Abstract
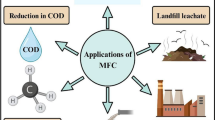
Petroleum, coal, and natural gas reservoir were depleting continuously due to an increase in industrialization, which enforced study to identify alternative sources. The next option is the renewable resources which are most important for energy purpose coupled with environmental problem reduction. Microbial fuel cells (MFCs) have become a promising approach to generate cleaner and more sustainable electrical energy. The involvement of various disciplines had been contributing to enhancing the performance of the MFCs. This review covers the performance of MFC along with different wastewater as a substrate in terms of treatment efficiencies as well as for energy generation. Apart from this, effect of various parameters and use of different nanomaterials for performance of MFC were also studied. From the current study, it proves that the use of microbial fuel cell along with the use of nanomaterials could be the waste and energy-related problem-solving approach. MFC could be better in performances based on optimized process parameters for handling any wastewater from industrial process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, one of the most critical threats faced by the world is the depletion of non-renewable energy sources and environmental pollution. However, the utilization of organic and inorganic waste can provide a means to resolve such issues. Various studies have successfully generated alternative energies by coupling anaerobic fermentation with other purification methods (Dai et al. 2020; Lu et al. 2020; Palanisamy et al. 2019). Nevertheless, sustainable energy solution must contain a wide variety of renewable energy technologies. In recent years, microbial fuel cell (MFC) has attracted much attention due to its potential functionality in wastewater treatment and bioenergy production. Furthermore, this technology has made it possible to convert embedded chemical energy within organic/inorganic waste into electrical energy via electrochemical reactions (Karthick and Haribabu 2020; Zhang et al. 2020). As a result, this technology could be a potential asset in wastewater treatment, bioremediation of heavy metals/toxic compounds, and other applications. MFCs mainly comprise 2 chambers: an anode chamber and a cathode chamber. These chambers are usually segregated by proton exchange membrane (Munjal et al. 2020; Yang et al. 2020). The mechanism of this technology includes microorganisms that act as biocatalysts to oxidize the substrate in an anode chamber from where electrons are directed to the cathode as a result of electrical flow (Walter et al. 2020). At the site of a cathode, the formation of water takes place due to reduction reaction. It can further be catalyzed using catalysts such as platinum; however, several microorganisms have shown promising and cost-friendly catalyst replacements (Do et al. 2020; Sonawane et al. 2020; Yadav et al. 2020). Such microorganisms as biocatalysts have shown exceptional characteristics such as mediating electrons to the surface of anode and catalyzing the reduction of electron acceptors, hence called exoelectrogens (Cao et al. 2019; Enamala et al. 2020; Ulusoy and Dimoglo 2018). These exoelectrogens are making MFC technology more useful and therefore applicable in variety of applications, for example, electricity generation. The mechanism involves generation of redox potential between the electrodes caused by oxidation of organic matter by exoelectrogens resulting in electron flow from anode to cathode. Numerous designs have been utilized and proposed in this regard (Chen et al. 2019b; Leiva-Aravena et al. 2019; Li et al. 2019). To produce electricity using MFC technology, either mixed or pure culture can be used (Kumar et al. 2016; Shehab et al. 2017). In this respect, various researchers have successfully enhanced the electricity output in MFC by incorporating methods such as surface modification with nanomaterials and microbial gene modification (Chiranjeevi and Patil 2020; Kaur et al. 2020; Liu et al. 2020). Furthermore, this technology can be useful in generating electricity and removing effluents from wastewater as the bacteria can degrade the organic matter (Sanjay and Udayashankara 2020). In addition, it is also possible to produce hydrogen gas by modifying MFC to microbial electrolysis cells (MEC). In order to produce hydrogen gas, a low voltage is needed by MEC which can be supplied by MFC. Various aspects of MFC have been investigated by researchers such as substrates (Pant et al. 2010), MFC configurations (Mohanakrishna et al. 2019), and removal of wastewater effluents (Chen et al. 2019a; Naik and Jujjavarappu 2019). The main objective of the current study is to review the effect of various parameters, which are affecting the performance of MFC along with uses of different wastewater as a substrate. Furthermore, in the current study, the use of advanced nanomaterials with respect to their application for wastewater treatment as well as for energy generation in MFC has been discussed.
Present energy scenario
Recently, a prosperous trend is seen in terms of energy consumption all over the world (Rahimnejad et al. 2009). Being one of the classifications of energy sources, non-renewable sources include fossil fuels and nuclear. Fossil fuels pose a negative impact in the environment by CO2 emissions, thereby adversely affecting human life and giving rise to global warming/atmospheric pollution (Rahimnejad et al. 2020).
Our globe is in the twenty-first century, and by stating this, it means that we are living in a high-standard and advanced society. If talking about energy, the available resources which generate energy and the methodologies in order to process them through suitable routes to utilize them in a desirable manner make a huge landmark in the history of science as it serves as a blessing for humanity and this is an undeniable truth. But everything has a dark side too because despite so much relaxation, the world faces an energy crisis and its sustainable solution puts a question mark. The major root causes which results in such global headache includes the past, existing, and prospective depletion of fossil fuel resources like coal, oil, and gas (Aziz et al. 2013). Moreover, to ensure equality, i.e., to fulfill the needs of a tsunami of the population throughout, this planet is a very much similar matter to solve a Rubik’s cube. This will not end up here because let say we have an alternative resource that produces energy and could serve us for a longer period but the point of the barrier is whether it is environmentally bearable or not? (i.e., it has enough potential to serve without contaminating or putting an adverse effect in our surrounding) and if not then up to what extent it would affect? On the opposite side, renewable energy resources, i.e., solar, hydropower, wind, tidal, wave, geothermal energy, etc., and even biomass come forward in resolving this issue to a greater extent and seem to be a possible remedial measure for the long-lasting energy production. Right now, scientists, engineers, and business corps are gathered to discover resources that support three kinds of “E,” i.e., it should be environmentally friendly, economically viable, and socially equitable.
