Abstract
This study aimed at investigating the photochemical behavior of sulfa drugs containing five and six-membered heterocyclic substituents (sulfamethoxazole (SMX) and sulfadiazine (SDZ), respectively), in an aqueous medium. Despite their importance, studies devoted to the use of photochemical models to predict the environmental phototransformation of pollutants in surface waters, by combining laboratory results and natural aquatic systems parameters, are still scarce in the scientific literature. In this work, the second-order reaction rate constants of SDZ and SMX with hydroxyl radicals (●OH), singlet oxygen (1O2), and triplet excited states of chromophoric dissolved organic matter (3CDOM*) were experimentally determined at pH 7, using the competition kinetics approach. The results show that ●OH and 3CDOM* are the key species involved in sulfonamide degradation, with anionic SMX, most prevalent at pH 6–9, being degraded much slower than the anionic form of SDZ. Moreover, SDZ and SMX photodegradation in natural water samples (spring-fed natural pond, public supply reservoir, and sea water) was significantly enhanced relative to depletion in pure water. Finally, from mathematical simulations of the sunlight-driven sulfonamide degradation, half-life times were predicted for these drugs varying from less than 2 to about 90 days, depending on the water depth, concentration of key species (DOC, HCO3−, NO2−, CO32−) in natural aqueous systems, as well as on the particular heterocyclic substituent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfonamide antibiotics are widely used in aquaculture, for veterinary purposes in the treatment and prevention of infectious diseases caused by bacteria, and for treating respiratory and urinary tract infections in humans (Boreen et al. 2004). Members of this class of pharmaceuticals contain an identical backbone structure, only differing in the N-bound substituent of the sulfonamide linkage. These antibiotics and their metabolites are not completely eliminated by conventional wastewater treatments; thus, they are frequently detected in various aqueous environments (Kolpin et al. 2002). The environmental persistence of sulfonamides is still unknown. Consequently, understanding the environmental fate of these antibiotics is important due to the possibility of increased bacterial resistance or other adverse effects on aquatic ecosystems.
In general, antibiotics may be transformed through direct photodegradation or through sensitized photoprocesses, including the reactions with singlet oxygen (1O2), hydroxyl radicals (●OH), and triplet excited states of chromophoric dissolved organic matter (3CDOM*), besides other reactive species formed in sunlit natural waters (Boreen et al. 2005). According to Vione (2020), these reactive photo-induced species (RPS) are mostly scavenged/quenched by natural water components. Reactions 1–11 summarize the main formation and scavenging processes involving RPS (Parizi et al. 2019; Vione 2020).
The photochemical behavior of model pollutants has been mainly focused on direct photolysis, while information regarding sensitized photoprocesses, including reactions with 1O2, ●OH, and 3CDOM*, are markedly more limited. In addition, the aqueous photochemical persistence of antibiotics from the same family, but with different structures, has not been much addressed in the literature. Similarly, the comparison of antibiotics photodegradation in natural water samples and pure water, to assess the importance of direct versus indirect photolysis processes, has received little attention. Finally, studies concerning the use of photochemical models to predict the environmental phototransformation of pollutants in surface waters, by combining laboratory results and natural aquatic systems parameters are still scarce in the scientific literature (Vione 2020).
On this basis, this study is aimed at investigating the aqueous phase photochemical behavior of the sulfonamide antibiotics sulfadiazine (SDZ) and sulfamethoxazole (SMX), selected as model pollutants of emerging concern, under simulated solar radiation. Thereby, the second-order reaction rate constants of these antibiotics with 1O2, ●OH, and 3CDOM* were experimentally determined at pH 7. Our experimental results and mathematical simulations were used for predicting sulfonamide persistence in sunlit natural waters and for discussing the conditions that favor or hinder SDZ and SMX photodegradation. Finally, the sunlight-driven photodegradation of SDZ and SMX in the presence of dissolved organic matter (DOM) and in different real aqueous matrices (spring-fed natural pond, public supply reservoir, and sea water) were investigated.
Materials and methods
Reagents
SDZ (4-amino-N-pyrimidine-2-ylbenzenesulfonamide, C10H10N4O2S) and SMX (4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide, C10H11N3O3S) were employed as model sulfonamide antibiotics of emerging concern. All the solutions were prepared using Milli-Q® water (18.2 MΩ cm), obtained from a Milli-Q® Direct-Q system (Merck Millipore). Solutions pH was adjusted to the initial desired value but not corrected overreaction time, due to the peculiarities of the experimental procedure. The reagents used in this study are detailed in Online Resource 1.
