Abstract
This work studies the degradation of seven representative antibiotics (ciprofloxacin, norfloxacin, levofloxacin, oxacillin, cloxacillin, cefalexin, and cefadroxil) by solar photo-Fenton process. The removal of antibiotics by the individual components (i.e., light, H2O2, or Fe (II)) and the complete photochemical system (light/H2O2/Fe (II)) was initially evaluated. Then, the effect of citric acid addition to the photo-Fenton system was assessed. In the third place, the primary transformation products for two illustrative cases (ciprofloxacin and oxacillin treated by photo-Fenton) were determined. Also, photo-Fenton in the presence of citric acid was applied to remove antibiotics from a simulated hospital wastewater. It was found that the solar light component induced degradation of ciprofloxacin, norfloxacin, and levofloxacin, but the rest of the considered antibiotics were not reduced by photolysis. In turn, the photo-Fenton system showed a degrading action on all the tested antibiotics. The addition of citric acid to the system significantly increased the removal of antibiotics. Initial degradation products indicated that hydroxyl radical attacked moieties of antibiotics responsible for their antimicrobial activity. Finally, the treatment of hospital wastewater evidenced the high potentiality of photo-Fenton process for degrading antibiotics in aqueous matrices containing elevated concentrations of citric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive use of antibiotics for preventing and treating human and animal diseases leads to release large amounts of such substances to the environment (Gothwal and Shashidhar 2015). These compounds are ubiquitous in wastewater from hospital, domestic, livestock, veterinary, and pharmaceutical industry activities (Rizzo et al. 2013; Botero-Coy et al. 2018), which are collected by municipal treatment plants or directly released into the environment.
Nowadays, it is well-known that conventional wastewater treatment plants are inefficient to remove antibiotics (Kümmerer 2009; Rivera-Utrilla et al. 2013); as a consequence, they reach natural water bodies (Khetan and Collins 2007; Kümmerer 2009; Watkinson et al. 2009; Martinez 2009; Sim et al. 2011; Brausch et al. 2012; Hernández et al. 2015). In natural media, antibiotics show low degradation rates by hydrolysis, biodegradation, and photo-degradation (Khetan and Collins 2007). Additionally, antibiotics can induce toxic effects and contribute to the development of antibiotic-resistant bacteria (Homem and Santos 2011). Due to the continuous input, persistence and negative impact in the aquatic ecosystems, these pharmaceuticals are considered emerging concern pollutants (Homem and Santos 2011), which demands the application of efficient processes to limit their input into the environment.
The photo-Fenton process has gained much attention as an alternative for degrading recalcitrant pollutants due to its high efficiency, operational easiness, and possibility of solar irradiation utilization (Pouran et al. 2015; Clarizia et al. 2017). Photo-Fenton system firstly involves reaction of Fe (II) with hydrogen peroxide (Eq. 1). Then, the Fe (III), produced in the first step, forms ferric aquo complexes (e.g., [Fe (OH)]2+). Such complexes have an important light absorption between 290 and 410 nm, and they can be reduced in the aqueous medium by the action of UV-light, producing extra hydroxyl radicals (Eq. 2) and making the system a photocatalytic process (Pignatello et al. 2006).
Some previous works have reported the application of photo-Fenton process for degrading antibiotics; even some of them have informed the use of iron-complexing substances to improve the process performance (Elmolla and Chaudhuri 2009; Trovó et al. 2009, 2011; De Lima Perini et al. 2013; Nogueira et al. 2017; Buitrago et al. 2020). Nevertheless, most of these publications were focused on the treatment of maximum three antibiotics; thus, information about the structural variety of pollutants is missed. Additionally, considerations about the antimicrobial activity and its connection with structural transformations of antibiotics are scarce.
In the present research, the removal of seven highly consumed antibiotics from water by solar photo-Fenton process, and its individual components (i.e., solar light, hydrogen peroxide, or ferrous ions) was initially evaluated. Special attention is paid on the structural aspects that determine the degradation of antibiotics. Secondly, the effect of citric acid addition to the photo-Fenton system was assessed. Thirdly, to better understand the action of the process on the antibiotics, primary transformation products for two illustrative cases (ciprofloxacin and oxacillin) were determined. Finally, the use of solar photo-Fenton improved by citric acid addition to remove representative antibiotics, and their associated antimicrobial activity from a simulated hospital wastewater (HWW) was examined.
Experimental
Reagents
Cloxacillin (CLO), cephalexin (CPX), and cefadroxil (CDX) were provided by Syntopharma laboratories (Bogotá, Colombia). Oxacillin (OXA) was purchased from Sigma-Aldrich (St. Louis, USA). Ciprofloxacin (CIP) and norfloxacin (NOR) were provided by Laproff laboratories (Medellín, Colombia). Levofloxacin (LEV) was obtained from Chemo laboratories (Sao Paulo, Brazil). Sodium chloride, sodium sulfate, potassium dihydrogen phosphate, calcium chloride dihydrate, potassium chloride, ammonium chloride, urea, acetonitrile, hydrogen peroxide (30%), and nutrient agar were provided by Merck (Darmstadt, Germany). Peptone, meat extract, and potato dextrose agar were purchased from Oxoid (Basingstoke, England). Formic acid was provided by Carlo-Erba (Val de Reuil, France). Iron (II) sulfate heptahydrate was purchased from PanReac (Barcelona, Spain). All chemicals were used as received. For the experiments, the concentration of antibiotics was 40 μmol L−1 each one.
Reaction system
Solutions of antibiotics (100 mL) were placed in a beaker. The irradiation of samples was carried out in an ATLAS SUNTEST CPS+ solar simulator, equipped with a Xe lamp and filters to allow the pass of wavelengths of light between 300 and 800 nm. The beakers, magnetically stirred, were placed under the SUNTEST lamp filters. The SUNTEST was operated at 500 W m−2 (51.1 W m−2 of UVA intensity determined using a SOLAR PMA2000 radiometer), and the temperature was kept constant at 35 °C (which was the lowest operative value allowed by the solar simulator). The antibiotics were individually treated. During treatments, aliquots were taken at regular interval times to perform the analyses.
