Abstract
In this research, fifteen potentially toxic elements (PTEs) (Al, As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Mo, Ni, Pb, Sb, Sc and Zn) were analysed and quantified in samples collected at 44 sites in an urban area of Iran. Sources were apportioned using enrichment factors (EFs), modified pollution index (MPI), principal component analysis (PCA), multivariate linear regression of absolute principal component scores (MLR-APCS) and speciation, with a focus on anthropogenic PTEs in the urban and industrial soils of the Arvand Free Zone area, an oil-rich zone in the country. Furthermore, the bioaccessibility and the human health risks of PTEs were investigated. The EF revealed a significant enrichment for elements such as Cd, Cu, Hg, Mo, Pb, Sb and Zn. Values of MPI showed that Abadan industrial district and Abadan petrochemical complex are the most polluted sites in the study area.
The PCA/MLR analysis revealed four main sources: natural sources, fossil fuel combustion, traffic and oil derivatives and petroleum waste. The relative contribution of each source to PTE concentration varied from 32.3% of the natural sources to 30.6% of traffic and from 20.1% of petroleum waste to 17% of fossil fuel combustion. The source apportionment of metals generated using MLR-APCS receptor modelling revealed that 85.0% of Hg was generated by oil products. Chemical speciation results were compatible with the results obtained from PCA. Bioaccessibility of PTEs decreased from gastric to intestinal phase except Mo and Sb due to their different geochemical characteristics. Hazard index (HI) for non-cancer risk of PTEs for both children and adults based on total element concentrations was estimated to range from 2-fold to more than 10-fold higher than that of bioaccessible phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among different kinds of environmental contaminants, potentially toxic elements (PTEs) represent a serious issue due to their ubiquity, toxicity and persistence in the ambient environment (Burges et al. 2015; Guney et al. 2010). Due to the fast development of industries, agriculture and urbanisation in many cities of the world, increasing inputs of PTEs into the environment have accelerated environmental problems (Farsani et al. 2018; Prabhakar et al. 2014; Soltani et al. 2017; Yang and Cattle 2018). In urban areas, PTEs and other contaminants can reach soils via natural and anthropogenic activities (Wei and Yang 2010), with anthropogenic inputs dominating over lithogenic inputs. Metals could originate from mining and smelting activities, vehicle exhaust, industrial wastes, coal and oil combustion and deposition of atmospheric dust and aerosols, depending on site characteristic (Farsani et al. 2018; Prabhakar et al. 2014; Soltani et al. 2017; Yang and Cattle 2018). This contamination could adversely impact soil biology and functioning, change soil physicochemical properties and cause other environmental issues (Papa et al. 2010), which ultimately lead to considerable risks for humans and the ecosystem (Siciliano et al. 2009).
The evaluation of characteristics and contamination levels of PTEs in urban soils is necessary to preserve the urban environment and human health due to their long half-lives in the human body (Keshavarzi et al. 2015). The PTEs exert their toxicity through ingestion and dermal contact; thus, hand-to-mouth activities of children may cause them to be more sensitive (Ali et al. 2013; Peña-Fernández et al. 2014; Ruby and Lowney 2012). In most of the studies on soils, the main pathway of toxicity was found to be ingestion of contaminated soils (Ruby and Lowney 2012; Shi et al. 2011; Siciliano et al. 2009; Wu et al. 2015); thus, in vitro tests simulating the human gastrointestinal tract have been developed for their precision, ease, rapidity and low cost (Koch et al. 2007). Different tests have been devised to assess the bioaccessibility of PTEs in soils, such as the Solubility Bioavailability Research Consortium (SBRC) test (Li et al. 2016), the in vitro gastrointestinal (IVG) and the Deutsches Institut für Normung e.V. (DIN) methods (Li et al. 2015; Xie et al. 2019) and the physiologically based extraction test (PBET) (Li et al. 2015; Ren et al. 2015). Some of them, such as the SBRC, have also been validated for one or more elements using in vivo results (Li et al. 2015, 2016).
