Abstract
A coal fire is one of the most serious disasters in coal mining. To improve the efficiency of an inert gas for extinguishing the fire, the adsorption behavior of coal in CO2/N2 mixed gas was investigated in this study. Proximate analysis, ultimate analysis, solid-state 13C nuclear magnetic resonance spectroscopy (NMR), X-ray photoelectron spectroscopy (XPS), and molecular dynamics (MD) were applied to analyze and establish the bituminous coal molecular model. The adsorption behavior of bituminous coal in mixed gas mixtures with different proportions was studied using the bituminous coal model and Materials Studio (MS) software. A self-built coal adsorption experimental system was used for experiments. The adsorption of bituminous coal to CO2 is stronger than that to N2, and there is a competitive adsorption relationship between them. The amount of CO2 adsorbed by the coal gradually increases as the CO2 partial pressure rises, consistent with the Langmuir model. With an increase in CO2 pressure, the total adsorption capacity, which is divided into the rapid increase stage, slow growth stage, and stable stage, also increases. The coal adsorbs 0.5050 cm3/g, 0.7455 cm3/g, 0.9450 cm3/g, 1.0715 cm3/g, and 1.2000 cm3/g for pure N2, 2%, 5%, 7%, and 10% CO2, respectively, in the experiment. The results of the simulation and experiment show the same trend, which means that the injection of a small amount of CO2 into pure N2 will greatly improve the gas adsorption volume of the coal, demonstrating that it is feasible to improve the ability of the coal to absorb mixed gases by changing the gas concentration and consequently to increase the efficiency of inert gas for fire extinguishing and suppression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal fire is one of the main threats to the safety of coal mines (Zhu et al. 2020; Song et al. 2020; Elick 2011; Song and Kuenzer 2014). Coal fires not only cause property damage to enterprises, but also threaten personal safety, which finally leads to the waste of resources and environmental pollution. Currently, the widely applied mature technologies for extinguishing fire domestically and internationally are mainly plugging and pressure equalizing fire extinguishing technology, grout fire extinguishing technology, inhibitor fire extinguishing technology (Hao et al. 2018), three-phase foam fire extinguishing technology (Zhou et al. 2006; Lu et al. 2019), inert gas for fire extinguishing technology (Lei et al. 2020), etc. The above fire extinguishing technologies all have their own advantages and have been applied popularly in practice, but there are still varying degrees of problems. The method of using an inert gas as the fire extinguishing technology is simple, low cost, easy to operate, and has the good effect of dilution and explosion suppression (Zhao et al. 2020; Mohalik et al. 2005). Therefore, this paper focuses on the analysis and discussion of the use of inert gas for fire extinguishing.
Inert gas as a fire extinguishing technology uses mainly nitrogen supplemented by carbon dioxide. Nitrogen production equipment is based mainly on pressure swing adsorption and membrane separation with ground-mounted and underground mobile nitrogen production equipment developed in tandem. Liquid nitrogen, liquid carbon dioxide direct injection and temperature-controlled perfusion fire extinguishing technology have been successfully applied (Shi et al. 2018; Zhang et al. 2019). CO2 fire extinguishing technology uses gaseous CO2 or liquid CO2 to extinguish fires in the pretreatment area, forming an inert gas layer in a certain area to cover the surface of the floating coal, thereby blocking the oxygen. Because the CO2 is denser than air, it can sink to the bottom layer, better effecting control on low-level fire sources, pressurizing and exhausting a certain amount of toxic and harmful gases. However, there are still some problems with inert gas for the fire extinguishing method. Strengthening the efficiency of inert gases for extinguishing fire in coal mines is among these problems.
