Abstract
This study aimed to investigate the concentration of 33 pesticide residues in 60 black tea samples collected from Iran, determine their transfer rate, and assess their health risk during brewing. Pesticide extraction and analysis were performed by using a quick, easy, cheap, effective, rugged, and safe (QuEChERS) method and gas chromatography/tandem mass spectrometry (GC-MS/MS), respectively. The limits of detection (LOD) and the limits of quantification (LOQ) of pesticides were ranged 0.1–7.26 and 0.8–24 μg/kg for dried tea leaves and 0.03–3.1 and 0.09–10 μg/L for the tea infusion, respectively. The levels of pesticide residue in 52 (86.67%) out of 60 tea samples were above the LOD (0.1–7.26 μg/kg). Twenty four (40%) of the samples contained pesticides in a concentration higher than the maximum residue limit (MRL) set by the European Commission (EC). Seven out of 33 validated pesticides were detected in dried tea leaf samples that only four of seven, including buprofezin, chlorpyrifos, hexaconazole, and triflumizole, were transferred into tea infusion, demonstrating that the concentrations of pesticides in infusion were raised during brewing. The risk assessment study for detected pesticides in the tea infusion samples indicated that this beverage consumption was safe for consumers, while the mean residue of some pesticides in positive samples was higher than the MRL; therefore, periodic control of these pesticides should be regularly implemented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, several investigations have indicated that tea’s daily drinking has beneficial effects on human health, including reducing the risk of heart diseases and various types of cancer like oral, pancreatic, and prostate (Feng et al. 2012; Ren et al. 2020). Green and black teas are the two most consumed types worldwide (Fatima and Rizvi 2011). After water, black tea is the most consumed drink among Iranian consumers, which is mainly imported from Sri Lanka into Iran (Gupta and Shanker 2009). The mean consumption of tea in Iran is 4.38 g/day for a person (Salahinejad and Aflaki 2010).
Nowadays, in modern agriculture, many farmers use different pesticides to protect products against pest attacks and increase efficiency to respond to the market’s growing demand (Gurusubramanian et al. 2008; Hamidi et al. 2019; Heshmati and Nazemi 2018). Since tea is consumed as an infusion, its consistent consumption may easily hold various pesticide residues and lead to tea consumers’ potential health risks. Due to differences in pesticides’ physiochemistry properties, the transfer rate of pesticide residue from dried tea leaves during brewing into the tea infusion is different (Jaggi et al. 2001).
Tea has many impurities; compounds, including carbohydrates, pigments, proteins, lipids, and alkaloids, cause many pesticide measurements (Karak and Bhagat 2010). Therefore, developing an extraction procedure that can remove this interference correctly is a great challenge (Shoeibi et al. 2013; Zhang et al. 2010). Because of the low concentration and different chemical properties of pesticides, using traditional extraction procedures has low efficiencies and may lead to various problems, such as long processing time and a large volume of solvents in identification processing. Thus, new extraction procedures are required. In current years, a quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction method has been abundantly used for pesticide analysis in tea due to short analysis times and high-performance extraction (Amirahmadi et al. 2013; Hu et al. 2008; Razzaghi et al. 2018; Shoeibi et al. 2013; Yadolahi et al. 2012).
Recently, some methods have been proposed for analysis and measurement of pesticide residues in tea and other crops, while gas chromatography and liquid chromatography/tandem mass spectrometry (LC-MS/MS and GC-MS/MS) have been used frequently due to stimulation analysis of multi-residue pesticide in one run, high identification power regarding the retention time, and the use of two or more transition ions for each pesticide in each sample (Abd El-Aty et al. 2014; Huang et al. 2019; Wang et al. 2019; Zongmao 2013).
The periodic regular check and control of pesticides in tea crops are necessary to increase their safety (Chen et al. 2016). There are various studies regarding residual pesticides in different teas and their leaching behavior during brewing (Chen et al. 2014; Gupta and Shanker 2008; Jaggi et al. 2001; Manikandan et al. 2009; Tewary et al. 2005; Tewary et al. 2004). According to previous studies, little information exists regarding pesticides in black tea samples consumed in the west of Iran and their infusion (Amirahmadi et al. 2013). Therefore, this study’s primary goal is to determine 33 pesticide residues in the black tea samples collected from Iran. The transfer rate of pesticide residues during various brewing times simultaneously (5, 10, and 15 min) was assessed. Furthermore, the risk assessment of pesticide residues in brewed tea was calculated.
