Abstract
The occurrence of 21 trace elements in native Mediterranean mussel (Mytilus galloprovincialis) from the Calich Lagoon, a typical brackish area of the northwest of Sardinia (Italy), was investigated. The seasonal variation of metals in bivalves was considered, and the highest values were found in spring and summer; in particular, a high significant (P < 0.001) temporal variation was reported for silver (Ag) and mercury (Hg). The highest and similar concentrations were registered for aluminium (Al, mean 32 mg kg−1wet weight), iron (Fe, mean 32 mg kg−1 w. w.), and zinc (Zn, mean 25 mg kg−1 w. w.). The maximum limits set by European Regulations for cadmium (Cd), mercury (Hg), and lead (Pb) were never exceeded. Speciation analysis revealed negligible risk related to inorganic arsenic (iAs). Therefore, M. galloprovincialis confirmed its role as suitable bioindicator to monitor the contamination of coastal environments. Although the recommended tolerable weekly intake (TWI) was not exceeded, the levels of aluminium should be carefully evaluated in monitoring plans in the studied lagoon.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals pollution is recognized all over as a public health hazard since these contaminants are ubiquitous in marine ecosystems (Lozano et al. 2010) and cannot be degraded or destroyed due to their persistence. Chemical contamination is a wide-ranging issue mostly in wetlands and coastal lagoons (Almeida and Soares 2012), sensitive environments with unique ecological characteristics determined by the interaction between land and sea (Castro et al. 1999; Cataudella et al. 2015). Coastal lagoons trap inorganic contaminants from anthropogenic activities in the flow of pollutants from land to sea (Usero et al. 2005); trace elements ultimately accumulate in the estuarine environments and potentially affect the health of these aquatic ecosystems (Kljaković-Gašpić et al. 2010). Shellfish could constitute a persistent risk for human consumption since they ingest metals directly from water or from particulate (van der Oost et al. 2003). Bivalve mollusks submerge themselves in sediments, lie on the seafloor or adhere to rocks, fewer can swim short distances. All these features make them excellent bioindicators of water quality on a local/regional scale (Laffon et al. 2006).
Elements such as copper (Cu), iron (Fe), manganese (Mn), selenium (Se), and zinc (Zn) have specific biological and physiological cellular roles at low concentrations and are considered essential for living beings (Oehlenschläger 2012; FAO 2014). Nevertheless, at high concentrations, these trace elements can exert hazard to aquatic organisms and represent a food safety concern (EFSA 2014a, b, c, 2015a, b, c). The nonessential elements Cd, Hg, and Pb are instead toxic even at low levels of exposure and have not recognized metabolic functions (Tchounwou et al. 2012). Synergy between essential and nonessential elements has been reported, moreover a high total metal content in mollusks could exert a potential hazard for human health (Has-Schön et al. 2006; Castro-Gonzalez and Mendez-Armenta 2008). Bioindicators were extensively used to monitor the presence of chemical in aquatic environments (Rainbow et al. 2004). Bivalves are ideal bioindicators among invertebrates due to their habits and ability to bioaccumulate and concentrate contaminants (Farrington et al. 2016). They have been used in biomonitoring of coastal aquatic ecosystems at different spatial-temporal scales (Chase et al. 2001; Emmanouil et al. 2008; Waykar and Deshmukh 2012; Zuykov et al. 2013; Sericano et al. 2014; González-Fernández et al. 2015; Farrington et al. 2016; Capolupo et al. 2017). The use of bivalves to estimate environmental quality is widespread, and the concentration of trace elements in these aquatic organisms has been investigated worldwide since shellfish are an important source of protein in the diet of coastal population (Pan and Wang 2012; Chen et al. 2014; Chandurvelan et al. 2015; Araújo et al. 2016; Conte et al. 2016; Baltas et al. 2017; Bilgin and Uluturhan-Suzer 2017; Cunha et al. 2017; Wang and Lu 2017; Bonnefille et al. 2018). Among bivalves, mussels are powerful filter feeders: each individual filter around 15 m3 of water every year. This means that they can accumulate toxic substances dissolved in sea water or that is associated with material in suspension by factors of 102–105 (Baltas et al. 2017). Investigation performed in the central-western Mediterranean Sea highlighted the use of mussels as the most suitable organisms to check the state of water contamination (Kljaković-Gašpić et al. 2007; Cardellicchio et al. 2008; Andral et al. 2011; Spada et al. 2012; Stanković and Jović 2012). In particular, the Mediterranean mussel Mytilus galloprovincialis, owing to its relative high frequency of consumption, provides the more complete information for the evaluation of human exposure to trace elements (Cirillo et al. 2010; Stanković et al. 2011).
The Calich Lagoon, a typical brackish area of Sardinia (Western Mediterranean Sea, Italy), was the subject of several studies covering different topics, from shellfish farming (Chessa et al. 2005; Pais et al. 2006, 2007; Cannas et al. 2011;) to fish fauna (Chessa et al. 2007) to fish fauna (Chessa et al. 2007), from ecotoxicology (Ielmini et al. 2014) to the ecology of different planktonic components (Pulina et al. 2017, 2018; Bazzoni et al. 2018; Satta et al. 2020), bacteria and biotoxins in shellfish (Sedda et al. 2016; Bazzoni et al. 2019).
The aims of the present study were:
-
To evaluate the bioaccumulation of 21 trace elements in samples of M. galloprovincialis collected from the natural beds of the Calich Lagoon.
-
To assess the seasonal accumulation of the analyzed trace elements and to evaluate the role of M. galloprovincialis as bioindicators of environmental quality.
-
To verify the compliance with the legal limits established by the in-force regulations for Cd, Hg, and Pb in shellfish.
