Abstract
Various spray adjuvants including surfactants are widely used in agricultural pesticide formulations, and some of them may remain in soils and waters and impose more adverse effects than active pesticide ingredients on organisms. However, previous studies are more focused on the active pesticide ingredients than the adjuvants. Thus, this study investigates the changes in toxic effects of surfactants during photodegradation, which is one way of naturally degrading contaminants in natural waters. Triton X-100, a water-soluble non-ionic surfactant, was degraded using different types of UV radiation (UVA, UVB, and UVC), and the changes in the toxic effects were determined using bioluminescent bacteria and water flea. The Triton X-100 removals were negligible with UVA within 24 h, while its removal was 81% with UVB and almost complete with UVC. The NMR spectra indicated possible molecule rearrangement after photolysis. On the other hand, the toxic effects based on the mortality of Daphnia magna and the bioluminescence of Aliivibrio fischeri increased (i.e., lower EC50 values) after photodegradation, suggesting the generation of photoproducts that are likely to have higher toxic effects or higher bioavailability. Furthermore, the sensitivities of D. magna and A. fischeri for Triton X-100 and the photodegraded Triton X-100 were different. This study suggests that the changes in the chemical composition of the Triton X-100 containing water with photodegradation can lead to changes in the relative toxic effects on different aquatic organisms. Therefore, not only the management of parent compound (i.e., Triton X-100) but also the photoproducts generated from the parent compound need to be considered when managing water environment subject to photodegradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various spray adjuvants including surfactants, dyes, and wetting agents are often used in application of agricultural pesticide formulations to enhance the performance of active ingredients in pesticides (Mullin et al. 2016). The pesticide adjuvants are also referred to as inert ingredients in pesticides. Numerous studies have reported that these adjuvants can harm non-target organisms (Krogh et al. 2003; Nobels et al. 2011; Ciarlo et al. 2012), and the toxic effects can often be more than the associated active gradients of pesticides (Mullin et al. 2016). Also, previous study reported that the toxicity of pesticide formulation is largely attributed to the formulation (i.e., mixture of chemicals) than the active ingredient of the formulation (Contardo-Jara et al. 2009; Druart et al. 2010). However, studies on the effects of spray adjuvants such as surfactants on agricultural soil or crops are limited (Mesnage and Antoniou 2018).
The pesticides applied on agricultural land can eventually reach nearby water bodies. The fate of the pesticides applied on agricultural soil can be affected by soil properties such as water holding capacity, texture, and soil organic matter; hence, the interaction between pesticides and humic substances has been studied to manage pesticides in soil environment (Das et al. 2019). Even if pesticide residues in soil environment are strongly sorbed on soil and are not bioavailable, they might be remobilized under appropriate conditions and become bioavailable (Zhu et al. 2019). Pesticide residue runoff can also reach surface water bodies, and this will lead to water contamination, which can be of great concern to aquatic ecosystems (Wang et al. 2019). In particular, surfactants, one of pesticide adjuvants, can have adverse effects on aquatic organisms (Borrely et al. 2018; Santos et al. 2019).
Recent studies on the treatment of surfactants in waters involved advanced oxidation processes (AOPs) since biological processes are often slow and not efficient (Arslan-Alaton et al. 2013). For example, biodegradation by activated sludge can be inhibited by the presence of surfactants (Karahan 2010). Although the biodegradability is limited, a previous study reported that the biodegradability of Triton X-100 can be controlled by controlling factors such as the types of microorganisms used, the initial Triton X-100 concentrations, and substrates given to microorganisms (Abu-Ghunmi et al. 2014). Among various AOPs, photolysis has been considered as a natural way of degrading contaminants in environment. In laboratories, different light sources such as natural light, simulated sunlight, ultraviolet A (UVA), and UVC have been used to study the degradation of various contaminants including surfactants (Ríos et al. 2017). The removal of water contaminants by photolysis is affected by many factors such as light intensity, types of UV, water pH, and presence of other chemicals (Cortés et al. 2011; Ríos et al. 2017; Mörtl et al. 2019). Also, the presence of oxidants (e.g., TiO2, H2O2) has influence on the photodegradation of surfactants such as Triton X-100 (Saien et al. 2011).