Materials and methods
Reagents, chemicals, and materials
All pesticides’ standard used in this research was provided by Dr. Ehrenstorfer Co. (Augsburg, Germany). MgSO4, NaCl, toluene, ethanol, and other chemicals in analytical grades were obtained from Merck (Darmstadt, Germany). Graphitized carbon black (GCB) and primary, secondary amine (PSA) used for pesticide extraction were purchased from Supelco (Bellefonte, USA). Triphenylmethane (TPM) was provided by Sigma-Aldrich (Buchs SG, Switzerland). The CAS number and purity of utilized materials are shown in Table S1.
Tea samples and infusion preparation
Sixty black tea samples from different brands were bought from local markets in Hamadan Province, Iran, from April to June 2018. The production data of tea samples ranged from January 2017 to May 2018. The size of the container of collected samples is approximately 500 g. For tea infusion preparation, 5 g of dried tea leaves was added to a glass teapot kettle (Pars Khazar Com, Tehran Iran) containing 100 mL of boiled water. Samples were brewed three times (5, 10, and 15 min). The pesticide concentration in dried tea leaves and its infusion both were determined.
Pesticide extraction
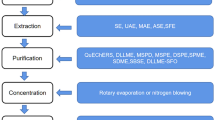
The extraction of pesticide residue was done similarly to our previous studies (Heshmati et al. 2020a; Heshmati et al. 2020b). The QuEChERS procedure, as shown in Fig. 1, was selected for pesticide extraction because it had high speed and efficiency of sample preparation and good recovery, and is environmental friendly (Chen et al. 2011; Hamidi et al. 2019).
Pesticide analysis
Pesticide analysis was performed by GC-MS/MS (7000 C, Agilent Technologies 7693, Wilmington, USA) equipped with a PAL RSI 85 autosampler (Agilent Technologies, USA). Pesticides separation was conducted on A HP-5 capillary column (30 m × 0.25 mm I.D., 0.25-μm film thicknesses). The initial temperature of the oven was 75 °C. It maintained at this temperature for 3 min, and then the temperature with a rate of 25 °C/min was increased to 120 °C. Furthermore, the temperature reached 300 °C with a 5 °C/min rate and remained for 11 min at this temperature.
The splitless mode was used. The ion source, injection port, quadrupole temperature, and ionization energy were 250, 230, 150 °C, and 70 eV, respectively. Helium (99.9999% purity and flow rate of 1 mL/min) was utilized as the carrier gas. The ionization mode used in the current study was the electron impact (EI). To prevent damage to the instrument, the MS was calibrated with perfluorotributylamine (PFTBA), and the delay time was set at 5.0 min. For obtaining the best response in pesticide identification, the MS system was set in the multi-reaction monitoring mode (MRM), and the better parent ions and fragment ions and the collision energies for each pesticide were selected. Table 1 presents the optimized GC-MS/MS parameters for all the analyzed pesticides in the current study. In addition, the chromatograms obtained from various samples including blank unspiked tea samples, spiked tea samples, and tea samples contaminated with pesticide residue are shown in Fig. S1.
Method validation
Method validation was studied based on the European SANCO Guidelines (SANTE/11813/2017 2017). First, stock solutions (1 mg/mL) of each pesticide and TPM as internal standards were prepared in ethyl acetate. A mixed working standard solution of 33 pesticides with a 10-μg/mL concentration was made in ethyl acetate and kept at − 20 °C until analysis. To overcome matrix effects, a spiked calibration standard method was applied. Therefore, calibration standards at various levels (10, 25, 50, 100, 200, and 400 μg/kg for dried tea leaves; 1, 2.5, 5, 10 15, 20, and 50 μg/kg for tea infusion) were prepared by addition of working standard solution and the internal standard (5 μL). The prepared calibration standards were spiked into 10 g of blank, dried tea leaves (blank sample not containing the pesticides), or blank tea infusion samples. The pesticide extraction and analysis were carried out according to the procedure mentioned above. In the final, the calibration curve of each pesticide was illustrated.
