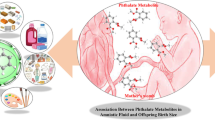
Abstract
Phthalates or phthalic acid esters (PAEs) are a group of compounds which they can be entered into the human body through the various pathways. The aim of this study was to examine associations between prenatal phthalates exposure with anthropometric measures of neonates. Urine samples were obtained from 121 Iranian pregnant women at their first trimester of pregnancy, and the levels of monobutyl phthalate (MBP), mono-benzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), and mono (2-ethyl-5hydroxyhexyl) phthalate (MEHHP) metabolites were determined by gas chromatography mass spectrometry (GC/MS). The correlations between the maternal urinary concentrations of phthalate metabolites with anthropometric measures of neonates as well as with the socio-demographic factors of participants (maternal education, age, family income, pre-pregnancy body mass index), their lifestyle variables (smoking habit, food pattern, and physical activity), and use of cleaning products (cosmetic and household cleaning products) were investigated. MBzP, MBP, MEHP, and MEHHP were detected in 100% of the participants with the concentration ranged 120 to 860 μg/g creatinine. Significant correlations were observed between the urinary levels of maternal MBzP (adjusted β = 0.3 (0.001), p = 0.03) and MEHHP (adjusted β = 0.3 (0.001), p = 0.04) with the birth weight of female neonates. MBP (adjusted β = -0.3 (0.02), p = 0.04) and MBzP (adjusted β = -0.3 (0.001), p = 0.02) had negative associations with the head circumference in male and female newborns, respectively. Furthermore, plastic packaging for pickle and passive smoking during pregnancy were identified to be significantly associated with low birth weight (p value < 0.05). Iranian pregnant women had higher concentrations of urinary phthalates compared to the other countries. Based on the findings, the higher prenatal exposure to phthalates could adversely impact the health status of newborns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalates are a group of compounds widely used in the production of industrial goods. The low molecular weight phthalates such as diethyl phthalate (DEP) and dibutyl phthalate (DBP) are commonly used as additives/stabilizing agents in cosmetics, perfumes, lotions, and pesticides, whereas high molecular weight phthalates (with ≥ 8 carbons in the alkyl chain) including di-2-ethyl hexyl phthalate (DEHP) and butyl benzyl phthalate (BBzP) are mainly applied as plasticizers in the manufacture of packaging materials, polyvinylchloride (PVC), and blood transfusion devices and catheters (Amin et al. 2018c, 2019a; Gao and Wen 2016). Phthalates can easily be released from materials containing these hazardous compounds; therefore, they can be entered into the body through inhalation, ingestion, or dermal adsorption (Amin et al. 2019a, b; Bui et al. 2016). However, dietary intake has been regarded to contribute as the main route of human exposure to phthalates (Bui et al. 2016; Moridzadeh et al. 2020). Upon the absorption, the diester phthalates are quickly hydrolyzed into the respective monoesters (mono-2-ethylhexyl phthalate, MEHP), which in turn are oxidized into the more simple products such as MEHHP (mono2-ethyl-5-hydroxyhexyl), and can be excreted into the urine and feces (Lee et al. 2017; Rafiee et al. 2018).
Previous studies have detected substantial amounts of phthalates in the cord blood and amniotic fluid, indicating that these compounds can cross the placenta and may harmfully affect the growing fetus. In this regard, several epidemiological studies have shown a potential relationship between maternal exposure to phthalates and the risk of poor birth outcomes (Li et al. 2018; Rafiee et al. 2019; Shahsavani et al. 2017). Due to these potential adverse effects, many developed countries have prohibited the use of di-ethylhexyl phthalate (DEHP), dibutyl phthalate (DBP), and butyl benzyl phthalate (BBzP) in industrial products (Becker et al. 2009). However, no strict regulation has been set up yet regarding the use of these chemicals in most of the developing countries, including Iran. So, pregnant women and their fetus are exposed to more negative health effects by the exposure to environmental chemicals (Amin et al. 2018b, 2019a, b; Mohammadi et al. 2019; Wenzel et al. 2018). To the best of our knowledge, no previous publication has investigated the association between exposure to phthalates during pregnancy and birth outcomes among Iranian pregnant women. Therefore, in the present study, we aimed to evaluate the urinary concentrations of phthalate metabolites in a sample of Iranian pregnant women at their first trimester, as an indicator of exposure to phthalates, and examine associations between prenatal phthalate exposures with the anthropometric measures of neonates. The findings of this study may also provide an insight into common sources of exposure to phthalates among Iranian pregnant women which can help decision-makers to take the appropriate measures.