When toxicity aspects are considered, the ecological toxicity of surfactants using microorganisms, water flea, fish, and plants has been extensively studied (Caux et al. 1988; Pettersson et al. 2000; Abu-Ghunmi et al. 2014; Mustapha and Bawa-Allah 2020); however, studies on the changes in the toxic effects during photodegradation are relatively limited. One previous study reported that the relative ecotoxicity effect of Triton X-45 on water flea (i.e., based on the dead or immobilized organisms) and bioluminescent bacteria (i.e., based on the bioluminescence) compared to a toxicant-free control was decreased during the degradation of Triton X-45 by peroxymonosulfate and UVC (Olmez-Hanci et al. 2015). Similarly, when surfactants in pesticide formulations reach surface water bodies, they can be photodegraded to some extent by sunlight that emits radiation at various wavelengths. However, the removal of surfactants by natural light in water bodies may not be as efficient as in the laboratory studies where the reaction conditions can be optimized to completely remove contaminants. Therefore, this study is set to investigate the changes in the removal and toxic effects of Triton X-100, a commonly used anionic surfactant for various purposes including pesticide formulations, after photodegradation by different types of UV radiation. Specifically, the Microtox® acute toxicity test using Aliivibrio fischeri and acute toxicity test using Daphnia magna, which are commonly used toxicity tests for water samples, were used to determine the relative changes in the Triton X-100 solution toxicity before and after photodegradation.
Materials and methods
Photolysis of Triton X-100
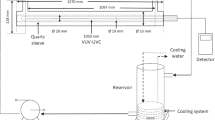
TritonTM X-100 (laboratory grade) was purchased from Sigma-Aldrich (Munich, Germany). Batch tests were carried out to study the changes in the Triton X-100 concentration after exposure to UV radiation. The samples under dark conditions were used as controls, and duplicate samples were prepared for each test condition. The initial concentrations of Triton X-100 used in the tests were 248 ± 11 mg L−1. Previous study used up to 100 mg L−1 of Triton X-100 to study the toxic effect of Triton X-100 on Tetrahymena pyriformis cells (Dias et al. 2003). Also, another study used a range of concentrations (1–100 mg L−1) of Triton X-100 to study the toxic effects on a freshwater planarian, Dugesia japonica (Li 2008). In this study, Triton X-100 is to be photodegraded; thus, the initial concentrations for the photodegradation experiments were set at higher concentrations than what were used in previous studies. Also, the effect of different UV radiation on the degradation of Triton X-100 can be clearly observed and compared by using the high initial concentrations. The test solutions (40 mL) were placed in quartz Petri dishes, and the distance between the UV lamps and the test solutions was maintained at 11.5 cm. The photolysis test was initially run for 35 days under UVA, UVB, and UVC radiation to determine the photodegradation period to use. The UV lamps (352 nm for UV-A, 306 nm for UV-B, and 253.7 nm for UV-C; 15 W) were purchased from the Sankyo Denki Co., Ltd. (Japan). With UV-A, the measured radiation intensity was 1.45 mW cm−2. The photolysis tests were also carried out for 24 h to determine the degradation rate constants and toxicity changes. At predetermined times, samples were taken to analyze the residual Triton X-100 concentrations.
Analysis of the residual Triton X-100
The Triton X-100 concentrations were measured by using HPLC (high-performance liquid chromatography; YL 9100, Republic of Korea) equipped with an AcclaimTM Surfactant Plus LC column (250 mm × 4.6 mm × 5 um; Thermo Fisher Scientific, USA). The Triton X-100 concentrations were determined at 225 nm. Ammonium acetate (10 mM; Purity > 97.0%, Kanto Chemical Co., Inc., Japan) and acetonitrile (HPLC Ultra Gradient Solvent, J.T. Baker, UK) were used as the mobile phase solutions (90:10 v/v) at the flow rate of 1 mL min−1. The column temperature was maintained at an ambient temperature (25 °C). The detection limit of Triton X-100 was 4.05 mg L−1.