-
To investigate the presence of inorganic arsenic in this bivalve.
Materials and methods
Study site
The Calich Lagoon is located along the north western coast (40° 35′ 47.5″ N; 8° 17′ 59.9″ E) of Sardinia (Italy) (Fig. 1). It is situated between the city of Alghero and the village of Fertilia and belongs to the Regional Natural Park of Porto Conte. The lagoon extends for 92 ha with a depth varying between about 0.5 and 1.5 m. A natural channel (60-m wide and 2-m deep) connects the lagoon to the sea; at the output of which are located a medium size shipyard and a tourist harbor, very popular during the dry period. The lagoon receives freshwater from two natural fluvial tributaries (Rio Calvia and Rio Barca) and one artificial canal (Oruni channel) which drains 70% of the catchment (Pulina et al. 2017). Water from a wastewater treatment plant enters in the Oruni channel, while waters from municipal sewage treatment plant enter in the Rio Barca. The Rio Calvia flows downstream of the drinking water treatment plant of the city of Alghero receiving water from areas of agricultural use (Fenza et al. 2014). Therefore, the lagoon is highly eutrophic (Bazzoni et al. 2018). Natural beds of bivalve mollusks of commercial interest (e.g., grooved carpet shell (Ruditapes decussatus), olive green cockle (Cerastoderma glaucum), and Mediterranean mussel (Mytilus galloprovincialis)) are present, but despite the abundance of highly valuable commercial species of bivalve mollusks, the Calich Lagoon has not yet been classified for shellfish production.
Sampling
To evaluate the effects of intensive inputs of human activities, and those deriving from the agricultural and livestock sectors, the study was conducted in three different seasons of 2017: spring, summer, and autumn (i.e., March, June, and September, respectively). Wild specimens of Mediterranean mussel (Mytilus galloprovincialis) were manually sampled at roughly 1-m depth from a single mollusks sampling point (MS) in a natural bed (Fig. 1). A total of 30 adult mussels per month were sampled (i.e., 90 in total) with comparable weight were selected (mean ± SD of total length 52.9 ± 9.5 mm and wet weight 5.0 ± 2.7 g). After sampling, mussels were washed with clean lagoon waters and immediately transported to the laboratory in insulated coolers. The samples were cleaned and measured, and the soft part of the shellfish was removed from the shell, washed with distilled water, and sent frozen to the chemical laboratory to be analyzed.
Detection of trace elements
A Direct Mercury Analyser (DMA80, Milestone, Shelton, CT, USA), based on thermal decomposition, amalgamation, and atomic absorption, was utilized for mercury (Hg) detection following a previous detailed protocol (Esposito et al. 2018). The limit of quantitation of the method (LOQ) was 0.010 mg kg−1.
The other 20 elements silver (Ag), aluminium (Al), arsenic (As), beryllium (Be), bismuth (Bi), cadmium (Cd), cobalt (Co), chrome (Cr), copper (Cu), iron (Fe), gallium (Ga), indium (In), manganese (Mn), nickel (Ni), lead (Pb), selenium (Se), tin (Sn), thallium (Tl), uranium (U), and zinc (Zn) were quantified by inductively coupled plasma mass spectrometry (ICP-MS Xseries II, Thermo Scientific, Bremen, Germany) according a method already described (Squadrone et al. 2016). Samples (1.0 g) were first homogenized then subjected to microwave acid (HNO3 and H2O2) mineralization process (oven ETHOS 1 from Milestone, Shelton, CT, USA). Recovery was checked by using the CRM–SRM 1566b from NIST and was in the range 82–104%. The limit of quantitation of the method (LOQ) was 0.010 mg kg−1.
Arsenic speciation analysis
Arsenic speciation was performed by high performance liquid chromatography coupled to an inductively coupled plasma mass spectrometer (HPLC-ICP-MS); ICP-MS Xseries II, Thermo Scientific, Bremen, Germany and HPLC Spectra System MCS 1000, Thermo Scientific, Bremen, Germany). Samples (about 0.30 g) were added with a solution at 1% v/v HNO3 and 1% v/v H2O2 briefly vortexed and stored overnight at room temperature. Extraction was performed in an ultrasonic bath at 55 °C for 2 h. After centrifugation, samples supernatants were filtered through a 0.20-μm mesh filter and diluted 1:1 with ultrapure water then analyzed to detect the following arsenic species: DMA (dimethylarsinic acid), MMA (monomethylarsonic acid), AB (arsenobetaine), and iAs (inorganic arsenic, sum of arsenite, and arsenate). The limit of quantitation of the method (LOQ) was 0.020 mg kg−1.
Results were reported on wet weight basis according to the European Regulation (1881/2006 UE and amendments).
Statistical analysis
R software (version 1.0.153 - RStudio, Inc.) was employed. The concentrations of trace elements in Mytilus galloprovincialis collected in the Calich Lagoon in relation to season were compared and analyzed with a linear regression model. The model was defined as:
where “y” is the trace elements concentration expressed as mg kgˉ1 of wet weight (w. w.); “season” is the effect of the month and “e” is the error term. Moreover, a multiple pairwise-comparison between the means of groups was compared with the Tukey HSD (Tukey honest significant differences). The results were considered statistically significant when P < 0.05 for all the tests performed.
Results and discussion
Metal concentrations are shown in Table 1 and were also graphically represented in Fig. 2 and Fig. 3. The highest values were found for Al (mean 32 mg kg−1 w. w.), Fe (mean 32 mg kg−1 w. w.), and Zn (mean 25 mg kg−1 w. w.). Be, Bi, Ga, In, Tl, and U are elements scarcely or not investigated in mussels, but we found values < LOQ in all the shellfish samples, according to another investigation reporting very low concentrations for some of these elements in Mytilus spp. (Rodríguez-Hernández et al. 2019). As speciation results was reported in Table 2, while the differences among the sampling periods (ANOVA and the Tukey test) in Table 3. A high significant difference (P < 0.001) between the three sampling seasons was found only for Ag and Hg (Table 3).