Materials and methods
Study population
This cross-sectional study was a part of PERSIAN birth cohort survey conducted between the years 2018 and 2019 on 121 pairs (mother-newborn) who lived in Isfahan city, Iran. The participants were selected randomly among those pregnant women at their first trimester who attended at the healthcare centers in Isfahan city. The purpose of the study was completely explained to the participants so that people were voluntarily involved in the study. The distributions of participants’ location are given in Fig. 1. All the participants were informed about the objectives, methodology, as well as the voluntary nature of the survey, and a signed consent letter was taken from them. It should be mentioned that the protocols and ethical issues related to this study were approved and observed by the Ethics Committees of Isfahan university of Medical sciences.
Urine sampling
The early morning urine samples were taken from the participants who visited the healthcare center, collected in borosilicate containers, and transferred to the laboratory to be kept at -20 °C for the future experiments. During the sample collection, the PERSIAN Birth Cohort questionnaires were used to gather the data on socio-demographic variables (maternal education, age, family income), lifestyle factors (pre-pregnancy BMI, smoking habit, and physical activity (PA)), and food habits and household cleaning products use (Kelishadi et al. 2012). The total physical activity (MET-minutes/week) score were computed by IPAQ (International Physical Activity Questionnaire) (Cleland et al. 2018). To obtain the data regarding food habits of the participants, food frequency questionnaire (FFQ) was applied. The questionnaire included the questions asking about the frequency of consuming fried foods (as daily, 1–2 per week, 1–3 per month, seldom, and never) and the use of plastics for packaging certain foods including bread, lemon juice, pickle, leftover, and water.
Measuring urinary metabolites of phthalate
All the measurements were done along with the standard solutions of MBP, MBzP, MEHP, and MEHHP. To measure the phthalate content of urine samples, the following steps were taken. At first, in order to extract MBP, MBzP, MEHP, and MEHHP metabolites, 10 ml of the urine samples were defrizzed and digested by 20 μl of β-glucuronidase enzyme for 18 h incubation at 37 °C. Then, 0.2 g of sodium chloride was added to the preparation and shaked for 24 h at 37 °C. Afterwards, 5 ml of the obtained mixture was diluted with the same volume of distilled water, and the solution pH was adjusted to 2 using 10% sulfuric acid solution. In the next step, 1 ml of acetone and 20 μl of chlorobenzene were added to the previously obtained mixture and centrifuged for 5 min at 5000 rpm. Then, the sediments were withdrawn from the bottom of the tubes using a micro-syringe and collected in different microtubes and dried under nitrogen gas stream. Thereafter, 10 μl of MSTFA was mixed with the sediment and centrifuged. At the final, 10 μl of the prepared solution was injected into the GC/MS (Model A 7890 of Agilent Technologies, USA) to measure the concentration of investigated phthalate metabolites (Amin et al. 2018a). To minimize the bias of the dilution difference between the samples, the phthalate concentrations were adjusted using creatinine levels. The limits of detection (LOD) were 0.017, 0.0126, 0.018, and 0.019 μg/L for MBP, MBzP, MEHP, and MEHHP, respectively. For the metabolite concentrations below the LOD, LODs divided by 2 were considered in the statistical analysis (Schuhmacher et al. 2009).
Anthropometric measures of neonates
Data on the anthropometric indices (weight, length, and head circumference) and other related information of neonates of corresponding mothers who participated in this study were retrieved from the hospital records which had been measured by experienced obstetric nurses using standardized procedures.
Quality assurance and quality control (QA/QC)
To confirm the reliability of the analytical data and to increase confidence in the relevance of obtained responses, the quality assurance and quality control (QA/QC) assessments were performed. Accordingly, the linear regression gave a good fit (R2 ≥ 0.98) with high precision (≤ 13.2 % RSD). The limit of detection (LOD) and limit of quantification (LOQ) were based on the signal-to-noise ratio of 3 and 10, respectively. For the metabolite concentrations below the LOD, LODs divided by 2 were considered in the statistical analyzes (Schuhmacher et al. 2009). Furthermore, to minimize the bias of the dilution difference between the samples, the phthalate concentrations were adjusted using creatinine levels. The R2, precision (% RSD), LOD, LOQ, and mean recovery are summarized in Table 1.