In this solar photo-Fenton system, a low Fe (II) concentration (i.e., 1 mg L−1) was selected to limit the formation iron precipitates at near-neutral pH. Besides, as a Fe:H2O2 ratio ~ 1:10 has shown to be very efficient for organic pollutant removal from water (Klamerth et al. 2010, 2013; De la Cruz et al. 2012, 2013); then, 10 mg L−1 of H2O2 was used in this work. Also, to favor the photo-regeneration of iron, the solar simulator was operated at a high irradiation intensity (i.e., 500 W m−2). Moreover, an initial pH of 6.5 was selected considering that hospital wastewater is typically ranged between 6 and 9 (Verlicchi et al. 2015).
Initially, the pharmaceuticals were individually treated by the solar photo-Fenton system to better analyze the effect of the structure of antibiotics. Also, it is important to indicate that such initial experiments were developed in distilled water spiked with the target pollutants. After understanding basic aspects (effects of structure of antibiotics, individual components of the process, and addition of citric acid), tests considering two representative antibiotics in the HWW were performed. Table SM1 (in Supplementary material) presents the composition of the HWW (taken from (Serna-Galvis et al. 2017)). Such water was selected because it contains both organic and inorganic substances, which allows to obtain an initial approach to the competence of matrix constituents during degradation of antibiotics.
Analyses
Antibiotic evolution was followed by liquid chromatography using a Thermo Scientific UHPLC (Dionex Ultimate 3000) equipped with a diode array detector (DAD) and an Acclaim™ 120 RP C18 column (5 μm, 4.6 × 150 mm). Conditions to follow the removal of antibiotics are presented in Table 1. The analyses of samples were carried out immediately after the experiments; then a quenching agent to eliminate residual H2O2 was not added.
The transformations of antibiotics were established through HPLC-MS techniques. For ciprofloxacin, the primary products were determined using the methodology performed in Villegas-Guzman et al. (2017c). A HPLC Agilent 1200 series coupled to an Agilent LC/MSD VL SQ mass spectrometer was used. The column and mobile phase were operated at the same conditions described for the quantitative analysis of this antibiotic in Table 1. The injection volume was 10 μL, and the mass spectrometer detector was operated in a positive ion mode.
In turn, the primary products of oxacillin were established using the chromatographic conditions presented in our previous work (Serna-Galvis et al. 2016). Such products were extracted from the treated water and concentrated using Strata X cartridges loaded with 50 mL of the sample. The byproducts were desorbed with 2 mL of 2% formic acid and then analyzed using a Thermo Scientific HPLC (Ultimate 3000)–MS (Orbitraps) instrument equipped with a Merck column LiChrospher RP-18 (5 μm, 250 × 4.5 mm). The mobile phase was a mixture of acetonitrile acidified with 0.1% of formic acid and water acidified with 0.1% formic acid in a linear gradient from 10 to 100% of acidified acetonitrile for 50 min and then these conditions for 5 min. The flow and injection volume were 0.4 mL min−1 and 20 μL, respectively. The mass spectrometer was operated in the electrospray positive ion mode.
The antimicrobial activity was determined by the inhibition zone measurement, using Staphylococcus aureus (S. aureus) as indicator microorganism. Briefly, sample solution (30 μL) was seeded on Petri dishes containing 5 mL of potato dextrose agar and 10 mL of nutrient agar inoculated with 10 μL of S. aureus (with an optical density of 0.600 at 580 nm). After 24 h at 37 °C in a Memmert Schwabach incubator, the diameter of the inhibitory halo was measured with a Vernier (Serna-Galvis et al. 2017).
Results and discussion
Antibiotics degradation under solar photo-Fenton system
Solar photo-Fenton and its component action
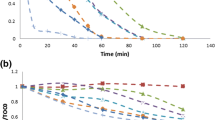
Figure 1 presents removal of the antibiotics under 5 min of treatment by the solar photo-Fenton system. It can be noted that at the considered treatment time, more than 30% of pollutant concentration was removed. Due to the photo-Fenton system has as components, solar light, hydrogen peroxide (H2O2), and ferrous ion (Fe (II)), the participation of such constituents was also tested (Fig. 1). Both H2O2 and Fe (II) showed low direct action (< 2% of degradation) on the antibiotics. Interestingly, the solar light induced a considerable removal of the fluoroquinolones, whereas the cephalosporins and penicillins had no degradation.
Antibiotics removal by solar photo-Fenton system and its components (solar light, Fe (II), and H2O2). Experimental conditions: [antibiotics]: 40 μmol L−1, [Fe (II)]: 1 mg L−1, [H2O2]: 10 mg L−1, intensity of the simulated solar light: 500 W m−2, and pHinitial: 6.5. Antibiotics were individually treated
An examination of UV-Vis spectra for the considered penicillins and cephalosporins (Figure SM1A-B, in the Supplementary material) reveals that these substances do not present significant light absorption at wavelengths above 280 nm. In contrast, ciprofloxacin, norfloxacin, and levofloxacin have significant light absorptions between 280 and 380 nm (Figure SM1C), which make these antibiotics susceptible to direct sunlight action (because the emission of this system starts at ~ 300 nm (Figure SM1D)). Indeed, the direct photo-degradation order for the fluoroquinolones was CIP > NOR > LEV.
Previous works about the photochemistry of fluoroquinolones (FQ) indicate that substitution is a main primary mechanistic pathway promoted by light for these antibiotics (Eq. 3–4) (Albini and Monti 2003). Also, it is recognized that electron-withdrawing substituents bonded to aromatic systems favor this pathway, but electron-donating effects disfavor it (Cornelisse and Havinga 1975; Sturini et al. 2012). From the structures of the three fluoroquinolones, it can be noted that LEV has an electron-donating alkoxy group bonded to the aromatic ring, whereas CIP and NOR have cyclopropyl and ethyl moieties, respectively (Table SM1). Due to straining in alkyl rings (as cyclopropyl), they may have lower electron-donating effects than linear alkyl groups (as ethyl). Thus, CIP has substituents with lower electron-donating capability than NOR and LEV. Additionally, CIP has a higher quantum yield than LEV for direct photolysis by solar light (Ge et al. 2010). These facts would explain the highest CIP degradation by direct sunlight action (Fig. 1).