Focusing on these objectives, microbial fuel cell technology grabs the attention of environmentalists and researchers for more than a decade. It is because of its specialty to deal with both power generation and waste water treatment simultaneously (Liu et al. 2004). It is based on the conversion of organic matter into electric current directly through the anaerobic degradation instead of indirect generation of electricity (for example, production of methane from animal’s manure along with biologically oriented hydrogen through underground anaerobic digestion) (Min et al. 2005a). Microbial fuel cells (MFCs) (originated from hydrogen fuel cells) are devices which, on behalf of the microbial activities as a biological catalyst, digest organic and some inorganic matter as well as in the absence of oxygen and release energy. The energy is in the form of electrons which results due to bacterial action over these substrates, which first moved towards the negative anodic terminal and then moves all the way to a positive cathodic terminal connected with conductive material and accompanied by a resistor, or load under which it operates (i.e., turns on a device when placed in between the connection) as shown in Fig. 1. They fall into two categories via electron transport phenomenon, i.e., a “mediator” one in which the electrons are first migrated to the anode from the bacterial respiratory enzyme. It is usually facilitated by chemicals such as neutral redoranthraquinone-2,6-disulfonate (AQDS) (Logan et al. 2006), and others like phenoxazine, phenothiazine, azophenylene, indophenol, and even derivatives of bipyridylium were also found to be very beneficial as redox mediators in MFC having Alcaligenes eutrophus, Bacillus subtilis, Escherichia coli, or Proteus vulgaris as the active biological species, and glucose or succinate as the oxidizable substrate (Delaney et al. 1984). In the case when electrons are transferred by a direct association of bacterial membrane to the anodic electrode or by other means, i.e., without any external reagent, then MFC is classified as “mediatorless.” Until now, numerous research works on microbial fuel cell have been conducted by the utilization of domestic-, sewage-, and industrial-released wastewaters as a substrate and microbes present in that water as a bio-oriented catalyst (Dannys et al. 2016). Wastewater treatment has conventionally been an energy-intensive process, acquiring between 950 and 2850 kJ/m3 of wastewater for the treatment (Al-Bsoul et al. 2020; Dannys et al. 2016). But on the other hand, some noteworthy studies show that wastewater encloses 9.3 times additional energy compared to that employed to treat an identical volume, so making the desire to capture this energy from the applicability of MFC (Shizas and Bagley 2004). Industrialism has completely changed the face of the world. It is because of day by day manufacturing of variety of products for humans’ ease and it is a true fact that in today’s world, we are not only dependent upon industries but we also cannot afford to live without benefits acquired from them. With these advantages, if we turn the picture upside down, the same industries create a lot of trouble in terms of pollution. If thinking about employment opportunities, economic growth, and exports, leather industry with no doubt has a distinct identity in the global market (Al-Othman et al. 2020; Mathuriya 2014). The various processes involved in leather tanning generate wastes in solid form and liquid effluent containing substantial amount of chromium and organic matters such as calcium, sodium, and potassium salts of fatty acids along with lime and sulfide which need to be treated. A large number of small-scale tanneries do not have an approach to a typical treatment plant, and hence, they end up throwing their wastes and residues in open fields or buried in landfill sites. The use of sludge as a kind of cheap manure in agricultural site is also not a very common practice. With these methods of disposal, the soil and water are contaminated, providing a straightforward route for such severe pollutants in the food chain (Raju and Tandon 1999).
Schematic diagram showing reasons for the increase in demand for energy requirement, resulting in depletion of non-renewable energy resources (Chaturvedi and Verma 2016)
Different wastewater for microbial fuel cell
For any microorganism to grow and evolve, the substrate plays an important role as it serves as a source of nutrients. In the same way, the substrate is considered to be the major biological factor in MFC upon which the electricity generation highly depends (Toczyłowska-Mamińska et al. 2020). Some of the most common substrates and their impact on MFC performance are discussed in detail below:
Numerous types of wastewater have been used by researchers to produce electricity using MFC such as domestic wastewater (Ditzig et al. 2007), swine wastewater (Min et al. 2005b), starch processing wastewater (Lu et al. 2009), food processing wastewater (Oh and Logan 2005), and chocolate industry wastewater (Noori and Najafpour Darzi 2016). In this regard, He et al. have explored the general features of MFC in detail along with the treatment of various wastewater from agricultural, municipal, and industrial resources.
In the past few years, MFC technology has witnessed a dramatic improvement in treatment efficiency and power density. MFCs have shown excellent results in contaminant removal such as chemical oxygen demand (COD) with a removal efficiency of greater than 90% (He et al. 2012). Advantageous features such as thermophilic metabolism and high-temperature cellulose biodegradability could be used in favor of MFC to achieve higher power densities (Guan et al. 2019; Lan et al. 2020). Microbial fuel cells are well-known for their operation regarding conversion of substrate into energy and decreasing environmental problem related to wastewater. Microbial fuel cells need some improvements with respect to different operational and design parameters coupled with current energy scenario. Current work focusses on the effect of process parameter on the removal of COD and biological oxygen demand (BOD) from distillery effluent used as substrate in anode. Generation of current directly linked to the oxidizing ability of substrate with respect to microorganism. Table 1 presents the current density (mA/cm2) at maximum power density (W/m2) achieved using various wastewater as substrates in MFCs.
The microbial fuel cell configuration
A microbial cell mainly comprises 2 electrodes (anode and cathode) and a membrane that separates these 2 compartments. Electrons and protons are generated at the site of the anode due to oxidation of microbes and hence start transferring to cathode. Electrons flow via the circuit whereas protons through the membrane. Upon reaching the cathode, electrons and protons give rise to water by reducing the oxygen. Being electrochemically inactive, most of the microbial cells are facilitated by mediators (Guan et al. 2019; Zhang et al. 2019). For evaluating the performance of MFC, different operational parameters have a significant impact. By viewing the current energy scenario, it is noticeable for taking a serious step towards renewable energy resources. MFC has such a potential impact on energy and wastewater treatment perspectives.
Electron transfer mechanism in microbial fuel cell
Microbial fuel cell working principle was based on the mechanism of electron transfer. Microscopic observations showed the anode of the fuel cell to be inhabited with thick biofilm on to its surface. Similarly, the power generation is affected and hence depends upon various factors including tendency of microbes to transfer electrons, surface area of electrodes, electrolytic resistance, and kinetic oxygen reaction. All these factors can be categorized into 3 groups, namely kinetic limitation, ohmic limitation, and transport limitations (Jadhav and Ghangrekar 2009) found limited power generation by cathode, but when electron mediator was added or dissolved oxygen was increased, the power output raised. Such limitations come under the category of ohmic and transport limitations. Other studies such as Dai et al. (2015) concluded that bacterial cell wall and electron mediator affected the electron transfer, hence showing the importance of identifying limiting factors and thereby adjusting to improve overall performance. Furthermore, the MFC can suitably be used to treat wastewater via batch or continuous feeds. However, when dealing with the large-scale wastewater, batch feed is impractical. Aiyer (2020) explored the effects of operational conditions of a mediator-free microbial fuel cell. The researchers concluded with the optimizing parameters as pH to be 7 and resistance to be higher than 500 V. On the other hand, when the resistance was lower than 200 V, limited proton and oxygen supply were observed; hence for a fuel cell to be efficient, it needs to have a high reducing activity (Fig. 2).
Electron transfer mechanism in microbial fuel cell (Aiyer 2020). Adopted with permission
Effect of various parameters on the performance of MFC
It is unfortunately true that Pakistan is among the countries facing the energy crisis in the world today. Hence, indigenous technologies based on renewable energy sources are required to cope up with this crisis. MFC technology has shown a significant potential towards resolving energy shortage as well as being friendly to the environment. Like many other technologies, the MFC is influenced by several factors that consequently decide the performance and efficiency, such as fuel oxidation, microbial electron transfer, circuit resistance, supply of oxygen, proton transfer via the membrane, reduction at the site of cathode, pH, and concentration (Woodward et al. 2010). Microbial fuel cell performance can be affected by various parameters which could be enhanced and varied time to time.
