Abstract
Liver fibrosis occurs in most types of chronic liver diseases and can develop into cirrhosis and liver failure. Bone marrow-derived mesenchymal stem cells (BMSCs) showed promising effects in the treatment of fibrosis. This study evaluated the possible role of Nrf2/HO-1 signaling in the ameliorative effect of BMSCs against carbon tetrachloride (CCl4)-induced liver fibrosis, oxidative stress, and inflammation in rats. Hepatic fibrosis was induced by subcutaneous injection of CCl4 twice per week for 6 consecutive weeks and rat BMSCs were administered intravenously. After 4 weeks, the rats were sacrificed, and samples were collected for analysis. CCl4-intoxicated rats showed elevated serum transaminases, ALP, γGT, bilirubin and pro-inflammatory cytokines, and decreased albumin. Hepatic NF-κB p65 and malondialdehyde (MDA) were significantly increased, and cellular antioxidants were decreased in CCl4-intoxicated rats. BMSCs ameliorated liver function markers, suppressed MDA, NF-κB p65, and inflammatory cytokines, and enhanced antioxidants in the liver of CCl4-intoxicated rats. BMSCs were engrafted within the liver tissue and prevented histological alterations and collagen accumulation induced by CCl4. In addition, BMSCs upregulated hepatic Nrf2 and HO-1 expression in CCl4-intoxicated rats. In conclusion, this study provides evidence that BMSCs suppress oxidative stress, inflammation, and liver fibrosis through a mechanism involving activation of the Nrf2/HO-1 signaling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
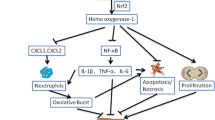
Chronic liver diseases are characterized by progressive deterioration of liver function and represent a significant problem worldwide with socioeconomic burden. The number of people suffering from chronic liver diseases has been estimated to exceed 800 million with 2 million deaths every year (Marcellin and Kutala 2018). Long-term hepatocyte injury provokes the loss of liver function and the excessive deposition of extracellular matrix (ECM), leading to the development of fibrogenesis. Hepatic fibrogenesis is a complex and multifactorial process characterized by inflammation and accumulation of ECM rich in collagen (Friedman 2008b), and can develop into an advanced stage of scarring and damage known as cirrhosis and hepatocellular carcinoma (HCC) (Tacke and Trautwein 2015). In chronic liver diseases, the excessive ECM is produced by the active hepatic stellate cells (HSCs). These cells are activated during chronic liver injury by different factors, including oxidative stress and inflammation (Gandhi 2012; Josan et al. 2015), and promote fibrogenesis by producing excess ECM (Friedman 2008a). Therefore, attenuation of inflammation and oxidative stress is critical for preventing fibrogenesis in liver disease. Accordingly, upregulation of the nuclear-factor erythroid 2-related factor 2 (Nrf2) ameliorated fibrosis in the lung of bleomycin- (Ni et al. 2015) and paraquat-induced rats (Tai et al. 2020). Nrf2 is a transcription factor that protects the cells against oxidative stress and inflammation by regulating the expression of antioxidant and defensive factors. It exists in the cytosol sequestered by Kelch-like ECH-associated protein 1 (Keap1), a protein which mediates the ubiquitination and proteasomal degradation of Nrf2 through adapting CUL-E3 ligase (Itoh et al. 1999). When reactive oxygen species (ROS) increase within the cells, Keap1 dissociates from CUL-E3 ligase, and Nrf2 translocates into the nucleus to stimulate the transcription of its target genes, including heme oxygenase-1 (HO-1) (Eggler et al. 2005).
Liver transplantation is a treatment option for cirrhosis and HCC; however, several problems, such as, the high cost, incompatibility, and surgical difficulties are the main obstacles precluding its use (Schuppan and Afdhal 2008). Thus, tissue repair via regenerative therapies represents an effective alternative to liver transplantation in patients with cirrhosis and liver failure. In this context, mesenchymal stem cells (MSCs) are multipotent stem cells reside in bone marrow and adipose tissue and possess the ability to differentiate to different lineages. Bone marrow-derived MSCs (BMSCs) have shown promising effects in the treatment of experimental hepatic and pulmonary fibrosis (Duman et al. 2019; Higashiyama et al. 2007; Ni et al. 2015). Human studies have also revealed the beneficial effects of BMSCs in patients with alcoholic cirrhosis (Suk et al. 2016) and hepatitis-related liver failure (Lin et al. 2017). In a rat model of bleomycin-induced pulmonary fibrosis, BMSCs showed beneficial effects associated with upregulation of Nrf2 expression (Ni et al. 2015). In addition, bleomycin-induced aged mice received BMSCs showed a reduction in pulmonary endoplasmic reticulum (ER) stress mediated via modulation of Nrf2 (Lee et al. 2020). Moreover, BMSCs have shown beneficial effects and improved liver function in animal models of hepatocarcinogenesis (Abdel aziz et al. 2011) and liver injury induced by carbon tetrachloride (CCl4) (Ahmed et al. 2014). However, the possible involvement of Nrf2 signaling in the ameliorative effect of BMSCs on liver fibrosis has not been demonstrated. Therefore, this study explored the ameliorative effect of BMSCs on CCl4-induced liver fibrosis, oxidative injury, and inflammation in rats, emphasizing the role of Nrf2/HO-1 signaling.
