substances (adjuvants) to enhance its action (Mertens et al. 2018). Therefore, glyphosate-based herbicides (GBHs) are formulations that mix adjuvants and glyphosate (the active ingredient) (Mesnage et al. 2013). There are more than 750 different GBHs that also contain other substances, most of which are classified as inert by regulatory agencies (Hanlon et al. 2013; Nagy et al. 2019). The most widely used GBH in agriculture is Roundup (a common name for a large number of GBHs), which has been produced since 1974 (Grube et al. 2011; Battaglin et al. 2014). Additionally, Roundup is the second most commonly used pesticide in an urban setting (Battaglin et al. 2014).
The mixture of glyphosate with various other ingredients that are considered inert is intended to change the physicochemical properties of glyphosate and increase its action. There are ingredients that are used to increase glyphosate adhesion to plants, such as polyglycosides, and others facilitate penetration into plant cells and tissues, such as ethoxylated tallow amines. However, the formulation of GBHs is a trade secret, so the composition of GBHs is unknown, which limits the available data on the dangers presented by different formulations. These formulations can have more potent effects than the effects of the ingredients alone (Zoeller et al. 2012). Studies have shown that GBHs are more toxic than glyphosate alone (Mesnage et al. 2013; Defarge et al. 2016).
Studies on exposure to GBHs in laboratory animals have shown altered reproductive development in male rats and in male and female fish, craniofacial and brain malformations in fish, hepatorrenal damage and cardiovascular damage, eye damage, oxidative stress induction and genotoxicity in vitro and in vivo (Vandenberg et al. 2017). Studies with rats and swine have shown effects on the gastric mucosa, liver, kidney and cardiovascular system as well as reproductive changes (Gill et al. 2018). There are few epidemiological studies evaluating the impact of glyphosate on human diseases (Vandenberg et al. 2017). In humans, glyphosate is considered a potent endocrine disruptor (Gill et al. 2018) and can cause lung, liver and kidney damage in acute exposures (Sribanditmongkol et al. 2012), and chronic nephropathy in chronic simultaneous exposure to heavy metals (Jayasumana et al. 2015). The International Agency for Research on Cancer (IARC) concluded in March 2015 that glyphosate is “probably carcinogenic to humans”, and epidemiological studies have shown an association of exposure to glyphosate and non-Hodgkin lymphoma, but evidence for this association in humans is still limited ( IARC Working Group 2015).
Given that glyphosate can be detected in our food and in our water, the digestive system is one of the organ systems affected by exposure to pesticides. The digestive tract can be affected both by occupational exposure through contact with mist microdroplets that can be swallowed, and by ingesting water and food contaminated by pesticides. Some studies have shown that gastritis and other gastrointestinal problems occur in the majority of the population exposed to pesticides (Myers et al. 2016; Landrigan and Belpoggi 2018).
Some studies have described the effects of exposure to glyphosate and GBH on parts of the digestive system, such as oesophageal corrosion on acute exposure in humans (Chen et al. 2013), alteration of intestinal motor activity on in vitro exposure (Chłopecka et al. 2014), changes in the rat gut microbiome Lozano et al. 2017) and changes in intestinal morphology, antioxidant capacity and barrier function in weaned piglets (Qiu et al. 2020).
The toxicity assessment of GBHs is performed for each substance separately, which leads to the failure to notice possible combined effects of these substances, resulting in inconsistent data on the toxic effects of glyphosate and GBHs on human cells and tissue (Nagy et al. 2019). Further evaluations of commercially used formulations of GBHs are still needed as herbicide formulations are likely to have effects that are due not only to exposure to glyphosate (Vandenberg et al. 2017).
GBH is the most widely used pesticide in the world for agricultural production, but there are few studies that have been carried out on exposure to glyphosate in the diet and evaluated the digestive tract, in the usual exposure concentrations that are used in crops. Reports of oral lesions caused by glyphosate are even scarcer.
The aim of this work was to evaluate the effects on the digestive tract in male and female rats of subchronic oral and inhalational exposure to a GBH formulation that is commonly used in our country as an herbicide in an agricultural and urban environment, using concentrations recommended by the manufacturer for use in crops. For this, we evaluated the cytopathological and histopathological changes associated with tissue exposure to chemical agents. In addition, we chose the micronucleus test to assess genotoxicity, as it is a widely used tool for research and assessment of the safety of numerous substances, thus providing our results with strong statistical support (Fenech et al. 1999).
Materials and methods
Animals, maintenance and exposure
The experiments were performed in 88 adult, 90-day-old male and female albino Wistar rats weighing 200–250 g housed in plastic cages (2 for cage) separated by sex in a vivarium at an average temperature of 22 ± 2°C and relative humidity of 50 ± 15% with 12-h light and dark cycle (National Research Council 2011). The animals went through 1 week of habituation to the vivarium before the beginning of the experiment.
Exposure to the glyphosate was performed using a glyphosate-based herbicide (GBH) (Roundup® Original DI, Monsanto do Brasil, São Paulo, Brazil) with the following formulation: diammonium salt of N-(phosphonomethyl)glycine (GLYPHOSATE): 445 g/L (44.5% m/v); N-(phosphonomethyl) glycine (GLYPHOSATE) acid equivalent: 370 g/L (37.0% m/v); other ingredients: 751 g/L (75.1% m/v).
After a 1-week adaptation period, oral and inhalation exposure was performed for 75 days. In the control groups, nebulization was performed using a sodium chloride solution (NaCl); in groups exposed to a low concentration, nebulization was performed using 3.71 × 10−3 grams of active ingredient per hectare (g.a.i./ha) of GBH diluted in 10 mL of sodium chloride [corresponding to 27.05 parts per million (ppm)]; in groups exposed to a medium concentration, nebulization was performed with 6.19 × 10−3 g.a.i./ha of GBH diluted in 10 mL of sodium chloride (corresponding to 45.27 ppm); and in groups exposed to a high concentration, nebulization was performed with 9.28 × 10−3 g.a.i./ha diluted of GBH in 10 mL of sodium chloride (corresponding to 67.54 ppm).
Various concentrations of the GBH corresponded to the concentrations that are used and described in the product insert of Roundup®. Each concentration was adjusted for the box area to simulate environmental exposure (occupational and food residue).
The animals were randomly distributed into eight groups (males: n = 5/group; females: n = 5/group): IC — inhalation control group; OC — oral control group; IL — low inhalation concentration group; OL — low oral concentration group; IM — medium inhalation concentration group; OM — medium oral concentration group; IH — high inhalation concentration group; OH — high oral concentration group.
In the inhalational exposure groups, all animals of the same group were exposed to nebulized GBH simultaneously, and in the orally exposed groups, the feed was exposed to nebulized GBH and then provided to the animals to simulate food contamination in crops.
For nebulization, two boxes (32×24×32 cm) were connected to an ultrasonic nebulizer (Pulmosonic Star®, Soniclear Ind. Com. Imp. and Exp. Ltda., São Paulo, Brazil) (Mello et al. 2018), which produces smaller and more uniform droplets, allowing the formation of mist simulating what occurs during the spraying of crops. The time required to nebulize the entire solution was 15 min for both the inhalational and oral exposure groups.
