Abstract
Human remains and corpses’ cremation is an increasing practice worldwide alternative to burials, which have increased their cost and reduced spaces in cemeteries. Alike to other combustion processes, cremation produces pollutant emissions that contribute to worsen air quality in modern cities. A 6-month sampling campaign was performed in order to characterize emissions from corpse cremation in three different crematorium ovens and develop emission factors which were used to determine the population exposure to those pollutants during cremation activities applying a dispersion model. The main difference among crematoria was the inclusion or non-inclusion of controlled air supply devices. Using isokinetic samplings in the chimneys crematoria, emissions were measured and characterized with different chemical analyses. No significant differences were found in arsenic and metal concentrations among different crematories, although carbon monoxide, particles, elemental carbon, organic carbon, and polycyclic aromatic hydrocarbon concentrations in facilities without controlled air supply were up to seven times higher than those with controlled air supply. Nevertheless, these pollutants exceeded standards in all crematoria. Except for elemental and organic carbon concentration that correlated with corpse weight, other recorded cadaver characteristics bear no relation with pollutant emissions. Emission factors among different ovens did not present significant differences; then, they were used for dispersion modeling of particles and mercury emissions over Mexico City when 35 crematoria operate simultaneously through an hour showing that PM2.5 and Hg increase 0.01–1 μg m−3 and 0.01–0.1 ng m−3, respectively, in that scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last two decades, cremation practice has increased around the world as an alternative to burials, mainly due to the lack of space for the deceased. Since 2008, Japan has the highest number of cremations in the world with 99.9% of corpses (Takaoka et al. 2010); in the UK, 75% of corpses are cremated in around 245 facilities (Wood et al. 2008), whereas in Latin America, cremation has been promoted in Colombia, Argentina, and Mexico, among other countries, as a more economical, modern, recently accepted by the catholic church, then funerary services that include incineration have increased in the last years (Klaufus 2014; Vatican 2016). The National Funeral Directors Association (NFDA) reported in 2015 that the rate of cremation is projected to be 56% in 2020 in the UK (NFDA 2016). According to the Mexico City Juridical Council and Legal Services Department, 50% of corpses are cremated in around 40 public and private facilities, then, from 2014 to 2016, 74,731 incinerations were carried out with an average of around 25,000 per year and an annual increased rate of 2000 cremations (Villegas 2019).
Cremation is the process by which corpse and human remains are subjected to high temperatures in order to reduce them to bone fragments and ashes. The process begins with the reception of the corpse that could be subsequently embalmed. Embalming’s main objectives are sanitization and preservation, and depending on locality or federal laws, it is required by many countries in services in funerals with public viewing, and it is usually a general legal requirement for transporting human remains out of a state or for international repatriation; embalming consists in extracting corporal fluids to add a solution with chemicals preservatives such as formaldehyde in water to keep the corpse in good condition until the actual firing exposure, which takes place in a high-temperature air-gas-mix-fired oven. The ashes produced during cremation are placed in an urn (Buschmann and Tsokos 2014; SEDEMA 2018).
Generally, crematorium ovens include a primary chamber reaching temperatures from 500 to 800 °C followed by a secondary chamber where gases are after burned up to 1200 °C with secondary air and LP gas or natural gas. Cremation actually lasts between one and 2 h depending on the oven type and the corpse weight; a new cremation process can begin when the first chamber is cooled to a temperature of 300 °C; usually, crematoria have three to six services per day and they have services per day, all year round.
During cremation processes, several pollutants are emitted to the atmosphere, such as particles (PM), carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxides (NOx), black carbon, or soot (Santarsiero et al. 2005; Wood et al. 2008), as well as different trace metals (Mari and Domingo 2010; De Angelis et al. 2017) that can produce adverse health effects (Alvarado-Cruz et al. 2017; Aschner et al. 2005; Lee et al. 2012). Among these, mercury (Hg) is a leading public health concern since it is toxic, highly volatile, and a persistent pollutant that accumulates in food chain (Pavlish 2009); mercury is in the silver amalgam dental fillings found in many dead human bodies and during cremation and is released to the atmosphere (Takaoka et al. 2010). A recent study reported that inhabitants’ hair in Mexico City presented between 1.74 and 2.89 μg Hg g−1, which is greater than that of U.S. Environmental Protection Agency (USEPA) standard of 1–2 μg g−1 for unexposed population (Fuentes and Martínez 2018). In the UK, it was estimated that crematoria would be one of the largest contributors to national mercury emissions by 2020 with up to 31% (Wood et al. 2008); in Japan, it is expected that mercury emissions increase by 2.6-fold from 2007 to 2037 (Takaoka et al. 2010).
Other researchers have reported also the emissions of organic toxic compounds such as polychlorated dibenzodioxines and dibenzofurans (Smith et al. 2012; Takeda et al. 2014), as well as polycyclic aromatic hydrocarbons (PAHs), which are ubiquitous compounds with two to seven aromatic rings produced during incomplete combustion at high temperatures (Xue et al. 2016). Unlike incinerators, studies about emission from crematoria are limited (Mari and Domingo 2010), especially those related to toxic compounds such as mercury, NO2, CO, SO2, and PAHs, among others (Tavares da Cruz et al. 2017); additionally, published emission factor is incomplete since they have not been developed for elemental or black carbon, organic carbon, and several metals. A previous study in Mexico City showed, on the one hand, that particle emissions are higher in crematorium ovens where air supply is not controlled and, on the other hand, that service number during the day did not influence emission concentrations (Gozález-Cardoso et al. 2018). Cremation emissions depend on different variables such as corpse characteristics, type of ovens, cremation duration, and regulations, among other factors. To know pollutant dispersion over cities and population exposure to pollutants emitted by point sources, emissions have been modeled under the Gaussian, Lagrangian, Eulerian methods, or a combination of them such as the Hysplit model v.4 which has been used in several countries (Bullock 2000; Chen et al. 2013; Draxler and Hess 1998; Yang et al. 2019). With the aim to determine accurate emission factors of different pollutants from cremation that can be applied for better emission inventories worldwide, emissions of CO, PM2.5, elemental carbon, organic carbon, PAHs, and metals were characterized and emission factors were estimated in three crematoria with different air supply conditions. Obtained data were used for modeling the dispersion of pollutants over Mexico City as well as to determine the atmosphere concentration increase of different assessed pollutants due to simultaneous cremation processes.