Electrode material
Several factors, i.e., electron transfer, electrochemical efficiency, and microbial adhesion, are highly dependent upon the material of electrodes. Many studies have used different material types to enhance the performance of electrodes such as carbon paper, carbon felt, and carbon fiber as described in Table 2. If MFC technology were to have diverse applications, it needs to use cost-efficient materials and enhanced power densities. Besides, the cathode should possess catalytic properties. Even though the criteria for each of the electrode are different, yet both of them should at least have the common traits of surface area and porosity. One of the reasons that results in lesser power output is the electrode resistance. This issue can be tackled by increasing the surface area of the electrodes while keeping the volume constant, thus improving the MFC efficiency and enhancing electrode kinetics. On the other hand, higher porosity of electrodes results in reduced electrical conductivity, thereby declining efficiency. When the electrons are generated, they travel to the cathode by passing through the anode; however, if the electrodes have higher porosity, they offer resistance to the moving electrons which reduces the conductivity and efficiency. Furthermore, cathode needs to have higher ionic conductivity to facilitate electron transfer and triple phase boundary reaction (Pocaznoi et al. 2012; Qiao et al. 2010)
Effect of cathode material
The power density was shown to improve from 660 to 1114 mW/m2 when carbon paper was replaced with carbon cloth electrode, bringing an overall upscale of 69%. This increase was mainly due to the cathode material replacement whereas the anode potential essentially stayed invariably the same. A similar increase was observed by other researchers such as from 17 to 45% using carbon paper cathode and from 22 to 52% by the use of carbon cloth cathode. However, using higher current densities, the carbon cloth cathode resulted in higher energy recovery (6.8–9%) than carbon paper cathode (4.6–8.8%) (Gao et al. 2018; Sonawane et al. 2017).
Aeration rate
Controlled aeration rate results in higher yield of current. When aeration rate was increased to 100 mL min−1, the maximum current increased as well; however, its value decreased when the aeration rate was 200 mL min−1, probably due to the disturbance caused by the shear force of immobilized microbes on the anode (Khan et al. 2019). As per the results, the generation of power increased with higher air flow rate but reached the threshold at the flow rate of 150 mL/min before declining. This shows that air flow rate above the threshold value resulted in reduced MFC generation capacity, probably due to the obstruction caused by oxygen in the way of microbial anaerobic activity (Jatoi et al. 2020; Jatoi et al. 2018).
Other researchers also discussed the importance of aeration rate on the cathodic chamber or on air cathode microbial fuel cell. Jatoi et al. (2020) discussed the effect of oxygen flow rate on power production during the running of MFC. Which were studied with different oxygen flow rates from 20 to 200 mL/min yielding in power production between 220 and 995 mV per L of the sewage treatment, respectively. These results suggested that power production increased as the air flow rate increased and reached a maximum of around 1 V at an oxygen flow rate of 150 mL/min before showing a decline afterwards. This indicates that at the higher air flow rate, the power generation capacity of MFC substantially reduced due to the higher rate of oxygen in the air diffused down to the vicinity of the anode, which probably disturbed the anaerobic microbes living on the surface of the anode (Aziz et al. 2013).
The performance of microbial fuel cells was measured in the form of a different aeration rates. Running of microbial fuel cell with different substrate concentrations coupled with different pH and aeration rates. The effect of oxygen flow rate on power generation during MFC operation was investigated, and power generation was generated between 220 and 995 mV/L using different oxygen flow rates of 20 to 200 mL/min. These results show that as the air flow rate increases, the amount of power generation increases and reaches a maximum of about 0.77 V at an oxygen flow rate of 150 mL/min, after which display is reduced. This indicates that at higher air flow rates, MFC can interfere with the anaerobic microorganisms present on the anode surface due to the higher oxygen rate in the air diffusing near the anode, resulting in a significant decrease in power generation capacity.
pH effect
The operational mechanism of MFC involves the generation of protons at an anode that moves towards the cathode to give water by reacting with oxygen. This continuous loop operation results in anode acidification, mainly due to incomplete diffusion of protons through the membrane. On the other hand, the cathode faces alkalization due to the reduced efficiency of proton replacement. These factors ultimately limit the performance of an MFC and hence give rise to a pH concentration gradient. An increase of pH in the cathode compartment decreases the current generation; hence, it is beneficial to reduce operational pH for achieving higher power output (Jatoi et al. 2018). Furthermore, bacterial growth plays a key role in pH adjustments. Bacteria usually grow optimally at pH close to neutral. Such pH variations to support bacterial growth may result in changing other parameters such as ion concentration, proton motility, the potential of the membrane, and biofilm formation (Rozendal et al. 2006). It has been observed that most of the prokaryotes and their enzymatic activity highly depend upon pH. Variations in pH could result in changes in reaction rates (He et al. 2008)
pH is a major factor affecting prokaryotic activity. At optimum pH, the microorganisms perform their biological activity of growth and metabolism at a maximum rate. This highlights the point that an enzyme that may be secreted by a microorganism at pH 8.5 will be the highest power yield at which the advantageous form of the ionic group at its active site will function properly. It is reported that a change in pH will result in a change in the ionic form of the active site, which will further alter enzyme activity leading to a change in reaction rate. The results also show that at pH 6 and below, electrochemical and cellulose activity may be lower than results obtained at higher pH. This may be due to the denaturation of cellulose, proteins, or active sites under acidic conditions. This finding is consistent with that reported by He et al. (2008). They observed that the neutral pH is suitable for cellulose degraders because the acidic conditions tend to inhibit the growth of most cellulose-degrading yeasts. By comparing the previous and current studies, the current maximum power output is 200 mL/min, with pH of 8.5.
Electrolyte
MFCs were operated using diluted wastewater with salt solutions to a COD value of 100 ppm. Fifty millimolars of phosphate buffer maintained at pH 7 was used with 100 mM NaCl. Phosphate buffer with NaCl resulted in higher current generation compared to buffer, NaCl, and distilled water alone, NaCl giving the lowest value. Similarly, in other studies, pH changes were measured using 2 set of experiments: one using wastewater diluted with water and another using control experiment 50 mM phosphate buffer with NaCl. It was observed that the pH change in the control experiment was significantly lower compared to the non-control experiment using wastewater coupled with distilled water. The cathode compartment of the fuel cell witnessed a gradual rise of pH up to 9.5 after the fuel supply while pH decreased on the anode. These results show the slow rate of proton transfer through the membrane than the production in the anode, which can be compensated by the buffer. As already mentioned, for the optimum performance of a fuel cell, neutral pH is required; hence, buffer is needed to maintain the optimum microbial environment and to compensate for the slower proton transfer rate through the membrane (Kumar et al. 2017; Margaria et al. 2017).