For recovery and precision studies, each pesticide’s standard solution in three levels of 75, 100, and 125 μg/kg was spiked into pesticide-free (previously examined for their existence) tea (blank sample not containing the pesticides) and water samples. Each recovery experiment was done in triplicate for three consecutive days to obtain the precision of the analysis method (SANTE/11813/2017 2017).
The extraction and analysis of pesticides were carried out according to the method, as mentioned above. The obtained concentration was divided into spiked concentrations and expressed as a percentage to show the recovery rate.
The limit of detection (LOD) and the limit of quantification (LOQ) were calculated as 3 and 10 times of spiked dried tea leaves/tea infusion samples’ signal-to-background noise (S/N), respectively.
Calculation of transfer rate
The transition percentage of each pesticide from dried tea leaves into the tea infusion was estimated using the following Equation:
where T% shows the transfer rate percentage, C1 and C2 are the amount of each pesticide in tea infusion (μg/L) and dried tea leaves (μg/kg), while M and V are dried tea leaves’ weight (5 g) and infusion volume (100 mL), respectively.
The health risk assessment of pesticide intake through tea infusion consumption
The chronic and acute risks of pesticide regarding dietary exposure for brewing tea were calculated according to some previous studies (Mehri et al. 2019; Nejad et al. 2019; Wang et al. 2018). The chronic risk was estimated using the following equations (Heshmati et al. 2020a; Heshmati et al. 2020b):
In the above equations, EDI indicates the estimated daily intake (mg/kg·bw); LP is per capita consumption of tea in Iran, i.e., 4.38 g/day or 0.00438 kg/day (Salahinejad and Aflaki 2010); MR denotes the mean amount of residue pesticide in dried tea leaves (mg/kg); TR represents transfer rate (in percentage) of pesticide of dried tea leaves into infusion; BW is the average body weight of an adult (60 kg); HQ indicates health hazard quotient, and ADI expresses the acceptable daily intake (mg/kg·bw).
The estimated short-term intake (ESTI) was obtained using the following equations:
HR shows the highest residue level in tea (mg/kg), aHI denotes an acute hazard risk index, and ARfD shows the acute reference dose (mg/kg·bw).
If the HQ or aHI level was lower than 100%, it presents that the risk level is acceptable. If HQ or aHI is higher than 100%, it indicates an unacceptable risk, and it threatens human health (Beneta et al. 2018); therefore, it is necessary to reduce exposure to pesticide residues (Amvrazi and Albanis 2009).
Statistics analysis
The SPSS software version 21.0 (SPSS Inc., Chicago, USA) was applied to analyze data. Each treatment was done in triplicate. The mean and standard deviation were reported. One-sample T test was utilized to show the difference between mean pesticide residue and maximum residue level (MRL). ANOVA and Tukey’s test determined the difference between pesticide concentrations during various brewing times. The significant level was considered P value < 0.05.
Results and discussion
Method validation
The method validation parameters, including linearity, calibration curve equation, R2, recovery, RSDs, LOD, and LOQ, are shown in Tables 2 and 3. LOD and LOQ ranged 0.1 to 7.26 μg/kg and 1 to 24 μg/kg in dried tea leaves and 0.03 to 1.37 μg/L and 0.9 to 7 μg/L in the tea infusion, respectively. The obtained LOQ was much lower than the MRLs proposed by the EU (European Commission 2005). As given in Tables 2 and 3, the recoveries were in the range of 72–120%, and RSDs obtained for all selected pesticides were in the range of 0.3–20%, entirely in agreement with the criteria set by SANTE/11813/2017 (2017). According to SANTE/11813/ 2017 requirements, recovery levels of a validated method for pesticide residue analysis should be in the range 70 to 120% and have relative standard deviation within ± 20%. The recovery range (72–120%) in this study was similar to the findings (77–116%) reported by Cho et al. (2014).