NMR analysis
After 24-h photodegradation test, duplicate samples for each condition (i.e., Dark, UVA, UVB, UVC) were combined and dried. Each dried sample was dissolved in 600 μL of DMSO-d6 (Merck, Darmstadt, Germany) and transferred to 5 mm NMR tube. 1H and 13C NMR spectra of the samples were acquired on a 600 MHz Varian NMR spectrometer (VNS-600, Palo Alto, CA, USA) at the Core Research Support Center for Natural Products and Medical Materials (CRCNM). The obtained NMR data was processed using the Mnova software (v.12.0.1, MestreLab Research S.L., Coruña, Spain). The 1H and 13C NMR chemical shift values (δ) were reported in ppm with reference to DMSO-d6 residual peaks at 2.50 and 39.5 ppm, respectively. Coupling constants (J) are reported in Hz. The NMR data analysis was performed based on signal integration, chemical shifts, and coupling constants. Along with 1D NMR, 2D-correlation spectroscopy (COSY), heteronuclear single quantum coherence spectroscopy (HSQC), and heteronuclear multiple-bond correlation spectroscopy (HMBC) data analysis were used to further confirm the structures.
Toxicity tests
The changes in the toxicity of the Triton X-100 solution before and after photodegradation for 24 h were determined by using the Microtox® acute toxicity test. The Microtox toxicity test using A. fischeri was carried out following the 81.9% basic test protocol that is described in the manufacturer’s protocol. The changes in the bioluminescence were measured by using the Microtox M500 (AZUR Environmental, USA) after exposing A. fischeri to the samples for 5, 15, and 30 min. The bioluminescence inhibition was determined using the Microtox Omni software (Azur Corp., ver. 4.1), and the half maximal effective concentration (EC50) was determined using the logistic regression analysis performed with SigmaPlot (v12.5).
The changes in the toxicity of the Triton X-100 solution before and after photodegradation for 24 h were also determined by using D. magna following the acute toxicity test method described in the Korea Standard Method for Water Quality (KMOE 2017). Four replicates with the total number of 20 D. magna were used for each test. D. magna was exposed to the Triton X-100 samples before and after photodegradation for 24 h. The 16-h light period with the 8-h dark period was used during the test period. The effective concentration at which 50% of D. magna is affected (i.e., EC50) for 24-h exposure was determined using the TOXCAL software (Tidepool Scientific Software, USA). The 24-h EC50 values for D. magna under different conditions were calculated using the linear interpolation analysis provided by the TOXCAL software.
Statistical analyses
The statistical significance of the changes in the Triton X-100 concentrations with time was determined by fitting the data to the linear regression model using GraphPad Prism (v8.0.1). The p value was used to determine whether the slope of the linear regression line fitted to the measured data is significantly non-zero at 95% confidence level. The toxic effects determined as EC50 under different conditions were compared using a one-way ANOVA test in SPSS (version 21).
Results and discussion
Effect of UV radiation types on Triton X-100 removal
With the 35-day photodegradation test, the Triton X-100 degradation was statistically insignificant (p value > 0.05) for 35 days under dark conditions (Fig. 1). With UVA, 25% of Triton X-100 was removed over 35 days, while complete Triton X-100 removal was observed within 21 days and 1 day with UVB and UVC, respectively (Fig. 1). Thus, the 24-h photodegradation test was carried out with more sampling points in 24 h. In the 24-h photodegradation test, the Triton X-100 degradation was negligible with UVA (Fig. 2). The Triton X-100 removal reached 81% within 24 h with UVB and 97% within 24 h with UVC (Fig. 2).