Data analysis
Continuous variables have been presented as mean ± SD and median (minimum-maximum), while categorical variables were expressed as percentages. Normality of continuous data was evaluated by using Kolmogorov-Smirnov test and Q-Q plot. Independent sample t-test was used to compare the mean values of birth outcomes across categories of possible demographic and lifestyle determinants, while Pearson or non-parametric correlation coefficients were used for the evaluation of bivariate associations between the maternal urinary phthalate concentrations with infants’ anthropometric measures and continuous determinants. Multiple linear regressions were used for evaluating the association of metabolites with infants’ anthropometric measures, and adjustment was done for mothers’ basic demographic and clinical characteristics. All statistical analyses were done using SPSS software version 23 (IBM SPSS Inc., Chicago, IL). A p value < 0.05 was considered statistically significant.
Results and discussion
The characteristics of the participants are summarized in Table 2. Descriptively, 59% (n = 71) of participants were ≥ 30 years old, and 41% (n = 50) were ≤ 30 years old. The majority of the participants (69%, n = 83) were categorized into overweight, while 29% of them (n = 35) had normal weight, and only 2 % (n = 3) were classified as underweight. Most of the participants were academically educated (n = 105, 86.8%). The majority of the participants had family income of 100–300 $ per month and grouped into the middle income category (n = 69, 57%). Household cleaning products and cosmetic products were found as the most common products used by 92.6% (112) and 100% (121) of the participants, respectively. Moreover, more than half (51.26%) of the pregnant women have reported the use of plastics for packaging of bread (n = 76, 62.8%), lemon juice (n = 67, 55.4%), pickle (n = 55, 45.5%), leftover (n = 52, 43%), and water (n = 60, 49.6%). The data showed that 31.4% (n = 38) of the subjects have used fried foods more than 1 time per week (1–2 times/week); however, 4.1% (n = 5) of them had never used fried food items. The majority of the participants did not have enough physical activity, where 53.7% of study population (n = 65) were grouped into the low physical activity category. Among the studied pregnant women, only 6.6% of them (n = 8) had high physical activity.
The mean (± SD) of birth weight, birth length, and head circumference of infants of the corresponding mothers who participated in this survey was 3204.04 ± 480.94 g, 50.35 ± 3.16 cm, and 34.5 ± 1.71 cm, respectively.
Table 3 shows the mean, minimum, and maximum concentration of phthalate metabolites adjusted by creatinine. All the urine samples (100%, n = 121) were positive for phthalate metabolites. MEHHP had the highest concentration (866.5 ± 307.6 μg/g creatinine), while MEHP was detected in the all samples with the lowest level (126.5 ± 118.3 μg/g creatinine). The mean concentration of MBP, MBzP, MEHP, and MEHHP found in this study were 13, 30, 20, and 44 times greater than those levels that have been reported by the studies in US and European countries (Berman et al. 2009; Valvi et al. 2015; Wenzel et al. 2018). These results are in accordance with the findings of Amin et al. who previously reported higher levels of exposure to phthalates in Iranian population (Amin et al. 2018a). Several factors including the socio-demographic, environmental and regional variables, as well as the size of study population, can be attributed to the different levels of exposure obtained in this survey and those that have been reported by other countries.
The results of correlation analysis between the creatinine-adjusted urinary levels of phthalate metabolites and birth outcome measures are presented in Table 4. None of the phthalate metabolites exhibited significant correlation with the birth outcomes, and only the correlation between MBzP with birth weight was of borderline significance (β = 0.2 (0.16), p = 0.06). Based on the results, it is obvious that the concentration of phthalates in the first trimester maternal urine was positively correlated to the birth weight of neonates; however, negative correlation was observed between urinary phthalate levels with birth length and head circumference of newborns.
In line with the results found in this study, Suzuki et al. (2010) and Philippat et al. (2011) have also found no statistically significant associations between prenatal phthalate exposure and birth outcomes. Furthermore, Huang et al. have reported negative association between the maternal urinary phthalate concentration with the head circumference of their newborns; however, the significant correlations were only noted in female neonates (Huang et al. 2014). In line with the results obtained in this study, no significant relationship was found between maternal urinary phthalates and birth by Zhu et al. (2018).
On the contrast, Shoaff et al. have shown that a ten-fold increase in the maternal urinary MEP levels is associated with a 0.23 standard deviation reduction (95% CI: −0.46, −0.01) in birth weight z-score; however, after adjustment for confounding factors, this relationship was attenuated towards the null (Shoaff et al. 2016). Casas Sanahuja et al. (2016) have assessed the effect of prenatal exposure to eight phthalates on fetal growth but found no significant association between the maternal urinary ∑DEHP concentration and any of the neonatal growth outcome measures. In another study conducted by Wolff et al., they investigated the association between maternal urinary phthalates concentration at third trimester with the body size measures of infants at birth. They found that low molecular weight phthalates had a positive, but not statistically significant association with the head circumference of newborns (Wolff et al. 2008).