Materials and methods
Experimental animals
This study included 6–8-week old male albino rats weighing 130–150 g. The animals were obtained from the National Research Center (Giza, Egypt) and were housed under standard laboratory conditions (12 h light/dark cycle and 23 ± 1 °C) with free access to water and a standard diet.
Preparation and characterization of BMSCs
BMSCs were isolated from 6-week-old male rats as previously described (Abdel aziz et al. 2011). Briefly, bone marrow was harvested from the femurs of rats by flushing with DMEM (GIBCO) supplemented with 10% fetal bovine serum (FBS; GE Healthcare, UK) and 1% penicillin/streptomycin. Mononuclear cells were isolated by centrifugation over a Ficoll gradient (Sigma, USA) suspended in complete DMEM. The cells were resuspended in complete medium and incubated at 37 °C in 5% CO2 in saturated humidity. The medium was changed every other day until the cells reached 80–90% confluency (day 12) and large colonies were developed. The cells were then trypsanized and subcultered in flasks until reached 90% confluency and then collected and filtered through a 0.22-μm filter. The cells were characterized by their fibroblast-like morphology and their differentiation capacity (Abdel aziz et al. 2011).
Labelling BMSCs with PKH26
To investigate the homing of BMSCs in the liver, the undifferentiated cells were labelled with PKH26 (Sigma, USA) following the manufacturer’s instructions. This red fluorochrome is physiologically stable and does not affect the biological and proliferating activity of BMSCs (Abdel aziz et al. 2011).
Experimental groups
Twenty-four male rats were assigned into 4 groups (n = 6) as follows (Fig. 1):
-
Group I (control): received subcutaneous (s.c.) injections of corn oil twice/week for 6 weeks followed by a single intravenous (i.v.) injection of phosphate-buffered saline (PBS).
-
Group II (CCl4): received s.c. injections of 3 ml/kg CCl4 (Sigma, USA) dissolved in corn oil twice/week for 6 weeks (Lin et al. 2018) followed by a single intravenous (i.v.) injection of PBS.
-
Group III (CCl4/BMSCs): received s.c. injections of 3 ml/kg CCl4 dissolved in corn oil twice/week for 6 weeks followed by a single i.v. injection of 3 × 106 BMSCs suspended in PBS (Abdel aziz et al. 2011).
-
Group IV (CCl4/-): received s.c. injections of 3 ml/kg CCl4 dissolved in corn oil twice/week for 6 weeks followed by a single i.v. injection of PBS.
Rats of group II were sacrificed 24 h after the i.v. administration of PBS, whereas rats in groups I, II, and IV were sacrificed 4 weeks after PBS or BMSCs administration. All rats were sacrificed under thiopental (Eipico, Egypt) anesthesia (50 mg/kg), and blood samples were collected via cardiac puncture for serum preparation. The blood was left to coagulate and centrifuged at 3000 rpm for 15 min and serum was separated. The animals were sacrificed by cervical dislocation, dissected, and the liver was removed and washed in cold PBS. Liver samples were fixed in 10% neutral buffered formalin for histological studies, and others were stored at − 80 °C for the assessment of malondialdehyde (MDA), antioxidants, and gene and protein expression.
Assay of liver function markers and cytokines
Serum transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), alkaline phosphatase (ALP) and γ-glutamyl transferase (γGT) were determined using reagent kits supplied by Biosystems (Spain). Serum bilirubin and albumin were assayed using kits purchased from Diamond Diagnostics (Egypt). Tumor necrosis factor (TNF)-α and interleukin (IL)-6 were determined in serum using R&D Systems (USA; Cat. No. RTA00 and R6000B, respectively) ELISA kits. All assays were carried out according to the manufacturers’ instructions.