Changes in Triton X-100 concentration during the 35-day photodegradation test using different types of UV radiation (a) UVA, (b) UVB, and (c) UVC. The p values indicate the p values determined by fitting the data obtained under the dark conditions to the linear regression model. The p values indicate that the slopes of the linear regression line fitted to the data are significantly non-zero at 95% confidence level
Changes in Triton X-100 concentration during the 24-h photodegradation test using different types of UV radiation. The p values for the data under the dark and UVA conditions were 0.53 and 0.90, respectively, and these indicate that the slopes of the linear regression line fitted to the data are significantly non-zero at 95% confidence level
Previous study used first-order kinetics to describe the photodegradation of Triton X-100 (Saien et al. 2011). The first-order rate constants for the Triton X-100 removal obtained in this study were 0.063 h−1 with UVB and 0.29 h−1 with UVC. The Triton X-100 degradation was approximately 4.6 times faster with UVC than with UVB. It can be expected as shorter wavelengths have stronger energy of the emitted photons (Maverakis et al. 2010). Similarly, the dye degradation was negligible with UVA, but it increased to 12% with UVB and to 58% with UVC (Cortés et al. 2011). Also, the degradation rate of bisphenol A by the photo-Fenton-like treatment was slower with UVA than with UVC (Molkenthin et al. 2013). Furthermore, the photodegradation of Triton X-100 by UV radiation can be enhanced when catalysts such as TiO2 or oxidants such as H2O2 are available in surface waters (Cortés et al. 2011; Saien et al. 2011).
The degradation rate of Triton X-100 by different types of UV radiation was further confirmed by NMR experiments. When the relative integration ratios of the individual peaks were compared, no change was observed in the sample exposed to UVA. However, for UVB- and UVC-exposed samples, it was observed that the resonances from an aromatic hydrophobic group (indicated in blue in Fig. 3) decreased. This indicates that while the sample exposed to UVA remained unchanged, the samples exposed to UVB and UVC were degraded with greater degradation by UVC. This observation of the lower peak intensity could be due to molecular rearrangement of Triton X-100 by UV radiation (Beyer 1982), but when the changes in the Triton X-100 concentration with UV radiation are considered, the lower peak intensity could be attributed to the decomposition of Triton X-100.
Changes in the toxic effects before and after photodegradation
Toxic effects of Triton X-100 after the 24-h degradation under different types of UV radiation on Aliivibrio fischeri are shown in Fig. 4. The data in Fig. 4 were fitted to the logistic regression model to determine the EC50 values. The photodegraded Triton X-100 solutions are likely to contain residual Triton X-100 and intermediate products generated; thus, the EC50 values were estimated based on the dilution percentage of the photodegraded solutions (Fig. 5a). The EC50 values were also estimated using the residual Triton X-100 concentrations in the photodegraded solutions, assuming that the Triton X-100 is the main component of the photodegraded solution (Fig. 5b). The trends in EC50 values in both figures were similar (Fig. 5).
At all exposure times (i.e., 5, 15, and 30 min), the toxic effects were statistically greater (i.e., lower EC50 values) in the UVB- and UVC-radiated samples than in the Dark control samples and UVA-radiated samples (p value < 0.05) (Fig. 5). The EC50 values of the Dark control samples and UVA-radiated samples were statistically similar (p value > 0.05). This can be expected as the Triton X-100 removal under UVA and Dark conditions was similar. Regardless of the exposure times, the EC50 values were statistically similar (p value > 0.05). Also, the EC50 values decreased as the UV wavelength decreased from UVA (i.e., 352 nm) to UVC (i.e., 237.7 nm) (p value < 0.05). For example, the EC50 values for the UVC-radiated samples were lower than the UVB-radiated samples (Fig. 5). The EC10 and EC20 values calculated from the toxic effects observed in Fig. 4 are shown in Online Resource 1.
