Abstract
This research was carried out with an objective to examine the efficacy of ultrafiltered xylano-pectinolytic enzymes in pulping of sugarcane bagasse. Maximum biopulping was achieved with enzyme dose of xylanase (175 IU / g bagasse) and pectinase (75 IU / g bagasse) at treatment period of 180 min. The temperature, pH, and bagasse to liquid ratio for biopulping experiments were kept constant at 55o C, 8.5, and 1:10 (g/ml), respectively. The ultrafiltered biopulping improved chemical pulping, resulted in 25.11%, 9.17% increase in brightness, unscreened pulp production and 11.81, 59.50, and 49.14% decrease in total solids, rejections. and kappa number, respectively. The bagasse biopulping also resulted in 15% decrease of alkali load to attain similar kappa number and optical properties as obtained under 100% alkali dosage. Ultrafiltered biopulped-unbleached samples showed significant increase in breaking length (13.55%), burst index (40.21%), tear index (19.04%), double fold (42.5%), Gurley porosity (28.21%) and viscosity (13.37%) in comparison with non-enzymatically treated control pulp samples. In comparison with non biotreated-bleached pulp samples, ultrafiltered biopulped-bleached samples also resulted in higher burst index (56.80%), breaking length (17.38%), double fold (39.58%), tear index (3.38%), viscosity (30.68%), and Gurley porosity (52.50%). This environmentally sustainable ultrafiltered biopulping approach for sugarcane bagasse has the potential to decrease the demand of chemicals, ultimately pollution along with enhance the quality of paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The market for pulp continues to grow rapidly across the globe. The boom in the paper production has shown no signs of slowing down, due to population explosion. It is expected that the demand for raw material would surpass the supply of pulp, from highly limited forest resources. This has led the researchers to look for suitable additional non-woody material resources for pulp and paper manufacturing. In addition to search for new raw materials, biodegradation of lignocellulosics has been steadily stressed (Fahmy et al. 2017), and much focus has now been given to the development of new, eco-friendly technology for the production of pulp and paper (Bajpai 2015; Söderholm et al. 2019). The use of agro-waste for the production of paper is an environment friendly approach (Sadh et al. 2018). Many types of non-wood lignocellulosic farming by-products have been studied; the most prominent one is sugarcane bagasse (Eugenio et al. 2019). In some countries, 70% of the pulp industry’s raw materials come from non-woody plants like bagasse and cereal straws (Laftah and Wan Abdul Rahman 2016).
Bagasse is a by-product after extraction of juice from sugarcane. Around 300 kg of bagasse is recovered from each ton of sugarcane (Khristova et al. 2006). Many pulp and paper mills also use bagasse for paper manufacturing. Sugarcane bagasse has been reported to possess approximately 42% cellulose, 22.5% xylan, and 20% lignin as structural polymers (Ferreira-Leitão et al. 2010; Kim and Day 2011). Mamaye et al. (2019) reported that high cellulose, along with fiber dimension characteristics, makes sugarcane bagasse suitable for paper industry.
In pulp and paper industry, lignin removal by chemical pulping and bleaching is done. Since these processes consume large amounts of chemicals, the paper production is allied with generation of large amount of pollution and consumption of water (Hubbe et al. 2016). The effluents generated from the paper industry contain many hazardous compounds like chlorinated phenolic compounds, resins acids, phenolic carboxylic acids, and hydrocarbons (Tripathi et al. 2020). The paper industry has been consistently looking for eco-friendly biological substitute, for chemicals used in the paper making process, in order to reduce consumption of pulping chemicals and also to get better quality pulp. The alternative and environmental friendly approach for pulping with bacterial enzymes can be used to improve the quality of paper as well as to reduce environmental pollution. Process of making pulp using ultrafiltered xylano-pectinolytic enzymes can be useful biotechnological approach. Biopulping shows the future direction of development of sustainable pulping technology.
In this study, emphasis was on the use of ultrafiltered xylano-pectinolytic treatment prior to chemical pulping of sugarcane bagasse, with an aim to reduce toxic pulping chemical consumption along with production of better quality paper. This type of work has not been reported in the literature till date.