Materials and methods
Crematorium ovens
The study was carried out during 5 months between August and December 2017 in three funeral service facilities at Mexico City that perform from two to four cremations per day, one located in downtown and the other two at the North. In Mexico, death corpses are cremated without coffin; in addition, shoes and accessories are removed before the process. The three crematorium ovens have a primary chamber with burners mixing LP gas and air, where corpses are introduced when the oven temperature is 300 °C; afterwards, combustion gases are fed into a secondary chamber supplied with secondary air; this chamber is heated up to 1100 °C to finish the combustion process; bones and ashes are cooled to room temperature, hammered into fine fragments, and placed in an urn. When the first chamber has cooled down to 300 °C, a subsequent cremation service can begin. Table 1 displays the crematorium ovens’ characteristics. According to our research, almost all crematoria in Mexico City are similar to these; most of them have air supply control, and only one has a pollutant control system consisting in a scrubber, but the others have no control systems, neither for gases nor for particles; all have some ventilation system to drive emissions to the chimney, which has sampling ports according to Mexico City Law; the main difference among crematoria is the chamber capacity.
The cremation services lasted 2 h in the first crematorium oven, whereas in the other two ovens, the time for complete cremation lasted 70 min, and measurements were performed during an hour according to the standard method described in the next section. According to the information provided at the crematorium facilities, the LP gas consumption is presented also in Table 1; differences in consumption can be due to oven 1 which is 13 years old with small burners into the chamber whereas the other two are around 30 years old with a big burner.
Cremation process variables
Number of service, gender, age, weight, and declared illness were registered in the 42 performed cremations. Corpse weights were obtained just before the process using a Justa balance model JS7516 with a JS10 indicator and 250 kg capacity; other data were obtained from death certificates such as gender, age, and declared illness of individuals. Measurements were carried out at different service order: 13 during the first service of the day, 15 during the second service, 12 during the third service, and 2 during the fourth service. The sampling dates and detailed information of these variables are presented in Appendix A of supplementary material.
Isokinetic sampling
The three crematorium ovens have a 5-m chimney with 0.70-m OD and a 0.5-m ID that warrant laminar flow at the gases and particles’ sampling point. Gas and particle measurements of 1 h were according to the standard Mexican method (NMX-AA-010-SCFI- 2001) corresponding to the United States Environmental Protection Agency (USEPA 1999a) methods 1, 2, 3, and 4 to determine ambient and chimney temperature, flow rate in the chimney, volumetric flow, gas moisture, and gases molecular weights. Measurements were carried out with isokinetic Seelin equipment, Model FF-0012, Series 0024, with certified gasometer and orifice plate. CO and CO2 simultaneous analyses were carried out with a Bacharach 24-7343 Fyrite Insight Plus equipment using Method 201A of Federal Code of Regulations No. 40, Part 60, of USEPA, that was calibrated and certified in Bacharach facilities prior the samplings. Quartz filters were used for collection and quantification of total suspended particles (TSP) following USEPA Method 5, whereas for PM2.5, a cyclone with 47-mm quartz filters was used applying the 201A-modified method of the Federal Code of Regulations No. 40, Part 60, of USEPA. A detailed description of employed methods can be found in Mugica-Álvarez et al. (2018) or in the corresponding methods. Particle concentrations were obtained by gravimetry using an analytical balance Mettler Toledo MT5 (Max. 5.1 g, d = 1 μg) dividing the PM mass by the total volume of air.
Determination of carbon, metals, and polycyclic aromatic hydrocarbons in collected particles
Thermo-optic method NIOSH 870 (Birch and Cary 1996; Santiago-De La Rosa et al. 2018) was used with the Carbon Sunset Laboratory Analyzer for quantification of elemental and organic carbon in PM2.5. According to Petzold et al. (2013), the term elemental carbon is commonly used instead of black carbon that is determined by an optical method. Polycyclic aromatic hydrocarbons (PAHs) were analyzed by USEPA method TO-13A with a gas chromatograph Agilent HP 6890 coupled with a mass spectrometer HP 5973 using a HP-5 MS capillary column (30 m long × 0.250 mm inner diameter × 0.25 μm thick stationary phase) (USEPA 1999b). Samples were analyzed in selected ion monitoring (SIM) by electron impact mode (EI) at 70 eV. The transfer line, ion source, and quadrupole temperatures were 300 °C, 230 °C, and 150 °C. The oven temperature was programed to ramp from 40 (for 1 min) to 120 °C (held for 1 min) at a rate of 50 °C min−1, then increased 5 °C min−1 to 305 °C and finally increased 20 °C min−1 to 330 °C for 10 min. Filters with particles were fortified with eleven deuterated PAHs (Chem Service and Ultra Scientific, USA) for further extraction with dichloromethane (HPLC grade, Burdick & Jackson) using an ultrasonic bath (Branson 2510) at 60 °C for two 30 min periods. Appendix B in supplementary material shows the abbreviations, target and secondary ions, and retention time of PAHs and deuterated PAHs. A rotary evaporator was used to concentrate the extracted solution down to 1 mL (Büchi 461, Water Bath) according to the methodology published elsewhere (Valle-Hernández et al. 2010; Mugica-Álvarez et al. 2018). Metal extractions were achieved mixing one filter section with 10 mL of ultrapure nitric acid and digested in a microwave oven CEM with turntable, whereas the analysis was made by atomic absorption spectrometry (GBC 932AA) coupled with a System 3000-graphite furnace system (GFAAS) with a GF3000 graphite power supply and PAL3000 furnace auto sampler both controlled by a computer. In the case of mercury (Hg) and arsenic (As), a hydride vapor generator (AAS-HVG) (Model GBC 932, GBC Scientific Equipment) was employed according to Gómez-Arroyo et al. (2018) methodology.
Quality assurance and quality control QA/QC
The certification of standard gases for isokinetic sampling was done by Praxair Mexico, following EPA Method CTM-34 at 0 °C, pressure 101.3 kPa, and 11% O2; only the measurements that met isokinetism between 90 and 110% were taken into account.