Temperature effect
Temperature affects MFCs significantly as its various kinetic/mass transfer (conductivity, mass transfer coefficient, activation energy) and thermodynamic (free Gibbs energy and electrode potentials) properties/characteristics highly depend upon it (Mohammed et al. 2019; Ren et al. 2017). Although the temperature effects have been explored in the past years, it lacks systematic information in MFC. It is observed that temperature directly influences MFC performance to remove COD and generate electricity. Power density rises with an increase in temperature whereas the ohmic resistances fall (Kakarla and Min 2019; Ren et al. 2017). It is further shown that membrane permeability bears an insignificant relationship with temperature rise or power output. On the other hand, a higher value of temperature results in lesser ohmic resistance, presenting a linear trend (Heidrich et al. 2018). This could possibly be due to the ionic conductivity created by temperature rise. Nevertheless, MFC performance with respect to temperature cannot be fully explained through the changes in ohmic resistance since these changes are exponential (Heidrich et al. 2018; Wang et al. 2018b). Similarly, the temperature effect on microbial activity is shown to have an exponential trend as well, hence affecting the power output of MFC. Such microbial activity could be explained in terms of biofilm developed on to the anodic compartment, thereby affecting the biocatalyst activity. Several studies have conclusively shown the relationship and effect of initial temperature on the biofilm generation, hence the MFC performance. In order for the optimized bioelectrolytic activity, the temperature range should be between 30 and 45 °C, thereby achieving the improved MFC performance. Differing temperatures than the mentioned yields lower biofilm development and MFC performance. It can also lead to irreversible denaturation processes that ultimately can deactivate the bacterial metabolic activity. Different species of bacteria require different temperatures to grow into biofilm; once this temperature is achieved, these species can adapt their metabolic activity accordingly (Song et al. 2017). It was surprising to see a slight drop in power density (9%) when the temperature was reduced from 32 to 20 °C. Usually, the coefficients of the chemical reaction rate double with each 10 °C temperature increase. The slight decrease in power output with respect to temperature drop can therefore be advantageous for wastewater treatment, especially under anaerobic conditions. However, further research is needed to explore the effect of temperature fluctuations over MFC performance (Jadhav and Ghangrekar 2009). This suggests the applicability of MFC over a wide range of temperatures, especially for wastewater treatment (Woodward et al. 2010). The above observations that the startup procedures affect the system performance are clearly illustrated in Table 3; therefore, using higher initial temperatures could result in improved MFC performance. After the startup, the temperatures can be lowered without compromising the performance.
Application of nanomaterials in microbial fuel cell
Electrode materials used in any low-cost industrial process (such as MFC) should primarily consist of low-cost materials that maintain chemical stability during the operating life cycle, have a large surface area, and are easy to upgrade the scale (Aiyer 2020). Due to the biological characteristics of microbial fuel cells, ideally the electrodes that interact with the biocatalysts should promote cell adhesion (or at least be harmless to bacteria in the anode) and have a limited tendency for chemical and biological contamination, improving their long-term functioning time. For these reasons stated, carbon electrodes are generally included as electrode materials. Considering the cost-efficiency and ease of production, coupled with the chemical stability of many carbon-based materials, good biocompatibility, and good electrical conductivity, their integration as large-scale electrode materials for fuel cells is of obvious research interest. However, compared to their metallic counterparts, carbonaceous electrodes have relatively low electrical conductivity and higher electrochemical surges. Considering the above situation, the application of nanomaterials and research in this field therefore cover many aspects; the most notable is the modification of the electrode and its potential impact (1) power transfer process of anode electrons, (2) mass transfer in the system, and (3) the process of cathodic electron transfer. Therefore, in this chapter, we describe how nanomaterials positively impact the key limitations in terms of electrode and surface materials, catalysts in oxygen reduction reactions, and media that increase efficiency of bacterial electron transfer.
Recent developments of MFC performance based on nanomaterial
Designing the electrodes is one of the main challenges in manufacturing microbial fuel cells, as it must be cost-effective and compatible with improved power generation. Therefore, the selection and manufacture of effective anode materials for fuel cell, which is essential for determining the final efficiency and power density of the fuel cell. By modifying various material parameters, MFC technology has made significant progress in the generation and transmission of electrons. Innovations in the design of anode materials have led to the formation of various materials with improved power density and efficiency. Carbon-based materials are widely used in the manufacture of anodes due to their high porosity, large surface area, and good electrical conductivity. In order to improve the performance of electrodes, various nanocomposites with improved properties (e.g., high mechanical resistance, electrical conductivity, thermal stability) have been proposed and developed in recent years. The main purpose of MFC research is to enhance the interaction of microorganisms and anode materials in wastewater treatment and determine their compatibility. However, further efforts are still needed to increase the amount of electricity generated by wastewater. Cost-effective materials are one of the main concerns of researchers because MFC must be sustainable and environmentally friendly. As mentioned above, stainless steel is very effective in improving the power density and coulombic efficiency of MFC. Therefore, significant progress has been made by incorporating stainless steel into various anode materials. For example, steel wool/PANI/polypyrrole nanocomposites have a higher power density of 2880 mW m2 (Sonawane et al. 2018). In addition, graphene has the advantages of large surface area and excellent electrical conductivity, which is why it is widely used as an anode material in MFC. The power density of graphene-modified stainless steel mesh anode in MFC is 2668 mW m2 (Yuan and He 2015). By N-doping TiO2 nanosheets, the electronic properties of carbon paper anodes can be improved. It has been noted that the adhesion of bacteria to the surface of the anode leads to an increase in energy production with minimal electron loss (produced by bacteria in the MFC). TiO2 nanofilms doped with carbon paper have been calcined in NH3 atmosphere at different temperatures (e.g., 400, 500, 600, and 700 °C) (Yin et al. 2017). It can be seen that at 600 ° C, the best performance of the electrode reaches 196% with the increase in the maximum power density (for example compared to bare carbon paper) (Yin et al. 2017). A modified anode based on multi-walled carbon nanotubes was also prepared to improve the performance of the MFC. Compared to bare carbon fabric anodes, the efficient growth of E. coli was obtained on the MWCNT-, MWCNT-COOH-, and MWCNT-NH2-doped anodes. Therefore, the maximum power density recorded with an anode modified by MWCNT-COOH is 560.4 mW/m2 (Fan et al. 2017). Therefore, these types of anode modifications help improve power generation and stability of the MFC. The purpose of this review is to propose detailed performance indicators for various wastewater as a substrate with respect to anode modifications, such as carbon-based composite materials and nanomaterials. Nanomaterial electrode materials in MFC provide a promising tool for high hydrogen production because MFC has the potential to generate electricity by treating wastewater. Although the yield and purity of hydrogen is still a difficult problem, there are still many opportunities in the development of electrode materials for the production of hydrogen (Zhao and Ci 2019). It is hoped that this research can provide various valuable information for further improvement of the MFC. Table 4 shows the comparison of various nanomaterial as electrode material for the performance of MFC.
Critical discussion
Microbial fuel cells (MFCs), as a partial solution to overreliance on fossil fuel–based electricity, are a potential way to be explored. Limitations limit the progress of MFC development, including low power generation, expensive electrode materials, and the inability to expand MFC to related industrial capabilities. However, the use of new advanced electrode materials (i.e., 2D nanomaterials) is expected to promote the development of electromicrobiology. New electrode materials, coupled with a more in-depth understanding of the mechanism by which the bacteria-generating bacteria participate in electron transfer, may greatly increase the power and may reach the upper limit of the theoretical limit. Continued research in electrochemistry and microbiology is essential to realize the industrial-scale development of MFC.
The current review addressed the different types of nanomaterials for use in microbial fuel cell and their performance to investigate the effect of various parameters. Recent investigation shows that nanomaterial has wide range of application for wastewater treatment to promote the application of microbial fuel cell. Apart from nanomaterial application, microbial fuel cell is one of the tremendous field of research in current world, due to their nature of converting wastewater into energy and their treatment efficiencies.