The PTEs in soils originate from various sources in urban areas, including natural (pedogenic processes) and different anthropogenic origins; therefore, it is important to distinguish pollution sources of PTEs that have become a significant environmental issue, to control pollution directly and define mitigation strategies (Hu and Cheng 2013; Luo et al. 2015; Szolnoki et al. 2013). Many approaches have been applied to identify different sources and quantitatively analyse the contributions of each source to the total pollution load, including receptor models such as chemical mass balance (CMB) model, principal component analysis (PCA)-related methods (absolute principal component scores (APCS); multiple linear regression (MLR), PCA-MLR; UNMIX model) and positive matrix factorisation (PMF) (Abbasi et al. 2020; Fei et al. 2019; Qu et al. 2018; Song et al. 2018). Among these receptor models, PCA-MLR or MLR-APCS has been widely applied for the source apportionment of contaminants in various media, such as soils and sediments, as it does not require a priori knowledge of the number of sources nor of the source profiles (Ashayeri and Keshavarzi 2019; Fei et al. 2019; Huang et al. 2018; Luo et al. 2015).
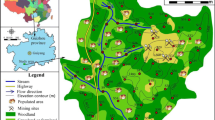
In recent years, several researches have focused on soil contamination in industrialised and urbanised areas of developed and developing countries, where there is specially great concern owing to ubiquity of dust and anthropogenic sources (Ajmone-Marsan and Biasioli 2010; Gulan et al. 2017; Li et al. 2017; Luo et al. 2015; Manta et al. 2002; Mehr et al. 2017; Qing et al. 2015; Tepanosyan et al. 2017; Xiong et al. 2016). Limited studies are available in urbanised and industrialised cities of Iran. The Arvand Free Zone, located in the Khuzestan Province, northwestern part of Persian Gulf (Fig. 1), is a long-established oil-rich zone of the country due to good transportation facilities, which include the Karoon and Arvand rivers, roads, rail, ports and airports. Lands of this zone have been allocated to industrial activities, in Khorramshahr and Abadan cities, such as the Abadan oil refinery, which is the oldest oil refinery in the Middle East, and other sectors as trade, tourism and office, ports, warehousing and transit.
Despite the importance of the Arvand Free Zone and the numerous possible sources of PTEs in the area, there is no available data on pollution of urban and industrial soils in the Arvand district nor data on the possible sources, and the environmental and related human health risks.
The primary goals of this work were (i) to evaluate the concentration and contamination degree of metals in urban and industrial soils, (ii) to determine the origins of PTEs using multivariate statistical analyses (PCA and PCA-MLR), (iii) to determine the bioavailability of PTEs using Community Bureau of Reference (BCR) method and the bioaccessibility of PTEs using both gastric and intestinal phases of SBRC method and (iv) to assess non-cancer health risks related to exposure to elements via various routes (ingestion, dermal contact and inhalation) based on total and bioaccessible concentrations for adults and children.
There is no comprehensive study on both urban and industrial soils in Iran, which investigates this number of bioaccessibility of PTEs in both gastric and intestinal fractions. Furthermore, this research focuses on investigating of Hg pollution resulting from different sources of Hg including chloralkali unit of Abadan petrochemical complex. So, this study constitutes the basis of a database to estimate the risk for the environment and humans as well as remediation of PTE-contaminated soils.
Materials and method
Study area
The Arvand Free Zone, with an area of 37,400 ha, is located at the anastomosis of the Karoon and Shatt al-Arab rivers. It is at the north-west of the Persian Gulf border, at a height of 3 m above sea level and in the vicinity of Iraq. The Arvand Free Zone consists of the cities of Abadan, Khorramshahr and Minushahr. The first oil refinery of the Middle East was established in Abadan, and the largest trade port of Iran is in Khorramshahr. This refinery started to operate in 1912 with a capacity of 2500 barrels/day. The Abadan petrochemical complex is another important industry in the Arvand Free Zone which was officially launched in 1969, producing mainly polyvinyl chloride (PVC) with the capacity of 20,000 tons per year (Shokrollahzadeh et al. 2008).
Due to numerous explosions at the Abadan oil refinery during Iran–Iraq war periods and other accidents, significant emissions of pollutants have occurred in different periods of time. Khorramshahr has a special importance in the province of Khuzestan owing to its economic impact and industrialisation, as well as its ports and associated cargo transit.
Sample collection
A total of 44 soil samples were collected in October 2017 (Fig. 1). These included thirty-two urban soils, nine samples in industrial areas and three samples as local background soil. Background soil samples were collected from zones far from population centres, industrial areas and other possible pollution sources at 50 cm deep to evaluate the geochemical background of elements. The study area is covered with quaternary sediments and recent alluvial deposits. According to pedological maps, Entisols and Aridisols are the soil types in the study area which do not reveal any profile development other than an A horizon (Moore and Keshavarzi 2014). Each surface sample consists of no less than five sub-samples from 1 to 10 cm surface soil. Different types of land use and possible sources of contaminants (industrial, commercial, high traffic and residential) were the factors which were considered for sampling site selection. Clean, self-sealed polyethylene bags were used for keeping them. The samples were dried at room temperature and passed via a 63-μm sieve for determination of the total concentration of PTEs, 200 μm for the SBRC test (Padoan et al. 2017) and a 2-mm sieve for physicochemical characteristics and BCR test in laboratory. The sieves were thoroughly washed and rinsed between samples by deionised water then oven-dried at 50 °C to exclude cross-contamination. Eventually, PTEs were analysed using inductively coupled plasma mass spectrometry (ICP-MS) at the accredited Acme Analytical Laboratory in Canada, using the digestion method of Liang and Grégoire (2000).