On the other hand, the low removal by Fe (II) is explainable considering that ferrous ions are reducing species in aqueous media. In fact, Fe (II) can evolve to Fe (III) by the action of dissolved oxygen, generating anion superoxide radical (O2•−, Eq. 5) (Morgan and Lahav 2007), which is a soft oxidizing agent (E°: 1.0 V, (Hayyan et al. 2016)). Additionally, anion superoxide radical undergoes disproportion reactions in aqueous media leading to formation of H2O2 (Sawyer and Valentine 1981). Thus, at a short period of treatment, O2•− species has no strong degrading effect on the antibiotics. Although H2O2 could act as degrading agent (Petri et al. 2011), due to its moderate redox potential (E°: 1.78 V, (Giraldo-Aguirre et al. 2018)), hydrogen peroxide showed low degrading action on the antibiotics. In contrast, the sunlight/Fe (II)/H2O2 combination (i.e., the solar photo-Fenton system), which produces hydroxyl radical (a strong oxidizing agent, E°: 2.80 V, Eq. 1–2) (Pignatello et al. 2006)), led to significant removals of antibiotics (Fig. 1).
At this point, it should be indicated that solar photo-Fenton has as a subsystem, the Fenton process (Fe (II)/H2O2, Eq. 1). Although the Fenton reaction (Fe2+/H2O2) is able to induce significant degradations on antibiotics at low times of treatment, the process rapidly reaches a plateau (as exemplified in Figure SM2). In the Fenton system, the interaction between Fe (II) and H2O2 to produce HO● is very fast (Eq. 1, k = 53–76 L mol−1 s−1), but the regeneration of Fe (II) is significantly slower (Eq. 6, k = 10−6–10−2 L mol−1 s−1) (Pignatello et al. 2006). For this reason, after the initial step (Eq. 1), the production of radicals is low (furthermore, the generated perhydroxyl radical (HOO•, E°: 1.46) is a weaker oxidizing agent than hydroxyl radical (E°: 2.80 V) (Armstrong et al. 2013)). Consequently, the degradation of antibiotics is practically stopped (Figure SM2). Meanwhile, in the photo-Fenton process, the light accelerates the Fe (II) regeneration and increases the amount of HO● available (Eq. 2) to degrade pollutants (Pignatello et al. 2006). Hence, the percentage of removal of antibiotics by solar photo-Fenton is higher than by Fenton (Figure SM2). Additionally, it is important to mention that although the initial pH was 6.5, in most of cases, during the experiments, the pH decreased up to 4.0 (± 0.2), which limits the iron precipitation as ferric hydroxides, thus favoring the performance of the solar photo-Fenton process (Clarizia et al. 2017).
From Fig. 1, it can be noted that, independently of the antibiotic nature (structure), the removals of antibiotics by the solar photo-Fenton process were > 35% after only 5 min of treatment. This evidenced the strong degrading ability of this AOP, which can be associated to the non-selective nature of HO●. In fact, the second-order reaction rate constants for the interaction of hydroxyl radical with fluoroquinolones, penicillins, or cephalosporins are very close (they have the same magnitude order 109 M−1 s−1) (An et al. 2010a; Dail and Mezyk 2010; Márquez et al. 2013; He et al. 2014; Mandal 2018; Wojnárovits et al. 2018), which evidences the high reactivity of HO● towards the considered antibiotics independently of their classes or structures.
Improvement of solar photo-Fenton process using citric acid
The enhancement of photo-Fenton treatment efficiency can be achieved by the use of organic ligands such as short-chain acids (de Lima Perini et al. 2017; Gomes Júnior et al. 2018). Moreover, the addition of these ligand agents has also allowed to overcome pH limitations of the Fenton process (De Lima Perini et al. 2013; de Lima Perini et al. 2017). Thereby, the seven antibiotics were degraded by the solar photo-Fenton system in the presence of citric acid. It must be mentioned that citric acid is typically used for washing dialysis machines (BCRenal Agency 2016); thus, such ligand can be found in hospital wastewater together with antibiotics. In Fig. 2a, the removals of pollutants by solar photo-Fenton in the presence and in the absence of citric acid are compared after 5 min of treatment.
Comparison of antibiotics removal by solar photo-Fenton in the absence and the presence of citric acid. a Antibiotics removal. b UV-Vis spectrum of the iron (III)-citric acid complex. Experimental conditions: [antibiotics]: 40 μmol L−1, [Fe (II)]: 1 mg L−1, [H2O2]: 10 mg L−1, [citric acid]: 40 μmol L−1, [Fe (III)]: 1 mg L−1, intensity of the simulated solar light: 500 W m−2 and pHinitial: 6.5
Figure 2 a shows the significant pollutant removal enhancement by the citric acid addition. Citric acid is able to complex ferric ion (Fig. 2b) (Silva et al. 2009). The interaction of iron with citric acid produces soluble ferric complexes (species with a high formation constant K = 2.0 × 106 (Hamm et al. 1954)), which could limit ferric hydroxide formation and keep the Fe (III) in soluble forms at pH higher than 3.0 (Villegas-Guzman et al. 2017a, b). Besides, it is reported that the interaction of citric acid-Fe (III) complexes with light is able to induce the regeneration of Fe (II) ions (Eq. 6). Indeed, the quantum yield for Fe (II) photo-regeneration from iron-citric acid complex (0.28 at 366 nm) is higher than the observed for iron-aquo complexes (0.017 at 360 nm) (Gomes Júnior et al. 2018). Therefore, the improvement of all antibiotics elimination by solar photo-Fenton in citric acid presence can be associated to an effective regeneration of Fe (II), which enhances the iron catalytic cycle. It should be indicated that during the process application in presence of citric acid, the experimental pH decreased up to values below 5.0. This favors the predominance of the iron-citrate complex, limiting the iron precipitation (Clarizia et al. 2017).
The above results evidence the high potential for actual water treatments using solar photo-Fenton. For example, hospital wastewater from renal sections, which typically contains citric acid and hydrogen peroxide from the dialysis machine washing (BCRenal Agency 2016). Such sewage could be mixed with water from intensive care unit or hospitalization sections (which can have high antibiotics content) to apply a photo-Fenton process for pharmaceuticals elimination.