Despite the mentioned studies, there are several other reports that have shown inverse (Lenters et al. 2015; Zhang et al. 2009) or negative associations (Botton et al. 2016; Ferguson et al. 2016) between maternal urinary levels of phthalate metabolites and birth outcomes. In this regard, Lenters et al. (2015) and Zhang et al. (2009) have shown that maternal urinary levels of some DEHP metabolites during pregnancy are associated with low birth weight or increased risk of low birth weight. The discrepancy between the results might be explained by the potential contamination with phthalate di-esters or the differences in the levels of exposure to these compounds among different populations (Kato et al. 2003).
The results of crude and adjusted associations between maternal urinary phthalate concentration with the birth outcomes in boys and girl neonates are presented in Table 5. According to these findings, MBP (adjusted β = -0.3(0. 2), p = 0.04) and MBzP (adjusted β = -0.3 (0.001), p = 0.02) were negatively associated with the head circumference in boys and girls, respectively. Furthermore, positive associations were observed between maternal urinary levels of MBzP (adjusted β =0.3 (0.001), p = 0.03) and MEHHP (adjusted β =0.3 (0.001), p = 0.04) with birth weight in girls after adjusting for potential confounding factors.
The correlation between prenatal exposure to MBzP and MEHHP with higher birth weight in female neonates may suggest that exposure to these chemicals during pregnancy can influence the function of feminine hormones and thus increase fetal growth or induce fat accumulation. Consistently, it has been demonstrated that phthalates have weak estrogenic activities (Ghisari and Bonefeld-Jorgensen 2009; Kiyama and Wada-Kiyama 2015) and can promote adipocyte differentiation by activation of peroxisome proliferator-activated receptor gamma (PPARs) (Hao et al. 2012). Furthermore, due to their estrogenic activities, phthalates may engage nuclear receptors and induce the expression of several other genes involved in obesity. Additionally, it is possible that prenatal phthalate exposure may affect thyroid axis function, disrupt energy balance and metabolism, and thus result in the fat accumulation (Boas et al. 2012). These compounds may also affect the pituitary-adrenal axis, which is crucial for fetal growth (Liu et al. 2014). Since we observed a sex difference in the associations of maternal urinary phthalate metabolite levels with birth weight of infants, hence it can be concluded that the most potential mechanism by which these compounds have resulted in higher birth weight might be related to their influence on sexual hormones or sex-related biological variables (Grün and Blumberg 2009). However, further studies are warranted to shed light on this claim.
We also examined the effects of some potential factors which may be associated with the prenatal exposure to phthalates including maternal education level, family income per month, and plastic packaging (bread, lemon juice, pickle, leftover, water) and might influence the neonatal birth outcomes (Table 6).
According to the results, maternal education level, family income per month, and physical activity had no significant influences on the investigated birth outcome measures (p value > 0.05). However, birth weight of infants was significantly different (p value < 0.05) among those participants who used plastic packaging for pickle and passively exposed to smoke. This can be explained by the fact that phthalates are still widely used as plasticizers in materials used for food and water packaging for many years; therefore, the plastic packaging of food items can be associated with higher exposure to these chemicals (European Food Safety Authority).
Although the main part of the participants in the present work were non-smokers, more than half of them (n = 65, 53.7%) were passively exposed to smoking. Likewise, previous studies have also linked smoking habits to higher exposure levels to phthalates (Arbuckle et al. 2014; Valvi et al. 2015). In this regard, Casas et al. observed that smoking has a direct association with the elevated urinary phthalates (Casas et al. 2011; Darvishmotevalli et al. 2019b). It has been demonstrated that cigarette smoke and filters contain phthalates in the form of di-2-methoxyethyl phthalate (Jackson Jr and Darnell 1985). Furthermore, it has been assumed that low-quality cigarettes may also further increase the urinary concentration of phthalates metabolites both in first- and second-hand smokers. Table 7 presents the results of correlations between quantitative determinants and birth outcome measures.
Here, we found positive significant correlations between prenatal BMI and the use of cosmetics and household cleaning products with the neonatal birth weight (p value < 0.05 and r = 0.18, r = 0.21, and r = 0.24, respectively). However, there were no significant relationships observed between maternal age, consumption of fried foods, and physical activity with the birth measures (p value > 0.05).