The urban-waste bio-organic substance (UW-BOS), identified by the acronym CVT230 (Arques and Bianco Prevot 2015), was isolated from home gardening and park trimming residue piles aerated for 230 days, according to the procedure previously reported by Montoneri et al. (2011). Stock solutions of the different types of organic matter (Suwannee River Natural Organic Matter-SRNOM; Aldrich humic acid sodium salt-AHA; and Urban-waste bio-organic substance-UW-BOS; see Online Resource 1) were prepared in a phosphate buffer solution (pH 7.1).
Photodegradation experiments under simulated sunlight
All photodegradation experiments were performed using a Newport solar simulator (Model 91160) equipped with a 450-W Xenon lamp and an air mass 1.5 global filter. The samples were stored in 2-mL Pyrex vials of 10-mm irradiated path length with no head space, placed in a water bath kept at 25 °C, positioned at 15 cm of the radiation source. The total irradiance provided by the solar simulator at this distance, measured by a spectroradiometer (Luzchem Research, SPR-02 model), was equal to 68 W m−2 in the wavelength range 290–800 nm. The experiments were performed in duplicates.
Kinetic study
The second-order kinetic rate constants between the sulfonamides (SDZ, SMX) and reactive photo-induced species (RPS), namely singlet oxygen (1O2), hydroxyl radicals (●OH), and triplet excited states of chromophoric dissolved organic matter (3CDOM*), were determined by the competition kinetics method (Shemer et al. 2006). Briefly, according to this method, the antibiotic (SDZ or SMX) competes for RPS (1O2, ●OH or 3CDOM*) with a reference compound (FFA, pCBA, or TMP, respectively), whose reactivity toward the RPS is known.
In this study, H2O2 (50 mmol L−1), methylene blue (31.3 μmol L−1), and anthraquinone-2-sulfonate (AQ2S) (30.5 μmol L−1) were used as the sources of ●OH, 1O2, and 3CDOM*, respectively. It is worth observing that due to the complex structure of CDOM, the nature of 3CDOM* is poorly known; for that reason, proxy molecules, such as AQ2S have been used to model the photoreactivity of CDOM (Marchetti et al. 2013; De Laurentiis et al. 2014). para-Chlorobenzoic acid (pCBA) (63.9 μmol L−1), furfuryl alcohol (FFA) (40.8 μmol L−1), and 2,4,6 trimethylphenol (TMP) (36.7 μmol L−1) were used as reference compounds for ●OH, 1O2, and 3CDOM*, respectively. When required, methanol (0.1 mol L−1) was added to quench ●OH radicals. All competition kinetic experiments were performed in duplicates. More details about the experimental conditions and the optimized concentrations can be found elsewhere (Lastre-Acosta et al. 2019; Parizi et al. 2019).
Analytical methods
The time evolution of SDZ, SMX, pCBA, FFA, and TMP concentrations were analyzed by high-performance liquid chromatography (HPLC), using a Shimadzu (LC20 model) equipped with a UV/Vis diode array detector (SPD20A model). The column was a Wakosil® C18 (250 mm × 4.6 mm; 5 μm), acquired from SGE. Temperature, injected volume, and mobile phase flow rate were 40 °C, 100 μL, and 1 mL min−1, respectively. Isocratic elution was used in all cases. The conditions used, along with the limits of detection (LOD) and quantification (LOQ) of each compound, are presented in Online Resource 2.
Real aqueous matrices
Additional experiments were carried out using different natural water samples: sea water, reservoir water for public supply, and surface water. Sea water was collected in Guarujá (SP, Brazil). Water for public supply was sampled at Guarapiranga Reservoir, the second largest source of water supply in the metropolitan region of São Paulo (MRSP). Finally, surface water was obtained from a spring-fed natural pond (Guarulhos, SP, Brazil). The characteristics of these water samples are shown in Online Resource 3.