In Table 4 a comparison of results with literature data was shown.
Nonessential trace elements
Maximum limits (ML) for Cd, Hg, and Pb in shellfish are set by European Regulations 1881/2006 UE and amendments (1.0, 0.5, 1.5 mg kg−1 w.w. for Cd, Hg, and Pb, respectively). The values for Cd, Hg, and Pb were found lower than the ML (Table 1; Fig. 1). Cd concentrations (Table 4) were lower than those registered in the genus Mytilus from Italy (Cubadda et al. 2006; Lafabrie et al. 2007; Desideri et al. 2010; Bille et al. 2015); Montenegro (Stanković et al. 2011; Perošević et al. 2018); Croatia (Bogdanović et al. 2014; Kanduč et al. 2018); France (Gueguen et al. 2011; Richir and Gobert 2014); Spain (Olmedo et al. 2013); Portugal (Anacleto et al. 2015); Ireland (Bennion et al. 2019); Norway (Schøyen et al. 2017); Morocco (Azizi et al. 2018; Mejdoub et al. 2018); South Africa (Firth et al. 2019); China (He and Wang 2013; Li and Gao 2014; Lu and Wang 2018); India (Sivaperumal et al. 2007); Trinidad and Tobago (Balgobin and Singh 2018); and New Zealand (Whyte et al. 2009), but similar to those recorded in the Italian Tyrrhenian coast (Papetti and Rossi 2009); in the Sardinian coast (Piras et al. 2013); in the Gulf of La Spezia, Italy (Squadrone et al. 2016).
No significant seasonal variation of Cd bioaccumulation was registered (Table 3).
Hg concentrations (0.011–0.021 mg kg−1 w. w.) were in the range or even lower of the levels reported in Italy (Cubadda et al. 2006; Lafabrie et al. 2007; Desideri et al. 2010; Brambilla et al. 2013; Piras et al. 2013; Bille et al. 2015; Squadrone et al. 2016); Montenegro (Stanković et al. 2011; Perošević et al. 2018); France (Gueguen et al. 2011); South Africa (Firth et al. 2019); China (Liu et al. 2013; Li and Gao 2014); and New Zealand (Whyte et al. 2009).
A study performed on M. galloprovincialis from Italy reported the presence of Hg in the toxic organic form of methyl mercury (MeHg) ranging from 17 to 49% to total mercury (Di Leo et al. 2010). Since the maximum concentration found in this investigation was 0.021 mg kg−1, MeHg should range from 0.0036 to 0.010 mg kg−1 and should not constitute any risk for consumers.
In this study, a high significant temporal variation in bioaccumulation of Hg was reported (P < 0.001) (Table 3). In fact, in summer Hg was found about twice the values of spring and autumn (0.021 mg kg−1 w. w.).
The concentrations of Pb recovered in the Calich Lagoon (range 0.071–0.20 mg kg−1w. w.) were in line with previous Italian findings (Cubadda et al. 2006). No significant seasonal variation was recovered for this element (Table 3). Higher levels of Pb were instead described in samples of M. galloprovincialis from the Moroccan coasts (Azizi et al. 2018) and in M. edulis samples collected in Ireland (Bennion et al. 2019).
Aluminium showed the highest values between the nonessential element (mean 32 mg kg−1w. w.) and different concentrations in the three seasons, with the highest found in spring (Table 1). The registered values were higher than those reported in South Africa (Firth et al. 2019) but lower than those described in previous studies from Italy (Squadrone et al. 2016) and Montenegro (Perošević et al. 2018). Interestingly, Richir and coauthors (Richir and Gobert 2014) reported similar values from the nearby Corsica island (Table 4). It is well known that Al has a low bioavailability, but as result of intensive industrialization acid rains may lead to increased Al concentration in natural waters. Dissolved aluminum ions were then absorbed by marine or freshwater biota entering the food chain. It is well known that the major source of human exposure to Al is via diet (EFSA 2008) and after ingestion this element reaches all tissues and accumulates, especially in bone. Al carrier is the Fe-binding protein transferrin; Al can enter the brain and reach the placenta and fetus (EFSA 2008).
The TWI recommended by the European Food Safety Authority (EFSA) for Al is 1 mg Al kg−1 b. w./w. (EFSA 2008), that would be exceeded if consuming 310 g of mussels per day.
Then, the levels of aluminium in mussels should be routinely monitored since its presence could be related to the downstream flow, in one of the natural fluvial tributaries of the Calich Lagoon (Rio Calvia), of the drinking water treatment plant of the city of Alghero, where Al-based flocculants are used and could be influence the seasonal variation.
The inorganic forms of As are highly toxic but bivalve are known to contain mostly total arsenic (tAs), in the organic forms arsenobetaine; dimethylarsinic acid; monomethylarsonic acid, and a low percentage of iAs (EFSA 2009, 2014b). In the present study, tAs was found at values ranging from 1.2 mg kg−1 w. w. in spring to 1.7 mg kg−1 w. w. in summer (Table 1). Similar concentrations were observed by He and Wang (2013) and Firth et al. (2019) in M. galloprovincialis samples and by Olmedo et al. (2013) and Li and Gao (2014) in M. edulis samples. Low values of tAs have been also reported in clams from the Calich Lagoon (Esposito et al. 2018). High levels from moderate contaminated environments have been described by Richir and Gobert (2014) in the Corsica Island and by Kanduč et al. (2018) in Croatia. As reported in Table 2, the level of iAs in M. galloprovincialis was low and corresponded to 0.012 mg kg−1 w. w. (iAs 0.80% of tAs).