Transformation of antibiotics by photo-Fenton process action
The determination of primary transformations contributes to better understand the process action on the antibiotics. Thus, as illustrative cases, the initial products of CIP (whose degradation involves the participation of light and radicals, Fig. 1) and OXA (mainly eliminated by radical attacks, Fig. 1) were established. Table 2 presents the chemical structures of the degradation products identified by the HPLC-MS analyses.
The products for CIP showed modifications at the piperazyl moiety and the quinolone system on the antibiotic. As above indicated, photolysis (direct solar light action) is able to induce the substitutions of fluorine on benzene ring of the ciprofloxacin by hydroxyl or hydrogen groups producing DP1 and DP2, respectively. Such transformations are typical in the photo-degradation of aromatic compounds as fluoroquinolone antibiotics (Fasani et al. 1999; Albini and Monti 2003; Niu et al. 2016). Meanwhile, hydroxyl radical attacked the amines on the piperazyl resulting in the formation of a hydroxylated product with the opening of this ring (DP3). In addition to the attacks on the amine groups, HO• reacted with the quinolone moiety (hydroxyl radical has high reactivity toward C-C π-systems (Pignatello et al. 2006)). Electron abstraction from the quinoline moiety followed by a reaction with water would generate DP4. Interestingly, the computational calculations in a previous work have evidenced that piperazyl moiety and quinolone ring have the highest electron density on CIP (Serna-Galvis et al. 2017)), which correspond to the regions on the fluoroquinolone attacked by hydroxyl radicals. Moreover, the oxidative action of hydroxyl radicals on piperazyl and quinolone groups has been also found in the CIP treatment by others AOP such as electro-Fenton and TiO2-photocatalysis (Paul et al. 2010; Salma et al. 2016; Villegas-Guzman et al. 2017c).
Regarding NOR, it can be mentioned that the norfloxacin elimination by a Fenton-like system (whose degradation is also based on oxidation by HO•) shows oxidative action of hydroxyl radical on piperazyl ring and substitution of F atom by OH moiety (Wang et al. 2018). Meanwhile, in the treatment of NOR and LEV by TiO2 photocatalysis, elimination of piperazyl group in fluoroquinolone molecules, HO• addition to quinolone ring, and ipso attack at the F atoms on the aromatic ring by hydroxyl radicals were observed (An et al. 2010b). Also, in the degradation of LEV by electro-Fenton, the hydroxyl radical firstly attacked the piperazyl moiety and induced a decarboxylation of this fluoroquinolone (Liu et al. 2017). Therefore, both our experimental results and literature indicate that primary transformations of the fluoroquinolone antibiotics by HO• start at piperazyl moiety.
On the other hand, the analysis of initial products for OXA showed that its degradation occurred through hydroxylation on its aromatic ring yielding DP5 intermediate, oxidation of the thioether group to form DP6, opening of the β-lactam to produce DP7 and DP8 (two stereoisomers of penicilloic acid), and rupture of the central secondary amine to generate DP9. Because the electron-rich character of the sulfur and aromatic ring on OXA (as also demonstrated by computational analysis in a previous work (Serna-Galvis et al. 2017)), the HO• attack to these moieties is favored. Indeed, the thioether oxidation to sulfoxide group has been previously reported during the OXA degradation by TiO2 photocatalysis (Giraldo-Aguirre et al. 2015) and sonochemistry (Serna-Galvis et al. 2018). This transformation route was also observed for the ampicillin (other structurally related β-lactam antibiotic) removal by photogenerated HO• (UVC/H2O2 process) (He et al. 2014). The high oxidative power of hydroxyl radical plus the low stability of the four-membered ring promote the β-lactam opening to produce the stereoisomers (Trovó et al. 2011; He et al. 2014). In fact, DP7 and DP8 were also reported for the OXA degradation by non-thermal plasma process (Magureanu et al. 2011). It must be mentioned that pathways of CLX transformation very similar to OXA have been described for the treatment by the photo-Fenton process. Thus, it can be indicated that OXA and CLX are primarily degraded by HO• attacks to the penicillin nucleus and benzene ring.
In the case of cephalosporins (i.e., CPX and CDX), based on their structural similarity to penicillins, analogous degradation pathways could be expected. For example, a previous work about cephalexin treatment by a Fenton-like process demonstrated that hydroxyl radicals promoted the central secondary amine cleavage plus further rupture of β-lactam on such cephalosporin (Bansal and Verma 2017). Additionally, the elimination of ceftriaxone (other cephalosporin antibiotic) by Fenton oxidation has indicated that the β-lactam ring is attacked in the first stages of degradation (Puddoo et al. 2017).
Considering that the solar photo-Fenton process induced strong transformations on the basic core of antibiotics (as above shown), the overall removal of antibiotic activity could be expected. It is recognized that the destruction of β-lactam ring and oxidation of thioether group on penicillins and cephalosporins leads to antimicrobial activity elimination (Serna-Galvis et al. 2016; Qian et al. 2018). Also, modifications of piperazyl and quinolone moieties produce a decreasing of the bactericidal action of fluoroquinolones (Paul et al. 2010). More details on the antimicrobial activity are discussed in the next subsection.
Application of the solar photo-Fenton process to HWW
The applicability of the solar photo-Fenton process to matrices more complex than distilled water (DW) was also tested. Thus, the degradation of LEV and OXA in HWW was considered. These antibiotics were selected because LEV showed the lowest degradation, and OXA had highest removal percentage (Fig. 1).
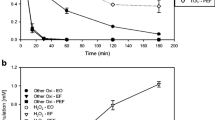
Figure 3 presents the treatment of LEV and OXA in HWW by solar photo-Fenton process. After 90 min of treatment, 54 and 15% of levofloxacin and oxacillin were removed, respectively. The comparison of antibiotics degradation in HWW and DW evidenced that LEV is little affected, whereas the removal of OXA is strongly inhibited by the matrix components (Figure SM3). This could be related to the fact that in the solar photo-Fenton process, LEV is degraded by direct photolysis plus action of hydroxyl radical (Ahmad et al. 2013; Nogueira et al. 2017), while OXA elimination is mainly due to HO● attacks. As the hydroxyl radical is not selective, this reacts with antibiotics and HWW matrix components (which are more concentrated than the target pollutants; Table SM1); thus, the degradation of OXA (whose removal occurs via radical) is more affected than LEV.