According to the principles of using inert gas for fire extinguishing, inert gas can replace a part of the oxygen in the surface of the coal fractures, thereby reducing the adsorption of oxygen on the coal surface and, to a certain extent, alleviating and inhibiting the oxidation of the coal remaining in the goaf. Therefore, the ability to adsorb coal gas is stronger, and the efficiency of the inert gas for fire extinguishing is higher. Adsorption refers to the entry of molecules into the exposed surface of solid substances through physical or chemical methods, including the outer and inner surfaces that are in contact with gas molecules. Former researchers have performed extensive research on coal gas adsorption. For example, Li investigated the adsorption pore structure and fractal characteristics of coals (0.69–2.79% R-o, R-m) using N2 adsorption/desorption and field emission scanning electron microscopy (Li et al. 2019). The results showed that the pore structure and coal composition at different coalification stages have a specific influences on the fractal characteristics of pore shape, surface and volume. The research results of Clarkson and Bustin (Clarkson and Bustin 2000) proved that moisture will reduce the adsorption performance of the coal. A new method was adopted to investigate the evolution characteristics of the crystallite structure of eight coals of different rank and the influence of these characteristics on the methane adsorption capacity by Meng et al. (2019). The results showed that methane adsorption is related to the crystallite structure characteristics of coal. The impact of coal composition and pore structure on gas adsorption was investigated by Sun et al. (2019). The gas adsorption capacity depends on the coal composition. Wang et al. (2019b) reviewed the current state of research regarding the temperature-dependent adsorption of methane in coal and cataloged the conclusions from experiments conducted on that subject by 28 researchers, in papers published between 1995 and 2017. The wettability and methane adsorption/desorption properties of coal were studied by Li et al. (2020). The adsorption pore structure and fractal characteristics of coals (0.69–2.79% R-o, R-m) were investigated by Li et al. through N2 adsorption/desorption and field emission scanning electron microscopy. However, these studies were based on experiments and showed the macro performance results of the coal. It is difficult to determine the microperformance situation, which cannot fully reflect the properties of the adsorbed gas in coal. Therefore, this paper combines the two methods of simulation and experiment to analyze the situation of coal adsorption gas from micro- and macroperspectives.
You et al. (2016) studied the adsorption behavior of CO2, CH4 and their mixtures in bituminous coal and established a bituminous coal model through molecular dynamics (MD) simulation. Under the conditions for CO2 adsorption, CO2 exhibits preferential adsorption over CH4, and as the pressure increases, the selectivity of CO2 adsorption changes significantly. At lower pressures, as the temperature rises, the selectivity for CO2 adsorption decreases rapidly, while at higher pressures, the selectivity for CO2 adsorption it is not sensitive to temperature. The adsorption isotherms of pure CO2 and CH4 on dry coal samples at temperatures of 45 °C and 60 °C were measured by Ottiger et al. (2006). Under any circumstances, the amount of CO2 adsorbed by coal is always greater than the amount of CH4 adsorbed by coal. The adsorption of CO2/CH4 mixed gas on different coal grades was studied by Mastalerz et al. (2004). The research showed that as the pressure increases, the adsorption rate of all coals for CO2 decreases, and the adsorption rate of coal to gas decreases with increasing of coal grade. The effect of pore structure on the combined adsorption of SO2 and typical benzene Volatile Organic Compounds (VOCs, i.e., toluene) was investigated systematically by Qie et al. (2019). The molecular dynamics simulation further demonstrates that the hierarchical pore structure enables the functional differentiation of pore spaces with different sizes so that micropores dominate SO2 adsorption and toluene molecules tend to be stored in meso-/macropores. These studies have provided a theoretical basis for establishing the molecular structure of coal and the adsorption of coal on gas.
At present, CO2 is one of the most effective inert gases to prevent spontaneous coal combustion. Previous studies have shown that the ability of coal to adsorb CO2 is greater than the ability of coal to adsorb N2. However, in actual applications, the use of a single gas cannot achieve an ideal extinguishing effect. The cost of CO2 is much higher than the cost of N2, and this cost is expensive when the gas is injected for a long time, making the economic benefit relatively poor. Therefore, based on previous research, the authors proposed the idea of using CO2 and N2 mixed gas to prevent spontaneous coal combustion. The authors built a coal molecular model and used Materials Studio (MS) software to simulate coal adsorption of N2 and CO2 mixed gas in different proportions and verified the adsorption by experiments using the self-built coal adsorption experimental system. The aim was to find a method for fire extinguishing that is both economical and efficient.
Experimental and simulation methods
Coal molecular model
The experimental coal was bituminous coal taken from Fangezhuang, Hebei Province, China, and its proximate analysis and ultimate analysis are shown in Table 1.