Materials and methods
Microorganism and other materials
In this investigation, the bacterial strain Bacillus pumilus AJK (MTCC Accession No. 10414) was used for simultaneous production of xylano-pectinolytic enzymes. All chemicals used in this work were of analytical reagent grade. Agro-residues, such as citrus peel and wheat bran used for enzymes production, were procured from local market. The sugarcane bagasse samples were collected from local market of Kurukshetra, India, and were depithed manually. Depithed bagasse samples were chopped in small pieces (2–3 in.), washed thrice times to clean dirt, air dried till constant weight was obtained, and were placed in plastic bags till further use.
Production of xylano-pectinolytic enzymes and activity estimation
For xylanase and pectinase production, medium (50 ml in 250 ml Erlenmeyer flasks) contained citrus peel (1 g), wheat bran (1 g), peptone (0.25 g), 10 mM MgSO4, and pH 7 and was autoclaved for sterilization. Inoculum (2% v/v) of 21-h old was used, and flasks were incubated at temperature 37 °C for 60 h at 200 rpm (Kaur et al. 2017). Crude enzyme preparations were obtained after centrifuging the inoculated material at 10,000 rpm for 10 min, and the crude enzymes were further used for ultrafiltration step.
Activity of xylanase and pectinase was measured as reported by Kaur et al. (2011), by estimating the reducing sugars released from substrates, 2% birchwood xylan, and 1% pectin, using Miller (1959).
Ultrafiltration of xylano-pectinolytic enzymes
Extraction of clear crude enzymes was done by microfiltration unit (Model Quixstand system from GE Healthcare Biosciences Ltd., Hongkong) using autoclavable and CIPable polysulfone membrane cartridge with a pore size of 0.2 μm, membrane area of 110 cm2, 30 cm nominal flow path length and operated at 5 psi transmembrane pressure. For preparation of ultrafiltered enzymes extract, microfiltered crude extract was passed through 1KDa NMCO ultrafiltered membrane and used further for experiments after discontinuous diafiltration and concentration.
Biopulping treatment
In order to find out the efficacy of ultrafiltered xylano-pectinolytic enzymes in biopulping, for effective removal of non-cellulosic impurities from the sugarcane bagasse, parameters such as retention time and enzymes dose were checked. All experiments were carried out at bagasse to liquid ratio of 1:10 (g/ml), pH 8.5, and temperature 55 °C. The different enzyme doses of xylanase and pectinase ranging from 50:21 to 250:107 (IU/g bagasse) and retention time ranging from 60 to 360 min were checked, so as to find out most effective conditions. In order to stop the enzymatic reaction, biopulped samples were washed three times with hot water and kept at 37 °C for drying. Similarly, the control samples were prepared by providing the same conditions to sugarcane bagasse samples, but without adding enzymes in them. Each experiment was repeated five times and in each repeat, each sample in triplicates was tested.
Evaluation of biopulping conditions
The effect of ultrafiltered enzymes during biopulping was evaluated by measuring the reducing sugar content in filtrates obtained after treating sugarcane bagasse samples with enzymes, by Miller’s method (1959). The release of lignin, phenolic compounds, and hydrophobic compounds was also checked in filtrate samples by measuring optical density at wavelengths 209, 280, and 320 nm for lignin (Nissen et al. 1992; Khandeparkar and Bhosle 2007), at 237 and 465 nm for phenolic (Gupta et al. 2000; Khandeparkar and Bhosle 2007) and hydrophobic compounds (Patel et al. 1993), respectively.
Each experiment was repeated five times, and in each repeat, each sample in triplicates was tested. Microscopic images were also taken for evaluating the effect of ultrafiltered biopulping on sugarcane bagasse samples as compared with raw samples.