Assay of lipid peroxidation (LPO) and antioxidants
Samples from the liver were homogenized (10% w/v) in cold PBS, and the homogenate was centrifuged, and clear supernatant was separated. MDA, a marker of LPO (Ohkawa et al. 1979), reduced glutathione (GSH) (Beutler et al. 1963), superoxide dismutase (SOD) (Marklund and Marklund 1974), and glutathione-s-transferase (GST) (Mannervik and Guthenberg 1981) were determined in the clear supernatant.
Histological examination
Liver samples fixed in neutral buffered formalin for 24 h were processed for paraffin embedding. Five micrometers of sections were cut using rotary microtome and stained with hematoxylin and eosin (H&E). Other sections were stained with Masson’s trichrome (MT) for the assessment of collagen deposition. Both H&E- and MT-stained sections were examined using a light microscope. The MT-positive area was quantified using ImageJ (version 1.32j, NIH, USA). To track the homing of PKH26-labelled BMSCs, sections from the liver were examined with a fluorescence microscope.
Gene expression analysis
Total RNA was isolated from liver samples using RNA isolation kit (Thermo Scientific, USA), and its quantity was determined by measuring optical density (OD) at 260 nm. RNA samples with OD26/OD280 ≥ 1.8 were reverse transcribed into cDNA using Revert AidTM First Strand cDNA Synthesis Kit (Thermo Scientific, USA). cDNA was amplified by SYBR Green master mix (Thermo Scientific, USA) in a total volume of 20 μl using the primer set listed in Table 1. The cycling conditions consisted of initial denaturation at 95 °C for 2 min followed by 40 cycles of denaturation at 95 °C for 5 s, annealing for 10 s, and extension at 72 °C for 30 s. The obtained data were analyzed using the 2-ΔΔCt method (Livak and Schmittgen 2001), normalized to β-actin and presented as % of control.
Western blotting
The changes in NF-κB p65, Nrf2, and HO-1 were evaluated in the liver samples using western blotting as previously described (Mahmoud et al. 2017a). Briefly, liver samples were homogenized in RIPA buffer with proteinase/phosphatase inhibitors and the homogenate was centrifuged. Protein concentration was assayed in the clear supernatant using Bradford reagent (Bradford 1976), and 50-μg protein was subjected to SDS-PAGE followed by electrotransfer to nitrocellulose membranes. After blocking with 5% skimmed milk in tris-buffered saline (TBS), the membranes were incubated overnight with primary antibodies for NF-κB p65, Nrf2, HO-1, and β-actin (Novos Biologicals, USA). The membranes were incubated with the secondary antibodies, washed with TBS/tween 20 and developed using an enhanced chemiluminescence kit (Thermo Scientific, USA). The band intensity was quantified with ImageJ (version 1.32j, NIH, USA) and the data were normalized to β-actin.
Statistical analysis
The results were represented as mean ± standard error of the mean (SEM). The differences between groups were determined by one-way ANOVA and Tukey’s test using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). A P value less than 0.05 was considered significant.
Results
Body weight changes
The changes in body weight of all groups throughout the experiment are summarized in Fig. 2. Body weight was steadily increased in the control group, and the increase was lower in the CCl4-induced groups as represented in Fig. 2a. At the end of the experiment, rats in groups 3 and 4 exhibited a significantly lower body weight gain when compared with the control group (Fig. 2b). Rats treated with BMSCs showed significant improvement of body weight gain when compared with the recovery group (Fig. 2b).
BMSCs ameliorate liver function in CCl4-intoxiacted rats
Transaminases, ALP, γGT, bilirubin, and albumin were determined to evaluate the ameliorative effect of BMSCs on CCl4-induced liver injury in rats (Fig. 3). S.c. administration of CCl4 caused liver injury manifested by elevated ALT, AST, ALP, γGT, and bilirubin along with decreased albumin (P < 0.001). Rats received BMSCs following CCl4 showed significant amelioration of all liver function markers (Fig. 3a–f). CCl4-intoxiacted rats left untreated for 4 weeks (recovery) showed a reduction in serum transaminases, ALP, γGT, and bilirubin and increased albumin when compared with the CCl4-intoxicated rats sacrificed after a 6-week challenge with CCl4. However, the rats treated with BMSCs showed significant reduction in ALP, γGT, and bilirubin levels when compared with the recovery group (P < 0.05).