Antibiotics degradation in simulated hospital wastewater (HWW). a Levofloxacin case (LEV). b Oxacillin case (OXA). c Antimicrobial activity evolution. Experimental conditions: [antibiotic]: 40 μmol L−1, [Fe (II)]: 1 mg L−1, [H2O2]: 10 mg L−1, [citric acid]: 40 or 4000 μmol L−1, intensity of the simulated solar light: 500 W m−2, and pHinitial: 6.5
It should be mentioned that components of HWW such as chloride, dihydrogen phosphate, ammonium/ammonia, and urea can act as quenchers of hydroxyl radical (because they have high rate constants, Table SM3), making slower the degradation of the antibiotics (Liao et al. 2001; Devi et al. 2013). Furthermore, in the HWW matrix, the Fe (III) cations may react with phosphate anions, forming insoluble compounds (Eq. 7), hampering iron photo-regeneration (Eq. 2) (Oller et al. 2006; Pignatello et al. 2006; Bacardit et al. 2007). This also affects the solar photo-Fenton performance in the HWW matrix.
To enhance the antibiotics degradation in HWW, the addition of citric acid (at two concentrations: 40 μM and 4 mM) to the solar photo-Fenton system was assessed (Fig. 3a–3b). For both antibiotics (LEV and OXA), the citric acid presence accelerated their removal. Furthermore, the increment in citric acid concentration also improved the antibiotics elimination. As indicated in the “Improvement of solar photo-Fenton process using citric acid” section, citric acid enhances the iron catalytic cycle favoring pollutant degradation (Abrahamson et al. 1994; Giannakis et al. 2016; Villegas-Guzman et al. 2017a), which explains its positive effect on the degradation of antibiotics in HWW.
Taking into account the high ability of solar photo-Fenton in the presence of citric acid at 4 mM to degrade LEV and OXA antibiotics in HWW, the evolution of antibiotic activity (AA) during such treatment was established (Fig. 3c). Interestingly, the AA decreased with the process application. In the case of LEV, the AA was completely diminished after 90 min of treatment. Meanwhile, in the same period, ~ 73% of antibacterial activity of HWW with OXA was removed. These results can be associated to the structural transformations of the antibiotics.
As previously mentioned, the action of hydroxyl radical on LEV can induce oxidative action on piperazyl ring and substitution of F atom plus decarboxylation. The piperazyl oxidation alters the acid/base speciation and consequently the cell permeability (Paul et al. 2010). The piperazyl modifications also reduce the binding to bacterial DNA topoisomerase or DNA gyrase (i.e., the action mechanism of fluoroquinolone antibiotics) (Alovero et al. 2000). The carboxylic group is involved in the fluoroquinolone-enzyme linkage. Meanwhile, the F atom on LEV favors the bacterial cell permeation (Paul et al. 2010), and this moiety also exerts a blocking effect on fundamental enzymes for DNA replication (Domagala 1994; Peterson 2001; Andersson and MacGowan 2003). Thus, the antibiotic activity from LEV is removed by the treatment with the solar photo-Fenton process.
In its turn, OXA experimented transformations on the penicillin core (i.e., β-lactam ring opening and sulfur oxidation, Table 2), which is responsible for the bactericidal action. The elimination of β-lactam ring from OXA (the antibiotic part with the main role against bacteria (Gringauz 1997; Konaklieva 2014)) produces the reduction of AA. After 90 min of treatment of the HWW containing OXA (Fig. 3c), the remaining AA may be associated to the residual OXA concentration or products conserving unmodified the penicillin core. Complete removal of the antibiotic activity could be achieved at longer treatment times. Finally, decreasing/removal of AA is a positive aspect of the solar photo-Fenton process application, which has repercussions in the limitation of antibiotic resistance proliferation. However, it can be indicated that complementary analyses of mineralization and biodegradability to better support the positive environmental impact of the treatment of antibiotics should be carried out in future researches.
Conclusions
The treatment of antibiotics showed that solar light only induced direct degradation of the fluoroquinolones due to their considerable absorption of UV-light component. Meanwhile, in the solar photo-Fenton system, all the target antibiotics experimented considerable degradation, which was mainly associated to action of generated hydroxyl radicals. The addition of citric acid significantly increased the removal of all antibiotics by formation of soluble ferric complexes, which are able to keep the Fe (III) in solution, favoring the catalytic cycle in the solar photo-Fenton process. From the analyses of initial products, it can be concluded that primary transformations of the fluoroquinolone antibiotics by HO● occur at piperazyl moiety. In turn, the β-lactam antibiotics were very susceptible to radical attacks on their sulfur atoms, central four-member ring and aromatic moiety. Hence, the process led to strong structural modifications (including active nuclei) of the antibiotics, which could be correlated with a decreasing of antimicrobial activity. Finally, the treatment of HWW evidenced the potential applicability of solar photo-Fenton process for the elimination of relevant antibiotics in actual aqueous matrices having high amounts of citric acid (e.g., hospital wastewater from renal sections).