Animals exposed by inhalation were nebulized for 5 consecutive days per week to simulate occupational exposure. The feed of the animals exposed orally was changed every 2 days throughout the experiment, and nebulization was performed 1 day before the feed was offered to the animals. Six hundred grams of feed per box was offered, and the uneaten feed was weighed at each change. The uneaten feed was subtracted from the total feed offered (600 g) to establish consumption per box. Then, consumption per box was divided by two, since in each box, there were two animals to assess consumption per animal.
A ninth group was established as a positive control group for the bone marrow micronucleus test: PC — positive control group (males: n = 8): cyclophosphamide at a single subcutaneous dose (50 mg/kg) on the first day of the experiment (MacGregor 1987).
Anaesthesia was performed with sodium thiopental (Syntec, USA) at a dose of 100 mg/kg body weight administered in the peritoneal cavity. Indicators of death included the absence of respiratory movements and heartbeat and loss of reflexes (National Research Council 2011).
Cytopathological analysis
After euthanasia, the material was collected with a cytological brush (Cytobrush; Medical Burs, Indústria e Comércio de Produtos Abrasivos Ltda, Cotia, São Paulo Brazil) from the dorsum of the tongue. After harvesting, the material was spread on glass slides and fixed by air drying, and the slides were stained with Giemsa stain (Merck KGaA, Darmstadt, Germany) (MacGregor 1987). The analysis was performed using a standard optical microscope (NIKON Labophot, Japan) at 400× magnification. The following nuclear changes were quantified in 100 epithelial cells: micronuclei [according to the criteria established by Tolbert et al. 1992], karyomegaly and binucleation.
Histopathological analysis
Subsequently, the tongue, oesophagus, stomach and small and large intestine of each animal were harvested. The fragments were fixed in 10% buffered formaldehyde (Cinética Indústria Química, São Paulo, Brazil) for 24 h and subjected to usual histological processing, including paraffin embedding (Dynamic Analytical Reagents, São Paulo, Brazil). Serial 5-μm slices were obtained by a LEICA RM2265 microtome (Leica Biosystems Nussoch GmbH, Germany) and stained using the haematoxylin-eosin (HE) method (Dolles, São Paulo, Brazil).
Additional sections of the tongue were stained with toluidine blue (Merck, Darmstadt, Germany) to identify mast cells. Deparaffinized sections of the tongue were also stained by silver impregnation according to Ploton et al. (1986) for the evaluation of NORs (nucleoli-organizing regions).
Histopathological analysis was performed in blinded fashion by a single experienced observer using a standard optical microscope (NIKON Labophot, Japan). The general parameters evaluated in the tongue, oesophagus, stomach and small and large intestine with their respective scores were as follows: interstitial inflammatory infiltrate (absent, mild, moderate or intense) and type of inflammatory cells present (polymorphonuclear and/or mononuclear); tissue congestion (absent, mild, moderate or severe); tissue necrosis (absent, present and focal or present and diffuse); vascular necrosis (absent or present); non-neoplastic changes in the mucosa (atrophy and hyperplasia and metaplasia: absent or present); dysplastic lesions (absent, mild dysplasia, moderate dysplasia or severe dysplasia); and presence of neoplastic lesions (absent, benign or malignant) (Pegoraro et al. 2018).
The following parameters were also specifically analysed: tongue: presence of hyperkeratosis, defined as thickening of the stratum corneum (absent, mild, moderate or marked), and presence of parakeratosis, defined as an abnormal process of epithelial cell maturation with retention of the nuclei in the corneal layer (absent, focal or diffuse) (Martelli et al. 2014); oesophagus: presence of hyperkeratosis (absent, mild, moderate or marked) and presence of parakeratosis (absent, focal or diffuse) (Nai et al. 2015); intestine: lymphoid hyperplasia (absent or present) (Pegoraro et al. 2018).
Histomorphometric analysis of the tongue
The following histomorphometric analyses were performed on the tongue: measurements of the thickness of the tongue epithelium were performed in two areas of the dorsal region and in two areas of the ventral surface (Martelli et al. 2014); mast cell count in 10 high-magnification fields (HPF), corresponding to approximately 1 mm2 (Parizi et al. 2010); NORs were counted in 10 cells/HPF, totalling 100 cells per animal and area (dorsum and ventral surface) (Ploton et al. 1986).
The measurements of the thickness of the tongue epithelium were performed using the ImageJ software from the National Institutes of Health (NIH) of the USA, freely available on the internet (http://rsbweb.nih.gov/ij/).
Micronucleus test on bone marrow cells
The bone marrow of one of the femurs was used for the micronucleus test. Bone marrow cells were collected from the femur in 3 mL of saline. After resuspension, the material was centrifuged for 5 min at 1000 rpm. The supernatant was discarded, and the precipitate was resuspended in 0.5 mL of saline. Smears were performed by extending drops of this suspension on a slide. The smears were air-dried and stained with Giemsa stain (Merck KGaA, Darmstadt, Germany). Two slides with smears were prepared per animal (MacGregor 1987).
Two thousand polychromatic erythrocytes per animal were counted at 400× magnification to determine the number of micronuclei (MacGregor 1987). Structures that had a surrounding halo suggestive of a membrane smaller than one-third of the diameter of the associated nucleus with intensity of staining similar to that of the nucleus and located in the same focal plane according to microscopy were considered micronuclei (Tolbert et al. 1992).
Statistical analysis
Variables expressed as scores were considered non-parametric. Continuous variables were evaluated by the Shapiro-Wilk test to validate the assumption of normality of the data. Homogeneity of variance was assessed by the Levene test. Non-parametric variables were compared between sexes using the Mann-Whitney test and between groups using the Kruskal-Wallis test with Dunn’s test for multiple comparisons. The variables weight and feed intake showed homogeneity of variances (Levene’s test, p> 0.05) and were analysed with an analysis of variance. For comparisons between Inhalation × Oral, the t-test (when the data showed normality and homogeneity of variance) or the Mann-Whitney test was used. For the qualitative variables, the likelihood ratio test was used. All analyses were performed in R at 5% level of significance (R Development Core Team 2019). The effect size was also evaluated by Cohen’s d, where values <0.19 are considered insignificant, between 0.20 and 0.49 are considered small, between 0.50 and 0.79 medium, between 0.80 and 1.29 large and >1.30 very large (Espírito-Santo and Daniel 2015).
Results
Mortality, animal weight and feed intake
One female of the oral control group died during the study due to ear canal infection.
The mean initial weight of the unexposed animals was 332.65 ± 71.3 g, and that of the animals exposed to GBH was 311.55 ± 60.78 g, while the mean final weight of the unexposed animals was 340.45 ± 81.76 g and that of the exposed animals was 331 ± 67.09 g (ANOVA F = 0.096; p = 0.29) (Table 1). With a Cohen’s d of 0.318495, exposed animals showed a small difference from those not exposed in their initial weight. With a Cohen’s d of 0.126361, exposed animals showed an insignificant difference from those not exposed in their final weight.