Silver and tin were found at trace level in the Calich Lagoon (Table 1), however significant seasonal variation has been highlighted in both elements (Table 3) since they were found higher in spring than in the other two seasons. The levels of Ag and Sn were in the range of concentrations found in the nearby Corsica Island (Richir and Gobert 2014) and in the Gulf of La Spezia (Squadrone et al. 2016), and do not pose any concern.
Essential trace elements
Essential trace elements levels are shown in Fig. 3 and Table 1. Cobalt was recorded in mussels from 0.042 mg kg−1 w. w. in spring to 0.067 mg kg−1 w. w. in summer (Table 1), levels comparable to those recorded in previous studies (Table 4) in Italy (Squadrone et al. 2016); France (Richir et al. 2014); Croatia (Kanduč et al. 2018); and Montenegro (Perošević et al. 2018). The high levels of Cr were found in spring (0.11 mg kg−1 w. w.) according to studies on the same species from Italy (Lafabrie et al. 2007; Squadrone et al. 2016); France (Richir and Gobert 2014); Montenegro (Perošević et al. 2018); South Africa (Firth et al. 2019); and China (He and Wang 2013). High levels of Cr have been instead registered in Mytilus galloprovincialis from Morocco (Azizi et al. 2018). The Cu values were quite different in the three seasons (Table 1) with the highest value in spring (mean 3.6 mg kg−1 w. w.) and the lowest value in summer (mean 1.4 mg kg−1 w. w.). These concentrations are comparable to those found in M. galloprovincialis from Croatia (Kanduč et al. 2018) and China (He and Wang 2013). Fe concentrations (Table 1) were lower than those reported in the same species from Italy (Squadrone et al. 2016); France (Richir and Gobert 2014); Montenegro (Perošević et al. 2018); and Morocco (Azizi et al. 2018) while Mn levels were in the range of published studies carried out in the Mediterranean Sea (Bogdanović et al. 2014; Richir and Gobert 2014; Squadrone et al. 2016; Kanduč et al. 2018; Perošević et al. 2018). Ni and Se values concentrations (Table 1) were homogeneous in the examined seasons, but Ni values were lower to those found from Mediterranean (Richir and Gobert 2014; Squadrone et al. 2016; Azizi et al. 2018; Kanduč et al. 2018; Mejdoub et al. 2018; Perošević et al. 2018).
Selenium values were comparable to concentrations registered in M. galloprovincialis from the Galician Coast (Olmedo et al. 2013), the Corsica Island (Richir and Gobert 2014), and the gulf of La Spezia, Italy (Squadrone et al. 2016), but lower to those found in clams from the Calich Lagoon (Esposito et al. 2018). Zn concentrations were found in the range 21–29 mg kg−1 w. w. (Table 1), according to other findings in M. galloprovincialis from different locations such as Italy, Montenegro, and South Africa (Squadrone et al. 2016; Perošević et al. 2018; Firth et al. 2019). Remarkably high levels of zinc in M. galloprovincialis (range 162–361 mg kg−1 w. w.) have been recently reported in two Moroccan studies (Azizi et al. 2018; Mejdoub et al. 2018).
It is well known that the levels of trace elements in mussels register fluctuations depending on the period of the year in which they are collected (Claisse 1992). In the period in which each species reaches sexual maturity, the bivalve pulp increases the mass of the organism, causing a relative decrease in metals concentrations. The highest metals concentrations are then usually recorded in winter and spring and the lowest in summer and autumn (Piras et al. 2013). According to this phenomenon in this investigation, the highest levels of Ag, Al, Cd, Fe, and Sn were recovered in spring; in addition, seasonal variation of physicochemical parameters such as temperature, salinity, dissolved oxygen, nutrients, and food availability are known to influence metals body burden.
Conclusions
The study provides useful information on the distribution of trace elements in native Mediterranean mussels (Mytilus galloprovincialis) from the Calich Lagoon (North Western Mediterranean Sea, Italy) that could constitute a baseline for future monitoring plans. The concentrations of metals in mussels did not pose concern for human consumption, but even if Cd, Hg, and Pb levels did not exceed the maximum limits set by European regulations, the high significant temporal variation registered for mercury suggests to constantly monitor this element in the different seasons. Moreover, although the recommended TWI was not exceeded, the levels of aluminium in mussels should be routinely monitored, since the presence of anthropogenic activities that could increase Al level in this valuable but fragile lagoon ecosystem.
Data availability
Data supporting the findings are available from the corresponding author upon reasonable request.