There are a large body of evidences indicating that urinary phthalate metabolites in pregnant women are positively associated with the amount and frequency of cosmetics and household cleaning products they use (Darvishmotevalli et al. 2019a, c). On contrast with our findings, Valvi et al. (2015) showed no association between the use of cosmetics and urinary levels of phthalates. The legislative actions regarding the production of cosmetics and care products in various countries are thoroughly different. For instance, the use of some phthalates including DEHP, di-n-butyl phthalate, di-iso-butyl phthalate, and BBP in the production of cosmetics have been prohibited by the EU (European Union) (Wittassek et al. 2011); however, there is no strict regulation regarding the use of these chemicals in the production of various industrial goods in developing countries, including Iran. Besides, Iran has been ranked as the second country in the Middle East with the highest use of personal care products (Volpe et al. 2012). This is in line with our observation that a large proportion of the participants in this study have reported higher amounts of cosmetics use. On the other hand, since the majority of the women participated in this survey were of low or middle income families, therefore it can be assumed that the main part of this population may use inexpensive with low-quality cosmetic products containing higher grades of phthalates.
Household cleaning products have also been regarded as another important source of phthalate exposure, especially among pregnant women (Harley et al. 2016). Accordingly, Valvi et al. (2015) have shown that the use of household chemical products is associated with the higher urinary concentrations of MEHHP, MEHP, and MBzP. In countries like Iran, females are mainly involved in household works; thus, they may expose to higher levels of phthalates through the use of chemical cleaning products. Furthermore, exposure to chemicals occurrs with more adverse effects for pregnant women and their growing fetus due to the physiological changes (Philips et al. 2017).
In this study, higher levels of MBzP, MEHHP, and MBP were found in urinary samples of obese individuals compared to women with normal body weight.
Prenatal maternal BMI is considered one of the most important determinants of fetal weight, growth, and body composition (Hajizadeh et al. 2020; Sewell et al. 2006). It is clear that the maternal BMI is not playing as a biological effector and the mechanism by which prenatal maternal BMI affects the fetal growth remains largely unknown (Hajizadeh et al. 2021; Roland et al. 2012). Several studies have demonstrated that fetal growth is largely influenced by placental capacity in transferring nutrients to fetus, the genetics of parents, as well as by maternal supply of nutrients. Since maternal BMI may influence nutrients supply and the capacity of placenta in transporting nutrients from mother to fetus, thus it can affect the fetal growth measures. Previous studies have also positively linked maternal BMI to the levels of circulating glucose. Additionally, higher maternal BMI and plasma glucose levels have been associated with the longer gestational age and greater body fat in newborns (Nathanielsz et al. 2007). In spite of influencing adipogenesis, phthalates have lipophilic properties enable these chemicals to be stored in adipose tissue. Therefore, it would be expected that obese mothers may exhibit higher urinary levels of phthalates and potentially have more affected newborns (Philips et al. 2017).
Limitations and strengths
One of the important limitations of the present study was its cross-sectional nature. Additionally, this study has assessed the maternal urinary phthalate concentration only at first trimester period as spot sampling. However, we tried to adjust the mean concentration of phthalate by creatine adjusting. To the best of our knowledge, no or only a limited number of studies have been conducted in Iran to address this issue; thus, these findings can provide a basis for the future studies and help decision-makers to implement proper actions. Taken together, higher exposure to neglected chemicals such as phthalates, especially during pregnancy, could threaten the health of both mothers and newborns. We recommend further investigations to evaluate the prenatal exposure to phthalates during second and third trimesters as well. Such data on the whole period of pregnancy can be more meaningful.
Conclusion
This study was conducted to determine the possible relationship between prenatal phthalate exposure and neonatal anthropometric measures in association with maternal lifestyle variables and characteristics of pregnant women. According to the findings, the following conclusions can be made:
-
MBzP, MBP, MEHP, and MEHHP were detected in 100% of urines obtained from pregnant women at their first trimester.
-
Investigated metabolites had higher concentration in the Iranian pregnant women urines compared to the other countries.
-
Our findings revealed a positive association between the maternal concentrations of MBzP and MEHHP with increased birth weight in girl neonates.
-
Urinary concentration of phthalates were significantly higher among those pregnant women who had higher pre-pregnancy BMI, as well as among the users of cosmetics and household cleaning products, and those subjects who routinely used plastic packaging for pickle storage.
-
Pregnant women who passively exposed to smoking had significantly higher levels of urinary phthalates.