Simulations using the APEX model
The photochemical environmental fate of the studied sulfonamides was simulated using the APEX model (Aqueous Photochemistry of Environmentally Occurring Xenobiotics), developed at the Department of Chemistry of the University of Torino (Italy) (Bodrato and Vione 2014). In brief, this photochemical model considers the pollutant photolysis quantum yield under sunlight, the experimental second-order reaction rate constants with RPS, the chemical composition of water, and irradiation depth to calculate pollutants half-life times. Further details regarding the model equations can be found at http://chimica.campusnet.unito.it/do/didattica.pl/Show?_id=4pyh. In our study, literature data for Brazilian surface waters were used in simulations (Silva et al. 2015), to define the range of environment-dependent concentrations: dissolved organic carbon (DOC = 0.5–10 mg L−1), nitrate ([NO3−] = 1.0–1.0 × 102 μmol L−1), nitrite ([NO2−] = 5.0 × 10−2–15 μmol L−1), and bicarbonate ([HCO3−] = 2.0 × 102–2.0 × 103 μmol L−1); the water depth was varied in the range 0.5–5 m, and simulations were carried out at pH 7.
Results and discussion
Hydrolysis of sulfonamides
Control experiments showed that no losses of SDZ ([SDZ]0 = 39.02 ± 0.02 μmol L−1) and SMX ([SMX]0 = 45.11 ± 0.25 μmol L−1) occurred due to hydrolysis at pH 7 in Milli-Q® water, which is in agreement with previous studies (Batista et al. 2016).
Direct photolysis of sulfonamides
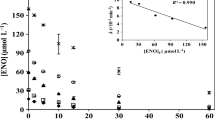
Figure 1 and Table 1 show the results for the solar-driven photolytic degradation of the antibiotics in Milli-Q® water at pH 7.
Sulfonamides decayed with specific photolysis rates of (8.06 ± 0.62) × 10−6 s−1 (R2 = 0.987) and (1.95 ± 0.34) × 10−6 s−1 (R2 = 0.955) for SDZ and SMX, respectively. In general, sulfonamides exist as different dissociated species, depending on the pH of the aqueous medium. Thereby, at the pH range 6–9 of natural waters, both SDZ and SMX are mostly in their ionized, negatively charged form. As observed in Fig. 1, the anionic form of SMX was degraded much more slowly at pH 7 compared to the anionic form of SDZ.
In our study, some differences in the direct photolysis of SDZ and SMX at pH 7 were observed, in spite of their structural similarities. According to Perisa et al. (2013), these differences are due to the heterocyclic group: sulfonamides with six-membered heterocyclic substituents (SDZ) contain a pyrimidine ring which is less stable under irradiation under basic conditions (pH 8) than the isoxazole ring present in sulfonamides containing five-membered heterocyclic substituents (SMX). According to these authors, the isoxazole derivative (SMX) showed less photostability under acidic conditions (pH 4). These findings are in good agreement with those of Boreen et al. (2005), who found a more pronounced direct photolysis of the anionic form of sulfa drugs containing six-membered heterocyclic substituents, such as SDZ. Other authors have observed similar behaviors and reported that sulfonamides containing six-membered heterocyclic groups degrade more quickly at pH 8 than at pH 4 (Perisa et al. 2013).
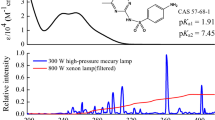
Figure 2 shows the overlap of aqueous SDZ and SMX absorption spectra, along with the spectral irradiance of the solar simulator. As observed, the sulfonamides exhibit low molar absorption coefficients above 290 nm (from which the solar simulator effectively emits). In general, our results showed little SDZ and SMX degradation when the antibiotics were spiked into Milli-Q® water; the percent removals after 12 h were (28.2 ± 1.5) % and (7.9 ± 1.9) % for SDZ and SMX, respectively (Fig. 1), which are a consequence of the low direct photolysis quantum yields depicted in Table 1 for these sulfonamides.
From these data, direct photolysis quantum yields (Φ) were calculated following the approach given by Schwarzenbach (2003), giving (1.96 ± 0.15) × 10−3 and (2.12 ± 0.37) × 10−3 mol Einstein−1, for SDZ and SMX, respectively (Table 1). Our values are in good agreement with the direct photolysis quantum yields measured under natural sunlight by Boreen et al. (2005). The authors obtained Φ ranging from 0.01 × 10−3 (neutral form of sulfadimethoxine) to 5 × 10−3 (anionic form of sulfamethazine) for sulfa drugs containing six-membered heterocyclic substituents. In another study, Boreen et al. (2004) determined the photolysis quantum yields of five sulfa drugs with varying five-membered heterocyclic substituents; the values of Φ obtained ranged from < 0.005 for the neutral state of sulfamethizole to 0.7 ± 0.3 for the protonated state of sulfisoxazole.