References
Almeida C, Soares F (2012) Microbiological monitoring of bivalves from the Ria Formosa Lagoon (south coast of Portugal): 20 years of sanitary survey. Mar Pollut Bull 64:252–262
Anacleto P, Maulvault AL, Nunes ML, Carvalho ML, Rosa R, Marques A (2015) Effects of depuration on metal levels and health status of bivalve molluscs. Food Control 47:493–501
Andral B, Galgani F, Tomasino C, Bouchoucha M, Blottiere C, Scarpato A, Benedicto J, Deudero S, Calvo M, Cento A, Benbrahim S, Boulahdid M, Sammari C (2011) Chemical contamination baseline in the Western Basin of the Mediterranean Sea based on transplanted mussels. Arch Environ Contam Toxicol 61:261–271
Araújo CFS, Lopes MV, Vasquez MR, Porcino TS, Ribeiro ASV, Rodrigues JLG, Menezes-Filho JA (2016) Cadmium and lead in seafood from the Aratu Bay, Brazil and the human health risk assessment. Environ Monit Assess 188:259
Azizi G, Layachi M, Akodad M, Yáñez-Ruiz DR, Martín-García AI, Baghour M, Mesfioui A, Skalli A, Moumen A (2018) Seasonal variations of heavy metals content in mussels (Mytilus galloprovincialis) from Cala Iris offshore (Northern Morocco). Marine Poll Bull 137:688–694
Balgobin A, Singh NR (2018) Impact of anthropogenic activities on mussel (Mytella guyanensis) in the Gulf of Paria, Trinidad. Marine Poll Bull 135:496–504
Baltas H, Sirin M, Dalgic G, Ylmaz Bayrak E, Akdeniz A (2017) Assessment of metal concentrations (Cu, Zn, and Pb) in seawater, sediment and biota samples in the coastal area of Eastern Black Sea, Turkey. Marine Poll Bull 122:475–482
Bazzoni AM, Mudadu AG, Lorenzoni G, Soro B, Bardino N, Arras I, Sanna G, Vodret B, Bazzardi R, Marongiu E, Virgilio S (2018) Detection of Dinophysis species and associated okadaic acid in farmed shellfish: a two-year study from the western Mediterranean area. J Vet Res 62:137–144
Bazzoni AM, Mudadu AG, Esposito G, Urru R, Ortu S, Mara L, Uda MT, Arras I, Lorenzoni G, Sanna G, Bazzardi R, Marongiu A, Virgilio S, Meloni D (2019) Bacteriological and viral investigation combined with determination of phytoplankton and algal biotoxins in mussels and water from a Mediterranean coastal lagoon. J Food Prot 82(9):1501–1511
Bennion M, Morrison L, Brophy D, Carlsson J, Cortiñas Abrahantes J, Graham CT (2019) Trace element fingerprinting of blue mussel (Mytilus edulis) shells and soft tissues successfully reveals harvesting locations. Sci Total Environ 685:50–58
Bille L, Binato G, Cappa V, Toson M, Dalla Pozza M, Arcangeli G, Ricci A, Angeletti R, Piro R (2015) Lead, mercury and cadmium levels in edible marine molluscs and echinoderms from the Veneto Region (North-Western Adriatic Sea-Italy). Food Control 50:362–370
Bilgin M, Uluturhan-Suzer E (2017) Assessment of trace metal concentrations and human health risk in clam (Tapes decussatus) and mussel (Mytilus galloprovincialis) from the Homa Lagoon (Eastern Aegean Sea). Environ Sci Pollut Res 24:4174–4184
Bogdanović T, Ujević I, Sedak M, Listeš E, Šimat V, Petričević S, Poljak V (2014) As, Cd Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic coast, Croatia. Food Chem 146:197–203
Bonnefille B, Gomez E, Courant F, Escande A, Fenet H (2018) Diclofenac in the marine environment: a review of its occurrence and effects. Mar Pollut Bull 131:496–506
Brambilla G, Abete MC, Binato G, Chiaravalle E, Cossu M, Dellatte E, Miniero R, Orletti R, Piras P, Roncarati A, Ubaldi A, Chessa G (2013) Mercury occurrence in Italian seafood from the Mediterranean Sea and possible intake scenarios of the Italian coastal population. Regul Toxicol Pharmacol 65(2):269–277
Cannas A, Manca S, Trentadue M, Fois N (2011) Population structure of carpet shell clam (Ruditapes decussates L.) in two coastal lagoons of Sardinia (Italy). Biol Mar Mediterr 18:298–299
Capolupo M, Franzellitti S, Kiwan A, Valbonesi P, Dinelli E, Pignotti E, Birke M, Fabbri E (2017) A comprehensive evaluation of the environmental quality of a coastal lagoon (Ravenna, Italy): integrating chemical and physiological analyses in mussels as a biomonitoring strategy. Sci Total Environ 598:146–159
Castro H, Aguilera PA, Martinez JL, Carrique EL (1999) Differentiation of clams from fishing areas an approximation to coastal quality assessment. Environ. Monitor Assess 54:229–237
Castro-Gonzalez I, Mendez-Armenta M (2008) Heavy metals: implications associated to fish consumption. Environ Toxicol Pharmacol 26(3):263–271
Cataudella S, Crosetti D, Massa F (2015) Mediterranean coastal lagoons: sustainable management and interactions among aquaculture, capture fisheries and the environment. Studies and Reviews General Fisheries Commission for the Mediterranean. No 95 pp 7–49. Rome, FAO
Cardellicchio N, Buccolieri A, Di Leo A, Giandomenico S, Spada L (2008) Levels of metals in reared mussels from Taranto Gulf (Ionian Sea, southern Italy). Food Chem 107:890–896
Chandurvelan R, Marsden I, Glover CN, Gaw S (2015) Assessment of a mussel as a metal bioindicator of coastal contamination: relationships between metal bioaccumulation and multiple biomarker responses. Sci Total Environ 511C:663–667