Future perspectives and challenges of MFC
MFC technology is shown to achieve promising results in terms of electricity generation, especially through organic materials/waste, but it does come with drawbacks that hinder its wide range applicability (Chaturvedi and Verma 2016). One of the first drawbacks is the low power density, which can be rectified by using potent microorganisms capable of efficiently transferring electrons to the anode or by genetically modifying microbes through recombinant DNA technology for their improved transfer rates. It is confirmed by various studies that consortium of many bacteria performs electron transfer rates faster. Similarly, many bacterial strains produce mediators to enhance this transfer rate; hence, new mediators can result in achieving improved MFC performance. Another drawback is the limited electrode surface area for the microorganisms to develop, thereby limiting biofilm production. To deal with this issue, several studies have suggested using air cathodes (Rossi et al. 2019), stacked reactors, and cloth electrode assemblies (Logan et al. 2019). Among these, air cathode has proved to be effective since it helps in efficient oxygen use from air and refrains from aerating water or using any chemical catholytes.
Conclusion
The depletion of fossil fuel enforced the study on the renewable energy source. Apart from these different wastewater generated from various industries, for treating these types of wastewater, significant techniques are available for treating such waste; among them, some are conventional and some are new entry. Among them, nowadays, one of the promising technologies puts this positive impact on bioenergy generation simultaneously treating wastewater, which is MFC technology. MFC energy generation has many advantages, including cleanliness, efficiency, and recyclability without generating harmful toxic by-products. In addition, microorganisms utilized in MFC are free and available in the environment. Despite many advances in MFC technologies, there are still challenges ahead the effectiveness of these technologies. The current review comprises different wastewater as a substrate in terms of electric current, and power outputs together with key factor affecting have been discussed. Currently, the growth of MFC technology is limited by the low efficiency of electrode material properties such as charge transport, surface, catalytic behavior, and cost. Indigenously developed electrode materials have better charge transfer properties, robustness, and high surface area which are thus expected to make efficient MFC electrodes.
Abbreviations
- MFC:
-
Microbial fuel cell
- COD:
-
Chemical oxygen demand
- BOD:
-
Biological oxygen demand
- OCP:
-
Open circuit potential
- OCV:
-
Open circuit voltage
- PEM:
-
Proton exchange membrane
- SCMFC:
-
Single-chamber microbial fuel cell
- DCMFC:
-
Dual-chamber microbial fuel cell
- OLR:
-
Organic loading rate
References
Ahn Y, Logan BE (2010) Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol 101:469–475
Aiyer KS (2020) How does electron transfer occur in microbial fuel cells? World J Microbiol Biotechnol 36:19
Al-Bsoul A, Al-Shannag M, Tawalbeh M, Al-Taani AA, Lafi WK, Al-Othman A, Alsheyab M (2020) Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Sci Total Environ 700:134576
Al-Othman A, Nancarrow P, Tawalbeh M, Ka’ki A, El-Ahwal K, El Taher B, Alkasrawi M (2020) Novel composite membrane based on zirconium phosphate-ionic liquids for high temperature PEM fuel cells. Int J Hydrog Energy
Asensio Y, Montes I, Fernandez-Marchante C, Lobato J, Cañizares P, Rodrigo M (2017) Selection of cheap electrodes for two-compartment microbial fuel cells. J Electroanal Chem 785:235–240
Aziz S, Memon AR, Shah SF, Soomro SA, Parkash A, Jatoi AS (2013) Electricity generation from sewage sludge using environment-friendly double chamber microbial fuel cell. Sci Int 25(1):57–61
Behera M, Murthy S, Ghangrekar M (2011) Effect of operating temperature on performance of microbial fuel cell. Water Sci Technol 64:917–922
Cao X, Zhang S, Wang H, Li X (2019) Azo dye as part of co-substrate in a biofilm electrode reactor–microbial fuel cell coupled system and an analysis of the relevant microorganisms. Chemosphere 216:742–748
Chandrasekhar K, Kumar G, Mohan SV, Pandey A, Jeon B-H, Jang M, Kim SH (2020) Microbial electro-remediation (MER) of hazardous waste in aid of sustainable energy generation and resource recovery. Environ Technol Innov 100997
Chaturvedi V, Verma P (2016) Microbial fuel cell: a green approach for the utilization of waste for the generation of bioelectricity. Bioresour Bioprocess 3:38
Chen F, Zeng S, Luo Z, Ma J, Zhu Q, Zhang S (2019a) A novel MBBR–MFC integrated system for high-strength pulp/paper wastewater treatment and bioelectricity generation. Sep Sci Technol 1–10. https://doi.org/10.1080/01496395.2019.1641519
Chen K-T, Bai M-D, Wu S-I, Chen C-C, Lu W-J, Wan H-P, Huang C (2019b) Electro-autotrophs induced the growth of exoelectrogens on the anode in a microbial fuel cell. Biochem Eng J 141:29–34
Cheng S, Xing D, Logan BE (2011) Electricity generation of single-chamber microbial fuel cells at low temperatures. Biosens Bioelectron 26:1913–1917
Chiranjeevi P, Patil SA (2020) Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies 11 Supplementary information (SI) available. Biotechnol Adv 39:107468
Choudhury P, Ray RN, Bandyopadhyay TK, Basak B, Muthuraj M, Bhunia B (2020) Process engineering for stable power recovery from dairy wastewater using microbial fuel cell. Int J Hydrog Energy
Cui H-F, Du L, Guo P-B, Zhu B, Luong JH (2015) Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J Power Sources 283:46–53
Dai J, Wang JJ, Chow AT, Conner WH (2015) Electrical energy production from forest detritus in a forested wetland using microbial fuel cells. GCB Bioenergy 7:244–252
Dai K, Sun T, Yan Y, Qian D-K, Zhang W, Zhang F, Jianxiong Zeng R (2020) Electricity production and microbial community in psychrophilic microbial fuel cells at 10 °C. Bioresour Technol 313:123680
Dannys E, Green T, Wettlaufer A, Madhurnathakam C, Elkamel A (2016) Wastewater treatment with microbial fuel cells: a design and feasibility study for scale-up in microbreweries. J Bioprocess Biotech 6:2
Delaney GM, Bennetto HP, Mason JR, Roller SD, Stirling JL, Thurston CF (1984) Electron-transfer coupling in microbial fuel cells. 2. performance of fuel cells containing selected microorganism—mediator—substrate combinations. J Chem Technol Biotechnol 34:13–27
Ditzig J, Liu H, Logan BE (2007) Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int J Hydrog Energy 32:2296–2304