References
Amin MM, Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, Poursafa P, Fallah Z, Rafiei N, Kelishadi R (2018a) Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere 211:547–556. https://doi.org/10.1016/j.chemosphere.2018.07.172
Amin MM, Parastar S, Ebrahimpour K, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, Kelishadi R (2018b) Association of urinary phthalate metabolites concentrations with body mass index and waist circumference. Environ Sci Pollut Res 25:11143–11151. https://doi.org/10.1007/s11356-018-1413-8
Amin MM, Rafiei N, Poursafa P, Ebrahimpour K, Mozafarian N, Shoshtari-Yeganeh B, Hashemi M, Kelishadi R (2018c) Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ Sci Pollut Res 25:34046–34052. https://doi.org/10.1007/s11356-018-3354-7
Amin MM, Tabatabaeian M, Chavoshani A, Amjadi E, Hashemi M, Ebrahimpour K, Klishadi R, Khazaei S, Mansourian M (2019b) Paraben content in adjacent normal-malignant breast tissues from women with breast cancer. Biomed Environ Sci 32:893–904. https://doi.org/10.3967/bes2019.112
Amin MM, Ebrahim K, Hashemi M, Shoshtari-Yeganeh B, Rafiei N, Mansourian M, Kelishadi R (2019a) Association of exposure to Bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int J Environ Health Res 29:94–106. https://doi.org/10.1080/09603123.2018.1515896
Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD (2014) Phthalate and bisphenol A exposure among pregnant women in Canada—results from the MIREC study. Environ Int 68:55–65. https://doi.org/10.1016/j.envint.2014.02.010
Becker K, Güen T, Seiwert M, Conrad A, Pick-Fuß H, Müller J, Wittassek M, Schulz C, Kolossa-Gehring M (2009) GerES IV: phthalate metabolites and bisphenol A in urine of German children. Int J Hyg Environ Health 212:685–692. https://doi.org/10.1016/j.ijheh.2009.08.002
Berman T, Hochner-Celnikier D, Calafat AM, Needham LL, Amitai Y, Wormser U, Richter E (2009) Phthalate exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environ Int 35:353–357. https://doi.org/10.1016/j.envint.2008.08.010
Boas M, Feldt-Rasmussen U, Main KM (2012) Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355:240–248. https://doi.org/10.1016/j.mce.2011.09.005
Botton J, Philippat C, Calafat AM, Carles S, Charles M-A, Slama R, Group EM-CCS (2016) Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ Res 151:601–609. https://doi.org/10.1016/j.envres.2016.08.033
Bui TT, Giovanoulis G, Cousins AP, Magnér J, Cousins IT, de Wit CA (2016) Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ 541:451–467. https://doi.org/10.1016/j.scitotenv.2015.09.036
Casas Sanahuja M, Valvi D, Ballesteros-Gomez A, Gascon Merlos M, Fernandez MF, García Esteban R, Iñiguez C, Martínez Muriano D, Murcia M, Monfort Mercader N (2016) Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell cohort. Environ Health Perspect 124:521–528. https://doi.org/10.1289/ehp.1409190
Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodríguez LSM, Riaño I, Tardón A (2011) Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int 37:858–866. https://doi.org/10.1016/j.envint.2011.02.012
Cleland C, Ferguson S, Ellis G, Hunter RF (2018) Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol 18:176. https://doi.org/10.1186/s12874-018-0642-3
Darvishmotevalli M, Bina B, Feizi A, Ebrahimpour K, Pourzamani H, Kelishadi R (2019a) Monitoring of urinary phthalate metabolites among pregnant women in Isfahan, Iran: the PERSIAN birth cohort. J Environ Health Sci Eng 17:969–978. https://doi.org/10.1007/s40201-019-00412-8
Darvishmotevalli M, Moradnia M, Noorisepehr M, Fatehizadeh A, Fadaei S, Mohammadi H, Salari M, Jamali HA, Daniali SS (2019b) Evaluation of carcinogenic risks related to nitrate exposure in drinking water in Iran. MethodsX 6:1716–1727. https://doi.org/10.1016/j.mex.2019.07.008
Darvishmotevalli M, Moradnia M, Asgari A, Noorisepehr M, Mohammadi H (2019c) Reduction of pathogenic microorganisms in an Imhoff tank–constructed wetland system. Desalin Water Treat 154:283–288. https://doi.org/10.5004/dwt.2019.24044
Ferguson KK, Meeker JD, Cantonwine DE, Chen Y-H, Mukherjee B, McElrath TF (2016) Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environ Int 94:531–537. https://doi.org/10.1016/j.envint.2016.06.013
Gao D-W, Wen Z-D (2016) Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ 541:986–1001. https://doi.org/10.1016/j.scitotenv.2015.09.148
Ghisari M, Bonefeld-Jorgensen EC (2009) Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189:67–77. https://doi.org/10.1016/j.toxlet.2009.05.004
Grün F, Blumberg B (2009) Endocrine disrupters as obesogens. Mol Cell Endocrinol 304:19–29. https://doi.org/10.1016/j.mce.2009.02.018
Hajizadeh Y, Feizabadi GK, Ebrahimpour K, Shoshtari-Yeganeh B, Fadaei S, Darvishmotevalli M, Karimi H (2020) Urinary paraben concentrations and their implications for human exposure in Iranian pregnant women. Environ Sci Pollut Res 27:1–12. https://doi.org/10.1007/s11356-020-07991-2
Hajizadeh Y, Moradnia M, Feizabadi GK, Rafiei N, Tahmasbizadeh M, Darvishmotevalli M, Fadaei S, Karimi H (2021) The sex-specific association between maternal urinary paraben levels and offspring size at birth. Environ Sci Pollut Res:1–10. https://doi.org/10.1007/s11356-021-13175-3
Hao C, Cheng X, Xia H, Ma X (2012) The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep 32:619–629. https://doi.org/10.1042/BSR20120042
Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, She J, Gavin Q, Zahedi R, Bradman A (2016) Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ Health Perspect 124:1600–1607. https://doi.org/10.1289/ehp.1510514
Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, Wang L, Tan Y, Luo J, Qiu Z (2014) Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One 9:e87430
Jackson Jr WJ, Darnell WR (1985) Process for foaming cellulose acetate rod. US Patent 4,507,256. Eastman Kodak.