The average feed intake of the unexposed groups was 380.5 ± 48.04 g, and that of the groups exposed to GBH was 398.4 ± 35.88 g (ANOVA F = 0.66; p = 0.703) (Table 1). With a Cohen’s d of 0.422188, exposed animals showed a small difference in food intake from those not exposed.
Tongue
Cytopathological analysis
No micronucleated cells were detected in the oral smears of the tested animals. A female of the group orally exposed to the medium concentration of GBH presented binucleated cells (Fig. 1).
Cytological smears of the tongue mucosa. A Normal squamous cells (female animal of the inhalation control group). B Binucleated cell (female animal of the medium oral concentration group). C Cells with karyomegaly (female animal of the medium oral concentration group). Giemsa stain, 400× magnification — scale bar: 50 μm
Karyomegaly was the predominant nuclear alteration in females exposed to GBH (p <0.05) (Fig. 1). Differences were detected between the medium oral concentration group × low and high oral concentration groups (Kruskal-Wallis chi-squared = 6.1923, df = 3, p = 0.0080) and between the low inhalation concentration group × the other groups exposed by inhalation (Kruskal-Wallis chi-squared = 13.29, df = 3, p = 0.0009).
Histopathological analysis
No hyperkeratosis, parakeratosis, dysplastic or benign or malignant neoplastic lesions were observed in the tongue epithelium.
The congestion was mild in animals that had congestion of the tongue. There were no sex-dependent differences between the animals (Kruskal-Wallis chi-squared = 10.95, df = 7, p = 0.070). There were differences between the inhalation control group × high inhalation concentration group (p = 0.016) and the medium inhalation concentration group × low and high inhalation concentration groups (p = 0.0174) (Kruskal-Wallis chi-squared = 12.0761, df = 3, p <0.05). There were no significant differences between the groups exposed orally (Figs. 2 and 3A, Table 2).
Percentage of cases that presented congestion and inflammation in the tongue according to the study groups regardless of sex of the animals (p = 79). Congestion — Kruskal-Wallis chi-squared = 12.0761, p <0.05: IC × IH; IM × (IL, IH). Inflammation — Kruskal-Wallis chi-squared = 36.5464, p = 0.0115: IC × (IL, IM, IH); OH × (OC, OL, OM) (t-test). IC, inhalation control; OC, oral control; IL, low inhaled glyphosate concentration; OL, low oral glyphosate concentration; MI, medium inhaled glyphosate concentration; OM, medium oral glyphosate concentration; IH, high inhaled glyphosate concentration; OH, high oral concentration of glyphosate
Photomicroscopy of the tongue mucosa. A Ventral surface with moderate tissue congestion. Note dilated and blood-filled capillary (male animal of the low inhalation concentration group). B Dorsal surface with a focus of inflammation of the submucosa, characterized by lymphocyte infiltrate (male animal of the low oral concentration group). Haematoxylin-eosin, 100× magnification — scale bar: 200 μm
The females of the groups exposed to low GBH concentrations and the males of the IM group did not present inflammation. In other groups, inflammation was mild and consisted of mononuclear cells (lymphocytes), and there were no differences between sexes (t-test t = 0.80179, df = 7.7859, p = 0.4463). The inhalation control group differed from the groups exposed by inhalation to GBH, and the high oral concentration group differed from the other groups exposed orally (Kruskal-Wallis chi-squared = 36.5464, df = 7, p = 0.0115) (Figs. 2 and 3B, Table 2).
The average number of mast cells of the unexposed groups was 71 ± 17.45, and that of the groups exposed to GBH was 78.5 ± 15.69 (ANOVA F= 2.706, df = 7, p = 0.0152). With a Cohen’s d of 0.451988, exposed animals showed a small difference from those not exposed in the mast cell count. The number of mast cells differed between sexes and was higher in females (t-test t = 2.8886, df = 5.5363, p = 0.02511). The groups exposed by the oral route had a higher number of mast cells than did animals exposed by the inhalational route (t-test t = 1.314, df = 3, p = 0.016) (Fig. 4, Table 2). The average number of mast cells in the oral GBH group was 82.9 ± 4.01, and that in the inhalation GBH group was 73.23 ± 8.76. With a Cohen’s d of 1.41947, animals exposed orally showed a very large difference from those exposed by inhalation in the mast cell count.
Mean number (± standard error) of mast cells (per mm2) per study group regardless of sex of the animals (p = 79). Inhalation vs. oral exposure (t-test t = 1.314, p = 0.016). IC, inhalation control; OC, oral control; IL, low inhaled glyphosate concentration; OL, low oral glyphosate concentration; MI, medium inhaled glyphosate concentration; OM, medium oral glyphosate concentration; IH, high inhaled glyphosate concentration; OH, high oral concentration of glyphosate
Measurement of the thickness of the tongue epithelium
The average epithelial thickness of the dorsum in the unexposed groups was 773,806 ± 36217.6 pixels, and that of the groups exposed to GBH was 813,334 ± 27996.1 pixels. The average epithelial thickness of the dorsum in the ventral surface of the unexposed groups was 400,090 ± 75,623 pixels, and that of the groups exposed to GBH was 471,819 ± 36678.2 pixels. There were no significant differences in epithelial thickness between the groups exposed or not exposed to GBH or between sexes on the dorsum (ANOVA F = −1.809, df = 7, p = 0.0988) or on the ventral surface (ANOVA F = 0.44, df = 7, p = 0.874) (Table 2). With a Cohen’s d of 0.012212 for the epithelial thickness of the dorsum and with a Cohen’s d of 0.002766 for the epithelial thickness of the ventral surface, exposed animals showed an insignificant difference from those not exposed to GBH for epithelial thickness.
Number of nucleoli-organizing regions on the tongue
Exposure to GBH caused an increase in NORs on the dorsum of the tongue, and the numbers differed between inhalation control group × the groups exposed by inhalation to GBH, between oral control group × the groups exposed orally to GBH, between low inhalation concentration group × medium and high inhalation concentration groups, between medium inhalation concentration group × high inhalation concentration group, between high oral concentration group × low and medium oral concentration groups, between low inhalation concentration group × low oral concentration group and between high inhalation concentration group × high oral concentration group (ANOVA F = 268.1, df = 7, p = 0.001) (Fig. 5, Table 2).
Mean number (± standard error) of nucleoli-organizing regions (NORs) (per mm2) in the epithelium of the dorsum and ventral surface of the tongue according to the study groups independent of animal sex (n = 79). Dorsum — ANOVA F = 268.1, p = 0.001: IC × (IL, IM, IH); OC × (OL, OM, OH), IL × (IM and IH); IM × IH; OH × (OL, OM); IL × OL; IH × OH. Ventral surface — ANOVA F = 770.9, p = 0.001: IC × (IL, IM, IH); IL × (IM, IH); IM × IH; OC × (OL, OM, OH); OL × (OM, OH); OM × OH. IC, inhalation control; OC, oral control; IL, low inhaled glyphosate concentration; OL, low oral glyphosate concentration; MI, medium inhaled glyphosate concentration; OM, medium oral glyphosate concentration; IH, high inhaled glyphosate concentration; OH, high oral concentration of glyphosate
The average number of NORs in the dorsum of the unexposed groups was 1005.65 ± 11.78, and that of the groups exposed to GBH was 1088.73 ± 9.89. With a Cohen’s d of 7.639021, exposed animals showed a large difference from those not exposed to GBH in their NOR count in the dorsum.