References
Abrahamson HB, Rezvani AB, Brushmiller JG (1994) Photochemical and spectroscopic studies of complexes, of iron (III) with citric acid and other carboxylic acids. Inorg Chim Acta 226:117–127. https://doi.org/10.1016/0020-1693(94)04077-X
Ahmad I, Bano R, Sheraz MA, Ahmed S, Mirza T, Ansari SA (2013) Photodegradation of levofloxacin in aqueous and organic solvents: a kinetic study. Acta Pharma 63:223–229. https://doi.org/10.2478/acph-2013-0011
Albini A, Monti S (2003) Photophysics and photochemistry of fluoroquinolones. Chem Soc Rev 32:238–250. https://doi.org/10.1039/b209220b
Alovero FL, Pan X, Morris JE et al (2000) Engineering the specificity of antibacterial fluoroquinolones: benzenesulfonamide modifications at C-7 of ciprofloxacin change its primary target in Streptococcus pneumoniae from topoisomerase IV to gyrase. Antimicrob Agents Chemother 44:320–325. https://doi.org/10.1128/AAC.44.2.320-325.2000
An T, Yang H, Li G, Song W, Cooper WJ, Nie X (2010a) Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl Catal B Environ 94:288–294. https://doi.org/10.1016/j.apcatb.2009.12.002
An T, Yang H, Song W, Li G, Luo H, Cooper WJ (2010b) Mechanistic considerations for the advanced oxidation treatment of fluoroquinolone pharmaceutical compounds using TiO2 heterogeneous catalysis. J Phys Chem A 114:2569–2575. https://doi.org/10.1021/jp911349y
Andersson MI, MacGowan AP (2003) Development of the quinolones. J Antimicrob Chemother 51(Suppl 1):1–11. https://doi.org/10.1093/jac/dkg212
Armstrong DA, Huie RE, Lymar S, Koppenol WH, Merényi G, Neta P, Stanbury DM, Steenken S, Wardman P (2013) Standard electrode potentials involving radicals in aqueous solution: inorganic radicals. Bioinorg React Mech 9:59–61. https://doi.org/10.1515/irm-2013-0005
Bacardit J, Stötzner J, Chamarro E, Esplugas S (2007) Effect of salinity on the photo-Fenton process. Ind Eng Chem Res 46:7615–7619. https://doi.org/10.1021/ie070154o
Bansal P, Verma A (2017) Synergistic effect of dual process (photocatalysis and photo-Fenton) for the degradation of Cephalexin using TiO2 immobilized novel clay beads with waste fly ash/foundry sand. J Photochem Photobiol A Chem 342:131–142. https://doi.org/10.1016/j.jphotochem.2017.04.010
BCRenal Agency (2016) Cleaning & disinfecting Hemodialysis machines & stations. BCRenal Agency Canada
Botero-Coy AM, Martínez-Pachón D, Boix C, Rincón RJ, Castillo N, Arias-Marín LP, Manrique-Losada L, Torres-Palma R, Moncayo-Lasso A, Hernández F (2018) An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater. Sci Total Environ 642:842–853. https://doi.org/10.1016/j.scitotenv.2018.06.088
Brausch JM, Connors K, Brooks BW, Rand GM (2012) Human pharmaceuticlas in the aquatic environment: a review of recent toxicological studies and considerations for toxicity testing. Rev Environ Contam Toxicol 218:1–99. https://doi.org/10.1007/978-1-4614-3137-4
Buitrago JL, Sanabria J, Gútierrez-Zapata HM, Urbano-Ceron FJ, García-Barco A, Osorio-Vargas P, Rengifo-Herrera JA (2020) Photo-Fenton process at natural conditions of pH, iron, ions, and humic acids for degradation of diuron and amoxicillin. Environ Sci Pollut Res 27:1608–1624. https://doi.org/10.1007/s11356-019-06700-y
Clarizia L, Russo D, Di Somma I et al (2017) Environmental homogeneous photo-Fenton processes at near neutral pH : a review. Appl Catal B Environ Environ 209:358–371. https://doi.org/10.1016/j.apcatb.2017.03.011
Cornelisse J, Havinga E (1975) Photosubstitution reactions of aromatic compounds. Chem Rev 75:353–388. https://doi.org/10.1021/cr60296a001
Dail MK, Mezyk SP (2010) Hydroxyl-radical-induced degradative oxidation of beta-lactam antibiotics in water: absolute rate constant measurements. J Phys Chem A 114:8391–8395. https://doi.org/10.1021/jp104509t
De la Cruz N, Giménez J, Esplugas S et al (2012) Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res 46:1947–1957. https://doi.org/10.1016/j.watres.2012.01.014
De la Cruz N, Esquius L, Grandjean D et al (2013) Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res 47:5836–5845. https://doi.org/10.1016/j.watres.2013.07.005
De Lima Perini JA, Perez-Moya M, Nogueira RFP (2013) Photo-Fenton degradation kinetics of low ciprofloxacin concentration using different iron sources and pH. J Photochem Photobiol A Chem 259:53–58. https://doi.org/10.1016/j.jphotochem.2013.03.002
de Lima Perini JA, Costa e Silva BC, Tonetti AL et al (2017) Photo-Fenton degradation of the pharmaceuticals ciprofloxacin and fluoxetine after anaerobic pre-treatment of hospital effluent. Environ Sci Pollut Res 24:6233–6240. https://doi.org/10.1007/s11356-016-7416-4
Devi LG, Munikrishnappa C, Nagaraj B, Rajashekhar KE (2013) Effect of chloride and sulfate ions on the advanced photo Fenton and modified photo Fenton degradation process of Alizarin Red S. J Mol Catal A Chem 374–375:125–131. https://doi.org/10.1016/j.molcata.2013.03.023
Domagala JM (1994) Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother 33:685–706. https://doi.org/10.1093/jac/33.4.685
Elmolla E, Chaudhuri M (2009) Degradation of the antibiotics amoxicillin , ampicillin and cloxacillin in aqueous solution by the photo-Fenton process. J Hazard Mater 172:1476–1481. https://doi.org/10.1016/j.jhazmat.2009.08.015
Fasani E, Barberis Negra FF, Mella M, Monti S, Albini A (1999) Photoinduced C-F bond cleavage in some fluorinated 7-amino-4-quinolone- 3-carboxylic acids. J Organomet Chem 64:5388–5395. https://doi.org/10.1021/jo982456t
Ge L, Chen J, Wei X, Zhang S, Qiao X, Cai X, Xie Q (2010) Aquatic photochemistry of fluoroquinolone antibiotics: kinetics, pathways, and multivariate effects of main water constituents. Environ Sci Technol 44:2400–2405. https://doi.org/10.1021/es902852v
Giannakis S, Polo López MI, Spuhler D et al (2016) Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction-part 2: a review of the applications for drinking water and wastewater disinfection. Appl Catal B Environ 198:431–446. https://doi.org/10.1016/j.apcatb.2016.06.007