Do MH, Ngo HH, Guo W, Chang SW, Nguyen DD, Liu Y, Varjani S, Kumar M (2020) Microbial fuel cell-based biosensor for online monitoring wastewater quality: a critical review. Sci Total Environ 712:135612
Enamala MK, Dixit R, Tangellapally A, Singh M, Dinakarrao SMP, Chavali M, Pamanji SR, Ashokkumar V, Kadier A, Chandrasekhar K (2020) Photosynthetic microorganisms (Algae) mediated bioelectricity generation in microbial fuel cell: concise review. Environ Technol Innov 19:100959
Fan M, Zhang W, Sun J, Chen L, Li P, Chen Y, Zhu S, Shen S (2017) Different modified multi-walled carbon nanotube–based anodes to improve the performance of microbial fuel cells. Int J Hydrog Energy 42:22786–22795
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880
Gao N, Qu B, Xing Z, Ji X, Zhang E, Liu H (2018) Development of novel polyethylene air-cathode material for microbial fuel cells. Energy 155:763–771
Guan Y-F, Zhang F, Huang B-C, Yu H-Q (2019) Enhancing electricity generation of microbial fuel cell for wastewater treatment using nitrogen-doped carbon dots-supported carbon paper anode. J Clean Prod 229:412–419
Harewood A, Popuri S, Cadogan E, Lee C-H, Wang C-C (2017) Bioelectricity generation from brewery wastewater in a microbial fuel cell using chitosan/biodegradable copolymer membrane. Int J Environ Sci Technol 14:1535–1550
He Y-R, Xiao X, Li W-W, Sheng G-P, Yan F-F, Yu H-Q, Yuan H, Wu L-J (2012) Enhanced electricity production from microbial fuel cells with plasma-modified carbon paper anode. Phys Chem Chem Phys 14:9966–9971
He Z, Huang Y, Manohar AK, Mansfeld F (2008) Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 74:78–82
Heidrich E, Dolfing J, Wade M, Sloan W, Quince C, Curtis T (2018) Temperature, inocula and substrate: contrasting electroactive consortia, diversity and performance in microbial fuel cells. Bioelectrochemistry 119:43–50
Heilmann J, Logan BE (2006) Production of electricity from proteins using a microbial fuel cell. Water Environ Res 78:531–537
Hou Y, Yuan H, Wen Z, Cui S, Guo X, He Z, Chen J (2016) Nitrogen-doped graphene/CoNi alloy encased within bamboo-like carbon nanotube hybrids as cathode catalysts in microbial fuel cells. J Power Sources 307:561–568
Huang L, Logan BE (2008) Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl Microbiol Biotechnol 80:349–355
Huang L, Li X, Ren Y, Wang X (2016) In-situ modified carbon cloth with polyaniline/graphene as anode to enhance performance of microbial fuel cell. Int J Hydrog Energy 41:11369–11379
Jadhav GS, Ghangrekar MM (2009) Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour Technol 100:717–723
Jang JK, Pham TH, Chang IS, Kang KH, Moon H, Cho KS, Kim BH (2004) Construction and operation of a novel mediator-and membrane-less microbial fuel cell. Process Biochem 39:1007–1012
Jatoi AS, Tunio M, Riaz S, Abro R, Wajahat MH, Qureshi K, Shah A, Nizamuddin S, Mubarak N (2018) Utilization of distillery effluent as substrate for power generation with optimized parametric conditions using microbial fuel cell. Eurasian J Anal Chem 13:1–8
Jatoi AS, Baloch A, Jadhav A, Nizamuddin S, Aziz S, Soomro SA, Nazir I, Abro M, Baloch HA, Ahmed J (2020) Improving fermentation industry sludge treatment as well as energy production with constructed dual chamber microbial fuel cell. SN Appl Sci 2:9
Kakarla R, Min B (2019) Sustainable electricity generation and ammonium removal by microbial fuel cell with a microalgae assisted cathode at various environmental conditions. Bioresour Technol 284:161–167
Karthick S, Haribabu K (2020) Bioelectricity generation in a microbial fuel cell using polypyrrole-molybdenum oxide composite as an effective cathode catalyst. Fuel 275:117994
Kaur R, Marwaha A, Chhabra VA, Kim K-H, Tripathi SK (2020) Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew Sust Energ Rev 119:109551
Khan A, Chen Z, Zhao S, Ni H, Pei Y, Xu R, Ling Z, Salama E-S, Liu P, Li X (2019) Micro-aeration in anode chamber promotes p-nitrophenol degradation and electricity generation in microbial fuel cell. Bioresour Technol 285:121291
Khilari S, Pandit S, Varanasi JL, Das D, Pradhan D (2015) Bifunctional manganese ferrite/polyaniline hybrid as electrode material for enhanced energy recovery in microbial fuel cell. ACS Appl Mater Interfaces 7:20657–20666
Kumar R, Singh L, Zularisam AW (2016) Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew Sust Energ Rev 56:1322–1336
Kumar SS, Basu S, Bishnoi NR (2017) Effect of cathode environment on bioelectricity generation using a novel consortium in anode side of a microbial fuel cell. Biochem Eng J 121:17–24
Lan L, Li J, Feng Q, Zhang L, Fu Q, Zhu X, Liao Q (2020) Enhanced current production of the anode modified by microalgae derived nitrogen-rich biocarbon for microbial fuel cells. Int J Hydrog Energy 45:3833–3839
Larrosa-Guerrero A, Scott K, Head I, Mateo F, Ginesta A, Godinez C (2010) Effect of temperature on the performance of microbial fuel cells. Fuel 89:3985–3994
Leiva-Aravena E, Leiva E, Zamorano V, Rojas C, Regan JM, Vargas IT (2019) Organotrophic acid-tolerant microorganisms enriched from an acid mine drainage affected environment as inoculum for microbial fuel cells. Sci Total Environ 678:639–646
Li J, Yang W, Zhang B, Ye D, Zhu X, Liao Q (2018) Electricity from microbial fuel cells, bioreactors for microbial biomass and energy conversion. Springer, pp 391–433
Li X, Zheng R, Zhang X, Liu Z, Zhu R, Zhang X, Gao D (2019) A novel exoelectrogen from microbial fuel cell: bioremediation of marine petroleum hydrocarbon pollutants. J Environ Manag 235:70–76
Liang P, Duan R, Jiang Y, Zhang X, Qiu Y, Huang X (2018) One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res 141:1–8
Liu D, Chang Q, Gao Y, Huang W, Sun Z, Yan M, Guo C (2020) High performance of microbial fuel cell afforded by metallic tungsten carbide decorated carbon cloth anode. Electrochim Acta 330:135243
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285
Liu Z, Liu J, Zhang S, Su Z (2009) Study of operational performance and electrical response on mediator-less microbial fuel cells fed with carbon-and protein-rich substrates. Biochem Eng J 45:185–191
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Logan BE, Call D, Merrill M, Cheng S (2019) Cathodes for microbial electrolysis cells and microbial fuel cells. Google Patents
Lu H, Yu Y, Xi H, Zhou Y, Wang C (2020) A quick start method for microbial fuel cells. Chemosphere 259:127323
Lu N, Zhou S-G, Zhuang L, Zhang J-T, Ni J-R (2009) Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem Eng J 43:246–251
Lv Z, Chen Y, Wei H, Li F, Hu Y, Wei C, Feng C (2013) One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim Acta 111:366–373