All quartz filters were pre-calcined at 500 °C, and filter blanks were taken every day, treated the same as samples, and analyzed for each parameter. Blanks results were subtracted from the samples. The accuracy of organic carbon and elemental carbon (OC/EC) method for measuring total carbon was performed analyzing a known quantity of sucrose applied in blank quartz filters which is part of the quality assurance program. Detection limits were 0.397 μg m−3 and 0.476 μg m−3 for EC and OC, respectively, whereas quantification limits were 0.514 μg m−3 and 0.719 μg m−3 for EC and OC, respectively.
Element calibration standards were prepared with the same acid concentration than the samples, using High-Purity Standards traceable to National Institute of Standards and Technology (NIST). Cross-check methods of standard additions were used. Ten replicated measurements of each metal showed that precision was 3.0, 4.3, 4.8, 6.0, 5.1, 5.3, 6.7, and 5.3% relative standard deviation (% RSD) for arsenic (As), cadmium (Cd), copper (Cu), mercury (Hg), nickel (Ni), lead (Pb), vanadium (V), and zinc (Zn), respectively, whereas recoveries from SRM-1649a of NIST were as follows: As 87.3 ± 3.6%, Cd 91.2 ± 3.8, Cu 97.3 ± 4.1, Hg 89.4 ± 3.8%, Ni 93.4 ± 4.5, Pb 98.7 ± 3.7%, V 96 ± 4.1%, and Zn 95.1 ± 2.9%. Limits of detection and quantification can be found in Appendix C of supplementary material.
PAH calibration plots were constructed using each PAH at eight concentrations, injected in triplicate. The concentration range of such a plot was in the 10 to 7000 pg μL−1. The linear regression coefficient for each compound was from 0.9734 for acenaphthene to 0.9942 for fluoranthene. The method quantification limits for PAHs were found between 6 (benzo [a]pyrene) and 120 pg m−3 (perylene) (Appendix C of supplementary material). The recovery efficiency determined with deuterated PAHs was found to be between 68 ± 2% (benzo [ghi]perylene) and 87 ± 4% (benzo [a]pyrene) (Appendix D of supplementary material).
Modified combustion efficiency and emission factor estimation
Modified combustion efficiency (MCE) is the quantity of carbon released as CO2, without considering the carbon content of hydrocarbons and particulate matter (Eq. (1), considering that all carbon is released as CO2 and CO. ΔCO2 and ΔCO are the concentrations of CO2 and CO measured during the test minus corresponding the background concentration of every species.
Emission factors (mg kg−1) from crematoria of different pollutants (P): carbon monoxide, particles, elemental, and organic carbon as well as polycyclic aromatic hydrocarbons, were estimated taking into account pollutant concentrations (Cp) in mg m−3, total volume of combustion gases (Vt) in m3, and burned corpse weight (Wb) in kg according to Eq. (2) (Mugica-Álvarez et al. 2018); nevertheless, as emission factor from cremation is usually reported as mass of pollutant per corpse, the mean values reported in this research were multiplied by the average weight corpse of 60 kg to obtain EF in (mg/corpse):
Statistical analysis
Statistical analyses were performed with the IBM SPSS Statistics 20 software to determine, means, maxima, minima, medians, and standard deviations. The nonparametric Mann-Whitney U test was employed for median comparison with significance of 95%, as well as Spearman correlation for relationship determinations among variables.
Modeled emissions
Hysplit model v.4 (Hybrid Single-Particle Lagrangian Integrated Trajectory) is a hybrid model for computing dispersion of complex trajectories and deposition simulations using either puff or particle dispersion that employs the Lagrangian method to model trajectories and the Eulerian method to calculate concentrations (Draxler and Hess 1998; Bullock 2000; Chen et al. 2013). This model was applied to know the crematorium emission dispersion over Mexico City. The assumption in the modeled scenario was that 35 crematoria located in Mexico City began cremation processes simultaneously at 10:00 am working during 2 h that is a common scenario in the city. A day with typical weather during dry-cold season 2018, in this case November 17, was determined under the deletion of atypical value criterion for the following meteorological parameters: temperature, relative humidity, UVa radiation, barometric pressure, and wind speed, all based on hourly data. Meteorology data were compiled in the meteorological module of Hysplit from forecast data available for NOAA’s Global Forecast System (GFS). Hysplit outputs were post-processed in a geographical information system to calculate accumulated concentrations. Emission factors obtained in this research were used as inputs for dispersion modeling.
Results
According to USEPA modified method 201 A, the acceptable isokinetism range during particle sampling is from 90 to 110%, and the average percentage for all the samplings was 106.4% and only the first one was out of range (112%).
Carbon monoxide concentrations and emission factors
Figure 1a shows the basic statistics of the CO emission concentrations determined during 1-h isokinetic sampling in the three crematorium ovens as well as the average of all ovens. The most efficient crematoria were 1 and 3 with MCE of 0.998 ± 0.001 and 0.996 ± 0.004, respectively, whereas the second crematorium oven had an MCE of 0.988 ± 0.015, showing the importance of controlled air supply; consequently, crematorium 2 presented the highest CO concentrations since air was insufficient for an efficient combustion. Although crematorium 1 exhibited the lowest CO concentrations, statistical analyses with Mann-Whitney U test showed no significant difference with crematorium 3 (α = 0.05; p = 0.157) with an average of 72 mg Nm−3 dry 11% O2; in opposite, significant differences were found between ovens 1 and 2 and 2 and 3 (α = 0.05; p = 0.007 and p = 0.01, respectively); oven 2 presented an average of 190 mg Nm−3 dry 11% O2. In comparison with other studies, the CO concentrations measured in this study were greater than the range of < 1–50 mg CO Nm−3 dry 11% O2 reported by Santarsiero et al. (2005).
Despite concentration differences between ovens with and without controlled air supply, no significant differences were found among the CO EF of the three ovens (α = 0.05; p = 0.396), probably due to the normalization by the corpse weight, but Mexico City has an emission crematorium standard published in 2018 (NADF-017-AIRE-2017) stating that concentrations should not be higher than 120 mg CO Nm−3 dry 11% O2. In this study, 72% of cremations carried out in crematorium ovens with controlled air supply accomplished the standard, but only 35% of crematorium oven without controlled air supply did it, meaning that all ovens should include at least air supply systems, improve combustion performance, and install catalytic devices.