The data regarding LOD, LOQ, recovery, and precision of analysis of pesticide residue in tea in previous studies is shown in Table S2 (Abd El-Aty et al. 2014; Beneta et al. 2018; Huang et al. 2007; Kivrak and Harmandar 2018; Manikandan et al. 2009; Yang et al. 2009). The maximum LOD (7.26 μg/kg) and LOQ (24 μg/kg) levels obtained in our study were lower than the previous studies (Beneta et al. 2018; Feng et al. 2012). The LOD and LOQ values for pesticide residue in tea analyzed by Feng et al. (2012) were reported as 0.3–34.96 μg/kg and 1.14–100.19 μg/kg, respectively. Besides, in a previous study done by Beneta et al. (2018), LOD and LOQ of pesticide residue in tea ranged from 0.018–40 μg/kg and 0.06–135 μg/kg, respectively. The maximum LOD (10 μg/kg) and LOQ (30 μg/kg) levels were reported by Cho et al. (2014) which were similar to our findings (Cho et al. 2014). Therefore, data regarding the validated method indicated that the analysis method setup in the current study was sensitive and suitable to determine 33 pesticide residues in tea.
Pesticide residues in black tea samples
The concentration of pesticides in different samples of dried tea leaves and their infusion samples are summarized in Table 4. Seven out of 33 validated pesticides were detected in dried tea leaves samples. Fifty-two (86.67%) of the analyzed samples were contaminated the least by one of the pesticides above the LOD. Ten (16.67%%) out of the analyzed samples contained three or more than three pesticides. The pesticide residue in 24 (40%) samples exceeded the MRL set by EC (European Commission 2005). The concentration of pesticide residues varied among studied samples. The highest and the lowest of obtained mean concentrations of pesticides were related to triflumizole (238 ± 0.005 μg/kg) and ethalfluralin (10 ± 0.020 μg/kg), respectively. The concentration of other pesticides, including quintozene and buprofezin, was detected as very low in dried tea leaves samples.
In studies performed among other countries, different pesticides were found in dried tea leaves samples. For example, fenpropathrin and lambda-cyhalothrin were the most abundant pesticides in tea samples from China (Xiao et al. 2017). Jaggi et al. (2001) demonstrated phosphamidon, monocrotophos, malathion, methyl parathion, quinalphos, and chlorpyrifos as the most common pesticides in tea samples from India, while difenoconazole, azoxystrobin, and triflumizole residues were found in tea samples from Korea (Cho et al. 2014). Moreover, carbendazim, acetamiprid, buprofezin, and triazophos were the main pesticides in tea samples of China (Chen et al. 2011; Hou et al. 2016; Hou et al. 2014; Huang et al. 2007; Huang et al. 2009; Zhang et al. 2010).
In an investigation conducted by Cao et al. (2018), the concentration of chlorpyrifos in dried tea leaves was 138 μg/kg, which was lower than that of the current study (154.564 μg/kg). The concentration of triflumizole (2271 μg/kg) in dried tea leaves samples collected in Korea was higher than the corresponded value (238.612 μg/kg) in our study (Cho et al. 2014). Discrepancy observed in concentration and also the pesticide occurrence in tea samples depended on pesticide type and its initial concentration in a farm, tea type, physical and chemical properties of pesticides, and environmental condition, besides geographical location and tea sample preparation and equipment applied for pesticide analysis (Cajka et al. 2012; Chen et al. 2007). Also, pesticide residues might be degraded by various environmental agents (Vasseghian et al. 2020).
Transfer behavior of pesticide into black and green tea samples during the brewing
In the current study, the transfer rates of pesticide residue during brewing from dried tea leaves to infusion in different time intervals (5, 10, and 15 min) are provided in Table 4. The increase in brewing time caused a significant increment in the transfer rate of pesticide (P value < 0.05). The results indicated that the transfer rate differed for various pesticides; the highest mean transfer rate during brewing was related to hexaconazole (23.79%), followed by triflumizole (22.35%) and chlorpyrifos (11.7%), while buprofezin (8.33%) had the lowest transfer rate. However, the concentrations of ethalfluralin and quintozene and residues in tea infusion were lower than the detection limit.