Ma D, Jiang Z-H, Lay C-H, Zhou D (2016) Electricity generation from swine wastewater in microbial fuel cell: hydraulic reaction time effect. Int J Hydrog Energy 41:21820–21826
Margaria V, Tommasi T, Pentassuglia S, Agostino V, Sacco A, Armato C, Chiodoni A, Schilirò T, Quaglio M (2017) Effects of pH variations on anodic marine consortia in a dual chamber microbial fuel cell. Int J Hydrog Energy 42:1820–1829
Mathuriya AS (2014) Enhanced tannery wastewater treatment and electricity generation in microbial fuel cell by bacterial strains isolated from tannery waste. Environ Eng Manag J 13(12):2945–2954. http://omicron.ch.tuiasi.ro/EEMJ
Mehdinia A, Ziaei E, Jabbari A (2014) Multi-walled carbon nanotube/SnO2 nanocomposite: a novel anode material for microbial fuel cells. Electrochim Acta 130:512–518
Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38:5809–5814
Min B, Cheng S, Logan BE (2005a) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686
Min B, Kim J, Oh S, Regan JM, Logan BE (2005b) Electricity generation from swine wastewater using microbial fuel cells. Water Res 39:4961–4968
Min B, Angelidaki I (2008) Innovative microbial fuel cell for electricity production from anaerobic reactors. J Power Sources 180:641–647
Min B, Román ÓB, Angelidaki I (2008) Importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance. Biotechnol Lett 30:1213–1218
Modestra JA, Velvizhi G, Krishna KV, Arunasri K, Lens PN, Nancharaiah Y, Mohan SV (2017) Bioelectrochemical systems for heavy metal removal and recovery, sustainable heavy metal remediation. Springer, pp 165–198
Mohammed H, Al-Othman A, Nancarrow P, Elsayed Y, Tawalbeh M (2019) Enhanced proton conduction in zirconium phosphate/ionic liquids materials for high-temperature fuel cells. Int J Hydrog Energy
Mohan SV, Mohanakrishna G, Reddy BP, Saravanan R, Sarma P (2008) Bioelectricity generation from chemical wastewater treatment in mediatorless (anode) microbial fuel cell (MFC) using selectively enriched hydrogen producing mixed culture under acidophilic microenvironment. Biochem Eng J 39:121–130
Mohanakrishna G, Al-Raoush RI, Abu-Reesh IM, Pant D (2019) A microbial fuel cell configured for the remediation of recalcitrant pollutants in soil environment. RSC Adv 9:41409–41418
Munjal M, Tiwari B, Lalwani S, Sharma M, Singh G, Sharma RK (2020) An insight of bioelectricity production in mediator less microbial fuel cell using mesoporous Cobalt Ferrite anode. Int J Hydrog Energy 45:12525–12534
Naik S, Jujjavarappu SE (2019) Simultaneous bioelectricity generation from cost-effective MFC and water treatment using various wastewater samples. Environ Sci Pollut Res:1–11
Noori P, Najafpour Darzi G (2016) Enhanced power generation in annular single-chamber microbial fuel cell via optimization of electrode spacing using chocolate industry wastewater. Biotechnol Appl Biochem 63:427–434
Oh S, Logan BE (2005) Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res 39:4673–4682
Palanisamy G, Jung H-Y, Sadhasivam T, Kurkuri MD, Kim SC, Roh S-H (2019) A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J Clean Prod 221:598–621
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543
Park IH, Christy M, Kim P, Nahm KS (2014) Enhanced electrical contact of microbes using Fe3O4/CNT nanocomposite anode in mediator-less microbial fuel cell. Biosens Bioelectron 58:75–80
Patil SA, Surakasi VP, Koul S, Ijmulwar S, Vivek A, Shouche Y, Kapadnis B (2009) Electricity generation using chocolate industry wastewater and its treatment in activated sludge based microbial fuel cell and analysis of developed microbial community in the anode chamber. Bioresour Technol 100:5132–5139
Patil SA, Harnisch F, Kapadnis B, Schröder U (2010) Electroactive mixed culture biofilms in microbial bioelectrochemical systems: the role of temperature for biofilm formation and performance. Biosens Bioelectron 26:803–808
Pocaznoi D, Calmet A, Etcheverry L, Erable B, Bergel A (2012) Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ Sci 5:9645–9652
Qiao Y, Bao S-J, Li CM, Cui X-Q, Lu Z-S, Guo J (2008) Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. ACS Nano 2:113–119
Qiao Y, Bao S-J, Li CM (2010) Electrocatalysis in microbial fuel cells—from electrode material to direct electrochemistry. Energy Environ Sci 3:544–553
Radha M, Kanmani S (2017) Performance of cathode catalysts for bio-electricity from paper recycling, wastewater-fed, microbial fuel cells. Curr Sci 113:468
Rahimnejad M, Mokhtarian N, Najafpour G, Daud W, Ghoreyshi A (2009) Low voltage power generation in a biofuel cell using anaerobic cultures. World Appl Sci J 6:1585–1588
Rahimnejad M, Asghary M, Fallah M (2020) Microbial fuel cell (MFC): an innovative technology for wastewater treatment and power generation. Bioremediation of Industrial Waste for Environmental Safety. Springer, pp 215–235
Raju M, Tandon S (1999) Operationally determined speciation of chromium in tannery sludges. Chem Speciat Bioavailab 11:67–70
Ren H, Jiang C, Chae J (2017) Effect of temperature on a miniaturized microbial fuel cell (MFC). Micro Nano Syst Lett 5:1–7
Rodrigo M, Canizares P, Lobato J, Paz R, Sáez C, Linares J (2007) Production of electricity from the treatment of urban waste water using a microbial fuel cell. J Power Sources 169:198–204
Rossi R, Cario BP, Santoro C, Yang W, Saikaly PE, Logan BE (2019) Evaluation of electrode and solution area-based resistances enables quantitative comparisons of factors impacting microbial fuel cell performance. Environ Sci Technol 53:3977–3986
Rozendal RA, Hamelers HV, Buisman CJ (2006) Effects of membrane cation transport on pH and microbial fuel cell performance. Environ Sci Technol 40:5206–5211
Sanjay S, Udayashankara TH (2020) Dairy wastewater treatment with bio-electricity generation using dual chambered membrane-less microbial fuel cell. Mater Today: Proc
Sarmin S, Ethiraj B, Islam MA, Ideris A, Yee CS, Khan MMR (2019) Bio-electrochemical power generation in petrochemical wastewater fed microbial fuel cell. Sci Total Environ 695:133820
Sayed ET, Alawadhi H, Elsaid K, Olabi A, Adel Almakrani M, Bin Tamim ST, Alafranji GH, Abdelkareem MA (2020) A carbon-cloth anode electroplated with iron nanostructure for microbial fuel cell operated with real wastewater. Sustainability 12:6538
Shehab NA, Ortiz-Medina JF, Katuri KP, Hari AR, Amy G, Logan BE, Saikaly PE (2017) Enrichment of extremophilic exoelectrogens in microbial electrolysis cells using Red Sea brine pools as inocula. Bioresour Technol 239:82–86
Shizas I, Bagley DM (2004) Experimental determination of energy content of unknown organics in municipal wastewater streams. J Energy Eng 130:45–53
Sonawane JM, Yadav A, Ghosh PC, Adeloju SB (2017) Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens Bioelectron 90:558–576