BMSCs engraft in the liver and attenuate tissue injury and fibrosis in CCl4-intoxiacted rats
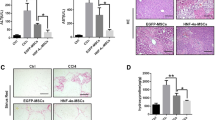
To track the homing of the cells to the liver, BMSCs were labelled with PKH26, a red fluorochrome fluorophore with no effect on the cells and suitable for in vivo long-term tracking. Examination of liver sections of rats received BMSCs revealed a strong red autofluorescence, confirming the engraft of these cells in the liver (Fig. 4A).
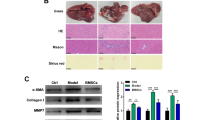
BMSCs engraft in the liver and attenuate tissue injury in CCl4-intoxiacted rats. A PKH26-labelled BMSCs showing red fluorescence in the liver of rats. B Photomicrograph of H&E-stained liver sections of (a) control rats showing the normal histological structures of hepatic lobules, central vein (CV), hepatocytes (H), and sinusoids (S). (b–d) CCl4-intoxiacted rats showing (b)dilated portal vein (P), hyperplasia of epithelial lining of bile duct (white star), newly formed bile ductules (black star), (c) steatosis of hepatocytes (white arrow), focal hepatic necrosis (black arrow), (d) perivascular fibrous connective tissue proliferation (F), inflammatory cells infiltration (arrow head), vacuolar degeneration of hepatocytes (V), and congestion in the central vein (C), (e) CCl4-intoxiacted rats treated with BMSCs showing nearly normal structure of the hepatic lobule and central vein (CV) with few inflammatory cells infiltration (arrow head), and (f) recovery group showing vacuolar degeneration of hepatocytes (V), inflammatory cells infiltration (arrow head), steatosis of hepatocytes (white arrow) and highly dilated central vein (CV). (Scale bar = 100 μm)
H&E-stained sections in the liver of control rats showed normal histological structure of the hepatic lobules (Fig. 4B (a)), whereas CCl4-intoxiacted rats exhibited highly dilated portal vein, hyperplasia of the epithelium the lining bile duct, newly formed bile ductules, steatosis, focal hepatic necrosis, perivascular fibrous connective tissue proliferation, inflammatory cells infiltration, vacuolar degeneration of hepatocytes, and central vein congestion (Fig. 4B (b–d)). Although the recovery group showed vacuolar degeneration of hepatocytes, inflammatory cells infiltration, steatosis of hepatocytes, and highly dilated central vein (Fig. 4B (f)), rats received BMSCs showed noticeable amelioration of the histological structure of the liver with only few inflammatory cells infiltration (Fig. 4B (e)).
The degree of fibrosis in hepatic tissues was detected using MT staining. The control group showed normal thin rims of collagen around the central vein and sinusoids (Fig. 5a), whereas the CCl4-intoxicated rats group showed heavy accumulation of perivascular collagen fibers (Fig. 5b–d). The CCl4-intoxicated rats left untreated showed significant accumulation of collagen (Fig. 5f), an effect that was significantly diminished in rats treated with BMSCs (Fig. 5e). Image analysis revealed the significant ameliorative effect of BMSCs on collagen accumulation (Fig. 5g).
BMSCs attenuate fibrosis in CCl4-intoxiacted rats. a–f MT staining of liver sections showing normal thin rims of collagen fibers in control rats (a), heavy accumulation of perivascular collagen fibers (arrow) in CCl4-intoxiacted rats (b–d), little collagen fibers in CCl4-intoxiacted rats treated with BMSCs (e), and accumulated collagen in untreated CCl4-intoxiacted rats (f). (scale bar = 100 μm]. Quantification of MT staining showing significant deposition of collagen and the ameliorative effect of BMSCs in CCl4-intoxiacted rats (g). Data are mean ± SEM, n = 6. ***P < 0.001 versus control and ###P < 0.001 versus CCl4
BMSCs mitigate inflammation in CCl4-intoxiacted rats
Given the role of inflammation in hepatic fibrogenesis (Koyama and Brenner 2017), we determined the expression of NF-κB and serum levels of TNF-α and IL-6 to evaluate the ameliorative effect of BMSCs on the inflammatory response in CCl4-intoxiacted rats. NF-κB mRNA abundance (Fig. 6a) and the protein expression of its p65 subunit (Fig. 6b) were significantly upregulated in the liver of CCl4-intoxiacted rats as well as the recovery group (P < 0.001). The inflammatory response was further evidenced by the significant elevation in serum TNF-α (Fig. 6c) and IL-6 (Fig. 6d). In contrast, treatment of the CCl4-intoxiacted with BMSCs downregulated NF-κB and reduced pro-inflammatory cytokines significantly when compared with the untreated and the recovery groups.