Production of chemical pulps
After biopulping of sugarcane bagasse, merging of biopulping with chemical pulping was done and exact reduction in alkali consumption after biopulping treatment was checked by decreasing pulping chemical dose gradually, step by step. For chemical pulping, ratio of bagasse to liquid 1:5 (g/ml), alkali dose (13.2–16%), temperature 168 °C, heating time 90 min, holding time 45 min and anthraquinone 0.05% were used. In order to study the effect of ultrafiltered enzymes on the pulpability of sugarcane bagasse samples, both non-biopulped (control) and biopulped sugarcane bagasse samples were further subjected to same pulping conditions (16% alkali load) and the reduction in alkali dose, in case of biopulped sugarcane bagasse samples were also checked from 100 to 83% as compared with control. Analysis of residual alkali content, pH, and total solids content in black liquor was done by method TAPPI T625 cm-14 (2014). Pulp samples were washed thrice with water and further used for measuring unscreened pulp yield (Young 1997) and Kappa number by TAPPI Method T-236 om-99 (2004). Screened pulp was used for evaluating pulp viscosity (TAPPI T230 om-99 1999) and physical and optical properties. Each experiment was repeated five times, and in each repeat, each sample in triplicates was tested.
Bleaching of enzymatically treated and control sugarcane bagasse soda-anthraquinone pulps
After pulping, both control samples (100% chemically treated) and biopulped samples (15% reduced alkali dose used) were bleached using conventional bleaching sequences (i.e., D-0, EP, D-1, D-2). Here D-0, D-1 and D-2 stages represent for chlorine dioxide bleaching and EP denotes for alkali peroxide bleaching stage. The chlorine dioxide dose was calculated at the various kappa factors by using equation, as given by Hise (1996). After completion of each stage of bleaching, bleached pulp liquor was analyzed for measuring the percentage consumption of ClO2. Pulp was washed at each stage, followed by analysis of optical properties, kappa number, and strength properties. Each experiment was repeated five times, and in each repeat, each sample in triplicates was tested.
Testing of biopulped-unbleached and biopulped-bleached handsheets
Formation of handsheets of 100% chemically treated pulp samples and biopulped samples (with 15% reduced alkali dose) was done by TAPPI method T205 sp-02 (2002), for measuring further various strength related studies. Schopper-Riegler number (oSR) of pulp was analyzed by using SCAN-C 19:65 (1999). Analysis of handsheets for physical properties like burst index, breaking length, tear index, Gurley porosity, double fold, and optical parameters was done by TAPPI T403 om-10 (2010), TAPPI T494 om-01 (2001), TAPPI T414 om-04 (2004), TAPPI T460 om-02 (2002), TAPPI T511 om-02 (2002), and TAPPI T217 wd-77 (2004), respectively.
Microscopic analysis of biopulped sugarcane bagasse
Raw and biopulped sugarcane bagasse samples were also microscopically examined (magnification 400X) for analyzing the effect of biopulping.
Results and discussion
Evaluation of biopulping
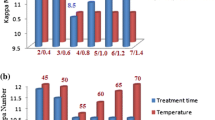
Ultrafiltered xylano-pectinolytic enzymes has been used for evaluation of biopulping conditions by measuring the reducing sugar content in biopulp-free filtrate samples, by Miller (1959). The release of lignin, phenolic, and hydrophobic compounds was checked in filtrates of biopulped samples by measuring optical density at λ 209 nm-λ 280 nm-λ 320 nm, λ 237 nm, and λ 465 nm, respectively. All experiments were performed at bagasse to liquid ratio of 1:10 g/ml, pH 8.5. and temperature 55 °C. Xylanase and pectinase dose of 175 and 75 IU/g of oven-dried sugarcane bagasse, respectively, and were adequate for efficient treatment (Fig. 1i). The best retention time for sugarcane bagasse pulping with ultrafiltered xylano-pectinolytic enzymes was found to be 180 min (Fig. 1ii). Release of reducing sugar content was nearly 80.39 + 4 mg/g of oven dried sugarcane bagasse. UV-Visible absorption profile of bio-pulp filtrates for lignin content, phenolic compounds, and hydrophobic compounds at λ 209 nm-λ 280 nm--λ 320 nm, λ 237 nm, and λ 465 nm, respectively, is shown in Fig. 2. The ultrafiltered enzymatic treatment also resulted in the release of impurities like lignin, hydrophobic, and phenolic compounds in biopulp filtrates, which is due to the degradation of xylan and pectin from sugarcane bagasse. Effectiveness of biopulping with ultrafiltered xylano-pectinolytic enzymes also supported by microscopic images of raw sugarcane bagasse and ultrafiltered biopulped sugarcane bagasse samples (Fig. 3).