Chase ME, Jones SH, Hennigar P, Sowles J, Harding GCH, Freeman K, Wells PG, Krahforst C, Coombs K, Crawford R, Pederson J, Taylor D (2001) Gulfwatch: monitoring spatial and temporal patterns of trace metal and organic contaminants in the Gulf of Maine (1991–1997) with the blue mussel, Mytilus edulis. L. Mar Pollut Bull 42:491–505
Chen H, Zha J, Liang X, Li J, Wang Z (2014) Effects of the humna antiepileptic drug carbamazepine on the behavior, biomarkers, and heat shock proteins in the Asian clam Corbicula fluminea. Aquat Toxicol 155:1–8
Chessa LA, Casola E, Lanera P, Pais A, Plastina N, Serra S, Scardi M, Valiante LM, Vinci D (2007) Is there a correspondence between dominant trophic group in benthic and fish fauna of the Calich Lagoon? Biol Mar Mediterr 14:290–291
Chessa LA, Paesanti F, Pais A, Scardi M, Serra S, Vitale L (2005) Perspectives for development of low impact aquaculture in a Western Mediterranean lagoon: the case of the carpet clam Tapes decussatus. Aquac Int 13:147–155
Cirillo T, Fasano E, Viscardi V, Arnese A, Amodio-Cocchieri R (2010) Survey of lead, cadmium, mercury and arsenic in seafood purchased in Campania, Italy. Food Addit Contam B 3(1):30–38
Claisse D (1992) Accumulation des métaux lourds et polluants organiques par les coquillages. In: Lesne J (ed) Coquillages et santé publique. Du risque à la prévention. ENSP ed, Nancy, pp 99–111
Conte F, Copat C, Longo S, Oliveri Conti G, Grasso A, Arena G, Dimartino A, Brundo MV, Ferrante M (2016) Polycyclic aromatic hydrocarbons in Haliotis tuberculata (Linnaeus, 1758) (Mollusca, Gastropoda): considerations on food safety and source investigation. Food Chem Toxicol 94:57–63
Cubadda F, Raggi A, Coni E (2006) Element fingerprinting of marine organisms by dynamic reaction cell inductively coupled plasma mass spectrometry. Anal Bioanal Chem 384(4):887–896
Cunha SH, Pena A, Fernandes JO (2017) Mussels as bioindicators of diclofenac contamination in coastal environments. Environ Pollut 225:354–360
Desideri D, Meli MA, Roselli C (2010) A biomonitoring study: 210Po and heavy metals in marine organisms from the Adriatic Sea (Italy). J Radioanal Nucl Chem 285:373–382
Di Leo A, Cardellicchio N, Giandomenico S, Spada L (2010) Mercury and methylmercury contamination in Mytilus galloprovincialis from Taranto Gulf (Ionian Sea, Southern Italy): Risk evaluation for consumers. Food Chem Toxicol 48(11):3131–3136
EC (Council Regulation) (2006) Commission Regulation (EC) No 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off J European Union L364:5e24
EFSA (2008) Safety of aluminum from dietary intake. EFSA J 754:1–34 Available online. www.efsa.europa.eu
EFSA (2009) Scientific opinion on arsenic in food. EFSA J 7(10):1351. https://doi.org/10.2903/j.efsa.2009.1351 (199 pp)
EFSA (2014a) Scientific opinion on dietary reference values for chromium. EFSA J 12(10):3845, 25 pp
EFSA (2014b) Dietary exposure to inorganic arsenic in the European population. EFSA J 12(3):3597
EFSA (2014c) Scientific opinion on dietary reference values for zinc. EFSA J 12(10):3844
EFSA (2015a) Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J 13(2):4002 202 pp
EFSA (2015b) Scientific opinion on dietary reference values for copper. EFSA J 13(10):4253 51 pp
EFSA (2015c) Scientific opinion on dietary reference values for iron. EFSA J 13(10):4254 115 pp
Emmanouil C, Kypriotakis S, Kungolos A, Machera K (2008) Effects of Thiram or MCPA acid on mussel gill DNA. Discussion Paper Series 14(24):455–468
Esposito G, Meloni D, Abete MC, Colombero G, Mantia M, Pastorino P, Prearo M, Pais A, Antuofermo E, Squadrone S (2018) The bivalve Ruditapes decussatus: a biomonitor of trace elements pollution in Sardinian coastal lagoons (Italy). Environ Poll 242:1720–1728
FAO (Food and Agriculture Organization). (2014). Species Fact Sheets: Mytilus galloprovincialis (Lamark, 1819). Available at http://www.fao.org/fishery/culturedspecies/Mytilus_galloprovincialis/en.FDA
Farrington JW, Tripp BW, Tanabe S, Subramanian A, Sericano JL, Wade TL, Knap AH, Edward D (2016) Goldberg’s proposal of “the mussel watch”: reflections after 40 years. Mar Pollut Bull 110:501–510. https://doi.org/10.1016/j.marpolbul.2016.05.074
Fenza A, Olla G, Salati F, Viale I (2014) Stagni e lagune produttive della Sardegna. Tradizioni sapori ed ambiente. Agenzia Regionale LAORE Sardegna, pp. 1-73
Firth DC, Salie K, O'Neil B, Hoffman LC (2019) Monitoring of trace metal accumulation in two South African farmed mussel species, Mytilus galloprovincialis and Choromytilus meridionalis. Marine Poll Bull 141:529–534
Ghidini S, Battaglia A, Canardi E, Campanili G (2001) Elementi in traccia in molluschi e crostacei dell’alto Adriatico. http://www.unipr.it/arpa/facvet/annali/2001/ghidini.pdf
González-Fernández C, Albentosa M, Campillo JA, Viñas L, Fumega J, Franco A, Besada V, González-Quijano A, Bellas J (2015) Influence of mussel biological variability on pollution biomarkers. Environ Res 137:14–31
Gueguen M, Amiard J, Arnich N, Badot P, Claisse D, Guerin T, Vernoux JP (2011) Shellfish and residual chemical contaminants: hazards, monitoring, and health risk assessment along french coasts. Rev Environ Contam Toxicol 213:55–111