BMSCs mitigate inflammation in CCl4-intoxiacted rats. BMSCs decreased hepatic NF-κB mRNA expression (a) and protein phosphorylation (b), and serum TNF-α (c) and IL-6 (d) in CCl4-intoxiacted rats. Data are mean ± SEM, n = 6. *P < 0.05 and ***P < 0.001 versus control. #P < 0.05, ##P < 0.01, and ###P < 0.001 versus CCl4
BMSCs alleviate oxidative stress in the liver of CCl4-intoxiacted rats
CCl4-intoxiacted rats exhibited significant increase in hepatic MDA (Fig. 7a) accompanied with a reduction in GSH (Fig. 7b), SOD (Fig. 7c), and GST (Fig. 7d). The group of rats left to recover after a 6-week challenge with CCl4 showed an oxidative stress status manifested by the increased MDA and decreased antioxidants when compared with the control rats. In contrast, treatment with BMSCs suppressed LPO and enhanced GSH and antioxidant enzymes when compared with the CCl4 and the recovery groups.
BMSCs upregulate Nrf2/HO-1 signaling in the liver of CCl4-intoxiacted rats
Both the gene and protein expression levels of hepatic Nrf2 and HO-1 were determined to assess the ameliorative effect of BMSCs on CCl4-induced oxidative stress and inflammation in rats. In comparison with the control group, CCl4-intoxiacted rats exhibited significant reduction in Nrf2 and HO-1 mRNA abundance (Fig. 8a, b) and protein expression (Fig. 8c, d). Similarly, the recovery group showed significant downregulation of hepatic Nrf2 and HO-1. BMSCs administration upregulated the expression of Nrf2 and HO-1 when compared with the CCl4-intoxiacted as well as the recovery groups.
Discussion
Hepatic fibrosis is a progressive disease characterized by excessive deposition of ECM and inflammation (Friedman 2008b). BMSCs are multipotent stem cells able to differentiate into a variety of lineages under suitable conditions and are therefore extensively applied in regenerative medicine. Recruitment of the bone marrow-derived cells occurs in the progression and regression of liver fibrosis (Higashiyama et al. 2007). In this study, we investigated the potential therapeutic effect of BMSCs against CCl4-induced fibrosis, oxidative stress, and inflammation in rats, pointing to the possible role of Nrf2 signaling.
Hepatic fibrosis was induced by CCl4 s.c. administration for 6 weeks. CCl4 provoked hepatic injury and fibrosis evidenced by the biochemical and histological findings. Circulating levels of transaminases, ALP, γGT, and bilirubin were elevated, whereas albumin was decreased in CCl4-intoxicated rats. Histological examination revealed vacuolar degeneration of hepatocytes, inflammatory cells infiltration, focal hepatic necrosis, highly dilated portal vein, central vein congestion, steatosis, perivascular fibrous connective tissue proliferation, hyperplasia of the epithelium lining the bile duct, and newly formed bile ductules. Additionally, MT staining of liver section showed heavy accumulation of collagen fibers perivascularly, demonstrating the development of hepatic fibrosis in CCl4-intoxicated rats. During chronic liver injury, the excessive accumulation of ECM rich in collagen I and III provokes scar deposition and hepatic fibrosis (Schuppan 2015). Accordingly, we have reported increased serum markers of liver function, multiple histopathological manifestations, particularly, focal necrosis, steatosis, and fibroblast proliferation, and increased expression of collagen in CCl4-intoxicated rats (Mahmoud et al. 2019). Interestingly, rats received BMSCs following a 6-week challenge with CCl4 showed significant amelioration of circulating liver function markers, collagen deposition, and tissue injury.
The beneficial role of BMSCs has been demonstrated in a rat model of bleomycin-induced pulmonary fibrosis (Ni et al. 2015). Treatment of bleomycin-induced rats with BMSCs for 3 days prevented all histological alterations and decreased pulmonary collagen deposition and hydroxyproline levels (Ni et al. 2015). In a rat model of hepatocarcinogenesis, administration of BMSCs ameliorated serum transaminases and exerted tumor suppressive effects (Abdel aziz et al. 2011). Improvement of serum transaminases has also been demonstrated in CCl4-induced rats that received BMSCs (Ahmed et al. 2014). Besides basic studies, the hepatoprotective efficacy of BMSCs has been reported in different studies and clinical trials (Lin et al. 2017; Suk et al. 2016). The mechanism underlying the beneficial effect of BMSCs against CCl4-induced fibrosis could be explained in terms of suppressing inflammation and ECM deposition. MSCs possess anti-inflammatory efficacies and have been reported to reduce hepatocyte injury (Forbes and Newsome 2012) and the activation of HSCs (An et al. 2017). MSCs can increase the production of matrix metalloproteinase (MMPs) to reduce the ECM, and change the polarity of macrophages towards an anti-inflammatory phenotype and increase their phagocytosis ability (Watanabe et al. 2019). In the current study, CCl4 administration provoked an inflammatory response marked be the significant upregulation of NF-κB p65 and increased pro-inflammatory cytokines as we previously reported (Mahmoud et al. 2019). Hepatic fibrosis and cirrhosis have been reported to be associated with chronic inflammation (Czaja and Carpenter 2004). In addition, injured hepatocytes due to inflammation release apoptotic bodies which activate HSCs and promote fibrogenesis (Friedman 2008a). Therefore, the anti-inflammatory activity of BMSCs represents a main part of their beneficial effects against hepatic fibrosis.