Effect of enzymes dose (i) and treatment time (ii) on sugarcane bagasse ultrafiltered biopulping. i These experiments were performed at treatment time of 360 min, bagasse to liquid ratio 1:10 g/ml, pH 8.5, and temperature 55 °C. ii These experiments were performed at enzyme dose of xylanase (175 IU/g of bagasse) and pectinase (75 IU/g of bagasse), bagasse to liquid ratio 1:10 g/ml, pH 8.5, and temperature 55 °C. Control = non-biopulped samples, test = biopulped samples
Absorbance profile of sugarcane bagasse filterate obtained after ultrafiltered biopulping. i These experiments were performed at treatment time of 360 min, bagasse to liquid ratio 1:10 g/ml, pH 8.5 and temperature 55 °C. ii These experiments were performed at enzyme dose of xylanase (175 IU/g of bagasse) and pectinase (75 IU/g of bagasse), bagasse to liquid ratio 1:10 g/ml, pH 8.5 and temperature 55 °C. Absorbance for all experiments was taken after 10X dilution in comparison to control
Comparison of biopulping with chemical pulping
To analyze the effect of enzymatic biopulping over chemical pulping, both non-biopulped (control) and biopulped samples of sugarcane bagasse were subjected to chemical pulping under the same conditions (Table 1). Reduction in kappa number (49.14%) along with 25.11% increase in brightness was obtained in biopulped samples. Residual active alkali of black liquor were also increased from 1.10 to 2.52 (g/l as NaOH), and total solids reduced to 11.81% in case of sugarcane bagasse ultrafiltered biopulped samples as compared with control pulp samples. Biopulping also resulted in screened pulp yield increase by 12.22%, rejects decrease by 59.50%, and viscosity values increased by 13.37%. These results illustrate that ultrafiltered xylano-pectinolytic enzymatic pulping could be an effective solution for reducing the alkali dose during chemical pulping. The exact reduction in alkali dose due to biopulping step was determined by treating biopulped samples with different alkali doses (i.e., 83, 85, 90, 95, and 100%) and 15% reduction in alkali dose along with higher pulp yield obtained (Table 1). These results concluded that biopulped samples require less alkali dose due to the removal of non-cellulosic impurities, which further increase the access of pulping chemicals to lignin layer, in enzymatically treated samples as compared with control samples.
To compare the physical properties, both pulp samples (100% chemically pulped and enzymes treated pulp with 85% pulping chemicals) were subjected to handsheets formation by using the method TAPPI T205 sp-02 (2002). Ultrafiltered biopulped-unbleached samples showed significant increase in breaking length (13.55%), burst index (40.21%), tear index (19.04%), double fold (42.5%), and Gurley porosity (28.21%) in comparison with control pulp samples (Table 1).
Effect of ultrafiltered biopulping on bleachability of pulp
After 15% reduction of alkali dose, pulp samples were subjected to bleaching in order to check the effect of ultrafiltered biopulping treatment on bleaching properties (Table 2). The ultrafiltered biopulped samples showed improvement in brightness (7.93%) along with less consumption of chlorine dioxide after D-0 stage of bleaching (Table 2). In EP stage of bleaching, increase of whiteness by 9.10% along with reduction in yellowness and kappa number by 17.22% and 17.44%, respectively, in biopulped samples was noticed, as compared with control pulp samples. Similarly, the ultrafiltered biopulped samples showed improvement in whiteness (3.10%) along with reduction in yellowness (10.19%) after D-1 stage of bleaching (Table 2). After D2 stage of bleaching, the decrease in yellowness by 22.42% was obtained in bleached biopulped samples in comparison with control samples. Consumption of chlorine dioxide was also reduced by 14.47% in biopulped samples. This may be due to the opening of structure during biopulping, which facilitates effective delignification by the pulping and bleaching chemicals, due to the removal of impurities like xylan and pectin, from the structure of cell wall. This approach also enhanced the overall optical quality of paper. The effect of ultrafiltered xylano-pectinolytic treatment on the physical properties of bleached pulps was also checked. Comparison with samples treated with 100% chemicals, the biopulped samples with 85% alkali dose had higher tear index (3.38%), burst index (56.80%), breaking length (17.38%), double fold (39.58%), Gurley porosity (52.50%), and viscosity (30.68%) under conventional bleaching conditions (Table 2). No report is available in the literature for comparison of data on sugarcane bagasse ultrafiltered biopulping. These results concluded that ultrafiltered xylano-pectinolytic treatment before the chemical pulping requires less alkali charge as well as bleaching chemicals. This enzymatic treatment also resulted in improved optical and physical properties of both unbleached and bleached pulp samples. So, this biotechnological approach seems to be the effective for reducing pollution along with producing better quality paper. A brief summary of manuscript is shown in Fig. 4.