Figure 6 compares the toxic effects (i.e., EC50) of the Triton X-100 photodegradation under different conditions on D. magna. The EC50 values were estimated using the residual Triton X-100 concentrations. The EC50 values of the Dark control samples and the UVA-radiated samples were statistically similar (p value > 0.05), while they were significantly different from the EC50 values of the UVB- and UVC-radiated samples (p value > 0.05) (Fig. 6). For the Dark control samples, the calculated 24-h EC50 values ranged from 55 to 90 mg L−1 (Fig. 6), and this is comparable to that observed in a previous study where the 24-h EC50 values ranged from 75.3 to 185.5 mg L−1 (i.e., average of 118.2 mg L−1) for Triton X-100 (Santos et al. 2019). They also reported the 48-h EC50 value of 72.2 mg L−1 (49.6–105.0 mg L−1); however, in another study, the 48-h EC50 value of 26.0 mg L−1 was reported (Mohammed 2007).
Half maximal effective concentration (EC50) of Triton X-100 after 24-h photodegradation under different types of UV radiation determined by using Daphnia magna. Four replicates were used to derive one EC50 value for each condition. EC50 values were calculated using the residual Triton X-100 concentrations after 24-h degradation for each condition
Furthermore, with the different model organism, different EC50 values were reported. With Dendrocephalus brasiliensis, the 24-h EC50 values and the 48-h EC50 values ranged from 38.8 to 75.3 mg L−1 (i.e., average of 54.1 mg L−1) and 30.1 to 63.2 mg L−1 (i.e., average of 43.6 mg L−1), respectively (Santos et al. 2019). This suggests that Triton X-100 is relatively more toxic to D. brasiliensis than D. magna. Also, Ceriodaphnia rigaudii was more sensitive than D. magna (Mohammed 2007). Similarly, based on the obtained EC50 values for the Dark control samples in this study, the relative toxic effects were lower for A. fischeri (i.e., higher EC50 values) than D. magna (Fig. 5b and Fig. 6). In other words, D. magna was more sensitive to the Dark control samples where only the parent compound (i.e., Triton X-100) is contained than A. fischeri, as the EC50 values were lower for D. magna.
Interestingly, the EC50 values of the UVB- and UVC-radiated samples where Triton X-100 removal was observed seemed to be lower for A. fischeri, suggesting that the toxic effects on A. fischeri were relatively greater than on D. magna. For D. magna, the EC50 values for UVB- and UVC-radiated samples were 6.9 ± 0.3 and 5.0 ± 0.6 mg L−1, respectively (Fig. 6). For A. fischeri, the EC50 values for UVB-radiated samples were 5.9, 6.4, and 6.8 mg L−1 for 5-, 15-, and 30-min exposures, respectively, while the EC50 values for UVC-radiated samples were 0.46, 0.46, and 0.46 mg L−1 for 5-, 15-, and 30-min exposures, respectively (Fig. 5b). Also, the differences in the toxic effects between the UVB-radiated samples and the UVC-radiated samples were greater for A. fischeri. With the greater removal of Triton X-100 under UVC (Fig. 2), the toxic effects on A. fischeri were lower than that under UVB (Fig. 5b). However, the toxic effects of UVB- and UVC-radiated samples on D. magna did not show a statistically significant difference (p value > 0.05) (Fig. 6). These results suggest that A. fischeri were more sensitive to the photodegraded Triton X-100 than D. magna.
During the UV photodegradation of Triton X-100, photoproducts including ethoxylated structure such as hydroxynonylphenol polyoxyethylene ether and honylphenol polyoxyethylene acid and other smaller structures such as polyoxyethylene alcohol were observed (Huang et al. 2020). In a previous study, toxic effects of some hydrophilic compounds on fish and Vibrio fischeri were different, while neutral hydrophobic compounds shared the same toxic mechanisms (Wang et al. 2016). They attributed such differences to the different movement of hydrophilic compounds through the cell membrane of V. fischeri and the gill of fish (Wang et al. 2016). Likewise, the photoproducts that are likely to be more hydrophilic than the parent compound (i.e., Triton X-100) may move more easily through the cell membrane of A. fischeri than the gill of D. magna, and this could lead to the greater toxic effects of the UVB- and UVC-radiated samples on A. fischeri than D. magna as well as the greater differences between the toxic effect of the UVB-radiated samples and UVC-radiated samples for A. fischeri.