Kato K, Silva MJ, Brock JW, Reidy JA, Malek NA, Hodge CC, Nakazawa H, Needham LL, Barr DB (2003) Quantitative detection of nine phthalate metabolites in human serum using reversed-phase high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J Anal Toxicol 27:284–289. https://doi.org/10.1093/jat/27.5.284
Kelishadi R, Majdzadeh R, Motlagh ME, Heshmat R, Aminaee T, Ardalan G, Esmaillzadeh A, Azadbakht L, Poursafa P, Movahedian M (2012) Development and evaluation of a questionnaire for assessment of determinants of weight disorders among children and adolescents: The CASPIAN-IV study. Int J Prev Med 3:699–705
Kiyama R, Wada-Kiyama Y (2015) Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ Int 83:11–40. https://doi.org/10.1016/j.envint.2015.05.012
Lee K-M, Kho Y, P-g K, S-h P, Lee J-H (2017) Urinary levels of phthalate metabolites and associations with demographic characteristics in Korean adults. Environ Sci Pollut Res 24:14669–14681. https://doi.org/10.1007/s11356-017-9068-4
Lenters V, Portengen L, Rignell-Hydbom A, Jönsson BAG, Lindh CH, Piersma AH, Toft G, Bonde JP, Heederik D, Rylander L, Vermeulen R (2015) Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ Health Perspect 124:365–372. https://doi.org/10.1289/ehp.1408933
Li X, Sun H, Yao Y, Zhao Z, Qin X, Duan Y, Wang L (2018) Distribution of phthalate metabolites between paired maternal–fetal samples. Environ Sci Technol 52:6626–6635. https://doi.org/10.1021/acs.est.8b00838
Liu T, Li N, Zhu J, Yu G, Guo K, Zhou L, Zheng D, Qu X, Huang J, Chen X (2014) Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reprod Toxicol 46:141–147. https://doi.org/10.1016/j.reprotox.2014.03.006
Mohammadi H, Alinejad A, Khajeh M, Darvishmotevalli M, Moradnia M, Mazaheri Tehrani A, Hosseindost G, Zaref MR, Mengelizadeh N (2019) Optimization of the 3D electro-Fenton processin removal of acid orange 10 from aqueoussolutions by response surface methodology. J Chem Technol Biotechnol 94(10):3158–3171. https://doi.org/10.1002/jctb.6122
Moridzadeh M, Dehghani S, Rafiee A, Hassanvand MS, Dehghani M, Hoseini M (2020) Assessing BTEX exposure among workers of the second largest natural gas reserve in the world: a biomonitoring approach. Environ Sci Pollut Res 27:44519–44527. https://doi.org/10.1007/s11356-020-10379-x
Nathanielsz PW, Poston L, Taylor PD (2007) In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Obstet Gynecol Clin N Am 34:201–212. https://doi.org/10.1016/j.clp.2007.09.005
Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA (2011) Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 120:464–470. https://doi.org/10.1289/ehp.1103634
Philips EM, Jaddoe VW, Trasande L (2017) Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod Toxicol 68:105–118. https://doi.org/10.1016/j.reprotox.2016.08.015
Rafiee A, Delgado-Saborit JM, Gordi E, Quémerais B, Moghadam VK, Lu W, Hashemi F, Hoseini M (2018) Use of urinary biomarkers to characterize occupational exposure to BTEX in healthcare waste autoclave operators. Sci. Total Environ 631:857–865. https://doi.org/10.1016/j.scitotenv.2018.03.090
Rafiee A, Delgado-Saborit JM, Sly PD, Amiri H, Hoseini M (2019) Lifestyle and occupational factors affecting exposure to BTEX in municipal solid waste composting facility workers. Sci. Total Environ 656:540–546. https://doi.org/10.1016/j.scitotenv.2018.11.398
Roland MCP, Friis CM, Voldner N, Godang K, Bollerslev J, Haugen G, Henriksen T (2012) Fetal growth versus birthweight: the role of placenta versus other determinants. PLoS One 7:e39324
Schuhmacher M, Nadal M, Domingo JL (2009) Environmental monitoring of PCDD/Fs and metals in the vicinity of a cement plant after using sewage sludge as a secondary fuel. Chemosphere 74:1502–1508. https://doi.org/10.1016/j.chemosphere.2008.11.055
Sewell MF, Huston-Presley L, Super DM, Catalano P (2006) Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 195:1100–1103. https://doi.org/10.1016/j.ajog.2006.06.014