Sulfonamide degradation by reactive photo-induced species
Table 2 shows the second-order kinetic rate constants of the sulfonamides with reactive photo-induced species (●OH, 1O2, 3AQ2S*), obtained through competition kinetics experiments carried out in Milli-Q® water at pH 7. The values of ksulfonamide,1O2 and ksulfonamide,●OH are consistent with previous values for this class of antibiotics (Boreen et al. 2004, 2005; Ge et al. 2019). Boreen et al. (2005) quantified the bimolecular rate constant for the reactions between different sulfa drugs and ●OH using the Fenton’s reagent with acetophenone as a reference compound, through the competition kinetics approach. For SDZ, the authors reported 3.7 ± 0.5 × 109 L mol−1 s−1. On the other hand, the rate constant for the neutral form of SMX with ●OH was 5.8 ± 0.2 × 109 L mol−1 s−1 (Boreen et al. 2004), measured with the Fenton’s reagent at pH 3. In another study, Ge et al. (2019) determined the bimolecular reaction rate constants of nine sulfonamides (sulfamethoxazole, sulfisoxazole, sulfamethizole, sulfathiazole, sulfamethazine, sulfamerazine, sulfadiazine, sulfachlorpyridazine, and sulfadimethoxine) with ●OH radicals, using a merry-go-round reactor irradiated by a 500-W high-pressure mercury vapor lamp. The authors generated hydroxyl radicals through the addition of H2O2 (100 mmol L−1) and acetophenone (10 μmol L−1) was used as the reference compound. The values of k ranged from (5.00 ± 0.77) × 109 L mol−1 s−1 to (9.16 ± 1.66) × 109 L mol−1 s−1 for sulfamerazine and sulfamethizole, respectively. The high reactivity of sulfa drugs with hydroxyl radicals is typical for ●OH-driven reaction with most substrates, given the low selectivity and high reactivity of these radicals.
Regarding the rate constants of the reactions with 1O2 in an aqueous medium for this class of antibiotics, the k values reported in the literature range over four orders of magnitude (104–108 L mol−1 s−1) (Boreen et al. 2004, 2005). According to the authors, this is due to the different structures of the sulfonamides, with the heterocyclic substituent being the likely site of reaction with singlet oxygen. In our study, the values obtained for ksulfonamide,1O2 were (6.22 ± 0.52) × 106 and (1.34 ± 0.34) × 106 L mol−1 s−1 for SDZ and SMX, respectively.
The values obtained in our study for ksulfonamide,3CDOM* are close to those determined for another antibiotic (Lastre-Acosta et al. 2019), using 3AQ2S* as a 3CDOM* proxy. On the other hand, Li et al. (2015) reported the second-order quenching constants of SDZ with the triplet state of 4-carboxybenzophenone (3CBBP*), as representative proxy for 3CDOM*. The k values found by these authors through laser flash photolysis (LFP) were 4.9 × 109 and 2.9 × 109 L mol−1 s−1 at pH 4 and 9, respectively. The values of these rate constants are high in comparison with our results, but according to Bianco et al. (2015), laser flash photolysis–derived quenching rate constants are upper limits for the actual reaction rate constants between triplet states and substrates.
To the best of our knowledge, the second-order reaction rate constants between the sulfonamides SDZ and SMX with 3CDOM* at pH 7, using anthraquinone-2-sulfonate (AQ2S) as CDOM proxy, are reported for the first time in the present study. Nevertheless, it is worth observing that the reactivity of organic pollutants with triplet excited states is greatly affected by the triplet nature. For example, Vione et al. (2011) used AQ2S and riboflavin (Ri) as CDOM proxies, for which they reported reaction rate constants with ibuprofen (IBP) at pH 8 of kIBP,3AQ2S* = (9.7 ± 0. 2) × 109 L mol−1 s−1 and kIBP,3Ri* = (4.5 ± 0.4) × 107 L mol−1 s−1, the latter being two orders of magnitude lower. While AQ2S was one of the first proxy molecules used to model the photoreactivity of CDOM, 3AQ2S* has a higher one-electron reduction potential (E° 3AQ2S*/AQ2S•− = 2.6 V) than typical 3CDOM* (1.4–1.9 V) and, therefore, the triplet state 3AQ2S* is more reactive than 3CDOM* (Minella et al. 2018). Thus, the values obtained in our study for ksulfonamide,3CDOM* are upper limits for the actual reaction rate constants between SDZ and SMX with 3CDOM*. Worthy of mention is that 4-carboxybenzophenone (CBBP) has been suggested as a proxy for CDOM instead of AQ2S, since the one-electron reduction potential of 3CBBP* (E° 3CBBP*/CBBP•− = 1.8 V) is in the range of the 3CDOM* reduction potentials. Consequently, 3CBBP* has more similar reactivity as average 3CDOM* compared to 3AQ2S* (Carena et al. 2019).