Conclusion
This study demonstrates that biopulping with ultrafiltered xylano-pectinolytic enzymes can produce better quality sugarcane bagasse pulp. This ultrafiltered biopulping approach has potential to improve the pulpability as well as bleachability, which lowers the demand of both pulping and bleaching chemicals in paper production. Less chemical consumption, would ultimately lead to reduction of environmental impact of paper making processes. Thus, biopulping using ultrafiltered xylano-pectinolytic treatment is dual benefit approach for paper and pulp industry in making better quality paper along with less pollution.
References
Bajpai P (2015) Green chemistry and sustainability in pulp and paper industry. Springer International Publishing, Cham, pp 217–246
Eugenio ME, Ibarra D, Martín-Sampedro R, Espinosa E, Bascón I, Rodríguez A (2019) Alternative raw materials for pulp and paper production in the concept of a lignocellulosic biorefinery. InCellulose. https://doi.org/10.5772/intechopen.90041
Fahmy Y, Fahmy TYA, Mobarak F, El-Sakhawy M, Fadl MH (2017) Agricultural residues (wastes) for manufacture of paper, board, and miscellaneous products: background overview and future prospects. Int J Chem tech Res 10(2):424–448
Ferreira-Leitão V, Perrone CC, Rodrigues J, Franke APM, Macrelli S, Zacchi G (2010) An approach to the utilisation of CO2 as impregnating agent in steam pretreatment of sugar cane bagasse and leaves for ethanol production. Biotechnol Biofuels 3(1):7
Gupta S, Bhushan B, Hoondal GS (2000) Isolation, purification and characterization of xylanase from Staphylococcus sp. SG-13 and its application in biobleaching of Kraft pulp. J Appl Microbiol 88:325–334
Hise R (1996) Pulp bleaching – principles and practice (Dence, C.W. & amp; Reeve, D. W. eds.), Tappi Press Atlanta, Georgia, USA, pp 241–259
Hubbe MA, Metts JR, Hermosilla D, Blanco MA, Yerushalmi L, Haghighat F, Lindholm-Lehto P, Khodaparast Z, Kamali M, Elliott A (2016) Wastewater treatment and reclamation: a review of pulp and paper industry practices and opportunities. BioResources 11(3):7953–8091
Kaur A, Mahajan R, Singh A, Garg G, Sharma J (2011) A novel and cost effective methodology for qualitative screening of alkalo-thermophilic cellulase free xylano-pectinolytic microorganisms using agricultural wastes. World J Microbiol Biotechnol 27(2):459–463
Kaur A, Singh A, Dua A, Mahajan R (2017) Cost-effective and concurrent production of industrially valuable xylano-pectinolytic enzymes by a bacterial isolate Bacillus pumilus AJK. Prep Biochem Biotechnol 47(1):8–18
Khandeparkar R, Bhosle NB (2007) Application of thermoalkalophilic xylanase from Arthrobacter sp. MTCC 5214 in biobleaching of Kraft pulp. Bioresour Technol 98(4):897–903
Khristova P, Kordsachia O, Patt R, Karar I, Khider T (2006) Environmentally friendly pulping and bleaching of bagasse. Ind Crop Prod 23(2):131–139
Kim M, Day DF (2011) Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J Ind Microbiol Biotechnol 38(7):803–807
Laftah WA, Wan Abdul Rahman WA (2016) Pulping process and the potential of using non wood pineapple leaves fiber for pulp and paper production: a review. J Nat Fibers 13(1):85–102
Mamaye M, Kiflie Z, Feleke S, Yimam A, Jabasingh SA (2019) Valorization of ethiopian sugarcane bagasse to assess its suitability for pulp and paper production. Sugar Tech 21(6):995–1002
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Nissen AM, Anker L, Munk N, Lange K (1992) Xylanase for the pulp and paper industry. In: Visser J, Beldman G, Kustersvan someren MA, Voragen AGJ (Eds.), Biotechnol Prog, pp 325–337