Has-Schön E, Bogut I, Strelec I (2006) Heavy metal profile in five fish species includedin human diet, domiciled in the end flow of river Neretva (Croatia). Arch Environ Contam Toxicol 50:545–551
He M, Wang WX (2013) Bioaccessibility of 12 trace elements in marine molluscs. Food Chem Toxicol 55:627–636
Ielmini SE, Piredda G, Mura S, Greppi GF (2014) Protein biomarkers as indicator for water pollution in some lagoons of Sardinia (Italy). Transit Waters Bull 8:32–52
Kanduč T, Šlejkovec Z, Falnoga I, Mori N, Budič B, Kovačić I, Pavičić-Hamere D, Hamere B (2018) Environmental status of the NE Adriatic Sea, Istria, Croatia: insights from mussel Mytilus galloprovincialis condition indices, stable isotopes and metal (loid)s. Marine Poll Bull 126:525–534
Kljaković-Gašpić Z, Ujević I, Zvonarić T, Barić A (2007) Biomonitoring of trace metals (Cu, Cd, Cr, Hg, Pb, Zn) in Mali Ston Bay (eastern Adriatic) using the Mediterranean blue mussel (1998–2005). Acta Adriat 48(1):73–88
Kljaković-Gašpić Z, Herceg-Romanić S, Kožul D, Vež J (2010) Biomonitoring of organochlorine compounds and trace metals along the Eastern Adriatic coast (Croatia) using Mytilus galloprovincialis. Marine Poll Bull 60(10):1879–1889
Lafabrie C, Pergent G, Kantin R, Pergent-Martini C, Gonzalez JL (2007) Trace metals assessment in water, sediment, mussel and seagrass species--validation of the use of Posidonia oceanica as a metal biomonitor. Chemosphere 68(11):2033–2039
Laffon B, Rábade T, Pásaro E, Méndez J (2006) Monitoring of the impact of prestige oil spill on Mytilus galloprovincialis from Galician coast. Environ Int 32:342–348
Li P, Gao X (2014) Trace elements in major marketed marine bivalves from six northern coastal cities of China: concentrations and risk assessment for human health. Ecotoxicol Environ Saf 109:1–9
Liang L, He B, Jiang G, Chen D, Yao Z (2004) Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci Total Environ 324(1–3):105–113
Liu J, Cao L, Huang W, Dou S (2013) Species- and tissue-specific mercury bioaccumulation in five fish species from Laizhou Bay in the Bohai Sea of China. Chin J Oceanol Limnol 31(3):504–513
Lozano G, Herraiz E, Hardisson A, Gutiérrez AJ, González-Weller D, Rubio C (2010) Heavy and trace metal concentrations in three rockpool shrimp species (Palaemon elegans, Palaemon adspersus and Palaemon serratus) from Tenerife (Canary Islands). Environ Monit Assess 168(1–4):451–460
Lu GY, Wang WX (2018) Trace metals and macroelements in mussels from Chinese coastal waters: national spatial patterns and normalization. Sci Total Environ 626:307–318
Majori L, Nedoclan G, Daris F, Modonutti GB (1991a) Metal distribution in Mytilus galloprovincialis Lmk in coast- and lagoon-areas in northern Adriatic. Revue Int d’Oceanogr Med 101-104:225–228
Majori L, Nedoclan G, Daris F, Modonutti GB (1991b) Mercury distribution in Mytilus galloprovincialis Lmk in northern Adriatic lagoons and coastal areas. Revue Int d’Oceanogr Med 101e104:214–217
Mejdoub Z, Zaid Y, Hmimid F, Kabine M (2018) Assessment of metals bioaccumulation and bioavailability in mussels Mytilus galloprovincialis exposed to outfalls pollution in coastal areas of Casablanca. J Trace Elem Med Biol 48:30–37
Oehlenschläger J (2012) Seafood: nutritional benefits and risk aspects. Int J Vitam Nutr Res 82:168–176
Olmedo P, Pla A, Hernández AF, Barbier F, Ayouni L, Gil F (2013) Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk Assess Consum Environ Int 59:63–72
Pais A, Chessa LA, Serra S, Ruiu A (2006) An alternative suspended culture method for the Mediterranean carpet clam, Tapes decussatus (L.), in the Calich Lagoon (north western Sardinia). Biol Mar Mediterr 13:134–135
Pais A, Chessa LA, Serra S, Ruiu A, Meloni G (2007) Suspended culture of Ostrea edulis in the Calich Lagoon (north western Sardinia, Italy): preliminary results. Ital J Anim Sci 6:810–810
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 3-16:421–422
Papetti P, Rossi G (2009) Heavy metals in the fishery products of low Lazio and the use of metallothionein as a biomarker of contamination. Environ Monit Assess 159(1–4):589–598
Perošević A, Joksimović D, Đurović D, Milašević I, Radomirović M, Stanković S (2018) Human exposure to trace elements via consumption of mussels Mytilus galloprovincialis from Boka Kotorska Bay. Montenegro J Trace Elem Med Biol 50:554–559
Piras P, Chessa G, Cossu M, Fiori G, Piras P, Ledda G (2013) Lead and other heavy metals (cadmium and mercury) accumulation in bivalve mollusks (Mytilus galloprovincialis, Ruditapes spp. and Crassostrea gigas) sampled in Sardinia in 2008-2012. Italian J Food Safety 2(e49):182–187
Pulina S, Satta CT, Padedda BM, Bazzoni AM, Sechi N, Lugliè A (2017) Picophytoplankton seasonal dynamics and interactions with environmental variables in three Mediterranean coastal lagoons. Estuar Coasts 40:469–478
Pulina S, Satta CT, Padedda BM, Sechi N, Lugliè A (2018) Seasonal variations of phytoplankton size structure in relation to environmental variables in three Mediterranean shallow coastal lagoons. Estuar Coast Shelf Sci 212:95–104