Considering the requirements of the experiment coal samples, large fresh coal samples were removed from the surface and pulverize into pulverized coal with a particle size of 0.25 mm. Hydrofluoric acid was used to demineralize the coal powder to reduce the influence of minerals in the coal on the experimental analysis results, and the demineralized pulverized coal was washed until the solution became neutral. The pulverized coal was put into a drying box, dried for 12 h, and then packaged quickly with a vacuum bag.
Solid-state 13C NMR spectroscopy
A Bruker AVANCE III 600 MHz nuclear magnetic resonance instrument was used to analyze the solid 13C NMR of the Fangezhuang bituminous coal, and the spectrum obtained was processed by peak fitting with Peakfit software (Bian et al. 2019).
X-ray photoelectron spectroscopy
A Thermo Scientific ESCALAB 250XI X-ray photoelectron spectrometer was used to analyze the chemical valence states of C, O, N, and S elements in the Fangezhuang bituminous coal, and Avantage software was used to perform peak fitting. According to the displacement of the photoelectron kinetic energy of each element, the chemical state of the element was determined (Wang et al. 2019a).
Adsorption simulation method
The Materials Studio (MS) software was used to simulate coal sample adsorption. The Compass II force field in the MS software strengthened support for polymers and heterocyclic systems compared with other force fields, and can be called in multiple modules such as Amophous Cell, Force Plus, Sorption, etc., applicable to the constructed coal molecular adsorption simulation in this article (Lin and MacKerell 2019). Therefore, in all the optimization processes below, Forcefield in Energy always uses the Compass II force field.
The geometric model of the built coal molecule was geometrically optimized to make the energy of the model reach a stable state, and the optimized molecular configuration was reversed and distorted. The optimized model was put into a cube box of the simulation system through the Amorphous Cell module, and set the density was set to 0.90.
In the process of model construction, to prevent fused aromatic hydrocarbons and other functional groups from overlapping, a low-density 3D model was first constructed, and then a series of compressions, decompressions and optimizations was performed. The details are as follows.
-
(1)
NVT optimization: The NVT system was used to keep the system temperature constant. The virtual thermal bath kept the kinetic energy of the system fixed. The atomic velocity was used to stabilize the system. The Temperature was set to 298 K, the time step to 1 fs, and the total simulation time to 100 ps.
-
(2)
NPT optimization: The NPT system was used to compress and decompress the system until the density remained the same. The set pressure was 0.1 MPa.
-
(3)
NVT optimization: The NVT system was adopted again to make the system stable under the new density to meet the actual density of bituminous coal;
-
(4)
The geometrically optimized low energy model was obtained.
At normal temperature, the pressure is proportional to the amount of substance. Therefore, the gas pressure ratio is the gas concentration ratio. In combination with subsequent experiments, the two gases were selected to have a total of 351 kPa, and the proportions were 90% N2, 10% CO2 to 100% N2, and 0 CO2. Eleven groups of gas ratios were used for adsorption simulation.
The Sorption module (Meng et al. 2018) and Compass II force field were used to mix coal molecules at 3.51 kPa – 35.1 kPa CO2 and 347.49 kPa – 315.9 kPa N2, respectively, so that 351 kPa mixed gas could physically adsorb the gas under the condition of fixed pressure. During the simulation process, multiple group simulations could be run simultaneously until all simulations were complete. The parameter settings are shown in Table 2.
Experimental method
Since the reaction reference tank for this experiment is small, the pressure in the tank must change after coal adsorption. Therefore, the adsorption volume in this experiment was calculated using the direct calculation method and the gas chromatography method. The direct observation method refers to directly observing the indication on the pressure gauge of the system before and after the adsorption, and the calculation is based on the pressure drop. The gas chromatography method refers to using the gas sample just filled in the reference tank and the gas sample obtained after the adsorption is completed. The content of each component is measured by a gas chromatograph.
The experimental device consists of a vacuum pumping system, an adsorption system, a high-pressure gas cylinder, and a data acquisition system. The vacuum pumping system includes a vacuum pump and a vacuum pressure gauge. The adsorption system is an independent experimental coal adsorption system. A schematic diagram of the device is shown in Fig. 1. The device is composed mainly of a gas sample tank, reference tank, pressure gauge and hose.