The mean concentration of hexaconazole (26.663 ± 3.846 μg/L) in infusion was approximately 65 times higher than buprofezin (0.413 ± 0.255 μg/L). The difference obtained between the transfer rates of pesticides could be related to the initial pesticide levels in dried tea leaves and pesticide properties, such as solubility rate in water and partition coefficient (Kow) (Abd El-Aty et al. 2014; Wang et al. 2019), as given in Table 5. Previous studies have reported a direct correlation between water’s solubility rate, Kow of pesticide residues, and transfer rates into tea infusion (Abd El-Aty et al. 2014). Pesticides with a high Kow value (i.e., ethalfluralin and quintozene) can be bonded to tea tissue and not transferred into the water, hence they are insoluble in the brewing process (Jaggi et al. 2001). In a study by Jaggi et al. (2001) on Indian tea samples, the transfer rate of chlorpyrifos was 3.14%. In another study performed by Manikandan et al. (2009), the transfer rate of chlorpyrifos from black tea into the infusion was 9.12%. Cho et al. (2014) indicated a lower transfer rate (3%) for triflumizole in tea samples in Korea, which was lower than our result (22.35%). They also showed that transfer rates of triflumizole reduced with increased water temperature (Abd El-Aty et al. 2014). The discrepancies of reports concerning the transfer rates in our study and previous investigations were associated with tea type, brewing procedure, brewing duration, water temperature, and tea/water ratio (Abd El-Aty et al. 2014; Wang et al. 2019; Xiao et al. 2017). As given in Table 3, our data indicated that concentrations of pesticides in the tea infusion were increased with the increase of the brewing time. These findings were in line with different authors’ results in other countries (Gupta and Shanker 2008; Manikandan et al. 2009; Tewary et al. 2005; Tewary et al. 2004). However, Chen et al. (2014) indicated that the concentrations of hexaconazole and other pesticides in the tea infusion decreased with increased infusion time. In this study, the data showed that pesticide residues in all tea infusions were lower than MRLs set by Regulation (EC) No. 396/2005 (European Commission 2005).
Risk assessment of pesticide
The calculated health risk of the pesticide residue in the tea infusion samples is presented in Table 6. For the detected pesticide in the tea infusion, EDI ranged from 5.29E-07 to 3.88E-04 mg/kg per day. The lowest and highest HQ values were related to buprofezin (0.0005%) and chlorpyrifos (0.0165%). The HQ value for chlorpyrifos (0.0165) in this study was lower than those reported by Beneta et al. (2018) (0.0773%) and Liu et al. (2016) (0.18%) in China. The results showed that the chronic risk hazard for all pesticides detected and infused into the brewed tea was much lower than 100%, indicating the acceptable risk and safe tea consumption for Iranians. The results regarding short-term risk evaluations showed the ESTI amounts to be less than the ARfD values. As seen in Table 5, ESTI, for the detected pesticide in the tea infusion, ranged from 6.21E-07 to 7.37E-06 mg/kg per day. The lowest aHI% value (0.0001%) was related to buprofezin. In a study performed by Liu et al. (2016) in China, a higher aHI% value (0.05%) was reported for buprofezin in comparison with our findings (Liu et al. 2016). In the present study, chlorpyrifos had the highest aHI% (0.05%), which was lower than the results (0.6598%) reported by Beneta et al. (2018) (Beneta et al. 2018). Therefore, for each defined pesticide, different aHI% values were reported in various studies; the causes of these discrepancies were related to the different original levels of pesticide in dried tea leaves, brewing times, temperature, and frequency, as well as the variation in per capita consumption of tea in different countries (Feng et al. 2015; Zongmao 2013).