The increase in the toxic effects with the UVB- and UVC-radiated samples suggests possible production of intermediate products with higher toxic effects, since the Triton X-100 concentrations were decreased. Also, the increased toxic effects after photodegradation could be attributed to the generation of molecules that can easily enter the bacterial cell and impose toxic effects (Yuan et al. 2011). The intermediate products (i.e., photoproducts) were not assessed in this study, so it is not clear whether the increased toxic effects are due to the generation of intermediate products with higher toxicity or increased bioavailability of intermediate products that do not necessarily have higher toxicity than Triton X-100. For example, when trimethoprim was subject to ozonation, the toxic effects on A. fischeri increased during the ozonation (Kuang et al. 2013). However, the predicted toxic effects of all the intermediates identified using the ECOSAR program were lower than the toxic effects of the parent compound (i.e., trimethoprim) (Kuang et al. 2013). Also, similar trends were observed with fluoxetine (Park et al. 2020). Thus, the increased toxic effects after ozonation can be largely attributed to the combined effects of these individual intermediates. However, this study emphasizes that the photodegradation of Triton X-100 that is released and present in natural waters could increase the toxic effects on aquatic organisms, and the sensitivity of different organisms may change during the photodegradation of Triton X-100. In other study, the reduced toxic effects on D. magna and A. fischeri were observed after photodegradation of Triton X-45 using peroxymonosulfate and UVC (Olmez-Hanci et al. 2015). However, they observed the initial increase in toxic effects on A. fischeri after 1-min treatment. This was attributed to the formation of early oxidation products that exerted higher toxic effects than the parent compound (Olmez-Hanci et al. 2015).
The photodegradation (60 min) of Triton X-100 was enhanced from 14 to 52% by adding TiO2, a catalyst, and H2O2, an oxidant, and to 78% by adding TiO2 and potassium persulfate (Saien et al. 2011). The enhancement in the Triton X-100 photodegradation can lead to greater Triton X-100 degradation and/or greater removal of photoproducts generated from Triton X-100. At this stage with only the basic acute toxicity test results, it is hard to predict how the toxicity of the photodegraded Triton X-100 will change. Thus, it would be necessary to characterize photoproducts and their toxicity with other toxicity assays such as chronic toxicity and genotoxicity in the future.
The photodegradation of Triton X-100 in waters change the composition of the water by generating photoproducts that are likely to have different chemical properties, and this may lead to increased toxic effects on aquatic organisms. Also, the sensitivity of different aquatic organisms can change during the photodegradation of Triton X-100 due to the changed water composition. Therefore, not only the presence of the parent compound but also the photoproducts that are likely to be generated during photodegradation need to be taken into account when ecological toxic effects are concerned.