The experimental process is shown in Fig. 2. First, the amount of gas filled into the connected experimental system was used to read the pressure indicator after the system was stable. The pressure indicator was read after 12 h. If the pressure indicator does not change, it indicates that the system has good tightness, and the experiment can be carried out. Second, the coal was ground into coal powder with a particle size of less than 0.250 mm, and the coal powder was put into a vacuum drying oven for 12 h. Then, 100 g coal powder was placed in the reaction tank, and all valves were closed and evacuated. The gas tank was flushed with CO2/N2 gas in different proportions, the cylinder was closed, the valves of the reaction tank were opened, the number of pressure gauges was counted when the system was stable, and the exhaust gas was detected using a gas chromatograph. The pressure indicator was read again, and the exhaust was checked after 24 h of adsorption. Then, different mixed gas mixtures were used to repeat the experiment.
Results and discussion
Coal molecule construction
13C NMR analysis
The 13C nuclear magnetic resonance analysis of the Fangezhuang bituminous coal sample was performed to obtain the combination and content of carbon atoms and other atoms in the coal sample. The 13C NMR peak results are shown in Fig. 3.
With reference to related literature (Chen et al. 2017), the assignment and proportion of C atoms in coal samples were determined according to the chemical shifts and peak areas of the spectra, and the 12 nuclear magnetic resonance carbon structure parameters in the coal samples were further determined. The results are shown in Table 3.
The ratio of aromatic bridge carbon to peripheral carbon (XBP) reflects the degree of condensation of aromatic rings in the coal molecules. Its size depends on the size of the aromatic nuclei in coal samples. The calculation method is XBP = faB/(faH + faP + faS) (Wang et al. 1996). XBP = 0.22 can be obtained from the bituminous coal sample from Fangezhuang in this paper through calculation.
According to the ultimate analysis, the carbon content of coal samples is 80.17%. According to previous studies, the average number of benzene rings in the condensed aromatic ring is 2, 3, and 3–5 when the carbon content of bituminous coal is 80%, 85%, and more than 90%, respectively (Li et al. 2010). Therefore, we inferred that the condensed aromatic ring structure in the coal sample is dominated by 2 rings, supplemented by 1 ring and 3 rings. According to the XBP, the combination of aromatic structures was adjusted until the XBP reached 0.22, and the coal-like aromatic structure combination was obtained, as shown in Table 4.
XPS analysis
X-ray photoelectron spectroscopy (XPS) uses X-rays to irradiate a sample so that the inner electrons or valence electrons of an atom or molecule are excited and emitted. Using the kinetic energy of the photoelectron as the abscissa and the relative intensity (pulse/s) as the ordinate, a photoelectron spectrum can be made to obtain the composition of the test object. XPS was used to analyze the chemical valences of C, O, N and S in the coal sample. The results are shown in Fig. 4 and Table 5.
Table 4 shows that the number of carbon atoms in the aromatic structure of the coal sample is 106, and the aromaticity of the coal sample is 0.68 according to the nuclear magnetic resonance test result, so the total number of carbon atoms in the coal molecule can be calculated to be 156. The numbers of H, O, N, and S atoms are 121, 10, 3, and 1, respectively, according to the results of the ultimate analysis. Therefore, the molecular formula of the sample is C156H121N3O10S.
Construction and correction of the coal molecular structure
The molecular formula of the bituminous coal was deduced to be C156H121N3O10S according to the above tests and analysis. The connection mode of some functional groups was obtained according to the XPS results analysis. A preliminary molecular structure of the bituminous coal was constructed according to each structural unit, analysis results and parameters etc. aromaticity. However, due to the large molecular structure of coal, numerous isomers can be composed according to the different connection methods of organic matter and the positions of various functional groups. Therefore, different molecular structure models need to be optimized.
The model was applied in Chemoffice and ACD/LABs software to predict the NMR carbon spectrum, and obtain a model with higher carbon spectrum similarity to the experimental test (Pagenkopf 2005). The plane model is shown in Fig. 5. The comparison between the predicted spectrum and the actual spectrum is shown in Fig. 6.