Numbers of NORs on the ventral surface of the tongue differed between inhalation control group × the groups exposed by inhalation to GBH, between low inhalation concentration group × medium and high inhalation concentration groups, between medium inhalation concentration group × high inhalation concentration group, between oral control group × the groups exposed orally to GBH, between low oral concentration group × medium and high oral concentration groups, and between medium inhalation concentration group × high inhalation concentration group (ANOVA F = 770.9, df = 7, p = 0.001) (Fig. 5, Table 2).
The average number of NORs on the ventral surface of the unexposed groups was 784.18 ± 3.19, and that of the groups exposed to GBH was 769.95 ± 11.03. With a Cohen’s d of 1.257674, exposed animals showed a large difference from those not exposed to GBH in their NOR count on the ventral surface.
However, there were no significant differences between sexes in the numbers of NORs on the dorsum (t-test t = 0.29564, df = 5.3079, p = 0.778) and on the ventral surface of the tongue (t-test t = 1.0896, df = 7.8888, p = 0.308) (Table 2).
Correlation between measurement of epithelial thickness and the numbers of nucleoli-organizing regions on the tongue
There were no correlations between epithelial thickness and the numbers of NORs on the dorsum (ρ = -0.229; p = 0.523) and on the ventral surface of the tongue (ρ = -0.018; p = 0.959) in all tested groups.
Oesophagus
No congestion, inflammation, hyperkeratosis, parakeratosis or malignant or benign neoplastic lesions were observed in the oesophagus. All animals exposed to GBH by inhalation had mild dysplasia, and all animals orally exposed to GBH had moderate epithelial dysplasia (Kruskal-Wallis chi-squared = 73.6921, df = 7, p = 0.00001), regardless of sex (t-test t = 0.80225, df= 5.6232, p = 0.371) (Fig. 6). The animals of the control groups did not present dysplastic lesions of the epithelium.
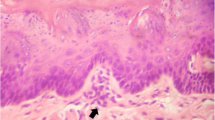
Photomicroscopy of the oesophagus. A Normal oesophageal epithelium (male animal of the inhalation control group). B Mild oesophageal dysplasia. There is a slight increase in the thickness of the epithelium, bulky nuclei with evident nucleoli and atypical mitosis figures (arrow) (male animal of the high inhalation concentration group). C Moderate oesophageal dysplasia. Note a marked increase in epithelial thickness, bulky nuclei with evident nucleoli and atypical mitosis figures (arrow) (male animal of the high oral concentration group). Haematoxylin-eosin, 200× magnification — scale bar: 100 μm
Stomach
No gastric changes were observed in the tested animals.
Small intestine
Animals of all groups showed mild congestion of the small intestinal mucosa. No benign or malignant neoplastic changes were observed in the tested animals. All animals exposed to GBH had a lymphocytic inflammatory process in the mucosa. Moderate inflammation was observed in 60% of the animals in the IL and OL groups. Other animals presented mild inflammation (Kruskal-Wallis chi-squared = 60.156, df = 7, p = 0.0001). There were no differences in the inflammation parameter between sexes (t-test t = −0.79856, df = 6.6208, p = 0.600) (Table 3, Fig. 8B). Most animals exposed to GBH, regardless of the route of exposure, presented lymphoid hyperplasia (Kruskal-Wallis chi-squared = 25.305, df = 7, p = 0.00067) with no differences between sexes (t-test t = −1.4953, df = 6.9976, p = 0.1785) (Table 3, Fig. 8C).
There were no differences in mucosal dysplasia between sexes (t-test t = −0.86035, df = 6.6522, p = 0.4195). Only the animals in the groups exposed to the medium and high concentrations of GBH had dysplasia, and all animals in the high oral concentration group had moderate dysplasia; other animals had mild dysplasia (Kruskal-Wallis chi-squared = 65.831, df = 7, p = 0.001). The medium inhalation concentration group had the lowest frequency of dysplasia among the groups that presented this alteration (Table 3, Figs. 7,8D and 8E).
Percentage of cases with mild and moderate dysplasia in the small intestine by study group (n = 79). Kruskal-Wallis chi-squared = 65.831, p = 0.001: (IC, IL) × (IM, IH); (OC, OL) × (OM, OH); OM × OH; IH × OH. IC, inhalation control; OC, oral control; IL, low inhaled glyphosate concentration; OL, low oral glyphosate concentration; MI, medium inhaled glyphosate concentration; OM, medium oral glyphosate concentration; IH, high inhaled glyphosate concentration; OH, high oral concentration of glyphosate
Photomicroscopy of the small intestine. A Normal intestinal mucosa (male animal of the inhalation control group) (haematoxylin-eosin, 200× magnification — scale bar: 100 μm). B Intestinal mucosa with moderate inflammation (arrow) (male animal of the low inhalation concentration group) (haematoxylin-eosin, 100× magnification — scale bar: 200 μm). C Lymphoid hyperplasia (arrow) (male animal of the high inhalation concentration group) (haematoxylin-eosin, 100× magnification — scale bar: 200 μm). D Mild mucosal dysplasia. Note hyperchromatic nuclei and mitosis figures (arrows) (male animal of the medium inhalation concentration group) (haematoxylin-eosin, 400× magnification — scale bar: 50 μm). E Moderate mucosal dysplasia. Note a decrease in the number of goblet cells, non-polarized and vesicular nuclei with nucleoli and atypical mitosis figures (arrow) (male animal of the high oral concentration group) (haematoxylin-eosin, 400× magnification — scale bar: 50 μm)
Large intestine
Animals in all groups showed mild congestion of the large intestinal mucosa. Only one female in the low oral concentration group, one female in the medium inhalation concentration group and three males had lymphoid hyperplasia (Fig. 10C). No benign or malignant neoplastic changes were observed in the animals.
The inflammation presented by the animals was mild and characterized by lymphocytic infiltration (Fig. 10B). There were significant differences in the presence of inflammation between the inhalation control group × medium and high inhalation concentration groups, between low inhalation concentration group × medium and high inhalation concentration groups, between oral control group × medium and high oral concentration groups and between low oral concentration group × medium and high oral concentration group (Kruskal-Wallis chi-squared = 14.229, df = 7, p = 0.04726); however, there were no differences between sexes (t-test t = −1.4115, df = 7.7235, p = 0.423) (Table 4).
All animals exposed by inhalation and orally to medium and high concentration of GBH showed dysplasia, with moderate dysplasia in one animal of the high inhalation concentration group and in all animals of the high oral concentration group (Kruskal-Wallis chi-squared = 65.482, df = 7, p = 0.001) (Table 4, Figs. 9, 10D and 10E). There were no differences for dysplasia between sexes (t-test t = −1.8284, df = 5.5068, p = 0.502) (Table 4).