Giraldo-Aguirre AL, Erazo-Erazo ED, Flórez-Acosta OA, Serna-Galvis EA, Torres-Palma RA (2015) TiO2 photocatalysis applied to the degradation and antimicrobial activity removal of oxacillin: evaluation of matrix components, experimental parameters, degradation pathways and identification of organics by-products. J Photochem Photobiol A Chem 311:95–103. https://doi.org/10.1016/j.jphotochem.2015.06.021
Giraldo-Aguirre AL, Serna-Galvis EA, Erazo-Erazo ED, Silva-Agredo J, Giraldo-Ospina H, Flórez-Acosta OA, Torres-Palma RA (2018) Removal of β-lactam antibiotics from pharmaceutical wastewaters using photo-Fenton process at near-neutral pH. Environ Sci Pollut Res 25:20293–20303. https://doi.org/10.1007/s11356-017-8420-z
Gomes Júnior O, Silva VM, Machado AEH, Sirtori C, Lemos CR, Freitas AM, Trovó AG (2018) Correlation between pH and molar iron/ligand ratio during ciprofloxacin degradation by photo-Fenton process: identification of the main transformation products. J Environ Manag 213:20–26. https://doi.org/10.1016/j.jenvman.2018.02.041
Gothwal R, Shashidhar T (2015) Antibiotic pollution in the environment : a review. Clean - Soil Air Water 43:479–489. https://doi.org/10.1002/clen.201300989
Gringauz A (1997) Antimicrobial drugs I. In: Introduction to medicinal chemistry, First. Wiley-VCH, New York, pp 191–263
Hamm RE, Shull CM, Grant DM (1954) Citrate complexes with iron(II) and iron(III)1. J Am Chem Soc 76:2111–2114. https://doi.org/10.1021/ja01637a021
Hayyan M, Hashim MA, Alnashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116:3029–3085. https://doi.org/10.1021/acs.chemrev.5b00407
He X, Mezyk SP, Michael I, Fatta-Kassinos D, Dionysiou DD (2014) Degradation kinetics and mechanism of β-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J Hazard Mater 279:375–383. https://doi.org/10.1016/j.jhazmat.2014.07.008
Hernández F, Ibáñez M, Botero-Coy A-M, Bade R, Bustos-López MC, Rincón J, Moncayo A, Bijlsma L (2015) LC-QTOF MS screening of more than 1,000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogotá, Colombia. Anal Bioanal Chem 407:6405–6416. https://doi.org/10.1007/s00216-015-8796-x
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices – a review. J Environ Manag 92:2304–2347. https://doi.org/10.1016/j.jenvman.2011.05.023
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev 107:2319–2364. https://doi.org/10.1021/cr020441w
Klamerth N, Rizzo L, Malato S, Maldonado MI, Agüera A, Fernández-Alba AR (2010) Degradation of fifteen emerging contaminants at ug L-1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res 44:545–554. https://doi.org/10.1016/j.watres.2009.09.059
Klamerth N, Malato S, Agüera A, Fernández-Alba A (2013) Photo-Fenton and modified photo-Fenton at neutral pH for the treatment of emerging contaminants in wastewater treatment plant effluents: a comparison. Water Res 47:833–840. https://doi.org/10.1016/j.watres.2012.11.008
Konaklieva M (2014) Molecular targets of β-lactam-based antimicrobials: beyond the usual suspects. Antibiotics 3:128–142. https://doi.org/10.3390/antibiotics3020128
Kümmerer K (2009) Antibiotics in the aquatic environment – a review – part I. Chemosphere 75:417–434. https://doi.org/10.1016/j.chemosphere.2008.11.086
Liao C, Kang S-F, Wu F-A (2001) Hydroxyl radical scavenging role of chloride and bicarbonate ions in the H2O2/UV process. Chemosphere 44:1193–1200. https://doi.org/10.1016/S0045-6535(00)00278-2
Liu X, Yang D, Zhou Y, Zhang J, Luo L, Meng S, Chen S, Tan M, Li Z, Tang L (2017) Electrocatalytic properties of N-doped graphite felt in electro-Fenton process and degradation mechanism of levofloxacin. Chemosphere 182:306–315. https://doi.org/10.1016/j.chemosphere.2017.05.035
Magureanu M, Piroi D, Mandache NB, David V, Medvedovici A, Bradu C, Parvulescu VI (2011) Degradation of antibiotics in water by non-thermal plasma treatment. Water Res 45:3407–3416. https://doi.org/10.1016/j.watres.2011.03.057
Mandal S (2018) Reaction rate constants of hydroxyl radicals with micropollutants and their significance in advanced oxidation processes. J Adv Oxid Technol 21:178–195. https://doi.org/10.26802/jaots.2017.0075
Márquez G, Rodríguez EM, Beltrán FJ, Álvarez PM (2013) Determination of rate constants for ozonation of ofloxacin in aqueous solution. Ozone Sci Eng 35:186–195. https://doi.org/10.1080/01919512.2013.771530
Martinez JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut 157:2893–2902. https://doi.org/10.1016/j.envpol.2009.05.051
Morgan B, Lahav O (2007) The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution – basic principles and a simple heuristic description. Chemosphere 68:2080–2084. https://doi.org/10.1016/j.chemosphere.2007.02.015
Niu X-Z, Busetti F, Langsa M, Croué J-P (2016) Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation. Water Res 106:214–222. https://doi.org/10.1016/j.watres.2016.10.002
Nogueira AA, Souza BM, Dezotti MWC, Boaventura RAR, Vilar VJP (2017) Ferrioxalate complexes as strategy to drive a photo-FENTON reaction at mild pH conditions: a case study on levofloxacin oxidation. J Photochem Photobiol A Chem 345:109–123. https://doi.org/10.1016/j.jphotochem.2017.05.020
Oller I, Gernjak W, Maldonado MI (2006) Solar photocatalytic degradation of some hazardous water-soluble pesticides at pilot-plant scale. J Hazard Mater 138:507–517. https://doi.org/10.1016/j.jhazmat.2006.05.075
Paul T, Dodd MC, Strathmann TJ (2010) Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: transformation products and residual antibacterial activity. Water Res 44:3121–3132. https://doi.org/10.1016/j.watres.2010.03.002
Peterson LR (2001) Quinolone molecular structure-activity relationships: what we have learned about improving antimicrobial activity. Clin Infect Dis 33:S180–S186. https://doi.org/10.1086/321846