The predicted carbon spectrum is in good agreement with the actual carbon spectrum, and the coal molecular model constructed is closer to the real situation so it can be used as the molecular model for this simulation.
Coal molecule adsorption simulation
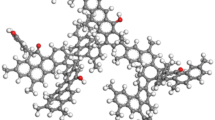
The optimized three-dimensional model is shown in Fig. 7. The optimized structure model is very compact and has a good three-dimensional structure, with aromatic carbons arranged almost in parallel.
The simulation results of the sorption module in MS software are presented in the form of the number of molecules. The results of this simulation showed how many gas molecules were adsorbed on one molecule. To have a good contrast effect, the following formula was used to convert the results in the simulation into consistent statistics.
where A represents the unit conversion results (mL/g); a represents the gas molecule number; b represents the coal molecule number; NA represents the Avogadro constant; V represents the gas volume in standard conditions; and M represents the relative molecular weight of coal.
The results of coal adsorption with different proportions after conversion are shown in Table 6.
The relationship between the CO2 partial pressure and the amount of adsorption is shown in Fig. 8 according to Table 6.
The adsorption volume of coal to CO2 is greater than N2 according to Fig. 8, because the gas adsorption by coal is essentially the interaction of gas molecules with the coal surface. Generally, the gas that is more easily liquefied adsorbs more easily. At the same time, solid adsorption of gas is essentially due to the interaction between atoms or ions on the solid surface and gas molecules. According to molecular thermodynamics and surface physical chemistry, these forces are divided into two categories: physical forces and chemical forces, which cause physical adsorption and chemical sorption, respectively. The adsorption of coal to N2 and CO2 is physical adsorption, which is caused by van der Waals forces, including electrostatic interaction, Debye induction, and London dispersion force. Polar gas molecules have a permanent dipole moment, and the polar groups on the coal surface will generate electrostatic interactions. CO2 also has a quadrupole moment, which also has a certain influence on the interaction between molecules. In the bituminous coal model, the electrostatic interaction between CO2 and heteroatom groups is stronger, so the adsorption performance of bituminous coal with CO2 is stronger. The amount of CO2 adsorbed by the coal gradually increases as the CO2 partial pressure rises, which is consistent with the Langmuir model. However, the adsorption of N2 by coal decreases with increasing CO2 partial pressure. The initial decrease is fast, but the decrease is basically unchanged afterwards, indicating that there is a competitive adsorption relationship between CO2 and N2. With an increase in CO2 pressure, the total adsorption capacity also increases in the curve in Fig. 8. The whole adsorption process is divided into a rapid decline stage, a slow growth stage and a stable stage. When the pressure reaches a certain level, the trend of gas adsorption increases slowly and eventually reaches saturation adsorption because there is a residual valence force on the surface of the coal, and adsorption occurs when gas molecules collide with an unabsorbed solid surface. With the occurrence of adsorption, the adsorption on the solid surface gradually reaches saturation, so that adsorption will not continue.
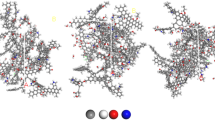
The state of atomic adsorption is shown in Fig. 9. N2 is in red and CO2 is in green in Fig. 9. N2 and CO2 adsorb at almost the same site in the coal molecular model, and these sites are all functional groups, indicating that functional groups have a great influence on the adsorption capacity of bituminous coal. The adsorption of N2 on coal molecules decreases with increasing CO2 because hydroxyl has a strong adsorption capacity for CO2, which also indicates that there is competitive adsorption between CO2 and N2, consistent with the results from the above adsorption curve.
Adsorption experiment
Direct observation method
The adsorption amount of coal on the mixed gas can be derived according to the ideal gas law. The specific derivation process is as follows:
where P1 represents gas pressure before adsorption (Pa). V1 represents the total volume of the gas before adsorption (m3). n1 represents the amount of the mixed gas before adsorption (mol). P2 represents the gas pressure after adsorption (Pa). V2 represents the total volume of the gas after adsorption (m3). n2 represents the amount of the mixed gas after adsorption (mol). T represents the system temperature (K). R represents the gas constant, which is approximately 8.314 J/(mol K). Since the temperature of 298.15 K and the reference tank volume were both constant during the experiment, V = V1 = V2.