Percentage of cases with mild and moderate dysplasia in the large intestine by study group (n = 79). Kruskal-Wallis chi-squared = 65.482, p = 0.001: (IC, IL) × (IM, IH); (OC, OL) × (OM, OH); OM × OH; IH × OH. IC, inhalation control; OC, oral control; IL, low inhaled glyphosate concentration; OL, low oral glyphosate concentration; MI, medium inhaled glyphosate concentration; OM, medium oral glyphosate concentration; IH, high inhaled glyphosate concentration; OH, high oral concentration of glyphosate
Photomicroscopy of the large intestine. A Normal intestinal mucosa (male animal of the oral control group) (haematoxylin-eosin, 200× magnification — scale bar: 100 μm). B Intestinal mucosa with mild inflammatory infiltration (arrow) (male animal of the medium inhalation concentration group) (haematoxylin-eosin, 100× magnification — scale bar: 200 μm). C Lymphoid hyperplasia (arrow) (male animal of the high inhalation concentration group) (haematoxylin-eosin, 200× magnification — scale bar: 100 μm). D Mild dysplasia. Note nuclei with loss of polarity, irregular chromatin and evident nucleoli and mitosis figures (arrow) (male animal of the medium oral concentration group) (haematoxylin-eosin, 400× magnification — scale bar: 50 μm). E Moderate dysplasia. Note a marked decrease in goblet cells, cells with vesicular nuclei with irregular chromatin and evident nucleoli and various mitosis figures (arrows) (female animal from the high oral concentration group) (haematoxylin-eosin, 400× magnification — scale bar: 50 μm)
Micronucleus test on bone marrow cells
The median number of micronuclei in the control groups was 0; in the low and medium inhalation concentration groups, it was 2; in the low and medium oral concentration groups, it was 2.5; in the groups exposed to high GBH concentrations, it was 3; and in the positive control group, it was 9 (p < 0.05). There were differences between sexes; a greater number of micronuclei were detected in females exposed to the low oral concentration, and the number was higher in males exposed to the high inhalation concentration (t-test t = −5, df = 8, p = 0.001053) (Figs. 11 and 12).
Median number (± standard error) of micronuclei in the bone marrow by study group and sex of the animals (n = 79). t-test t = −5, p = 0.001053: OL females vs. OL males; IH females vs. IH males. IC, inhalation control; OC, oral control; IL, low inhaled glyphosate concentration; OL, low oral glyphosate concentration; MI, medium inhaled glyphosate concentration; OM, medium oral glyphosate concentration; IH, high inhaled glyphosate concentration; OH, high oral concentration of glyphosate
Discussion
In this study, we observed tissue congestion, inflammation, an increase in the number of nucleoli-organizing regions (NORs) not associated with increased thickness of the epithelium in the tongue mucosa and dysplasia of the oesophageal epithelium and small and large intestine in animals exposed to GBH. With the exception of an increase in the number of mast cells in the tongue and the number of micronuclei in polychromatic erythrocytes in the bone marrow, no parameters were influenced by the sex of the animals. The gastric mucosa did not suffer damage upon exposure to GBH.
The kidney and gastrointestinal tract have been identified as the target organs of glyphosate in ruminants. In the case of the digestive tract, mucosal irritation was detected by histopathological examination (EFSA 2015). Thus, in this study, we investigated the digestive tract and possible impact of inhalation exposure (more common in occupational exposure) and oral exposure to food contaminated by GBH (more common in paraoccupational exposure) on this organ system.
Burning, erosion, ulceration and haemorrhage of the oral mucosa have been reported in cases of incidental acute exposure to glyphosate (Mui 1993; Sribanditmongkol et al. 2012; Deo and Shetty 2012). Our study detected karyomegaly in cytological smears, which can be a reaction to tissue damage or even to a chronic inflammatory process. However, the group exposed to low concentration by inhalation had the highest number of cases with karyomegaly, highest intensity of congestion and lowest number of cases of inflammation. This result demonstrates direct damage caused by the effects of GBH on the epithelium; however, probably due to the low dosage of exposure, an inflammatory process was not detected. However, a greater number of cases with congestion and low inflammation were observed in animals exposed to high concentration of GBH (regardless of the route of exposure). Tissue congestion is the initial change in the inflammatory process in the tissue (Punchard et al. 2004). High concentration of GBH can provoke a reaction in the tissue that stimulates more profound vascular alteration than that resulting from recruitment of inflammatory cells. A greater number of cases of inflammation were detected among animals exposed to low and medium oral concentrations, showing that the oral route and lower concentrations provide direct contact of GBH with the mucosa and can cause a local inflammatory response.
Mast cells reside in normal connective tissue and are associated with various pathological processes, such as allergies, tissue angiogenesis (formation of new vessels) and the inflammatory process (Parizi et al. 2010). We observed a greater number of mast cells in the oral mucosa in females and in groups exposed orally. These data show that GBH can stimulate an oral allergic reaction, especially after direct contact and in females. Studies evaluating other herbicides demonstrated that herbicides may be systemic allergens (Cushman and Street 1982; Yasunaga et al. 2015) and are allergenic to the skin, respiratory tract (Fukuyama et al. 2009) and oral cavity (Parizi et al. 2020).
NORs are markers of cell proliferation (Parizi et al. 2020). An increase in the number of NORs in the mucosa of the tongue observed in the groups exposed to GBH indicates toxicity to the oral epithelium that stimulated cell proliferation to repair possible cell destruction caused by GBH. However, in the group exposed to high oral concentration, the number of NORs was decreased compared to that in the control group. This decrease may be due to severe damage to the epithelium caused by a high oral concentration that prevents the stimulation of regenerative cell proliferation. We observed an increase in NORs in most groups exposed to GBH; however, this increase was insufficient to increase the thickness of the tongue epithelium. These data confirm that GBH causes a high level of cell destruction and that cell proliferation does not increase the thickness of the epithelium at this level of damage.
Case reports in humans described bleeding from the gastric mucosa after deliberate ingestion of glyphosate (Sribanditmongkol et al. 2012; Deo and Shetty 2012); however, another study showed ulceration of the oesophageal mucosa without gastric lesions in two cases of incidental ingestion of glyphosate (Chen et al. 2013). The authors speculated that the absence of gastric lesions in these cases may be due to the type of epithelium, presence of acid or mucus or other local factors and suggested that glyphosate may be considered a caustic agent of medium intensity (Chen et al. 2013). In our study, lesions of the gastric epithelium were not detected, possibly because even oral concentrations used in the present study were considerably lower, and GBH was inhaled or ingested along with feed rather than ingested alone in large quantities, as described in other studies. This consideration may also explain the absence of oesophageal lesions, such as erosions, ulcerations and inflammation, in the exposed animals.