Rainbow PS, Fialkowski ΖW, Sokolowski ΖA, Smith BD, Wolowicz ΖM (2004) Geographical and seasonal variation of trace metal bioavailabilities in the Gulf of Gdansk, Baltic Sea using mussels (Mytilus trossulus) and barnacles (Balanus improvisus) as biomonitors. Mar Biol 144:271–286
Richir J, Gobert S (2014) The effect of size, weight, body compartment, sex and reproductive status on the bioaccumulation of 19 trace elements in rope-grown Mytilus galloprovincialis. Ecol Indic 36:33–47
Rodríguez-Hernández Á, Zumbado M, Henríquez-Hernández LA, Boada LD, Luzardo OP (2019) Dietary intake of essential, toxic, and potentially toxic elements from mussels (Mytilus spp.) in the Spanish population: a nutritional assessment. Nutrients 17(4):864
Satta CT, Pulina S, Reñé A, Padedda BM, Caddeo T, Fois N, Lugliè A (2020) Ecological, morphological and molecular characterization of Kryptoperidinium sp. (Dinophyceae) from two Mediterranean coastal shallow lagoons. Harmful Algae 97:101855
Schøyen M, Allan IJ, Ruus A, Håvardstun J, Hjermann DØ, Beyer J (2017) Comparison of caged and native blue mussels (Mytilus edulis spp.) for environmental monitoring of PAH, PCB and trace metals. Mar Environ Res 130:221–232
Sedda T, Baralla E, Varoni MV, Pasciu V, Lorenzoni G, Demontis MP (2016) Determination of microcystin-LR in clams (Tapes decussatus) of two Sardinian coastal ponds (Italy). Mar Pollut Bull 108:317–320
Sericano JL, Wade TL, Sweet ST, Ramirez J, Lauenstein GG (2014) Temporal trends and spatial distribution of DDT in bivalves from the coastal marine environments of the continental United States, 1986–2009. Mar Pollut Bull 81:303–316
Sivaperumal P, Sankar TV, Viswanathan Nair PG (2007) Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-à-vis international standards. Food Chem 102(3):612–620
Spada L, Annicchiarico C, Cardellicchio N, Giandomenico S, Di Leo A (2012) Mercury and methylmercury concentrations in Mediterranean seafood and surface sediments, intake evaluation and risk for consumers. Int J Hyg Environ Health 215(3):418–426
Squadrone S, Brizio P, Stella C, Prearo M, Pastorino P, Serracca L, Ercolini C, Abete MC (2016) Presence of trace metals in aquaculture marine ecosystems of the northwestern Mediterranean Sea (Italy). Environ Pollut 215:77–83
Stanković S, Jović M, Milanov R, Joksimović D (2011) Trace elements concentrations (Zn, Cu, Pb, Cd, As and Hg) in the Mediterranean mussel (Mytilus galloprovincialis) and evaluation of mussel quality and possible human health risk from cultivated and wild sites of the southeastern Adriatic Sea. Montenegr J Serb Chem Soc 76(12):1725–1737
Stanković S, Jović M (2012) Health risks of heavy metals in the mediterranean mussels as seafood. Environ Chem Lett 10:119–130
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology, volume 3 environmental toxicology. Springer, New York, pp 133–164
Usero J, Morillo J, Gracia I (2005) Heavy metal concentrations in molluscs from the Atlantic coast of southern Spain. Chemosphere 59:1175–1181
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149
Wang WX, Lu G (2017) Heavy metals in bivalve mollusks. In: Schrenk D, Cartus A (eds) Chemical contaminants and residues in food, 2nd edn. Woodhead Publishing Series in Food Science, Technology. Nutrition, pp 553–594
Waykar B, Deshmukh G (2012) Evaluation of bivalves as bioindicators of metal pollution in freshwater. Bull Environ Cont Toxicol 88:48–53
Whyte ALH, Raumati Hook G, Greening GE, Gibbs-Smith E, Gardner JPA (2009) Human dietary exposure to heavy metals via the consumption of greenshell mussels (Perna canaliculus Gmelin 1791) from the Bay of Islands, northern New Zealand. Sci Total Environ 407(14):4348–4355
Zuykov M, Pelletier E, Harper DAT (2013) Bivalve mollusks in metal pollution studies: from bioaccumulation to biomonitoring. Chemosphere 93:201–200
Funding
This research was funded by the Italian Health Ministry Research Grant (Project no. IZS PLV 01/15RC).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. Material preparation, data collection were performed by Giuseppe Esposito, Alessandro Graziano Mudadu, Sergio Ortu, and Anna Maria Bazzoni. Statistical analysis was carried out by Giuseppe Esposito. Resources were provided by Maria Cesarina Abete and Domenico Meloni. Methodology optimization and chemical analysis were performed by Sabina Pederiva, Alessandra Griglione, and Caterina Stella. The first draft of the manuscript was written by Giuseppe Esposito, and all authors commented the manuscript. Review and editing of the final manuscript was performed by Stefania Squadrone; activity planning and execution was in charge of Domenico Meloni. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esposito, G., Mudadu, A.G., Abete, M.C. et al. Seasonal accumulation of trace elements in native Mediterranean mussels (Mytilus galloprovincialis Lamarck, 1819) collected in the Calich Lagoon (Sardinia, Italy). Environ Sci Pollut Res 28, 25770–25781 (2021). https://doi.org/10.1007/s11356-021-12380-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12380-4


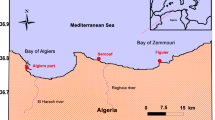

 ”) and the sampling location (red asterisk “*”)
”) and the sampling location (red asterisk “*”)