As observed in the present study, the reactions with ●OH and 3CDOM* should play a more important role in sulfonamide degradation in comparison with 1O2, since ksulfonamide,1O2 is much lower than ksulfonamide,●OH and kantibiotic,3CDOM*. According to Vione et al. (2018), direct photolysis and the reaction with ●OH can be important transformation pathways of both neutral (HSDZ) and anionic (SDZ−) forms of SDZ, in sunlit surface waters with low dissolved organic carbon contents (DOC < 1 mg L−1). At high DOC (DOC > 3–4 mg L−1), the main pathways involved in SDZ photodegradation in water would be the direct photolysis for SDZ− and the reaction with 3CDOM* for HSDZ (Vione et al. 2018).
Sulfonamide photodegradation in the presence of dissolved organic matter
SDZ and SMX photodegradation was studied at pH 7 in the presence of 10 mg L−1 of different types of organic matter (Table 3). The experiments were performed using the solar simulator and the solutions were irradiated over 9 h. In general, dissolved organic matter (DOM) can present photosensitizing or inhibitory effects; i.e., DOM can improve contaminants photodegradation through the formation of reactive photo-induced species (●OH, 1O2, 3CDOM*), or may also retard their removal due to inner-filter effect and/or reactive species scavenging/quenching.
For SDZ, the addition of CVT230 and AHA had a beneficial effect on sulfonamide photodegradation. In contrast, for SMX, the presence of CVT230 and SRNOM decreased pollutant removal, probably due to sunlight screening and reactive species scavenging. Oliveira et al. (2019) observed that SMX photodegradation under simulated solar radiation in ultrapure water ([SMX]0 = 100 μg L−1) was more pronounced than in the presence of humic substances at a concentration of 20 mg L−1 (humic acids, fulvic acids, and XAD-4) at both pH evaluated (5.0 and 7.3). The authors concluded that the inhibitory effect of humic substances upon SMX photodegradation is higher than their photosensitizing capacity.
In our study, for both antibiotics, the photodegradation efficiency was more prominent in the presence of AHA. Batista et al. (2016) correlated the degradation rate constants of the sulfonamide sulfamerazine (SMR) with the chemical and spectroscopic characteristics of organic matter of different origins (Suwannee River natural organic matter (SRNOM), Suwannee River humic acid (SRHA), Suwannee River fulvic acid (SRFA), and Aldrich humic acid (AHA)) and reported that the efficiency of SMR degradation was highest in AHA-containing solutions. According to the authors, AHA exhibited relatively high steady-state concentrations of 3NOM*, besides high total fluorescence intensity, SUVA254 and aromatic content in comparison with the other natural organic matters investigated.
Sulfonamide photodegradation in natural waters
SDZ and SMX solutions were prepared in different natural water samples and irradiated over 12 h under simulated solar radiation. The results detailed in Online Resource 4 and Fig. 3 indicate that the degradation of the sulfa drugs was enhanced in real water matrices in comparison with that observed in Milli-Q® water.
Time evolution of SDZ ([SDZ]0 = 42.90 ± 4.30) and SMX ([SMX]0 = 41.00 ± 3.20 μmol L−1) concentrations during photodegradation experiments in different natural water samples. White circle represents Milli-Q® water; black square represents spring-fed natural pond (Guarulhos); white triangle represents public supply reservoir (Guarapiranga); white diamond represents sea water (Guarujá). All locations in SP, Brazil. For the characteristics of each sample, see Online Resource 3
These results suggest the occurrence of additional indirect photochemical processes in these aqueous media. For SDZ, this effect is more noticeable, which is probably due to the higher values of kSDZ,1O2 and kSDZ,3CDOM*. Also, higher photodegradation rate constants were observed for SDZ at neutral pH in Milli-Q® water, while the values of kSDZ increased with increasing pH of the natural water samples, namely 7.5 (Guarujá), 6.8 (Guarapiranga), and 5.8 (Guarulhos). For SMX, an increase in the pH of the natural waters contributed to a decrease in the values of kSMX. A similar behavior was reported by Trovo et al. (2009), who observed that SMX photodegradation ([SMX]0 = 10 mg L−1) under simulated sunlight was faster in distilled water (pH 4.8) than in seawater (pH 8.1). Nevertheless, natural water constituents can also play an important role in sulfonamide photodegradation.