Conclusions
The ecological toxic effects of Triton X-100 changed with UV-induced photodegradation. The photodegradation of Triton X-100 in waters can impose greater toxic effects on aquatic organisms when the parent compound is degraded to generate a mixture of photoproducts. Also, the changes in the chemical composition of the Triton X-100 containing water with photodegradation can lead to changes in the relative toxic effects on different aquatic organisms. Therefore, such changes in the ecological toxic effects need to be considered when managing water environment exposed to surfactants such as Triton X-100.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abu-Ghunmi L, Badawi M, Fayyad M (2014) Fate of Triton X-100 applications on water and soil environments: a review. J Surfactant Deterg 17(5):833–838
Arslan-Alaton I, Olmez-Hanci T, Genç B, Dursun D (2013) Advanced oxidation of the commercial nonionic surfactant octylphenol polyethoxylate Triton™ X-45 by the persulfate/UV-C process: effect of operating parameters and kinetic evaluation. Front Chem. https://doi.org/10.3389/fchem.2013.00004
Beyer K (1982) Phase structures, water binding, and molecular dynamics in liquid crystalline and frozen states of the system Triton X-100-D2O: a deuteron and carbon NMR study. J Colloid Interface Sci 86(1):73–89
Borrely S, Rosa JM, Boiani NF, Garcia VSG, Sousa AL (2018) Emerging pollutants, related toxicity, and water quality decreasing: tannery, textile, and pharmaceuticals load pollutants. Biol Eng Med 3(6):1–6
Caux PY, Weinberger P, Carlisle DB (1988) A physiological study of the effects of triton surfactants on Lemna minor L. Environ Toxicol Chem 7(8):671–676
Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS One 7(7):e40848
Contardo-Jara V, Klingelmann E, Wiegand C (2009) Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes. Environ Pollut 157(1):57–63
Cortés JA, Alarcón-Herrera MT, Villicaña-Méndez M, González-Hernández J, Pérez-Robles JF (2011) Impact of the kind of ultraviolet light on the photocatalytic degradation kinetics of the TiO2/UV process. Environ Prog Sustain Energy 30(3):318–325
Das R, Sahoo S, Singh H, Nath S (2019) Humus pesticide interaction: the fate of pesticide in soil environment: an overview. Int J Chem Stud 7(3):3117–3123
Dias N, Mortara RA, Lima N (2003) Morphological and physiological changes in Tetrahymena pyriformis for the in vitro cytotoxicity assessment of Triton X-100. Toxicol in Vitro 17(3):357–366
Druart C, Scheifler R, De Vaufleury A (2010) Towards the development of an embryotoxicity bioassay with terrestrial snails: screening approach for cadmium and pesticides. J Hazard Mater 184(1-3):26–33
Huang K, Liang J, Wang J, Ouyang Y, Wang R, Tang T, Luo Y, Tao X, Yin H, Dang Z, Lu G (2020) Effect of nitrate on the phototreatment of Triton X-100 simulated washing waste containing 4, 4′-dibromodiphenyl ether: kinetics, products and toxicity assessment. Sci Total Environ 732:139247
Karahan Ö (2010) Inhibition effect of linear alkylbenzene sulphonates on the biodegradation mechanisms of activated sludge. Bioresour Technol 101(1):92–97
KMOE (Korea Ministry of Environment) (2017). Act on environmental testing and inspection article 6 official test methods for water quality. Acute toxicity test method ES 04704.1a
Krogh KA, Halling-Sørensen B, Mogensen BB, Vejrup KV (2003) Environmental properties and effects of nonionic surfactant adjuvants in pesticides: a review. Chemosphere 50(7):871–901
Kuang J, Huang J, Wang B, Cao Q, Deng S, Yu G (2013) Ozonation of trimethoprim in aqueous solution: identification of reaction products and their toxicity. Water Res 47(8):2863–2872
Li MH (2008) Effects of nonionic and ionic surfactants on survival, oxidative stress, and cholinesterase activity of planarian. Chemosphere 70(10):1796–1803
Maverakis E, Miyamura Y, Bowen MP, Correa G, Ono Y, Goodarzi H (2010) Light, including ultraviolet. J Autoimmun 34(3):J247–J257
Mesnage R, Antoniou MN (2018) Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front Public Health. https://doi.org/10.3389/fpubh.2017.00361