Besides inflammation, oxidative stress is implicated in the pathophysiology of different diseases, including hepatic fibrosis (Cheng et al. 2019; Gandhi 2012; Li et al. 2017; Mahmoud et al. 2017b). Thus, we assumed that suppression of oxidative stress might be involved in the ameliorative effect of BMSCs against CCl4-induced hepatic fibrosis. In the liver, activation of HSCs and excessive accumulation of ECM rich in collagen were provoked by surplus ROS generation (Cheng et al. 2019; Gandhi 2012). In the same context, hepatocyte injury due to ROS-mediated LPO can promote fibrogenesis via activating HSCs (Friedman 2008a; Sun et al. 2018). Here, oxidative stress in the liver of CCl4-intoxicated rats was evidenced by increased LPO and decreased antioxidant defenses as previously demonstrated (Mahmoud et al. 2019). CCl4 is metabolized by cytochrome P450 within the liver producing trichloromethyl and peroxyl radicals, highly reactive mediators that provoke LPO and bind covalently to the cellular macromolecules, leading to cell death (Weber et al. 2003). In addition to its derived damaging radicals, CCl4 induces surplus ROS production by activating NADPH oxidase (NOX) in the liver of rats (Cheng et al. 2019). In agreement with these findings, we have reported increased hepatic LPO along with decreased GSH and antioxidant enzymes in CCl4-induced fibrosis in rats (Mahmoud et al. 2019). ROS can activate NF-κB and increase the release of TNF-α and IL-6, effects reported in the present study. Therefore, attenuation of oxidative stress is an effective strategy to suppress inflammation and hepatic fibrogenesis. Interestingly, rats received BMSCs following CCl4 showed a significant decrease in MDA and enhanced hepatic antioxidant defenses. The role of BMSCs in ameliorating oxidative stress has been previously reported in the lung of bleomycin-induced rats (Ni et al. 2015), where treatment with BMSCs decreased MDA and increased SOD activity. Therefore, it is noteworthy assuming that the dual ability of BMSCs to suppress oxidative stress and inflammation is the main mechanism underlying their anti-fibrogenesis efficacy.
To further explore the protective mechanism of BMSCs, we evaluated their effect on Nrf2/HO-1 signaling in the liver of rats. Nrf2 is a transcription factor that activates the transcription of antioxidant defenses and protects against the deleterious effects of ROS (Satta et al. 2017). Under conditions of surplus production of ROS, the expression of Nrf2 has been reported to decrease in the liver of rats (Aladaileh et al. 2019). In this study, Nrf2 mRNA abundance and protein expression were decreased in CCl4-challenged rats, pinpointing the surplus levels of ROS and downregulation of Nrf2/HO-1 signaling which was further confirmed by the decreased HO-1 expression. These findings were in consistent with the declined activities of antioxidant enzymes by CCl4. Intravenous injection of BMSCs significantly increased the expression of Nrf2 and HO-1 in the liver of CCl4-intoxicated rats. These findings along with the suppressed oxidative stress and inflammation demonstrated the role of Nrf2/HO-1 signaling in the ameliorative effect of BMSCs on CCl4-induced hepatic fibrosis. In support of this notion, Ni et al have reported increased Nrf2 expression in the lung of bleomycin-induced rats following BMSCs administration (Ni et al. 2015). In addition, a recent study by Lee et al showed the ability of BMSCs to reduce ER stress in the lung of bleomycin-induced aged mice via modulating the PERK-Nrf2 pathway (Lee et al. 2020). Besides the anti-inflammatory role of BMSCs associated with changing the polarity of macrophages (Watanabe et al. 2019), their anti-fibrotic effect could be attributed to their ability to activate Nrf2/HO-1 signaling and subsequent upregulation of antioxidant and anti-inflammatory defenses. Nrf2 can suppress inflammatory responses through attenuating NF-ĸB activation (Pan et al. 2012; Wardyn et al. 2015). In addition to these mechanisms, the ability of exogenously administered BMSCs to engraft in the liver contributed to the repair of injured tissue. Accordingly, BMSCs can engraft injured liver and differentiate into hepatocytes (Li et al. 2013), and upregulate hepatocyte growth factor, MMPs, and stem cell factor-1 (Liedtke et al. 2013). The homing of BMSCs to the injured liver was confirmed by observing the presence of PKH26-labelled cells in the histological sections from the liver of CCl4-induced rats. The anti-fibrotic effect of BMSCs has been also associated with the expansion of intrahepatic natural killer cells in a rodent model of surgically induced fibrosis (Duman et al. 2019). Thus, multiple mechanisms seem to be involved in the protective effect of BMSCs against fibrosis. Our study introduced new information on the involvement of Nrf2/HO-1 signaling activation in the protective mechanism of BMSCs on CCl4-induced hepatic fibrosis.