An in vitro study with muscle fibres of the jejunum of rats detected a decrease in the motor activity even if glyphosate was applied at very low concentrations (Chłopecka et al. 2014). Dilation of the small intestine was observed after incidental ingestion of glyphosate in humans (Sribanditmongkol et al. 2012; Deo and Shetty 2012). In another study in weaned piglets, no change in intestinal morphology was detected after ingesting water containing glyphosate added in various concentrations (Qiu et al. 2020). A study in Sprague-Dawley rats demonstrated that long-term exposure to glyphosate in tap water was toxic to the intestinal microbiome, and dysbiosis was manifested only in treated females (Lozano et al. 2017). Dysbiosis of the intestinal microbiota is associated with a number of clinical conditions, such as inflammatory bowel disease or colorectal cancer (Cho and Blaser 2012). Induction of intestinal dysbiosis by glyphosate may explain the inflammation in the intestinal mucosa detected in exposed animals in the present study; however, we did not observe differences between sexes in this parameter reported in other studies. Greater intensity of inflammation and higher incidence of animals with lymphoid hyperplasia in the small intestine may be explained by higher concentrations of glyphosate detected in the small intestine after oral exposure (Chłopecka et al. 2014); a similar phenomenon may occur in inhalation exposure, since we did not detect differences between routes of exposure.
The carcinogenicity of glyphosate is a matter of controversy in the literature. IARC of World Health Organization (WHO) considers glyphosate to be “probably carcinogenic to human” (category 2A) ( IARC Working Group 2015), and some studies in rodents have detected benign and malignant neoplasms in the thyroid, liver, kidney, pancreas, testis and pituitary gland (Greim et al. 2015); however, reviews of the toxicological profile of this herbicide in other studies suggested a lack of carcinogenic risk for glyphosate (Williams et al. 2016; Tarazona et al. 2017). EFSA considered that human studies were too limited to associate glyphosate with neoplasms (Portier et al. 2016). The grade of precancerous lesions in epithelial dysplasia is associated with a lower likelihood (mild dysplasia) or greater likelihood (moderate and severe dysplasia) of progression to carcinoma. Our study is the first to demonstrate that GBH has carcinogenic potential in the oesophagus and small and large intestine, especially at higher concentrations, and that the oral exposure route can lead to moderate dysplasia, which corresponds to a higher risk of progression to carcinoma.
The oesophageal epithelium is similar to the oral cavity epithelium, i.e. stratified squamous epithelium; however, dysplasia was detected only in the oesophagus. This phenomenon may be due to mechanical washing of the oral cavity by saliva, which reduces the time of exposure to glyphosate in the mouth.
Similar to carcinogenicity, the genotoxicity of glyphosate is also a matter of controversy according to the literature. Some studies have shown that glyphosate does not present a significant genotoxic risk under normal conditions of human or environmental exposure (Heydens et al. 2008; Kier and Kirkland 2013). Other studies have shown that products containing glyphosate can be genotoxic in reptiles and mammals (Prasad et al. 2009; Lopez Gonzalez et al. 2013), increase DNA damage scores in the comet test in the erythrocytes and gill cells of fish (Moreno et al. 2014) and induce DNA strand breaks (also observed in the comet assay) in the gills and liver cells of eels exposed to glyphosate in water (Guilherme et al. 2012). Additionally, an increase in the incidence rate of chromosomal aberrations and induction of micronuclei depended on the concentration and time of exposure (1 to 3 days) to glyphosate in mammals (Prasad et al. 2009). This variability in the results may be due to differences in the purity of the active agent and the nature of the tested inert components, which can increase the toxicity of the herbicide (Prasad et al. 2009). Our study used the most common commercial worldwide brand and not the pure herbicide because it is not used in pure form to spray crops. Thus, comparison with the control groups in the present study detected an increase in the micronuclei in the groups exposed to GBH regardless of the route of exposure. This finding is in agreement with detection of dysplasia in the oesophagus and small and large intestine, indicating that GBH has genotoxic and carcinogenic potential at concentrations relevant to human exposures.
Gender can interact with exposure to xenobiotic agents and influence the toxicokinetics, toxicodynamics and results of the exposure. Sex-specific differences in response to xenobiotics can result from the differences in behaviour, exposure, anatomy, physiology, biochemistry and genetics, which can influence the responses to environmental chemicals and adverse reactions to drugs (Gochfeld 2017). Our study detected differences in some parameters between the sexes, including a higher incidence of cells with karyomegaly and mast cells in the oral cavity of females. These data show that females are more reactive and have a greater risk of oral allergic reaction upon exposure to GBH. Men and women were shown to differ in susceptibility to the development of immunological and allergy disorders and in the ability to fight infections. Women are more susceptible to the development of allergies and autoimmune diseases for multiple reasons, possibly associated with sex hormones and X chromosome factors (Laffont and Guéry 2019). These considerations may explain the higher cellular reactivity and greater number of mast cells in females exposed to GBH detected in the present study. We also observed that females had a higher number of micronuclei upon exposure to low oral GBH concentration, as micronuclei were not detected in males under these conditions; however, exposure to high concentrations by inhalation resulted in an increase in micronucleus formation in males. This result suggests that low concentrations are sufficient to cause DNA damage in females but not in males and that high concentrations can lead to cell destruction, explaining that the formation of micronuclei in females has not been detected. Other genotoxicity tests should be performed to provide a better explanation of various sex-dependent effects of various concentrations of GBH.
It is necessary to consider that pesticide formulations are mixtures of adjuvants and active ingredients (which in our case is glyphosate), and adjuvants may be as or more toxic than the active ingredient itself, or may potentiate the damage caused by the active ingredient (Mesnage et al. 2013). There is no description of the inert ingredients (adjuvants) added to the formulation in the package insert of the product that we use, so we cannot exclude the action of these products on the tissue damage observed in our study.
Further studies that evaluate the internal dose of glyphosate for each animal associated with the evaluation of the glyphosate concentration in the feed, as well as different concentrations of environmental exposure, may provide a better understanding of the effects on the digestive tract, in addition to a better definition of the dose response.
Conclusions
The data in our study are based on the standard conditions and time of exposure indicate that GBH stimulates the inflammatory process in the digestive tract, can cause oral allergies (mainly in females) and dysplastic lesions in the oesophagus and small and large intestine, and has genotoxic potential, characterized by an increase in the micronuclei in exposed animals. In general, the oral route causes more pronounced changes in the digestive tract than the inhalation route. The stomach is not damaged by prolonged exposure to the concentrations corresponding to environmental GBH exposures.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Authority EFS (2015) Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J 13(11):4302
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. JAWRA 50(2):275–290. https://doi.org/10.1111/jawr.12159
Benbrook C (2016) Trends in the use of glyphosate herbicide in the U.S. and globally. Environ Sci Eur 28(1):3. https://doi.org/10.1186/s12302-016-0070-0
Chen HH, Lin JL, Huang WH, Weng CH, Lee SY, Hsu CW, Chen KH, Wang IK, Liang CC, Chang CT, Yen TH (2013) Spectrum of corrosive esophageal injury after intentional paraquat or glyphosate-surfactant herbicide ingestion. Int J Gen Med 6:677–683. https://doi.org/10.2147/IJGM.S48273
Chłopecka M, Mendel M, Dziekan N, Karlik W (2014) Glyphosate affects the spontaneous motoric activity of intestine at very low doses - in vitro study. Pestic Biochem Physiol 113:25–30. https://doi.org/10.1016/j.pestbp.2014.06.005
Cho I, Blaser MJ (2012) The human microbiome: at the interface of health and disease. Nat Rev Genet 13:260–270. https://doi.org/10.1038/nrg3182
Conrad A, Schroter-Kermani C, Hoppe HW, Ruther M, Pieper S, Kolossa-Gehring M (2017) Glyphosate in German adults - time trend (2001 to 2015) of human exposure to a widely used herbicide. Int J Hyg Environ Health 220(1):8–16. https://doi.org/10.1016/j.ijheh.2016.09.016
Cushman JR, Street JC (1982) Allergic hypersensitivity to the herbicide 2,4-D in BALB/c mice. J Toxicol Environ Health 10(4-5):729–741
Defarge N, Takács E, Lozano VL, Mesnage R, Spiroux de Vendômois J, Séralini GE, Székács A (2016) Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int J Environ Res Public Health 13(3):264. https://doi.org/10.3390/ijerph13030264
Deo SP, Shetty P (2012) Accidental chemical burns of oral mucosa by herbicide. JNMA J Nepal Med Assoc 52(185):40–42
R Development Core Team (2019) R Software: a language and environment for statistical computing. Vienna. http://www.r-project.org. Accessed 19 March 2020.