Mohammed A (2007) Comparative sensitivities of the tropical cladoceran, Ceriodaphnia rigaudii and the temperate species Daphnia magna to seven toxicants. Toxicol Environ Chem 89(2):347–352
Molkenthin M, Olmez-Hanci T, Jekel MR, Arslan-Alaton I (2013) Photo-Fenton-like treatment of BPA: effect of UV light source and water matrix on toxicity and transformation products. Water Res 47(14):5052–5064
Mörtl M, Takács E, Klátyik S, Székács A (2019) Aquatic toxicity and loss of linear alkylbenzenesulfonates alone and in a neonicotinoid insecticide formulation in surface water. Sci Total Environ 652:780–787
Mullin CA, Fine JD, Reynolds RD, Frazier MT (2016) Toxicological risks of agrochemical spray adjuvants: organosilicone surfactants may not be safe. Front Public Health. https://doi.org/10.3389/fpubh.2016.00092
Mustapha DS, Bawa-Allah KA (2020) Differential toxicities of anionic and nonionic surfactants in fish. Environ Sci Pollut Res 27:16754–16762
Nobels I, Spanoghe P, Haesaert G, Robbens J, Blust R (2011) Toxicity ranking and toxic mode of action evaluation of commonly used agricultural adjuvants on the basis of bacterial gene expression profiles. PLoS One 6(11):e24139
Olmez-Hanci T, Arslan-Alaton I, Dursun D, Genc B, Mita DG, Guida M, Mita L (2015) Degradation and toxicity assessment of the nonionic surfactant Triton™ X-45 by the peroxymonosulfate/UV-C process. Photochem Photobiol Sci 14(3):569–575
Park J, An S, Jho EH, Bae S, Choi Y, Choe JK (2020) Exploring reductive degradation of fluorinated pharmaceuticals using Al2O3-supported Pt-group metallic catalysts: catalytic reactivity, reaction pathways, and toxicity assessment. Water Res, p.116242
Pettersson A, Adamsson M, Dave G (2000) Toxicity and detoxification of Swedish detergents and softener products. Chemosphere 41(10):1611–1620
Ríos F, Olak-Kucharczyk M, Gmurek M, Ledakowicz S (2017) Removal efficiency of anionic surfactants from water during UVC photolysis and advanced oxidation process in H2O2/UVC system. Arch Environ Prot 43(1):20–26
Saien J, Ojaghloo Z, Soleymani AR, Rasoulifard MH (2011) Homogeneous and heterogeneous AOPs for rapid degradation of Triton X-100 in aqueous media via UV light, nano titania hydrogen peroxide and potassium persulfate. Chem Eng J 167(1):172–182
Santos VSV, Silveira E, Pereira BB (2019) Ecotoxicological assessment of synthetic and biogenic surfactants using freshwater cladoceran species. Chemosphere 221:519–525
Wang XH, Yu Y, Huang T, Qin WC, Su LM, Zhao YH (2016) Comparison of toxicities to Vibrio fischeri and fish based on discrimination of excess toxicity from baseline level. PLoS One 11(2):e0150028
Wang R, Yuan Y, Yen H, Grieneisen M, Arnold J, Wang D, Wang C, Zhang M (2019) A review of pesticide fate and transport simulation at watershed level using SWAT: current status and research concerns. Sci Total Environ 669:512–526
Yuan F, Hu C, Hu X, Wei D, Chen Y, Qu J (2011) Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J Hazard Mater 185(2-3):1256–1263
Zhu X, Dsikowitzky L, Kucher S, Ricking M, Schwarzbauer J (2019) Formation and fate of point-source nonextractable DDT-related compounds on their environmental aquatic-terrestrial pathway. Environ Sci Technol 53(3):1305–1314
Funding
This study was funded by the National Research Foundation of Korea (NRF-2018R1C1B6002702). The funding body was not involved in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SHY and EHJ were involved in the design of the study and collection and analysis of data. PT and JWN performed the NMR analysis and interpretation. EHJ analyzed and interpreted all the data obtained from this study and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Jho, E., Yun, S.H., Thapa, P. et al. Changes in the aquatic ecotoxicological effects of Triton X-100 after UV photodegradation. Environ Sci Pollut Res 28, 11224–11232 (2021). https://doi.org/10.1007/s11356-020-11362-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11362-2