From (2)–(3), the following equation can be derived:
The molar volume of gas is 24.5 mol/L at normal temperature and pressure, and the gas adsorption volume of coal can be expressed as
Gas chromatography method
Since the gas chromatograph has two independent chromatographic columns for measuring N2 and CO2, the test results for mixed gases need to be processed.
The calculation process of the adsorption amount according to the ideal gas law by gas chromatography is as follows:
where Pi represents the gas pressure before adsorption (Pa). Vi represents the volume of the gas before adsorption (m3). ni represents the amount of the mixed gas before adsorption (mol). Pk represents the gas total pressure in the reference tank (Pa). Vk represents the gas total volume (m3). nk represents the total amount of mixed gases (mol). T represents the system temperature (K). R represents the gas constant, which is approximately 8.314 J/(mol K).
The volume and temperature of the system remain unchanged in this experiment. Then Vi = Vk, T = 293.15 K, and the gas pressure ratio and the gas molecular ratio are the same and are expressed as
The amount of the mixed gas before adsorption can be calculated by substituting the above formula into the ideal gas law:
The amount of the mixed gas after adsorption can be obtained in the same way. Furthermore, the adsorption amount per gram of coal can be expressed as
Calculation results
The adsorption capacity calculated by the two methods is shown in Table 7.
To improve the accuracy and avoid the inaccuracy caused by methods and operation errors, the two methods are used to calculate the adsorption volume. Due to the instrument error, subjective error, accidental error and the different precision of the equipment used in the two methods, the small differences are produced in the measurement process. However, the error of the two methods is very small, which does not affect the final result. Therefore, both methods for calculating adsorption capacity are feasible, and the accuracy of the experimental results is also confirmed.
Analysis of experiment and simulation
Due to the small range of variables in the simulation, it is impossible to accurately manipulate their changes during the experiment, so the range of variables was expanded in the experiment, aiming to verify the variation trend of the simulation through experiments.
The adsorption capacity of the experiment and simulation is shown in Fig. 10. The adsorption capacity of the experiment and simulation increases gradually, as shown in Fig. 10, and the increasing rate of adsorption decreases with increasing CO2 proportion. MS software has proven to be feasible to simulate the adsorption capacity of CO2 and N2 in bituminous coal because the simulation and experiment showed the same upward trend. However, there are still some differences between the experimental adsorption amount and the simulated adsorption amount, which may be caused by the following aspects:
-
(1)
In real life, coal is a kind of macromolecular material that has some heteroatomic groups in addition to the main carbon skeleton. However, the simulation only constructs the carbon skeleton of coal, which is different from the real life, leading to the difference in adsorption amount between the experiment and the simulation. However, the carbon skeleton of the coal molecule is in a dominant position, so it has little impact on the overall result.
-
(2)
Only one molecular model was used for the adsorption simulation, but in the experiment, 100 g of pulverized coal was used, which contains a large number of coal molecules, possibly leading to the difference in the adsorption amount between the simulation and the experiment. However, these differences do not affect the overall trend the results.
-
(3)
The adsorption of gas on coal occurs mainly in the solid pores, and the simulation is only for coal molecules. However, this effect is only reflected in the difference in adsorption amount and has little influence on the overall adsorption trend.
In addition, molecular simulation and adsorption have also been studied. The molecular model of coal was constructed and the adsorption of methane to a single molecular model of coal was studied by Meng et al. (2018). Xia et al. (2020) used the graphite sheets as the hydrophobic mineral, and the force characteristics and interfacial adsorption structures of undecane (a model collector) on graphite surface with different hydrophobicities were investigated by chemical force microscopy and molecular dynamics simulations. Xiang et al. (2014) studied the adsorption characteristics of CH4/CO2/H2O to coal by molecular simulation. These studies have shown that it is feasible to study adsorption characteristics by using molecular simulation. Generally, the simulation and experiment have proven to some extent that the adsorption capacity of coal to mixed gas increases gradually with increasing of CO2 ratio. To better understand the principle behind this phenomenon, much work needs to be invested, and further studies need to be carried out in the future.