Conclusion
In this study, a simple and accurate extraction method joined with GC-MS/MS was validated for the simultaneous determination of 33 pesticides in the dried tea leaves. The method showed excellent linearity and acceptable recovery, besides low LOD and LOQ. From 33 analyzed pesticides, only seven were detected in dried tea leaves. Based on the obtained results, the pesticide level in 86.7% of dried tea leaves samples was higher than the LOD, while pesticide residue in 40% of samples was greater than the MRL level set by EC. From 7 detected pesticides in dried tea leaves, four (buprofezin, hexaconazole, chlorpyrifos, and triflumizole) leached into tea infusion. The highest transfer rate of detected pesticides was related to hexaconazole, followed by triflumizole. The results of acute and chronic risk assessment indicated that pesticide intake through tea could not threaten the health of Iranian consumers. However, the mean residue of some pesticides in positive samples was higher than the MRL; therefore, the periodic control of these pesticides should be regularly implemented to minimize tea levels.
Data availability
Not applicable.
References
Abd El-Aty A, Choi J-H, Rahman MM, Kim S-W, Tosun A, Shim J-H (2014) Residues and contaminants in tea and tea infusions: a review. Food Additives Contaminants: Part A 31:1794–1804
Amirahmadi M, Shoeibi S, Abdollahi M, Rastegar H, Khosrokhavar R, Hamedani MP (2013) Monitoring of some pesticide residue in consumed tea in Tehran market. Iran J Environ Health Sci Eng 10:9
Amvrazi EG, Albanis TA (2009) Pesticide residue assessment in different types of olive oil and preliminary exposure assessment of Greek consumers to the pesticide residues detected. Food Chem 113:253–261
Beneta A, Mutavdžić Pavlović D, Periša I, Petrović M (2018) Multiresidue GC-MS/MS pesticide analysis for evaluation of tea and herbal infusion safety. Int J Environ Anal Chem 98:987–1004
Cajka T, Sandy C, Bachanova V, Drabova L, Kalachova K, Pulkrabova J, Hajslova J (2012) Streamlining sample preparation and gas chromatography–tandem mass spectrometry analysis of multiple pesticide residues in tea. Anal Chim Acta 743:51–60
Cao P, Yang D, Zhu J, Liu Z, Jiang D, Xu H (2018) Estimated assessment of cumulative dietary exposure to organophosphorus residues from tea infusion in China. Environ Health Prev Med 23:7
Chen S, Shi L, Shan Z, Hu Q (2007) Determination of organochlorine pesticide residues in rice and human and fish fat by simplified two-dimensional gas chromatography. Food Chem 104:1315–1319
Chen G, Cao P, Liu R (2011) A multi-residue method for fast determination of pesticides in tea by ultra performance liquid chromatography–electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure. Food Chem 125:1406–1411
Chen L, Chen J, Guo Y, Li J, Yang Y, Xu L, Fu F (2014) Study on the simultaneous determination of seven benzoylurea pesticides in Oolong tea and their leaching characteristics during infusing process by HPLC–MS/MS. Food Chem 143:405–410
Chen H, Hao Z, Wang Q, Jiang Y, Pan R, Wang C, Liu X, Lu C (2016) Occurrence and risk assessment of organophosphorus pesticide residues in Chinese tea. Human and Ecological Risk Assessment: An International Journal 22:28–38
Cho S-K, El-Aty AA, Rahman MM, Choi J-H, Shim J-H (2014) Simultaneous multi-determination and transfer of eight pesticide residues from green tea leaves to infusion using gas chromatography. Food Chem 165:532–539
European Commission (2005) Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off J Eur Union 70:1–50
Fatima M, Rizvi SI (2011) Health beneficial effects of black tea. Biomedicine 31:3–8
Feng L, Li J, Ng TP, Lee TS, Kua EH, Zeng Y (2012) Tea drinking and cognitive function in oldest-old Chinese. J Nutr Health Aging 16:754–758