Three different emission factors were estimated for all emitted pollutants: crematorium ovens with air supply, crematorium ovens without air supply, and an average of crematorium ovens when the type of air supply is unknown (Table 2). Average measured CO emission factor is around 1.5 times higher than that reported by the European Environmental Agency (EEA 2016).
Concentrations and emission factors of PM2.5, elemental, and organic carbon
PM2.5 concentrations are exhibited in Fig. 1b where it is observed that concentrations are up to 2.5 times higher in the crematorium ovens without controlled air supply, showing significant differences (α = 0.05; p = 0.0147) highlighting, by a large, the need to have devices to improve combustion process. Mexico City emission standard (NADF-017-AIRE-2017) establishes that total suspended particles (TSP) should not been higher than 40 mg TSP Nm−3 dry 11% O2. Although PM2.5 concentrations are lower than TSP, only 15% of cremations in crematoria without controlled air supply (15.2–96.7 mg Nm−3 dry 11% O2) and 64% of cremations in crematoria with controlled air supply (8.5–71.7 mg Nm−3 dry 11% O2) achieved the Mexican standard. This means that all crematorium ovens should include air control systems for combustion improvement, but particle control systems must be installed also. In comparison with other studies, results found in this research are similar to the reported emissions of 44.5 to 72.2 mg PM2.5 Nm−3 dry 12% O2 from Japanese crematoria (Kato et al. 2017). The carbonaceous fraction in PM2.5 was around 0.4% which is very low in comparison with concentrations found in ambient air. Chan (2002) carried out a study where is shown that to ensure complete combustion and minimize black smoke formation, preheat, secondary and primary air, and burners all have to be properly controlled; OC and EC concentrations are shown in Fig. 1 (c) following a similar trend than PM2.5 concentrations. OC is only 39% of total carbon (OC + EC), because the temperatures during the process were greater than 500 °C; thus, most organic compounds were volatilized. No other study was found reporting concentrations or emission factors of carbonaceous pollutants from crematoria to compare with this study.
PM2.5, EC, and OC concentrations from crematoria are in the range of those measured in industrial boilers using diesel and gas oil as well as LPG furnaces reported in a study that analyzed 67 boilers and 25 furnaces in Costa Rica (Murillo et al. 2017a), but the emissions in this study were lower than those measured in boilers using fuel oil and biomass; in the case of EC and OC crematoria emissions, these were lower from two to three magnitude orders than the recorded for all boilers and furnaces from that study, due to the high temperatures reached in crematoria. This means that in opposite to other emission sources, carbon content of particles emitted by crematoria is less than 1%, suggesting that most particles are mainly constituted by silicates, aluminum oxides, and alkaline salts and oxides as can be seen in Fig. 2 examples.
Figure 2 shows some SEM micrographs of particles collected in quartz filters, as well as their elemental PM composition determined by EDS. In general, there is a significant diversity of morphologies in the particles collected in chimneys. Figure 2a shows two plate-like aluminum-rich particles while Fig. 2b shows a translucent carbonaceous particle containing zinc copper and iron, maybe as oxides or carbides formed at high temperatures, plus other elements associated with the oven materials. Lastly, Fig. 2c displays some light-colored, shapeless fragments with relatively high zinc content.
Regarding emission factors, statistical analyses showed no significant difference among the three crematorium ovens (α = 0.05; p = 0.548), maybe due to the normalization with the corpse weight (Table 3). All EF are between 1.7- and twofolds higher than the reported by the European Environment Agency (EEA 2016). The determinations of EC and OC concentrations as well as their emission factors were carried out eliminating results of the cremated corpse weighting 150 kg, since the EC and OC concentrations were so high that the equipment could not measure them. Such behavior was not observed in the CO- and PM2.5-measured concentrations, because even when the values were high, they remained in the range of the other cremations.
Particle-bounded polycyclic aromatic compound concentrations and emission factors
PAH concentrations in TSP also presented significant differences between concentrations measured in ovens with and without controlled air supply, as can be observed in Table 4. PAHs with two and three rings contributed only 1 to 2% to particulate mass, since the lightest PAHs are mainly found in the gas phase, which was not sampled. PAHs of 5 and 6 rings, like benzo [ghi] perylene and indeno[1,2,3-cd] pyrene, were the most abundant PAHs present in particles emitted by ovens with controlled air supply. Nevertheless, in the oven without controlled air supply, PAHs of four rings such as fluoranthene, pyrene, and benzo [a] anthracene contributed with 36%, opposite to the ovens with controlled air supply where the contribution of those three PAHs was only 5.8%. Carcinogenic compounds contributed with 45% of total PAHs in average ovens. Benzo [a] pyrene is of especial concern for its great carcinogenic potential (Mastandrea et al. 2005), contributing between 14 and 16% of total PAHs mixture. Average ∑PAHs are in the range reported for 71 boilers and 22 furnaces of 1–592 μg m−3 (Murillo et al. 2017b).
Table 5 displays the emission factors determined for every PAHs. The USEPA (1990) reported an emission factor of 189 mg/corpse for the sum of 16 PAHs (alike this study, excepting 2-methylnaphtalene) that is greater than the average emission factor of 139 mg/corpse determined in this study for ovens with controlled air supply. Conversely, the determined emission factor of the PAH sum emitted by the oven without air supply control was 1.7 times greater than the USEPA’s one, showing again the need of controlled air injection as well as particle control devices. In the case of European standards, the emission factors for benzo [a] pyrene, benzo [b] fluoranthene, benzo [k] fluoranthene, and indeno[1,2,3-cd] pyrene are 13.2, 7.21, 6.44, and 6.99 mg/corpse, respectively. The emission factors obtained in this study for the average ovens were around 30% greater for the first three compounds and more than two times greater for the indeno[1,2,3-cd]pyrene.