Analogously, Boreen et al. (2005) observed that the photodegradation of sulfa drugs containing six-membered heterocyclic substituents in a natural water sample was significantly enhanced relative to degradation in deionized water. For sulfa drugs with five-membered heterocyclic groups, photodegradation in natural water samples from two lakes was attributed exclusively to direct photolysis (Boreen et al. 2004). Recently, Oliveira et al. (2019) reported a decrease in SMX photodegradation rate ([SMX]0 = 100 μg L−1) in environmental water samples (estuarine water, riverine water and sewage treatment plant effluent), when compared with ultrapure water.
Simulations of antibiotics photochemical fate
The predicted half-life times (t1/2) of SDZ and SMX as a function of water depth, DOC, [NO2−], and [HCO3−], under summertime irradiation conditions at pH 7, was obtained using the APEX model. The results are shown in Table 4. According to the mathematical simulations, the values of t1/2 vary from 1 to 22 days for SDZ and from 4 to 90 days for SMX.
Despite nitrate and bicarbonate being related to the generation and scavenging of hydroxyl radicals, their impact on the antibiotic persistence is small. In fact, DOC and water depth are more important in SDZ and SMX photodegradation compared to NO3− and HCO3−. Regarding SDZ, t1/2 increases only 2.6% and 0.2% for the minimum and maximum DOC levels, respectively, for a 10-fold increase in HCO3− concentration. In the case of SMX, similar trends were observed, with SMX half-life times increasing 15.1% and 1.2% for the minimum and maximum DOC levels, respectively. On the other hand, a 100-fold increase in nitrate concentration contributes to a 0.94% and 0.48% decrease in the t1/2 of SDZ, for the minimum and maximum depth levels, respectively. For the same increase in [NO3−], APEX simulations indicated a slight decrease in SMX half-life time at any depth level. In the case of nitrite, the impact of a 300-fold increase in [NO2−] is small for sulfadiazine, with SDZ half-life times decreasing 4.9% and 2.4% for the minimum and maximum depth levels. In contrast, for SMX, the half-life decreases 42.1% and 23.6% for the minimum and maximum depth levels, respectively.
As expected, DOC and water depth have the highest impacts on sulfonamide half-life times, with faster degradation in shallow water bodies and/or at low DOC levels (Fig. 4). Thereby, deep water bodies and those poorly lit by sunlight contribute to an increase in sulfonamide persistence. In such a scenario, lower ●OH formation rates are expected as a consequence. On the other hand, sulfonamide half-life times increase with increasing DOC concentration, i.e., higher DOM and CDOM contents. While hydroxyl radicals can be generated by CDOM, DOM can act as an important scavenger of these radicals (Fabbri et al. 2015; Passananti et al. 2014). In addition, CDOM can compete with the substrate for sunlight photons, affecting the direct photolysis of the pollutant.
High organic matter content affects contaminants degradation as discussed, but can also enhance back-reduction processes (Vione et al. 2018). In this case, the reactions between the antibiotics with 3CDOM* yields, e.g., SDZ•+ (and SMX•+) and CDOM•−, then the antioxidant moieties of DOM (mostly phenolic) may reduce SDZ•+ (and SMX•+) back to the parent sulfonamides, while CDOM•− reacts with oxygen to give O2•−. As a consequence, SDZ and SMX degradation mediated by 3CDOM* may be slowed down (Wenk et al. 2011; Vione et al. 2018), and their reactivity with 3CDOM* estimated in the present study should be viewed as an upper limit. Nonetheless, as pointed out by Vione et al. (2018), 3CDOM* takes part in key photodegradation reactions of many xenobiotics in high-DOC waters, despite the back-reduction phenomenon.
Finally, as clearly observed in Table 4 and Fig. 4, the trends for both SDZ and SMX are quite similar, although higher values of t1/2 are observed in the case of SMX. Therefore, the photochemical fate of these sulfonamides depends on the water depth, composition of key species (DOC, HCO3−, NO2−, CO32−) in natural aqueous systems, and the heterocyclic substituent of these antibiotics. In particular, SDZ and SMX photodegradation kinetics depends strongly on the DOC levels.