Sonawane JM, Patil SA, Ghosh PC, Adeloju SB (2018) Low-cost stainless-steel wool anodes modified with polyaniline and polypyrrole for high-performance microbial fuel cells. J Power Sources 379:103–114
Sonawane JM, Ezugwu CI, Ghosh PC (2020) Microbial fuel cell-based biological oxygen demand sensors for monitoring wastewater: state-of-the-art and practical applications. ACS Sensors 5:2297–2316
Song X, Jiang Q, Liu J, He W, Feng Y (2018) Enhanced antifouling performance for modified carbon nanotubes filtration cathode by the electric field. J Power Sources 400:493–501
Song Y, An J, Chae KJ (2017) Effect of temperature variation on the performance of microbial fuel cells. Energy Technol 5:2163–2167
Subha C, Kavitha S, Abisheka S, Tamilarasan K, Arulazhagan P, Banu JR (2019) Bioelectricity generation and effect studies from organic rich chocolaterie wastewater using continuous upflow anaerobic microbial fuel cell. Fuel 251:224–232
Tang J, Yuan Y, Liu T, Zhou S (2015) High-capacity carbon-coated titanium dioxide core–shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J Power Sources 274:170–176
Toczyłowska-Mamińska R, Pielech-Przybylska K, Sekrecka-Belniak A, Dziekońska-Kubczak U (2020) Stimulation of electricity production in microbial fuel cells via regulation of syntrophic consortium development. Appl Energy 271:115184
Ulusoy I, Dimoglo A (2018) Electricity generation in microbial fuel cell systems with Thiobacillus ferrooxidans as the cathode microorganism. Int J Hydrog Energy 43:1171–1178
Walter XA, Greenman J, Ieropoulos IA (2020) Microbial fuel cells directly powering a microcomputer. J Power Sources 446:227328
Wang Q, Wang P, Yang Q (2018a) Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci Total Environ 621:990–999
Wang S, Zhao J, Liu S, Zhao R, Hu B (2018b) Effect of temperature on nitrogen removal and electricity generation of a dual-chamber microbial fuel cell. Water Air Soil Pollut 229:244
Wang X, Feng Y, Ren N, Wang H, Lee H, Li N, Zhao Q (2009) Accelerated start-up of two-chambered microbial fuel cells: effect of anodic positive poised potential. Electrochim Acta 54:1109–1114
Wang Y-Q, Huang H-X, Li B, Li W-S (2015) Novelly developed three-dimensional carbon scaffold anodes from polyacrylonitrile for microbial fuel cells. J Mater Chem A 3:5110–5118
Wang Y, Li B, Cui D, Xiang X, Li W (2014) Nano-molybdenum carbide/carbon nanotubes composite as bifunctional anode catalyst for high-performance Escherichia coli-based microbial fuel cell. Biosens Bioelectron 51:349–355
Wen Q, Wu Y, Cao D, Zhao L, Sun Q (2009) Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour Technol 100:4171–4175
Woodward L, Perrier M, Srinivasan B, Pinto R, Tartakovsky B (2010) Comparison of real-time methods for maximizing power output in microbial fuel cells. AICHE J 56:2742–2750
Wu D, Yi X, Tang R, Feng C, Wei C (2018a) Single microbial fuel cell reactor for coking wastewater treatment: simultaneous carbon and nitrogen removal with zero alkaline consumption. Sci Total Environ 621:497–506
Wu X, Shi Z, Zou L, Li CM, Qiao Y (2018b) Pectin assisted one-pot synthesis of three dimensional porous NiO/graphene composite for enhanced bioelectrocatalysis in microbial fuel cells. J Power Sources 378:119–124
Xia T, Zhang X, Wang H, Zhang Y, Gao Y, Bian C, Wang X, Xu P (2019) Power generation and microbial community analysis in microbial fuel cells: a promising system to treat organic acid fermentation wastewater. Bioresour Technol 284:72–79
Yadav RK, Chiranjeevi P, Sukrampal PSA (2020) Integrated drip hydroponics-microbial fuel cell system for wastewater treatment and resource recovery. Bioresour Technol Rep 9:100392
Yang F, Ren L, Pu Y, Logan BE (2013) Electricity generation from fermented primary sludge using single-chamber air-cathode microbial fuel cells. Bioresour Technol 128:784–787
Yang G, Wang J, Zhang H, Jia H, Zhang Y, Cui Z, Gao F (2019) Maximizing energy recovery from homeostasis in microbial fuel cell by synergistic conversion of short-chain volatile fatty acid. Bioresour Technol Rep 7:100200
Yang Y, Zhao Y, Tang C, Xu L, Morgan D, Liu R (2020) Role of macrophyte species in constructed wetland-microbial fuel cell for simultaneous wastewater treatment and bioenergy generation. Chem Eng J 392:123708
Yin T, Su L, Li H, Lin X, Dong L, Du H, Fu D (2017) Nitrogen doping of TiO2 nanosheets greatly enhances bioelectricity generation of S. loihica PV-4. Electrochim Acta 258:1072–1080
Yong X-Y, Gu D-Y, Wu Y-D, Yan Z-Y, Zhou J, Wu X-Y, Wei P, Jia H-H, Zheng T, Yong Y-C (2017) Bio-Electron-Fenton (BEF) process driven by microbial fuel cells for triphenyltin chloride (TPTC) degradation. J Hazard Mater 324:178–183
Yuan H, He Z (2015) Graphene-modified electrodes for enhancing the performance of microbial fuel cells. Nanoscale 7:7022–7029
Zhang C, Liang P, Yang X, Jiang Y, Bian Y, Chen C, Zhang X, Huang X (2016) Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens Bioelectron 81:32–38
Zhang L, Fu G, Zhang Z (2019) Simultaneous nutrient and carbon removal and electricity generation in self-buffered biocathode microbial fuel cell for high-salinity mustard tuber wastewater treatment. Bioresour Technol 272:105–113
Zhang L, Wang J, Fu G, Zhang Z (2020) Simultaneous electricity generation and nitrogen and carbon removal in single-chamber microbial fuel cell for high-salinity wastewater treatment. J Clean Prod 276:123203
Zhao W, Ci S (2019) Nanomaterials as electrode materials of microbial electrolysis cells for hydrogen generation, nanomaterials for the removal of pollutants and resource reutilization. Elsevier, pp 213–242
Zhong D, Liao X, Liu Y, Zhong N, Xu Y (2018) Enhanced electricity generation performance and dye wastewater degradation of microbial fuel cell by using a petaline NiO@ polyaniline-carbon felt anode. Bioresour Technol 258:125–134
Zou L, Qiao Y, Zhong C, Li CM (2017) Enabling fast electron transfer through both bacterial outer-membrane redox centers and endogenous electron mediators by polyaniline hybridized large-mesoporous carbon anode for high-performance microbial fuel cells. Electrochim Acta 229:31–38
Author information
Authors and Affiliations
Contributions
Abdul Sattar Jatoi, Faheem Akhter, Shaukat Ali Mazari ,Nizamuddin Sabzoi, Shaheen Aziz, Suhail Ahmed Soomro, Nabisab Mujawar Mubarak, Humair Baloch, Abdul Qayoom Memon, and Shoaib Ahmed contributed in the review and article writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jatoi, A.S., Akhter, F., Mazari, S.A. et al. Advanced microbial fuel cell for waste water treatment—a review. Environ Sci Pollut Res 28, 5005–5019 (2021). https://doi.org/10.1007/s11356-020-11691-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11691-2