Patel RN, Grabski AC, Jeffries TW (1993) Chromophore release from Kraft pulp by purified Streptomyces roseiscleroticus xylanases. Appl Microbiol Biotechnol 39:405–412
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5(1):1–15
SCAN-C 19:65 (1999) Pulps Determination of drainability-Part 1 (Schopper-Riegler method)
Söderholm P, Bergquist AK, Söderholm K (2019) Environmental regulation in the pulp and paper industry: impacts and challenges. Curr For Rep 5(4):185–198
TAPPI T205 sp-02 (2002) Forming handsheets for physical tests of pulp. TAPPI Press, Atlanta
TAPPI T217 wd-77 (2004) Brightness of pulp. TAPPI Press, Atlanta
TAPPI T230 om-99 (1999) Viscosity of pulp (capillary viscometer method). TAPPI Press, Atlanta
TAPPI T236 om-99 (2004) Kappa number of pulp. TAPPI Press, Atlanta
TAPPI T403 om-10 (2010) Burst strength of paper. TAPPI Press, Atlanta
TAPPI T414 om-04 (2004) Internal tearing resistance of paper (Elmendorf-type method). TAPPI Press, Atlanta
TAPPI T460 om-02 (2002) Air resistance of paper. TAPPI Press, Atlanta
TAPPI T494 om-01 (2001) Tensile breaking properties of paper and paperboard. TAPPI Press, Atlanta
TAPPI T511 om-02 (2002) Folding endurance of paper (MIT tester). TAPPI Press, Atlanta
TAPPI T625 cm-14 (2014) Analysis of soda and sulfate 379 black liquor. TAPPI Press, Atlanta
Tripathi SK, Bhardwaj NK, Ghatak HR (2020) Effect of different elemental chlorine-free bleaching sequences on pulp, effluent properties and their impact on index of global pollution. Environ Sci Pollut Res 27(5):4917–4926
Young RA (1997) Processing of agro-based resources into pulp and paper. Paper and composites from agro-based resources. CRC Press/Lewis Publishers, New York, pp 137–245
Acknowledgments
The authors thankfully acknowledge the financial support provided by Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India (Grant Number: BT/ PR 20438 / BCE /8 /1220 / 2016 for 3 years). The authors are also thankful to Director, Avantha Centre for Industrial Research and Development, Yamuna Nagar for allowing to carry out this research work.
Availability of data and materials
Not Applicable.
Funding
The financial support was provided by Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India (Grant Number: BT/ PR 20438 / BCE /8 /1220 / 2016 for 3 years).
Author information
Authors and Affiliations
Contributions
1. Ritu Mahajan (corresponding author)
Idea of concept, planning, and designing of various experiments done for this manuscript. Experimental work done by the first authors for this manuscript under the corresponding author’s supervision.
2. Libin Mathew Varghese (first author)
All enzymatic experimental work mentioned in this manuscript done, at Research laboratory level, and manuscript written by him under the supervision of corresponding author.
3. Raksha Nagpal (first author)
All paper mill experimental work mentioned in this manuscript was done, at Paper Industry level, and also revised manuscript under the supervision of corresponding author.
4. Avtar Singh
Microscopic analysis of the samples was done by him.
5. Om Prakash Mishra
Guided the research work done, by Raksha Nagpal, at Paper Industry.
6. Nishi Kant Bhardwaj
Research work done, by Raksha Nagpal, at Paper Industry level under his supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
Not Applicable.
Consent to publish
Not Applicable.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varghese, L.M., Nagpal, R., Singh, A. et al. Ultrafiltered biopulping strategy for the production of good quality pulp and paper from sugarcane bagasse. Environ Sci Pollut Res 27, 44614–44622 (2020). https://doi.org/10.1007/s11356-020-11102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11102-6