EPA (1993) Re-registration Eligibility Decision (RED) glyphosate: EPA-738-R-93-014. US Environmental Protection Agency, Office of Pesticide Programs and Toxic Substances, Washington
Espírito-Santo H, Daniel F (2015) Calculating and reporting effect sizes on scientific papers (1): p < 0.05 limitations in the analysis of mean differences of two groups. Port J Behav Soc Res 1(1):3–16
Fenech M, Holland N, Chang WP, Zeiger E, Bonassi S (1999) The HUman MicroNucleus Project - an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res 428:271–283
Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T, Kosaka T (2009) Allergic reaction induced by dermal and/or respiratory exposure to low-dose phenoxyacetic acid, organophosphorus, and carbamate pesticides. Toxicol 261:152–161
Gill JPK, Sethi N, Mohan, A, Datta S, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16:401–426. https://doi.org/10.1007/s10311-017-0689-0
Gochfeld M (2017) Sex differences in human and animal toxicology. Toxicol Pathol 45(1):172–189. https://doi.org/10.1177/0192623316677327
Greim H, Saltmiras D, Mostert V, Strupp C (2015) Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit Rev Toxicol 45:185–208. https://doi.org/10.3109/10408444.2014.1003423
Grube A, Donaldson D, Kiely T, Wu L (2011) Pesticide industry sales and usage: 2006 and 2007 market stimates. US EPA, Washington, DC
Guilherme S, Gaivão I, Santos MA, Pacheco M (2012) DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide - elucidation of organ-specificity and the role of oxidative stress. Mutat Res 743(1-2):1–9. https://doi.org/10.1016/j.mrgentox.2011.10.017
Hanlon SM, Lynch KJ, Parris MJ (2013) Mouthparts of southern leopard frog, Lithobates sphenocephalus, tadpoles not affected by exposure to a formulation of glyphosate. Bull Environ Contam Toxicol 91(6):611–615. https://doi.org/10.1007/s00128-013-1117-1
Heydens WF, Healy CE, Hotz KJ, Kier LD, Martens MA, Wilson AG, Farmer DR (2008) Genotoxic potential of glyphosate formulations: mode-of-action investigations. J Agric Food Chem 56(4):1517–1523. https://doi.org/10.1021/jf072581i
IARC Working Group (2015) Glyphosate. In: Some organophosphate insecticides and herbicides: diazinon, glyphosate, malathion, parathion, and tetrachlorvinphos. Vol 112 IARC Monogr Prog, pp 1–92
Jayasumana C, Gunatilake S, Siribaddana S (2015) Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol 16:103. https://doi.org/10.1186/s12882-015-0109-2
Kier LD, Kirkland DJ (2013) Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit Rev Toxicol 43(4):283–315. https://doi.org/10.3109/10408444.2013.770820
Krüger M, Schledorn P, Schrödl W, Hoppe HW, Lutz W, Shehata AA (2014) Detection of glyphosate residues in animals and humans. J Environ Anal Toxicol 4:210. https://doi.org/10.4172/2161-0525.1000210
Laffont S, Guéry JC (2019) Deconstructing the sex bias in allergy and autoimmunity: from sex hormones and beyond. Adv Immunol 142:35–64. https://doi.org/10.1016/bs.ai.2019.04.001
Landrigan PJ, Belpoggi F (2018) The need for independent research on the health effects of glyphosate-based herbicides. Environ Health 17:51. https://doi.org/10.1186/s12940-018-0392-z
Lopez Gonzalez EC, Latorre MA, Larriera A, Siroski PA, Poletta GL (2013) Induction of micronuclei in broad snouted caiman (Caiman latirostris) hatchlings exposed in vivo to Roundup_ (glyphosate) concentrations used in agriculture. Pestic Biochem Physiol 105:131–134. https://doi.org/10.1016/j.pestbp.2012.12.009
Lozano VL, Defarge N, Rocque LM, Mesnage R, Hennequin D, Cassier R, de Vendômois JS, Panoff JM, Séralini GE, Amiel C (2017) Sex-dependent impact of Roundup on the rat gut microbiome. Toxicol Rep 5:96–107. https://doi.org/10.1016/j.toxrep.2017.12.005
MacGregor JT (1987) Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutat Res 189(2):103–112
Martelli BKL, Melo DM, Nai GA, Parizi JLS (2014) Influence of water pH in oral changes caused by cadmium poisoning: an experimental study in rats. Rev Odontol UNESP 43(3):1–5
Mello FA, Quinallia G, Marion AL, Jorge FC, Marinelli LM, Salge AKM, Fagiani MAB, Mareco EA, Favareto APA, Rossi e Silva RC (2018) Evaluation of the nasal cavity mice submitted to the inhalation exposure to the herbicide 2,4-dichlorophenoxyacetic acid. Medicina (Ribeirão Preto, Online) 51(4):247–253. https://doi.org/10.11606/issn.2176-7262.v51i4p00-00
Mertens M, Höss S, Neumann G, Afzal J, Reichenbecher W (2018) Glyphosate, a chelating agent-relevant for ecological risk assessment? Environ Sci Pollut Res Int 25(6):5298–5317. https://doi.org/10.1007/s11356-017-1080-1
Mesnage R, Bernay B, Séralini GE (2013) Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 313(2-3):122–128 https://doi.org/10.1016/j.tox.2012.09.006
Moreno NC, Sofia SH, Martinez CB (2014) Genotoxic effects of the herbicide Roundup Transorb and its active ingredient glyphosate on the fish Prochilodus lineatus. Environ Toxicol Pharmacol 37(1):448–454. https://doi.org/10.1016/j.etap.2013.12.012
Mui PC (1993) Endoscopic evaluation of paraquat-induced upper gastrointestinal injury. Gastrointest Endosc 39(1):105–106. https://doi.org/10.1016/s0016-5107(93)70032-2
Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, Vom Saal FS, Welshons WV, Benbrook CM (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15:19. https://doi.org/10.1186/s12940-016-0117-0
Nagy K, Tessema RA, Budnik LT, Ádám B (2019) Comparative cyto- and genotoxicity assessment of glyphosate and glyphosate-based herbicides in human peripheral white blood cells. Environ Res 179(Pt B):108851. https://doi.org/10.1016/j.envres.2019.108851
Nai GA, Filho MAG, Estrella MPS, Teixeira LDS (2015) Study of the influence of the ph of water in the initiation of digestive tract injury in cadmium poisoning in rats. Toxicol Rep 2:1033–1038. https://doi.org/10.1016/j.toxrep.2015.07.012
National Research Council (2011) Guide for the care and use of laboratory animals, eighth edition, vol 10. The National Academies Press, Washington, DC, p 17226/12910