Feng J, Tang H, Chen D, Li L (2015) Monitoring and risk assessment of pesticide residues in tea samples from China. Human and Ecological Risk Assessment: An International Journal 21:169–183
Gupta M, Shanker A (2008) Persistence of acetamiprid in tea and its transfer from made tea to infusion. Food Chem 111:805–810
Gupta M, Shanker A (2009) Fate of imidacloprid and acetamiprid residues during black tea manufacture and transfer into tea infusion. Food Addit Contam 26:157–163
Gurusubramanian G, Rahman A, Sarmah M, Roy S, Bora S (2008) Pesticide usage pattern in tea ecosystem, their retrospects and alternative measures. J Environ Biol 29:813–826
Hamidi M, Nili-Ahmadabadi A, Heshmati A (2019) Evaluation of different preparation methods of edible mushroom (Agaricus bisporus, strains H737) on reduction of health hazards caused by deltamethrin residue. Iran J Nutr Sci Food Technol 14:95–104
Heshmati A, Nazemi F (2018) Dichlorvos (DDVP) residue removal from tomato by washing with tap and ozone water, a commercial detergent solution and ultrasonic cleaner. Food Sci Technol 38:441–446
Heshmati A, Komacki H, Nazemi F, Khaneghah AM (2020a) Persistence and dissipation behavior of pesticide residues in parsley (Petroselinum crispum) under field conditions. Quality Assurance Saf Crops Foods 12:55–65
Heshmati A, Nili-Ahmadabadi A, Rahimi A, Vahidinia A, Taheri M (2020b) Dissipation behavior and risk assessment of fungicide and insecticide residues in grape under open-field, storage and washing conditions. J Clean Prod 270:122287
Hou X, Lei S, Qiu S, Guo L, Yi S, Liu W (2014) A multi-residue method for the determination of pesticides in tea using multi-walled carbon nanotubes as a dispersive solid phase extraction absorbent. Food Chem 153:121–129
Hou X, Lei S, Guo L, Qiu S (2016) Optimization of a multi-residue method for 101 pesticides in green tea leaves using gas chromatography–tandem mass spectrometry. Rev Bras 26:401–407
Hu B, Song W, Xie L, Shao T (2008) Determination of 33 pesticides in tea by accelerated solvent extraction-gel permeation and solid-phase extraction purification-gas chromatography-mass spectrometry. Se pu= Chinese journal of chromatography 26:22–28
Huang Z, Li Y, Chen B, Yao S (2007) Simultaneous determination of 102 pesticide residues in Chinese teas by gas chromatography–mass spectrometry. J Chromatogr B 853:154–162
Huang Z, Zhang Y, Wang L, Ding L, Wang M, Yan H, Li Y, Zhu S (2009) Simultaneous determination of 103 pesticide residues in tea samples by LC-MS/MS. J Sep Sci 32:1294–1301
Huang Y, Shi T, Luo X, Xiong H, Min F, Chen Y, Nie S, Xie M (2019) Determination of multi-pesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS. Food Chem 275:255–264
Jaggi S, Sood C, Kumar V, Ravindranath S, Shanker A (2001) Leaching of pesticides in tea brew. J Agric Food Chem 49:5479–5483
Karak T, Bhagat R (2010) Trace elements in tea leaves, made tea and tea infusion: a review. Food Res Int 43:2234–2252
Kivrak Ş, Harmandar M (2018) Development and validation of modified QuEChERS method coupled with GC-MS/MS for 123 pesticide residues in food. International Journal of Secondary Metabolite 5:130–139
Liu Y, Shen D, Li S, Ni Z, Ding M, Ye C, Tang F (2016) Residue levels and risk assessment of pesticides in nuts of China. Chemosphere 144:645–651
Manikandan N, Seenivasan S, Ganapathy MNK, Muraleedharan NN, Selvasundaram R (2009) Leaching of residues of certain pesticides from black tea to brew. Food Chem 113:522–525
Mehri F, Heshmati A, Moradi M, Khaneghah AM (2019) The concentration and health risk assessment of nitrate in vegetables and fruits samples of Iran. Toxin Rev:1–8. https://doi.org/10.1080/15569543.2019.1673424
Nejad ASM, Heshmati A, Ghiasvand T (2019) The occurrence and risk assessment of exposure to aflatoxin M1 in ultra-high temperature and pasteurized milk in Hamadan Province of Iran. Osong Public Health Res Perspect 10:228–233