Concentrations and emission factors of particle-bonded arsenic and metals
During cremation, metals, and metalloids contained in the corpse are released in the particles emitted to the atmosphere; consequently, their availability is increased in the environment. The living beings need essential elements such as iron (Fe), copper (Cu), and zinc (Zn) that are involved in biochemical processes for the life development, although these metals can become toxic if doses are high enough (Zoroddu et al. 2019). Zn is part of around 200 enzymes involved in several metabolic reactions, and Cu is necessary to fix calcium in the bones and to build all connective tissues (Rubio et al. 2007; Ashish et al. 2013; Al-Fartusie and Mohssan 2017). Nickel (Ni) and vanadium (V) are presumed to be necessary but not ascertained to be essential for humans since no biochemical function has been defined yet (Duda-Chodak and Błaszczyk 2008; Assem and Oskarsson 2015). Essential metals can become toxic if doses are high enough. In addition to mentioned metals, people is exposed to toxic non-essential elements like arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) by drinking contaminated water, food ingestion, and inhalation. Several studies have shown that accumulations of metals can cause toxic reactions in humans, animals, and plants due to alterations of their biochemical processes and the induction of oxidative stress (Gómez-Arroyo et al. 2018).
As, Cd, and Ni are well-known carcinogenic affecting several organs (Selene et al. 2003; Lewis et al. 2012; Al-Fartusie and Mohssan 2017; WHO 2018). Pb and Hg can affect digestive and immune systems and several organs like lungs, liver, and kidneys, but the nervous system is the most sensitive to poisoning by those metals (Al-Fartusie and Mohssan 2017; Fuentes and Martínez 2018).
As was expected, arsenic and metal emission concentrations were not affected by the air supply control, then the concentrations measured in the TSP from the three ovens did not present significant differences (α = 0.05; p = 0.643); for that reason, they were not depicted by oven in Fig. 1d that illustrates the basic statistics of As and metal emissions from all the ovens, with the exception of Hg and Zn, which presented very high emissions compared with other elements. The abundance order of elements in particles was Hg > Zn > V > Pb > Ni > As > Cu > Cd. Santarsiero et al. (2005) reported concentrations of 0.6–5.88 mg Zn Nm−3 dry 11% O2, similar to those found in this study with 0.55–3.21 mg Zn Nm−3 dry 11% O2; in the case of Pb, values measured were 0.07–0.18 mg Nm−3 dry 11% O2, which is also into the range reported in that manuscript of < 0.01–0.69. Finally, Hg concentrations in the present study were 0.13–0.37 mg Nm−3 dry 11% O2, which are higher than the reported values of < 0.02–0.29 mg Hg Nm−3 dry 11% O2 by Santarsiero et al. (2005), although the median (0.23 mg Hg Nm−3) is in agreement with the data of Buschmann and Tsokos (2014) reporting that several studies found emissions as large as 200 μg Hg m3 during cremation of each corpse with dental amalgam fillings, but are lower than the emission limit of 0.4 mg m−3 for crematories in China (Xue et al. 2016). It is worrying that Cu and Pb emission concentrations measured in this study were up to 10 times and 50 times greater than those reported from industrial boilers and furnaces using all kinds of fuel, while V and Ni were up to 50 times higher in boilers that use gas oil and biomass and LPG furnace. In addition to the metal content in the corpses, these results can be due to their clothing with which they are cremated that contain metal items like buttons, zippers, and ornaments.
As and metal emission factors are presented in Table 6; in comparison with those from the European Environmental Agency, EF for As, Cd, Cu, and Ni determined in this study are lower, with the exception of zinc, although all of them resulted much greater than those reported by Xue et al. (2016) in China. In the case of Hg, the determined emission factor is around 25.9% than the EF proposed by the European Environment Agency (EEA. 2016) of the 1.49 g/corpse. This could be explained because the EEA and Xue et al. (2016) mercury emission factors include gaseous and particle-bonded mercury, while only the metal bonded to particles is reported in this research, although most of the mercury could be emitted in the flue gases as evaporated mercury, since at 600 °C, Hg° is the dominant species contributing with 70%, as reported by Takaoka et al. (2010), who also assumed that most Hg emissions in the gas stack originated from silver dental amalgams.
Then, it would be necessary to determine the volatilized Hg° to have better estimations of the Hg released from each corpse. Other studies reported a large range from 0.7 to 362 mg/corpse (Takaoka et al. 2010), and an average 240 mg Hg/body release in the UK; and USEPA reported that the emission was 456 mg/body from nine cremations (Rahill 2008). In the UK, a standard of 150 mg/four cremations was proposed as a regulatory criterion by Defra (2004). Mercury emissions from crematories today are in significant decline due primarily to changes in dental practices. Comparing the Hg emissions with other sources such as factories or waste incinerators (municipal, hazardous, and medical), emissions from crematories are significantly lower and generally non-threatening to the environment, but cremation-related emissions of mercury should not be underestimated: mercury is unstable at cremation temperatures and free mercury metal is highly volatile (Buschmann and Tsokos 2014).
Relationships among variables
Positive high correlations were found between PM and 4–6 ring PAHs (r = 0.95) as well as between CO and EC (r = 0.795), see Appendix E of supplementary material. A total of 23 female corpses and 19 male corpses were cremated, 15 corpses belonged to people from 39 to 69 years old and 27 to people from 70 to 99 years old. Corpses’ weight varied from 30 to 150 kg. The most common decease causes were diabetes and hypertension as well as a combination of both, contributing with 21%, 16%, and 14%, respectively. Other reported illnesses were heart disease, cancer, renal disease, pneumonia, and hepatic cirrhosis. The Spearman correlation at 95% significance was applied to determine relationships among different variables, but they were not found, with the exception of the only positive relationship (r = 0.91 and r = 0.78, α = 0.05) among EC and OC emission concentrations with body weight, respectively (Appendix E), having higher emissions of total carbon for males.
No significant differences in PM, CO, and PAH emissions were found due to age (α = 0.05; p = 0.648) or weight (α = 0.05; p = 0.567) or due to corpse gender (α = 0.05; p = 0.570). Additionally, higher levels of copper were found in women cremations compared with males, since it has been reported that women tend to have higher levels of copper than men due to hormonal changes, then women also have more symptoms related to copper imbalance (Ashish et al. 2013).