Conclusions
The photochemical behavior of sulfamethoxazole and sulfadiazine, two sulfonamide antibiotics containing five- and six-membered heterocyclic substituents respectively, was investigated in an aqueous medium. In spite of the structural similarities of these sulfonamides, the anionic form of SMX, most prevalent at pH 6–9, is degraded much more slowly than the anionic form of SDZ. The different behavior regarding direct photolysis at pH 7 is related to the heterocyclic group: SDZ contains a pyrimidine ring which is less stable under irradiation in a basic medium in comparison with the isoxazole ring present in SMX. According to our study, the prevailing pathways involved in the sunlight-driven degradation of the studied sulfonamides are the reactions with ●OH and 3CDOM*. In fact, SDZ and SMX photodegradation in natural water samples (spring-fed natural pond, public supply reservoir, and sea water) was significantly enhanced relative to degradation in Milli-Q® water. For SDZ, the highest photodegrdation rate constant was observed in sea water, while SMX photodegradation was faster in the water sampled from the spring-fed natural pond.
Finally, mathematical simulations indicate that SDZ and SMX half-life times can vary from less than 2 days to about 90 days, depending on the range of environmental conditions, and the antibiotics persistence is favored in shallow, nitrite-rich, and DOM-poor environments. The trends for both SDZ and SMX are quite similar, although higher values of t1/2 are expected for SMX. Structural dissimilarities, mainly related to the heterocyclic substituents, are thus able to influence the environmental photochemical behavior of these sulfonamides.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Arques A, Bianco Prevot A (2015) Soluble bio-based substances isolated from urban wastes: environmental applications. Springer International Publishing
Batista APS, Teixeira A, Cooper WJ, Cottrell BA (2016) Correlating the chemical and spectroscopic characteristics of natural organic matter with the photodegradation of sulfamerazine. Water Res 93:20–29
Bianco A, Fabbri D, Minella M, Brigante M, Mailhot G, Maurino V, Minero C, Vione D (2015) New insights into the environmental photochemistry of 5-chloro-2-(2,4-dichlorophenoxy)phenol (triclosan): reconsidering the importance of indirect photoreactions. Water Res 72:271–280
Bodrato M, Vione D (2014) APEX (aqueous photochemistry of environmentally occurring xenobiotics): a free software tool to predict the kinetics of photochemical processes in surface waters. Environ Sci-Proc Imp 16:732–740
Boreen AL, Arnold WA, McNeill K (2004) Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ Sci Technol 38:3933–3940
Boreen AL, Arnold WA, McNeill K (2005) Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: identification of an SO2 extrusion photoproduct. Environ Sci Technol 39:3630–3638
Carena L, Puscasu CG, Comis S, Sarakha M, Vione D (2019) Environmental photodegradation of emerging contaminants: a reexamination of the importance of triplet-sensitised processes, based on the use of 4-carboxybenzophenone as proxy for the chromophoric dissolved organic matter. Chemosphere 237:124476
De Laurentiis E, Prasse C, Ternes TA, Minella M, Maurino V, Minero C, Sarakha M, Brigante M, Vione D (2014) Assessing the photochemical transformation pathways of acetaminophen relevant to surface waters: transformation kinetics, intermediates, and modelling. Water Res 53:235–248
Fabbri D, Minella M, Maurino V, Minero C, Vione D (2015) A model assessment of the importance of direct photolysis in the photo-fate of cephalosporins in surface waters: possible formation of toxic intermediates. Chemosphere 134:452–458
Ge LK, Zhang P, Halsall C, Li YY, Chen CE, Li J, Sun HL, Yao ZW (2019) The importance of reactive oxygen species on the aqueous phototransformation of sulfonamide antibiotics: kinetics, pathways, and comparisons with direct photolysis. Water Res 149:243–250
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Lastre-Acosta AM, Barberato B, Parizi MPS, Teixeira A (2019) Direct and indirect photolysis of the antibiotic enoxacin: kinetics of oxidation by reactive photo-induced species and simulations. Environ Sci Pollut Res 26:4337–4347
Li YJ, Wei XX, Chen JW, Xie HB, Zhang YN (2015) Photodegradation mechanism of sulfonamides with excited triplet state dissolved organic matter: a case of sulfadiazine with 4-carboxybenzophenone as a proxy. J Hazard Mater 290:9–15