Razzaghi N, Ziarati P, Rastegar H, Shoeibi S, Amirahmadi M, Conti GO, Ferrante M, Fakhri Y, Khaneghah AM (2018) The concentration and probabilistic health risk assessment of pesticide residues in commercially available olive oils in Iran. Food Chem Toxicol 120:32–40
Ren G, Fan Q, He X, Li W, Tang X (2020) Applicability of multifunctional preprocessing device for simultaneous estimation of spreading of green tea, withering of black tea and shaking of oolong tea. J Sci Food Agric 100:560–569
Salahinejad M, Aflaki F (2010) Toxic and essential mineral elements content of black tea leaves and their tea infusions consumed in Iran. Biol Trace Elem Res 134:109–117
SANTE/11813/2017 (2017) A guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. European Commission, Brussels. https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf. Accessed 20 July 2020
Shoeibi S, Amirahmadi M, Rastegar H, Khosrokhavar R, Khaneghah AM (2013) An applicable strategy for improvement recovery in simultaneous analysis of 20 pesticides residue in tea. J Food Sci 78:T792–T796
Tewary DK, Kumar V, Shanker A (2004) Leaching of pesticides in herbal decoction. Chem Health Saf 11:25–29
Tewary DK, Kumar V, Ravindranath S, Shanker A (2005) Dissipation behavior of bifenthrin residues in tea and its brew. Food Control 16:231–237
Vasseghian Y, Moradi M, Pirsaheb M, Khataee A, Rahimi S, Badi MY, Khaneghah AM (2020) Pesticide decontamination using UV/ferrous-activated persulfate with the aid neuro-fuzzy modeling: a case study of malathion. Food Res Int 137:109557
Wang S, Zhang Q, Yu Y, Chen Y, Zeng S, Lu P, Hu D (2018) Residues, dissipation kinetics, and dietary intake risk assessment of two fungicides in grape and soil. Regul Toxicol Pharmacol 100:72–79
Wang X, Zhou L, Zhang X, Luo F, Chen Z (2019) Transfer of pesticide residue during tea brewing: understanding the effects of pesticide’s physico-chemical parameters on its transfer behavior. Food Res Int 121:776–784
Xiao J-J, Li Y, Fang Q-K, Shi Y-H, Liao M, Wu X-W, Hua R-M, Cao H-Q (2017) Factors affecting transfer of pyrethroid residues from herbal teas to infusion and influence of physicochemical properties of pesticides. Int J Environ Res Public Health 14:1157
Yadolahi M, Babri M, Sharif AAM, Khaneghah AM (2012) Pesticide residue determination in Shahr-e-Rey tomatoes using Quechers method. Adv Environ Biol 6:2434–2438
Yang X, Qiu JW, Zhang H, Zhang YC, Dong AJ, Ma Y, Wang J (2009) Simultaneous determination of 118 pesticide residues in Chinese teas by gas chromatography-mass spectrometry. Chem Pap 63:39–46
Zhang X, Mobley N, Zhang J, Zheng X, Lu L, Ragin O, Smith CJ (2010) Analysis of agricultural residues on tea using d-SPE sample preparation with GC-NCI-MS and UHPLC-MS/MS. J Agric Food Chem 58:11553–11560
Zongmao C (2013) Pesticide residue in tea and its risk assessment. Tea Research Institute, Chinese Academy of Agricultural Sciences, Hangzhou, China
Funding
This study was financially supported by Hamadan University of Medical Sciences, Hamadan, Iran (project number: 970218781).
Author information
Authors and Affiliations
Contributions
Ali Heshmati: Investigation, data curation, resources, supervision, project administration, conceptualization, methodology writing, original draft. Fereshteh Mehri: Conducting risk assessment, writing and editing. Amin Mousavi Khaneghah: Supervision, review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
The authors declare their consent to participate for this article.
Consent to publish
The authors declare their consent to publish for this article.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heshmati, A., Mehri, F. & Mousavi Khaneghah, A. Simultaneous multi-determination of pesticide residues in black tea leaves and infusion: a risk assessment study. Environ Sci Pollut Res 28, 13725–13735 (2021). https://doi.org/10.1007/s11356-020-11658-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11658-3