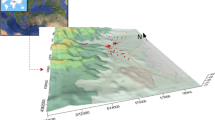
Pollutant dispersion from crematories
Outputs from the Hysplit v.4 model are presented in Fig. 3, where crematoria were georeferenced showing the affected area by the PM2.5 and Hg emissions on the first 50 m of the atmospheric layer at the end of 2-h cremation processes. An overlap of the contaminant puffs with the consequent pollutant accumulation is observed during the 35 simultaneous cremation processes. Overlapping puffs cover large areas with high concentrations of pollutants. It is a worrisome matter that most crematoria generally operate the equipment continuously, so that once the crematorium is idle, a new cremation process begins; consequently, concentrations of ambient pollutants rise gradually throughout the day. In this scenario, the simultaneous crematoria operation impacted 20 municipalities with PM2.5-increased concentrations between 0.0001 and 1 μg m−3.
Potential increments for all pollutants are shown in Table 7 and more information about people exposure can be found in Appendix F of supplementary material.
While the rise in PM2.5 may not be significant, the increase in Hg concentrations could result in significant exposure to this metal, since around 456,000 inhabitants (32% children under 12 and adults over 60 years) are exposed to increases greater than 0.1 ng Hg m−3 and more than 9450 people would be exposed to a Hg concentration increase of 1 ng m−3. Maximum Hg concentrations are two times higher than the estimated Hg potential increase of 2 to 3 ng Hg m−3 by Green et al. (2014) who, in the worst scenery, applied AERMOD model using a greater Hg EF (3 g per corpse), in a residential neighborhood close to a crematory operating 13 h per day in California.
Comparisons among different researches are difficult since models are very sensitive to meteorology, crematorium number, cremation time, and EF among other conditions as can be observed in other studies that report increments between 0.009 and 0.07 μg PM2.5 m−3 versus the 0.0001 to 1 μg PM2.5 m−3 obtained in this research (Heggies Pty 2009). The benzo [a] pyrene increase is also of concern since the European Directive has established a value of 1 ng m−3 of benzo [a] pyrene equivalent as an annual standard to protect the population’s health, then the crematoria emissions could represent a 10% increase of the daily concentration showing once again the need of particles control devices in crematoria facilities; in the case of Hg emitted in the vapor phase, several control methods have been proposed (Wang et al. 2014; Yan et al. 2003). To date, the impact of these emissions has been minimized; nevertheless, the results of this study show that they should be monitored especially if the number of cremations increases.
Conclusions
USEPA method 201-A for PM2.5 was applied in crematoria oven chimneys for identification and quantification of different pollutants in order to develop emission factors of toxic pollutants. CO, PM2.5, EC, OC, individual PAHs, As, Cd, Cu, Hg, Ni, Pb, V, and Zn emission factors were estimated from more than 40 corpse cremations in two types of crematoria facilities, whereas EC and OC emission factors were determined for the first time in crematoria. These emission factors can be applied for emission inventories estimations worldwide. CO, PM2.5, EC, OC, and PAH emission differences were noticeable between crematorium ovens without controlled air supply, which presented concentrations up to six times higher than ovens with air control supply, showing the need that all crematoria have controlled air supply devices for combustion improvement. PAHs with 5 and 6 rings were the most abundant in ovens with controlled air supply, while PAHs with 4 rings were the most abundant in ovens without air control supply. Carcinogenic species represented 45% of total PAHs in average ovens. Hg was the most abundant metal bonded to particles with concentrations up to 0.37 mg Nm−3 dry 11% O2, whereas Cu, Pb, Ni, and V emissions were greater than those reported for other combustion facilities using diesel, gas oil, biomass, and LPG furnaces. The emission factor of particle-bonded Hg was 386.4 ± 45.6 mg/corpse, but recommended also the measurement of vaporized Hg to get the EF of total emitted mercury. Controlled air supply devices were not enough to control CO and PM2.5, since cremations bearing such kind of control exceeded Mexicans and international emissions standards by 28% and 36%, respectively; then, emissions control systems should be installed in all crematoria. EC and OC emission concentrations had a high relationship with body weight, presenting higher emissions of total carbon for men, and Cu emissions were greater for women. The other emission factors or emission concentrations did not present correlation with any other corpse variables. Emissions modeling showed large nearby areas impacted by crematoria emissions; although the increase of PM2.5 in those locations is relatively small (up to 1.0 μg m−3), Hg particle-bonded and PAH increases can represent a risk for the surrounding population since more than 9450 people would be exposed to a Hg concentration increase of 1 ng m−3 and around 4999 people would be exposed to a benzo [a] pyrene concentration increase of 0.1 ng m−3. To date, the impact of these emissions has been minimized; nevertheless, the results of this study show the convenience of continuous surveillance of crematoria emissions since they will grow.