Shahsavani S, Dehghani M, Hoseini M, Fararouei M (2017) Biological monitoring of urinary 1-hydroxypyrene by PAHs exposure among primary school students in Shiraz, Iran. Int Arch Occup Environ Health 90:179–187. https://doi.org/10.1007/s00420-016-1184-9
Shoaff JR, Romano ME, Yolton K, Lanphear BP, Calafat AM, Braun JM (2016) Prenatal phthalate exposure and infant size at birth and gestational duration. Environ Res 150:52–58. https://doi.org/10.1016/j.envres.2016.05.033
Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H (2010) Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int 36:699–704. https://doi.org/10.1016/j.envint.2010.05.003
Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M (2015) Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health 218:220–231. https://doi.org/10.1016/j.ijheh.2014.11.003
Volpe M, Nazzaro M, Coppola R, Rapuano F, Aquino R (2012) Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA. Microchem J 101:65–69. https://doi.org/10.1016/j.microc.2011.10.008
Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, Somerville SE, Kucklick JR: Prevalence and predictors of phthalate exposure in pregnant women in Charleston (2018) Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 193:394-402. https://doi.org/10.1016/j.chemosphere.2017.11.019
Wittassek M, Koch HM, Angerer J, Brüning T (2011) Assessing exposure to phthalates–the human biomonitoring approach. Mol Nutr Food Res 55:7–31. https://doi.org/10.1002/mnfr.201000121
Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM (2008) Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 116:1092–1097. https://doi.org/10.1016/j.jpeds.2009.04.007
Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge R-S (2009) Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J Pediatr 155:500–504. https://doi.org/10.1016/j.jpeds.2009.04.007
Zhu Y, Wan Y, Zhang B, Zhou A, Huo W, Wu C, Liu H, Jiang Y, Chen Z, Jiang M (2018) Relationship between maternal phthalate exposure and offspring size at birth. Sci Total Environ 612:1072–1078. https://doi.org/10.1016/j.scitotenv.2017.08.207
Acknowledgements
The authors are grateful to the staff at the laboratory of the Department of Environmental Health Engineering, Isfahan University of Medical Sciences (IUMS).
Data Availability and material
The material and raw data are available upon request.
Code availability
Not.
Funding
This research was supported by the Isfahan University of Medical Sciences. The main cohort study is funded by the Ministry of Health with the project number IR.MUI.REC.1394.1.354.
Author information
Authors and Affiliations
Contributions
M. Darvishmotevalli , M. Moradnia, and R. Hosseini surveyed the studies for data extraction, inclusion, and assessing the study quality and wrote the first draft of the manuscript. B. Bina supervised this study. A Feizi performed the data analysis. K Ebrahimpour, H Pourzamani, G Kiani Feizabadi provided the critical input for the manuscript. R Kelishadi did critical revision of the manuscript. All authors have contributed considerably, and all authors are in agreement with respect to the manuscript content. The authors read and confirmed the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.RESEARCH.REC.1397.441) with project number #397573.
Consent to participate
All participants voluntarily agree to participate in this research study.
Consent for publication
The studied participant consent for publication of their identifiable details.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Darvishmotevalli, M., Moradnia, M., Hosseini, R. et al. Association between prenatal phthalate exposure and anthropometric measures of newborns in a sample of Iranian population. Environ Sci Pollut Res 28, 50696–50706 (2021). https://doi.org/10.1007/s11356-021-14182-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14182-0