Conclusions
In this paper, a molecular model of bituminous coal was established. MS software was used to simulate and design experiments to explore the relationship between the adsorption capacity of CO2 and N2 in bituminous coal.
The molecular structures of bituminous coal are composed mainly of aromatic rings with a ring number of 2 and a lower degree of condensation. Aliphatic carbon exists mainly in the form of methyl side chains and a few naphthenes. The molecular heteroatoms in the coal include mainly O, N, and very few S. The molecular formula of the sample is C156H121N3O10S.
Simulation results showed that the adsorption rate of bituminous coal for CO2 is greater than the adsorption rate for N2, and there is a competitive adsorption relationship between them. In the mixed gas, the adsorption volume increases with increasing of CO2 concentration. In the rapid increase stage, when a small amount of CO2 is added to N2, the adsorption capacity of bituminous coal for mixed gas is greatly increased. The experiment used two methods to calculate the adsorption volume and the specific adsorption volume is the average of the results of the two methods. The coal has adsorption amounts of 0.5050 cm3/g, 0.7455 cm3/g, 0.9450 cm3/g, 1.0715 cm3/g, and 1.2000 cm3/g for pure N2, 2%, 5%, 7%, and 10% CO2, respectively. The experimental results showed the same trend as the simulation.
Combining the simulation and the experimental results, the injection of a small amount of CO2 into pure N2 will greatly improve the gas adsorption volume of coal, showing that the mixture of N2 and CO2 has a better fire prevention effect than pure N2 and is cheaper than pure CO2. Therefore, mixed inert gas has a higher economy and practicability, which is of great significance to the prevention and control of coal fires.
References
Bian et al (2019) Construction and evaluation of a medium-rank coal molecular model using a hybrid experimental-simulation-theoretical method. Energy Fuel 33(12):12905–12915
Chen L et al (2017) Modification of structural parameters of solid nuclear magnetic carbon and its application in coal structure analysis. J Fuel Chem Technol 10:1153–1163
Clarkson CR, Bustin RM (2000) Binary gas adsorption/desorption isotherms: effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int J Coal Geol 42(4):241–271
Elick JM (2011) Mapping the coal fire at Centralia, Pa using thermal infrared imagery. Int J Coal Geol 87(3-4):197–203
Hao C, Chen Y, Wang J, Deng C, Xu G, Dai F, Si R, Wang H, Wang H (2018) Study on the effect of iron-based deoxidizing inhibitors for coal spontaneous combustion prevention. Energies 11(4):789
Lei B, He B, Xiao B, du P, Wu B (2020) Comparative study of single inert gas in confined space inhibiting open flame coal combustion. Fuel 265:116976
Lin F, MacKerell ADJ (2019) Force fields for small molecules. Methods Molec Biol (Clifton, NJ) 2022:21–54
Li P, Ma D, Zhang J, Huo Z (2020) Wettability modification and its influence on methane adsorption/desorption: a case study in the Ordos Basin, China. Energy Sci Eng 8(3):804–816
Li X et al (2010) Influence of tectonic deformation on the macromolecular structure of coal rocks: a case study of tectonic coal vitrinite separation. J China Coal Soc S1:150–157
Li Z, Liu D, Cai Y, Wang Y, Teng J (2019) Adsorption pore structure and its fractal characteristics of coals by N2 adsorption/desorption and FESEM image analyses. Fuel 257:116031
Lu X et al (2019) Research on a noble extinguish material for the underground fire prevention. Fire Mater 44(2):230–241
Meng J, Zhong R, Li S, Yin F, Nie B (2018) Molecular model construction and study of gas adsorption of Zhaozhuang coal. Energy Fuel 32(9):9727–9737
Meng J, Li S, Niu J (2019) Crystallite structure characteristics and its influence on methane adsorption for different rank coals. ACS OMEGA 4(24):20762–20772
Mastalerz M, Gluskoter H, Rupp J (2004) Carbon dioxide and methane sorption in high volatile bituminous coals from Indiana, USA. Int J Coal Geol 60(1):43–55