References
Al-Fartusie FS, Mohssan SN (2017) Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci 5:127–136. https://doi.org/10.22607/IJACS.2017.503003
Alvarado-Cruz I, Sánchez-Guerra M, Hernández-Cadena L, De Vizcaya-Ruiz A, Mugica V, Pelallo-Martínez NA, de Jesus Solís-Heredia M, Byun HM, Baccarelli A, Quintanilla-Vega B (2017) Increased methylation of repetitive elements and DNA repair genes is associated with higher DNA oxidation in children in an urbanized, industrial environment. Mutat Res Genet Toxicol Environ Mutagen 813:27–36. https://doi.org/10.1016/j.mrgentox.2016.11.007
Aschner M, Erikson KM, Dorman DC (2005) Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol 35:1–32. https://doi.org/10.1080/10408440590905920
Ashish, Neeti K, Khajuria H (2013) Copper toxicity: a comprehensive study. Res J Recent Sci 2:58–67
Assem FL, Oskarsson A (2015) Vanadium. Handb Toxicol Met 1347–1367. https://doi.org/10.1016/B978-0-444-59453-2.00060-3
Birch ME, Cary RA (1996) Elemental carbon-based method for monitoring occupational exposures to particulate diesel exhaust. Aerosol Sci Technol 25:221–241. https://doi.org/10.1080/02786829608965393
Bullock OR (2000) Modeling assessment of transport and deposition patterns of anthropogenic mercury air emissions in the United States and Canada. Sci Total Environ 259:145–157. https://doi.org/10.1016/S0048-9697(00)00578-7
Buschmann CT, Tsokos M (2014) Cremation. Handb Forensic Med:134–137. https://doi.org/10.1002/9781118570654.ch8
Chan KF (2002) Minimising black smoke emission from existing light duty flat bed cremators. HKIE Trans Hong Kong Inst Eng 9:56–60. https://doi.org/10.1080/1023697X.2002.10667893
Chen B, Stein AF, Maldonado PG, Sanchez de la Campa AM, Gonzalez-Castanedo Y, Castell N, de la Rosa JD (2013) Size distribution and concentrations of heavy metals in atmospheric aerosols originating from industrial emissions as predicted by the HYSPLIT model. Atmos Environ 71:234–244. https://doi.org/10.1016/j.atmosenv.2013.02.013
De Angelis D, Collini F, Muccino E, Cappella A, Sguazza E, Mazzucchi A, Cattaneo C (2017) Science and justice analysis of metallic medical devices after cremation : the importance in identification. Sci Justice 57:128–135. https://doi.org/10.1016/j.scijus.2016.11.003
Defra UK (2004) Mercury emissions from crematoria: Second consultation, July 2004, 26 pages. Department for Environment, Food and Rural Affairs. Accessed on the Internet on March 13, 2020 http://www.defra.gov.uk/environment/quality/pollution/ppc/old-consultations/crematoria-two/consultation.pdf
Draxler RR, Hess GD (1998) An overview of the HYSPLIT_4 modeling system for trajectories, dispersion, and deposition. Aust Meteorol Mag 47(4):295–308
Duda-Chodak A, Błaszczyk U (2008) The impact of nickel on human health. J Elem 13:685–696
EEA. (2016) European Environment Agency. Cremation
Fuentes I, Martínez R (2018) Mercury concentration in hair due to environment on two populations in Mexico. In: Leal Filho W, Noyola-Cherpitel R, Medellín-Milán P, Ruiz Vargas V (eds) Sustainable Development Research and Practice in Mexico and Selected Latin American Countries. Springer International Publishing, Cham, pp 241–255. https://doi.org/10.1007/978-3-319-70560-6_15
Gómez-Arroyo S, Barba-García A, Arenas-Huertero F, Cortés-Eslava J, Grutter M, Mora D, García-Martínez R (2018) Indicators of environmental contamination by heavy metals in leaves of Taraxacum officinale in two zones of the metropolitan area of Mexico City. Environ Sci Pollut Res 25:4739–4749
Gozález-Cardoso G, Hernández-Contreras JM, Santiago-de la Rosa N, Gutiérrez M, Mugica-Álvarez V, (2018). PM2.5 emissions from urban crematoriums, en: 5th International Conference on Energy and Environment Research, ICEER 2018. Energy Procedia, pp. 359–363. https://doi.org/10.1016/j.egypro.2018.10.047
Green LC, Crouch EAC, Zemba SG (2014) Cremation, air pollution, and special use permitting: a case study. Hum Ecol Risk Assess 20:559–565. https://doi.org/10.1080/10807039.2012.719391
Heggies Pty LTD, 2009. Air quality impact assessment proposed crematorium Tuggeranong, ACT
Kato N, Mastui Y, Takaoka M, Yoneda M (2017) Measurement of nanoparticle exposure in crematoriums and estimation of respiratory deposition of the nanoparticles by number and size distribution Nobuhuki. J Occup Health 59:572–580. https://doi.org/10.1539/joh.17-0008-FS
Klaufus C (2014) Deathscapes in Latin America’s metropolises: urban land use, funerary transformations, and daily inconveniences. Eur Rev Lat Am Caribb Stud 96:99–111. https://doi.org/10.18352/erlacs.9469
Lee CS, Lim YW, Kim HH, Yang JY, Shin DC (2012) Exposure to heavy metals in blood and risk perception of the population living in the vicinity of municipal waste incinerators in Korea. Environ Sci Pollut Res 19:1629–1639. https://doi.org/10.1007/s11356-011-0677-z
Lewis AS, Reid KR, Pollock MC, Campleman SL (2012) Speciated arsenic in air: measurement methodology and risk assessment considerations. J Air Waste Manage Assoc 62:2–17. https://doi.org/10.1080/10473289.2011.608620
Mari M, Domingo JL (2010) Toxic emissions from crematories: a review. Environ Int 36:131–137. https://doi.org/10.1016/j.envint.2009.09.006
Mastandrea C, Chichizola C, Ludueña B, Sánchez H, Álvarez H, Gutiérrez A (2005) Hidrocarburos aromáticos policíclicos. Riesgos para la salud y marcadores biológicos. Acta Bioquímica Clínica Latinoam 39:27–36
Mugica-Álvarez V, Hernández-Rosas F, Magaña-Reyes M, Herrera-Murillo J, Santiago-De La Rosa N, Gutiérrez-Arzaluz M, de Jesús Figueroa-Lara J, González-Cardoso G (2018) Sugarcane burning emissions: characterization and emission factors. Atmos Environ 193:262–272. https://doi.org/10.1016/j.atmosenv.2018.09.013
Murillo JH, Marín JFR, Mugica-Alvarez V, Arias DS, Guerrero VHB (2017a) Polycyclic aromatic hydrocarbons in filterable PM2.5emissions generated from regulated stationary sources in the metropolitan area of Costa Rica. Atmos Pollut Res 8:843–849. https://doi.org/10.1016/j.apr.2017.02.002