Petri BG, Watts RJ, Teel AL, et al (2011) Fundamentals of ISCO using hydrogen peroxide. In: Siegrist RL et al. (ed) In situ chemical oxidation for groundwater remediation. Springer Science + Business Media, pp 33–87
Pignatello JJ, Oliveros E, Mackay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84. https://doi.org/10.1080/10643380500326564
Pouran SR, Aziz ARA, Mohd W, Wan A (2015) Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J Ind Eng Chem 21:53–69. https://doi.org/10.1016/j.jiec.2014.05.005
Puddoo H, Nithyanandam R, Nguyenhuynh T (2017) Degradation of the antibiotic ceftriaxone by Fenton oxidation process and compound analysis. J Phys Sci 28:95–114. https://doi.org/10.21315/jps2017.28.3.7
Qian Y, Gao P, Xue G, Liu Z, Chen J (2018) Oxidation of cefalexin by permanganate: reaction kinetics, mechanism, and residual antibacterial activity. Molecules 23:2015. https://doi.org/10.3390/molecules23082015
Rivera-Utrilla J, Sánchez-Polo M, Ferro-García MÁ, Prados-Joya G, Ocampo-Pérez R (2013) Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 93:1268–1287. https://doi.org/10.1016/j.chemosphere.2013.07.059
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360. https://doi.org/10.1016/j.scitotenv.2013.01.032
Salma A, Thoröe-boveleth S, Schmidt TC, Tuerk J (2016) Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J Hazard Mater 313:49–59. https://doi.org/10.1016/j.jhazmat.2016.03.010
Sawyer DT, Valentine JS (1981) How super is superoxide? Acc Chem Res 14:393–400. https://doi.org/10.1021/ar00072a005
Serna-Galvis EA, Silva-Agredo J, Giraldo-Aguirre AL, Flórez-Acosta OA, Torres-Palma RA (2016) High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason Sonochem 31:276–283. https://doi.org/10.1016/j.ultsonch.2016.01.007
Serna-Galvis EA, Ferraro F, Silva-Agredo J, Torres-Palma RA (2017) Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV254 and UV254/persulfate processes. Water Res 122:128–138. https://doi.org/10.1016/j.watres.2017.05.065
Serna-Galvis EA, Montoya-Rodríguez DM, Isaza-Pineda L et al (2018) Sonochemical degradation of antibiotics from representative classes-considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason Sonochem 50:157–165. https://doi.org/10.1016/j.ultsonch.2018.09.012
Silva AMN, Kong X, Parkin MC, Cammack R, Hider RC (2009) Iron(III) citrate speciation in aqueous solution. Dalton Trans:8616–8625. https://doi.org/10.1039/b910970f
Sim W, Lee J-W, Lee E-S et al (2011) Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 82:179–186. https://doi.org/10.1016/j.chemosphere.2010.10.026
Sturini M, Speltini A, Maraschi F, Pretali L, Profumo A, Fasani E, Albini A, Migliavacca R, Nucleo E (2012) Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water Res 46:5575–5582. https://doi.org/10.1016/j.watres.2012.07.043
Trovó AG, Nogueira RFP, Agüera A, Fernandez-Alba AR, Sirtori C, Malato S (2009) Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res 43:3922–3931. https://doi.org/10.1016/j.watres.2009.04.006
Trovó AG, Pupo Nogueira RF, Agüera A, Fernandez-Alba AR, Malato S (2011) Degradation of the antibiotic amoxicillin by photo-Fenton process – chemical and toxicological assessment. Water Res 45:1394–1402. https://doi.org/10.1016/j.watres.2010.10.029
Verlicchi P, Aukidy MA, Zambello E (2015) What have we learned from worldwide experiences on the management and treatment of hospital effluent? — An overview and a discussion on perspectives. Sci Total Environ 514:467–491. https://doi.org/10.1016/j.scitotenv.2015.02.020
Villegas-Guzman P, Giannakis S, Rtimi S et al (2017a) A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl Catal B Environ 219:538–549. https://doi.org/10.1016/j.apcatb.2017.07.066
Villegas-Guzman P, Giannakis S, Torres-Palma RA, Pulgarin C (2017b) Remarkable enhancement of bacterial inactivation in wastewater through promotion of solar photo-Fenton at near-neutral pH by natural organic acids. Appl Catal B Environ 205:219–227. https://doi.org/10.1016/j.apcatb.2016.12.021
Villegas-Guzman P, Hofer F, Silva-Agredo J, Torres-Palma RA (2017c) Role of sulfate, chloride, and nitrate anions on the degradation of fluoroquinolone antibiotics by photoelectro-Fenton. Environ Sci Pollut Res 24:28175–28189. https://doi.org/10.1007/s11356-017-0404-5
Wang G, Zhao D, Kou F, Ouyang Q, Chen J, Fang Z (2018) Removal of norfloxacin by surface Fenton system (MnFe2O4/H2O2): Kinetics , mechanism and degradation pathway. Chem Eng J 351:747–755. https://doi.org/10.1016/j.cej.2018.06.033
Watkinson AJ, Murby EJ, Kolpin DW, Costanzo SD (2009) The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Sci Total Environ 407:2711–2723. https://doi.org/10.1016/j.scitotenv.2008.11.059
Wojnárovits L, Tóth T, Takács E (2018) Critical evaluation of rate coefficients for hydroxyl radical reactions with antibiotics: a review. Crit Rev Environ Sci Technol 48:575–613. https://doi.org/10.1080/10643389.2018.1463066
Funding
The authors received support provided to their research group through “Programa de Sostenibilidad” from Universidad de Antioquia UdeA and financing from MINCIENCIAS COLOMBIA (before named COLCIENCIAS) through the project No. 111577757323 (Convocatoria 777 de 2017). EA Serna-Galvis also received his doctoral scholarship (Convocatoria 647 de 2014) from MINCIENCIAS COLOMBIA provided from July 2015 to June 2019.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 112 kb)
Rights and permissions
About this article
Cite this article
Serna-Galvis, E.A., Cáceres-Peña, A. & Torres-Palma, R.A. Elimination of representative fluoroquinolones, penicillins, and cephalosporins by solar photo-Fenton: degradation routes, primary transformations, degradation improvement by citric acid addition, and antimicrobial activity evolution. Environ Sci Pollut Res 27, 41381–41393 (2020). https://doi.org/10.1007/s11356-020-10069-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10069-8