Mohalik NK et al (2005) Application of nitrogen as preventive and controlling subsurface fire - Indian context. J Sci Ind Res India 64(4):273–280
Ottiger S, Pini R, Storti G, Mazzotti M, Bencini R, Quattrocchi F, Sardu G, Deriu G (2006) Adsorption of pure carbon dioxide and methane on dry coal from the Sulcis Coal Province (SW Sardinia, Italy). Environ Prog 25(4):355–364
Pagenkopf B (2005) ACD/HNMR predictor and ACD/CNMR predictor. J Am Chem Soc 127(9):3232
Qie Z, Zhang Z, Sun F, Wang L, Pi X, Gao J, Zhao G (2019) Effect of pore hierarchy and pore size on the combined adsorption of SO2 and toluene in activated coke. Fuel 257:116090
Shi B, Zhou G, Ma L (2018) Normalizing fire prevention technology and a ground fixed station for underground mine fires using liquid nitrogen: a case study. Fire Technol 54(6):1887–1893
Song Z, Huang X, Jiang J, Pan X (2020) A laboratory approach to CO2 and CO emission factors from underground coal fires. Int J Coal Geol 219:103382
Song Z, Kuenzer C (2014) Coal fires in China over the last decade: a comprehensive review. Int J Aust Coal Geol 133:72–99
Sun Y et al (2019) Impact of coal composition and pore structure on gas adsorption: a study based on a synchrotron radiation facility. Greenhouse Gases: Sci Technol 10(1):116–129
Wang L et al (1996) Study on the structure of high sulfur coal in China by solid state NMR and electron spectroscopy. J Fuel Chem Technol 06:539–543
Wang X et al (2019a) Molecular structure of kerogen in the Longmaxi Shale: insights from solid state NMR, FT-IR, XRD and HRTEM. Acta Geol Sin-Engl 93(4):1015–1024
Wang Z et al (2019b) The influence of temperature on methane adsorption in coal: a review and statistical analysis. Greenhouse Gases: Sci Technol 10(1):116–129
Xia Y, Xing Y, Li M, Liu M, Tan J, Cao Y, Gui X (2020) Studying interactions between undecane and graphite surfaces by chemical force microscopy and molecular dynamics simulations. Fuel 269:117367
Xiang et al (2014) Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci China Earth Sci 57(8):1749–1759
You J, Tian L, Zhang C, Yao H, Dou W, Fan B, Hu S (2016) Adsorption behavior of carbon dioxide and methane in bituminous coal: a molecular simulation study. Chin J Chem Eng 24(9):1275–1282
Zhang Y, Lei B, Wu B, Meng Y, He B (2019) An experimental study on the heat and mass transfer of liquid nitrogen in a loose medium. Energies 12(18):3464
Zhao T, Yang S, Hu X, Song W, Cai J, Xu Q (2020) Restraining effect of nitrogen on coal oxidation in different stages: non-isothermal TG-DSC and EPR research. Int J Min Sci Technol 30(3):387–395
Zhou FB, Ren W, Wang D, Song T, Li X, Zhang Y (2006) Application of three-phase foam to fight an extraordinarily serious coal mine fire. Int J Coal Geol 67(1-2):95–100
Zhu H, Zhao HR, Wei HY, Wang W, Wang HR, Li K, Lu XX, Tan B (2020) Investigation into the thermal behavior and FTIR micro-characteristics of re-oxidation coal. Combust Flame 216:354–368
Acknowledgments
The financial and infrastructure support of the China University of Mining and Technology (Beijing) Research Program of the School of Emergency Management and Safety Engineering is gratefully acknowledged.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51774290).
Author information
Authors and Affiliations
Contributions
Hongqing Zhu: funding acquisition, supervision, and methodology
Song Guo: wrote this paper, conducted the simulation research, carried out the experiments, and processed the experimental data
Yuyi Xie: built the model and conducted the simulation research
Hongru Zhao: conceived the conceptualization and carried out the experiments
Corresponding authors
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
Not applicable
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, H., Guo, S., Xie, Y. et al. Molecular simulation and experimental studies on CO2 and N2 adsorption to bituminous coal. Environ Sci Pollut Res 28, 15673–15686 (2021). https://doi.org/10.1007/s11356-020-11722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11722-y