Murillo JH, Marín JFR, Mugica-Alvarez V, Arias DS, Guerrero VHB, Rojas Marín JF, Mugica-Alvarez V, Solórzano Arias D, Guerrero VHB (2017b) Chemical characterization of filterable PM2.5emissions generated from regulated stationary sources in the Metropolitan Area of Costa Rica. Atmos Pollut Res 8:709–717. https://doi.org/10.1016/j.apr.2017.01.007
NFDA, 2016. 2016 NFDA cremation and burial report released: rate of cremation surpasses that of burial in 2015, NFDA News Releases
NMX-AA-010-SCFI-2001, 2001. NMX-AA-010-SCFI-2001 Atmospheric pollution - stationary sources - determination of particles in the flue gases flowing through a duct - isokinetic sampling method. Mexico
Pavlish JH (2009) Preface to the AQVI special issue of fuel processing technologies entitled: air quality VI: mercury, trace elements, SO3, particulate matter, and greenhouse gases. Fuel Process Technol. 90:1327–1332. https://doi.org/10.1016/j.fuproc.2009.09.006
Petzold A, Ogren JA, Fiebig M, Laj P, Li S, Baltensperger U, Holzer-Popp T, Kinne S, Pappalardo G, Sugimoto N, Wehrli C, Wiedensohler A, Zhang X (2013) Recommendations for reporting “black carbon” measurements. Atmos Chem Phys 13:8365–8379. https://doi.org/10.5194/acp-13-8365-2013
Rahill P (2008) Mercury Emissions and the Cremation Process–2008. Available at: http://www.matthewscremation.com/pdf/MercuryEmissions&CremProcess.pdf
Rubio C, Weller DG, Revert C, Hardisson IRA (2007) Zinc: an essential oligoelement. Nutr Hosp 22:101–107
Santarsiero A, Settimo G, Cappiello G, Viviano G, Andrea ED, Gentilini L (2005) Urban crematoria pollution related to the management of the deceased. Microchem J 79:307–317. https://doi.org/10.1016/j.microc.2004.10.015
Santiago-De La Rosa N, González-Cardoso G, Figueroa-Lara JDJ, Gutiérrez-Arzaluz M, Octaviano-Villasana C, Ramírez-Hernández IF, Mugica-Álvarez V (2018) Emission factors of atmospheric and climatic pollutants from crop residues burning. J Air Waste Manage Assoc 68:849–865. https://doi.org/10.1080/10962247.2018.1459326
SEDEMA, 2018. Aviso por el que se da a conocer la norma ambiental para el distrito federal NADF-017- AIRE-2017 - equipos de cremación e incineración - límites máximos permisibles de emisiones a la atmósfera y condiciones de operación. Gac Of La Ciudad México 25–33
Selene CH, Chou J, De Rosa T (2003) Case studies- arsenic. Int J Hyg Environ Health 206:381–386. https://doi.org/10.1078/1438-4639-00234
Smith TO, Gitsham P, Donell ST, Rose D, Hing CB (2012) The potential dangers of medical devices with current cremation practices. Eur Geriatr Med 3:97–102. https://doi.org/10.1016/j.eurger.2012.01.013
Takaoka M, Oshita K, Takeda N, Morisawa S (2010) Mercury emission from crematories in Japan. Atmos Chem Phys 10:3665–3671. https://doi.org/10.5194/acp-10-3665-2010
Takeda N, Takoaka M, Oshita K, Eguchic S (2014) PCDD/DF and co-planar PCB emissions from crematories in Japan. Chemosphere 98:91–98. https://doi.org/10.1016/j.chemosphere.2013.09.114
Tavares da Cruz NJ, Rojas Lezana ÁG, Freire dos Santos da Cruz P, Santana Pinto Bittencourt IM, Zancan C, Silva de Souza GH (2017) Environmental impacts caused by cemeteries and crematoria, new funeral technologies, and preferences of the Northeastern and Southern Brazilian population as for the funeral process. Environ Sci Pollut Res 24:24121–24134. https://doi.org/10.1007/s11356-017-0005-3
USEPA (1990) Emission inventory section 112. Appendix b documentation of 7-pah and 16-pah national emission estimates
USEPA (1999a) Method CTM-034. Determination of oxygen, carbon monoxide and oxides of nitrogen from stationary sources for periodic monitoring
USEPA (1999b) Compendium method TO-13A determination of polycyclic aromatic hydrocarbons (PAHs) in ambient air using gas chromatography/mass spectrometry (GC/MS)
Valle-Hernández BL, Mugica-Álvarez V, Salinas-Talavera E, Amador-Muñoz O, Murillo-Tovar MA, Villalobos-Pietrini R, De Vizcaya-Ruíz A (2010) Temporal variation of nitro-polycyclic aromatic hydrocarbons in PM10and PM2.5collected in Northern Mexico City. Sci Total Environ 408:5429–5438. https://doi.org/10.1016/j.scitotenv.2010.07.065
Vatican, C.F.D., 2016. Instruction Ad resurgendum cum Christo. Vatican City
Villegas H (2019) Primer Informe de Actividades. Consejería Jurídica y de Servicios Legales, Mexico City, p 190 Consulted on 10-10-2019. https://consejeria.cdmx.gob.mx/storage/app/uploads/public/5d9/b43/0c4/5d9b430c4fdd1609449797.pdf
Wang S, Zhang L, Wang L, Wu Q, Wang F, Hao J (2014) A review of atmospheric mercury emissions, pollution and control in China. Front Environ Sci Eng 8:631–649. https://doi.org/10.1007/s11783-014-0673-x
WHO, (2018). Arsenic
Wood MD, Punt A, Leah RT, 2008. Assessment of the mercury concentrations in soil and vegetation , including crops , around crematoria to determine the impact of mercury emissions on food safety
Xue Y, Tian H, Yan J, Xiong C, Pan T, Nie L (2016) Present and future emissions of HAPs from crematories in China. Atmos Environ 124:28–36. https://doi.org/10.1016/j.atmosenv.2015.10.079
Yan R, Liang DT, Tay JH (2003) Control of mercury vapor emissions from combustion flue gas. Environ Sci Pollut Res 10:399–407. https://doi.org/10.1065/espr2003.04.149
Yang K, Li Q, Yuan M, Guo M, Wang Y, Li S, Tian C, Tang J, Sun J, Li J, Zhang G (2019) Temporal variations and potential sources of organophosphate esters in PM2.5 in Xinxiang, North China. Chemosphere 215:500–506. https://doi.org/10.1016/j.chemosphere.2018.10.063
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorg Biochem 195:120–129. https://doi.org/10.1016/j.jinorgbio.2019.03.013
Acknowledgments
The authors thank the support by the CONACYT project number 181231 as well as to H-Ramirez-Guzmán and O-Talavera by their help with particle micrographs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 98 kb)
Rights and permissions
About this article
Cite this article
González-Cardoso, G., Hernández-Contreras, J.M., Valle-Hernández, B.L. et al. Toxic atmospheric pollutants from crematoria ovens: characterization, emission factors, and modeling. Environ Sci Pollut Res 27, 43800–43812 (2020). https://doi.org/10.1007/s11356-020-10314-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10314-0