Marchetti G, Minella M, Maurino V, Minero C, Vione D (2013) Photochemical transformation of atrazine and formation of photointermediates under conditions relevant to sunlit surface waters: laboratory measures and modelling. Water Res 47:6211–6222
Minella M, Rapa L, Carena L, Pazzi M, Maurino V, Minero C, Brigante M, Vione D (2018) An experimental methodology to measure the reaction rate constants of processes sensitised by the triplet state of 4-carboxybenzophenone as a proxy of the triplet states of chromophoric dissolved organic matter, under steady-state irradiation conditions. Environ Sci Process Impacts 20:1007–1019
Montoneri E, Boffa V, Savarino P, Perrone D, Ghezzo M, Montoneri C, Mendichi R (2011) Acid soluble bio-organic substances isolated from urban bio-waste. Chemical composition and properties of products. Waste Manag 31:10–17
Oliveira C, Lima DLD, Silva CP, Calisto V, Otero M, Esteves VI (2019) Photodegradation of sulfamethoxazole in environmental samples: the role of pH, organic matter and salinity. Sci Total Environ 648:1403–1410
Parizi MPS, Acosta AML, Ishiki HM, Rossi RC, Mafra RC, Teixeira A (2019) Environmental photochemical fate and UVC degradation of sodium levothyroxine in aqueous medium. Environ Sci Pollut Res 26:4393–4403
Passananti M, Temussi F, Iesce MR, Previtera L, Mailhot G, Vione D, Brigante M (2014) Photoenhanced transformation of nicotine in aquatic environments: involvement of naturally occurring radical sources. Water Res 55:106–114
Perisa M, Babic S, Skoric I, Fromel T, Knepper TP (2013) Photodegradation of sulfonamides and their N (4)-acetylated metabolites in water by simulated sunlight irradiation: kinetics and identification of photoproducts. Environ Sci Pollut Res 20:8934–8946
Schwarzenbach RP (2003) Environmental organic chemistry, 2nd edn. Wiley & Sons, Inc., Hoboken
Shemer H, Sharpless CM, Elovitz MS, Linden KG (2006) Relative rate constants of contaminant candidate list pesticides with hydroxyl radicals. Environ Sci Technol 40:4460–4466
Silva MP, Mostafa S, McKay G, Rosario-Ortiz FL, Teixeira A (2015) Photochemical fate of amicarbazone in aqueous media: laboratory measurement and simulations. Environ Eng Sci 32:730–740
Trovo AG, Nogueira RFP, Aguera A, Sirtori C, Fernandez-Alba AR (2009) Photodegradation of sulfamethoxazole in various aqueous media: persistence, toxicity and photoproducts assessment. Chemosphere 77:1292–1298
Vione D (2020) A critical view of the application of the APEX software (aqueous photochemistry of environmentally-occurring xenobiotics) to predict photoreaction kinetics in surface freshwaters. Molecules 25:1–34
Vione D, Maddigapu PR, De Laurentiis E, Minella M, Pazzi M, Maurino V, Minero C, Kouras S, Richard C (2011) Modelling the photochemical fate of ibuprofen in surface waters. Water Res 45:6725–6736
Vione D, Fabbri D, Minella M, Canonica S (2018) Effects of the antioxidant moieties of dissolved organic matter on triplet-sensitized phototransformation processes: implications for the photochemical modeling of sulfadiazine. Water Res 128:38–48
Wenk J, von Gunten U, Canonica S (2011) Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical. Environ Sci Technol 45:1334–1340
Acknowledgments
The authors are thankful to Prof. N. Y. M. Iha (Laboratory of Photochemistry and Energy Conversion, Institute of Chemistry, University of São Paulo, Brazil), for the disposal of the solar simulator, and to Prof. J. A. S. Tenório (Laboratory of Recycling, Waste Treatment and Extraction-LAREX, Department of Chemical Engineering, Escola Politécnica, University of São Paulo, Brazil) for the characterization of natural water samples.
Funding
This work was financially supported by São Paulo Research Foundation (FAPESP) grant #2016/03695-8; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES) Finance Code 001 and post-doc grant #88887.340964/2019-00; and National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Contributions
AMLA: conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, visualization
BSC: methodology, investigation
MPSP: conceptualization, methodology
CAON: resources, supervision, funding acquisition
ACSCT: conceptualization, validation, resources, writing—original draft, writing—review and editing, supervision, funding acquisition
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Lastre-Acosta, A.M., Cristofoli, B.S., Parizi, M.P.S. et al. Photochemical persistence of sulfa drugs in aqueous medium: kinetic study and mathematical simulations. Environ Sci Pollut Res 28, 23887–23895 (2021). https://doi.org/10.1007/s11356-020-11715-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11715-x