Conclusions
This study demonstrates the ameliorative efficacy of BMSCs on hepatic fibrosis induced by CCl4 in rats. BMSCs engrafted the injured liver and attenuated oxidative stress, inflammation, liver injury, and collagen deposition in CCl4-intoxicated rats. The anti-fibrotic efficacy of BMSCs was associated with upregulation of Nrf2/HO-1 signaling which plays a significant role in attenuating inflammation and oxidative stress. These findings support the promising therapeutic role of BMSCs in tissue repair and their anti-fibrotic efficacy. However, dissection of the mechanisms by which BMSCs ameliorate hepatic fibrosis would be critical for understanding and developing MSCs-based therapies.
References
Abdel aziz MT, El Asmar MF, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Rashed LA, Sabry D, Hassouna AA, Taha FM (2011) Efficacy of mesenchymal stem cells in suppression of hepatocarcinorigenesis in rats: possible role of Wnt signaling. J Exp Clin Cancer Res 30:49–49
Ahmed SK, Mohammed SA, Khalaf G, Fikry H (2014) Role of bone marrow mesenchymal stem cells in the treatment of CCL4 induced liver fibrosis in albino rats: a histological and immunohistochemical study. Int J Stem Cells 7:87–97
Aladaileh SH, Abukhalil MH, Saghir SAM, Hanieh H, Alfwuaires MA, Almaiman AA, Bin-Jumah M, Mahmoud AM (2019) Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 9:346
An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, Park JY, Park SY, Kim JH, Do BR, Han C, Park HK, Kim OH, Song MJ, Kim SJ, Kim JH (2017) Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology 152:1174–1186
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med. 61:882–888
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheng Q, Li C, Yang C-f, Zhong Y-J, Wu D, Shi L, Chen L, Li Y-W, Li L (2019) Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-β1/Smad and NOX4/ROS pathways. Chemico-Biological Interactions 299:131–139
Czaja AJ, Carpenter HA (2004) Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology 39:1631–1638
Duman DG, Zibandeh N, Ugurlu MU, Celikel C, Akkoc T, Banzragch M, Genc D, Ozdogan O, Akkoc T (2019) Mesenchymal stem cells suppress hepatic fibrosis accompanied by expanded intrahepatic natural killer cells in rat fibrosis model. Molecular biology reports 46:2997–3008
Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD (2005) Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proceedings of the National Academy of Sciences of the United States of America 102:10070–10075
Forbes SJ, Newsome PN (2012) New horizons for stem cell therapy in liver disease. Journal of hepatology 56:496–499
Friedman SL (2008a) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiological reviews 88:125–172
Friedman SL (2008b) Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669
Gandhi CR (2012) Oxidative stress and hepatic stellate cells: a paradoxical relationship. Trends Cell Mol. Biol. 7:1–10
Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, Okazaki I (2007) Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology (Baltimore, Md.) 45:213–222
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & development 13:76–86
Josan S, Billingsley K, Orduna J, Park JM, Luong R, Yu L, Hurd R, Pfefferbaum A, Spielman D, Mayer D (2015) Assessing inflammatory liver injury in an acute CCl4 model using dynamic 3D metabolic imaging of hyperpolarized [1-(13)C]pyruvate. NMR in biomedicine 28:1671–1677
Koyama Y, Brenner DA (2017) Liver inflammation and fibrosis. The Journal of clinical investigation 127:55–64
Lee EJ, Cárdenes N, Álvarez D, Sellarés J, Sembrat J, Aranda P, Peng Y, Bullock J, Nouraie SM, Mora AL, Rojas M (2020) Mesenchymal stem cells reduce ER stress via PERK-Nrf2 pathway in an aged mouse model. Respirology 25:417–426
Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y, Fan D (2013) In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PloS one 8:e62363
Li X, Wang L, Chen C (2017) Effects of exogenous thymosin β4 on carbon tetrachloride-induced liver injury and fibrosis. Scientific Reports 7:5872
Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, Weiskirchen R (2013) Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis & tissue repair 6:19
Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Wing WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL (2017) Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology (Baltimore, Md.) 66:209–219
Lin Y, Luo H, Wang X, Zheng M, Jin Q, Chen H, Pan P, Zhang J (2018) Flavanones from Sedum sarmentosum Bunge Alleviate CCl(4)-Induced Liver Fibrosis in Rats by Targeting TGF-β1/TβR/Smad Pathway In Turn Inhibiting Epithelial Mesenchymal Transition. Evid Based Complement Alternat Med 2018:3080837
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods. 25:402–408
Mahmoud AM, Germoush MO, Alotaibi MF, Hussein OE (2017a) Possible involvement of Nrf2 and PPARgamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother 86:297–306
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017b) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARgamma and TGF-beta1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Mahmoud AM, Hozayen WG, Hasan IH, Shaban E, Bin-Jumah M (2019) Umbelliferone Ameliorates CCl4-Induced Liver Fibrosis in Rats by Upregulating PPARgamma and Attenuating Oxidative Stress, Inflammation, and TGF-beta1/Smad3 Signaling. Inflammation 42:1103–1116
Mannervik B, Guthenberg C (1981) Glutathione transferase (human placenta). Methods Enzymol 77:231–235
Marcellin P, Kutala BK (2018) Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver international : official journal of the International Association for the Study of the Liver 38(Suppl 1):2–6
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474
Ni S, Wang D, Qiu X, Pang L, Song Z, Guo K (2015) Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. International journal of clinical and experimental pathology 8:7752–7761
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry 95:351–358
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm 2012:217580
Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ (2017) The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longev 2017:9237263
Schuppan D (2015) Liver fibrosis: common mechanisms and antifibrotic therapies. Clinics and research in hepatology and gastroenterology 39(Suppl 1):S51–S59
Schuppan D, Afdhal NH (2008) Liver cirrhosis. Lancet (London, England) 371:838–851
Suk KT et al (2016) Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology (Baltimore, Md.) 64:2185–2197
Sun J, Wu Y, Long C, He P, Gu J, Yang L, Liang Y, Wang Y (2018) Anthocyanins isolated from blueberry ameliorates CCl4 induced liver fibrosis by modulation of oxidative stress, inflammation and stellate cell activation in mice. Food and Chemical Toxicology 120:491–499
Tacke F, Trautwein C (2015) Mechanisms of liver fibrosis resolution. J Hepatol 63:1038–1039
Tai W, Deng S, Wu W, Li Z, Lei W, Wang Y, Vongphouttha C, Zhang T, Dong Z (2020) Rapamycin attenuates the paraquat-induced pulmonary fibrosis through activating Nrf2 pathway. J Cell Physiol 235:1759–1768
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 43:621–626
Watanabe Y, Tsuchiya A, Seino S, Kawata Y, Kojima Y, Ikarashi S, Starkey Lewis PJ, Lu WY, Kikuta J, Kawai H, Yamagiwa S, Forbes SJ, Ishii M, Terai S (2019) Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Rransl Med 8:271–284
Weber LW, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33:105–136
Acknowledgments
The authors extend their appreciation to Faculty of Science, Beni-Suef University for supporting this research.
Availability of data and materials
The authors confirm that the data of this study are available within the article.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, data curation, investigation, and funding acquisition: A.M.M., S.M.K, and H.M.M. Original draft preparation: A.M.M. and S.M.K. Review and editing, validation and formal analysis: A.M.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The experimental protocol and procedures were approved by the Institutional Research Ethics Committee of Beni-Suef University (2018/0728).
Consent to participate
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khadrawy, S.M., Mohamed, H.M. & Mahmoud, A.M. Mesenchymal stem cells ameliorate oxidative stress, inflammation, and hepatic fibrosis via Nrf2/HO-1 signaling pathway in rats. Environ Sci Pollut Res 28, 2019–2030 (2021). https://doi.org/10.1007/s11356-020-10637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10637-y