Parizi ACG, Barbosa RL, Parizi JLS, Nai GA (2010) A comparison between the concentration of mast cells in squamous cell carcinomas of the skin and oral cavity. An Bras Dermatol 85(6):811–818
Parizi JLS, Tolardo AJ, Lisboa ACG, Barravieira B, Mello FA, Rossi RC, Nai GA (2020) Evaluation of buccal damage associated with acute inhalation exposure to 2,4-dichlorophenoxyacetic acid (2,4-D) in mice. BMC Vet Res 16:244. https://doi.org/10.1186/s12917-020-02461-w
Pegoraro CMR, Nai GA, Garcia LA, Serra FM, Alves JA, Chagas PHN, Oliveira DG, Zocoler MA (2018) Protective effects of Bidens pilosa on hepatoxicity and nephrotoxicity induced by carbontetrachloride in rats. Drug Chem Toxicol 5:1–11. https://doi.org/10.1080/01480545.2018.1526182
Ploton D, Menager M, Jeannensson P, Himberg G, Adnet JJ PF (1986) Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region of the optical level. Histochem J 18:5–14
Portier CJ, Armstrong BK, Baguley BC, Baur X, Belyaev I, Bellé R, Belpoggi F, Biggeri A, Bosland MC, Bruzzi P, Budnik LT, Bugge MD, Burns K, Calaf GM, Carpenter DO, Carpenter HM, López-Carrillo L, Clapp R, Cocco P, Consonni D, Comba P, Craft E, Dalvie MA, Davis D, Demers PA, De Roos AJ, DeWitt J, Forastiere F, Freedman JH, Fritschi L, Gaus C, Gohlke JM, Goldberg M, Greiser E, Hansen J, Hardell L, Hauptmann M, Huang W, Huff J, James MO, Jameson CW, Kortenkamp A, Kopp-Schneider A, Kromhout H, Larramendy ML, Landrigan PJ, Lash LH, Leszczynski D, Lynch CF, Magnani C, Mandrioli D, Martin FL, Merler E, Michelozzi P, Miligi L, Miller AB, Mirabelli D, Mirer FE, Naidoo S, Perry MJ, Petronio MG, Pirastu R, Portier RJ, Ramos KS, Robertson LW, Rodriguez T, Röösli M, Ross MK, Roy D, Rusyn I, Saldiva P, Sass J, Savolainen K, Scheepers PT, Sergi C, Silbergeld EK, Smith MT, Stewart BW, Sutton P, Tateo F, Terracini B, Thielmann HW, Thomas DB, Vainio H, Vena JE, Vineis P, Weiderpass E, Weisenburger DD, Woodruff TJ, Yorifuji T, Yu IJ, Zambon P, Zeeb H, Zhou SF (2016) Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J Epidemiol Community Health 70(8):741–745. https://doi.org/10.1136/jech-2015-207005
Prasad S, Srivastava S, Singh M, Shukla Y (2009) Clastogenic effects of glyphosate in bone marrow cells of Swiss albino mice. J Toxicol 2009:1–6. https://doi.org/10.1155/2009/308985
Punchard NA, Whelan CJ, Adcock I (2004) The Journal of Inflammation. Editorial J Inflamm 1:1. https://doi.org/10.1186/1476-9255-1-1
Qiu S, Fu H, Zhou R, Yang Z, Bai G, Shi B (2020) Toxic effects of glyphosate on intestinal morphology, antioxidant capacity and barrier function in weaned piglets. Ecotoxicol Environ Saf 187:109846. https://doi.org/10.1016/j.ecoenv.2019.109846
Sanchis J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, GarridoT FM (2012) Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by online solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 402(7):2335–2345
Sribanditmongkol P, Jutavijittum P, Pongraveevongsa P, Wunnapuk K, Durongkadech P (2012) Pathological and toxicological findings in glyphosate-surfactant herbicide fatality: a case report. Am J Forensic Med Pathol 33(3):234–237
Tarazona JV, Court-Marques D, Tiramani M, Reich H, Pfeil R, Istace F, Crivellente F (2017) Glyphosate toxicity and carcinogenicity: a review of the scientific basis of the European Union assessment and its differences with IARC. Arch Toxicol 91(8):2723–2743. https://doi.org/10.1007/s00204-017-1962-5
Tolbert PE, Shy CM, Allen JW (1992) Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res 271:69–77
Vandenberg LN, Blumberg B, Antoniou MN, Benbrook CM, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vom Saal FS, Welshons WV, Myers JP (2017) Is it time to reassess current safety standards for glyphosate-based herbicides? J Epidemiol Community Health 71(6):613–618. https://doi.org/10.1136/jech-2016-208463
Williams GM, Berry C, Burns M, de Camargo JLV, Greim H (2016) Glyphosate rodent carcinogenicity bioassay expert panel review. Crit Rev Toxicol 46(sup1):44–55. https://doi.org/10.1080/10408444.2016.1214679
Yasunaga S, Nishi K, Nishimoto S, Sugahara T (2015) Methoxychlor enhances degranulation of murine mast cells by regulating Fc#RI-mediated signal transduction. J Immunotoxicol 12:283–289. https://doi.org/10.3109/1547691X.2014.962122
Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS (2012) Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153(9):4097–4110. https://doi.org/10.1210/en.2012-1422
Acknowledgements
The authors would like to thank the technicians of the Laboratory of Surgical Pathology and Cytopathology at UNOESTE, Carlos Alexandre Santana de Oliveira, Mariana Fonseca Motta Borges and Talita Rizo Pereira, for the histological processing of the specimens.
Funding
This study was financed with research funds from the Universidade do Oeste Paulista (UNOESTE). P. H. N. Chagas received a scholarship from the Scientific Initiation Scholarship Program of the National Council for Scientific and Technological Development (PIBIC/CNPq).
Author information
Authors and Affiliations
Contributions
FMS: performed the experiments, performed the analysis and interpretation of data and drafted the manuscript; JLSP: made substantial contributions to research design and performed the experiments; GASM, GMRHS, IBP and PHNC: performed the experiments; FMA: made substantial contributions to research design and performed the experiments; GAN: made substantial contributions to research design, performed histological analysis, prepared the histological images for publication and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee on Animal Use of the Universidade do Oeste Paulista (UNOESTE) (protocol no. 3792).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Maria Serra, F., Parizi, J.L.S., Odorizzi, G.A.S. et al. Subchronic exposure to a glyphosate-based herbicide causes dysplasia in the digestive tract of Wistar rats. Environ Sci Pollut Res 28, 61477–61496 (2021). https://doi.org/10.1007/s11356-021-15051-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15051-6