Physiochemical characteristics
The procedure of hydrometer described by Gee and Bauder (1986) was utilised for determination of the grain size distribution (sand, silt and clay fraction), and the USDA classification system was used for determination of soil texture. Electrical conductivity (EC) and pH were measured on 1:2 soil-to-water suspensions. Cation exchange capacity (CEC) was measured by the sodium acetate–ammonium acetate (NaOAc-NH4OAc) method (Ryan et al. 2007). Upon further drying at 105 °C, the organic matter (OM) content was assessed by measuring the loss on ignition (LOI) to constant mass at 550 °C for 4 h. The total organic carbon (TOC) value was calculated using a correction factor of 1.724 (TOC = OM / 1.724) (Schumacher 2002).
Extraction and analysis of PTEs
The total content of the elements was determined by weighing approximately 0.25 g of sieved samples (< 63 μm) to which a mixture of concentrated HF−HNO3−HClO4 was added and, afterward, the solutions were warmed and dried. A 3:1 mixture of concentrated HCl and HNO3 (aqua regia) was applied for the residue digestion and then diluted with deionised water up to 25 mL. The dissolved solution was analysed by ICP-MS.
The analysis of reagent blanks, analytic duplicates and certified reference material (CRM) such as multi-element STD OREAS45EA and OREAS45c and an internal standard STD DS11 were utilised for QA/QC determination. The CRM recovery percentages were always within 88 to 106%, showing a good agreement between the measured and certified values (Table S1). The analytical precision was determined by evaluating the relative standard deviation (Aquisman et al. 2019); it was below 7%, which is within the acceptable range (Table S1).
Modified BCR sequential extraction procedure
Twelve samples (S7, S8, S11, S15, S24, S25, S29, S31, S33, S34, S35, S39) out of 44 samples were selected for metal speciation. This sub-group of samples was carefully selected based on the different pollution loads (low, medium and high) and on the spatial distribution, so that it was possible to extend their results to the entire area.
The extraction method of this study was the “modified BCR” (Rauret et al. 2000). In the first step of BCR, ionic form of PTEs and those that were precipitated or coprecipitated with carbonate were leached in 0.11 mol/L acetic acid at pH 2.8. In the second step, PTEs which were adsorbed to amorphous Fe and Mn (hydro)oxides were separated in 0.1 mol/L hydroxylamine hydrochloride at pH 2. In the third step, PTEs incorporated to organic matter and sulfides were released undergoing oxidation in acid-stabilised 30% hydrogen peroxide and leaching in 1 mol/L ammonium acetate at pH 2 regulated with nitric acid. The residue from the third step was dissolved using aqua regia (fourth step) (Rauret et al. 2000). Standards, blank and duplicated samples were analysed in each analytical step (Ko et al. 2005), and results were accepted when the coefficient of variation was under 5%. Accuracy was verified using certified reference materials for aqua regia–soluble contents (CRM 141R, Community Bureau of Reference, Geel, Belgium).
The sum of the fractions extracted by the four steps was compared with the aqua regia (AR) digestion (extraction) of 1-g sample results. This represents a pseudo-total content. Recovery was computed as the ratio of the sum of the fractions to the AR, expressed as percentage. The recovery of all elements except Hg were between 70 and 130%, considering the sum of the 4 extracted fractions, which is acceptable (Cuong and Obbard 2006; Dehghani et al. 2017; Franklin et al. 2016; Sutherland and Tack 2002). The low recovery of Hg shows that BCR is not a suitable method for Hg fractionation as the addition of aqueous solutions and the heating may cause Hg volatilisation (Sladek and Gustin 2003).
Assessment of the bioaccessibility of PTEs
In this study, the SBRC in vitro assay (Kelley et al. 2002), including both gastric and intestinal simulations, was utilised for the assessment of the bioaccessibility of PTEs in the soils. In this method, 10 mL of simulated gastric fluid containing glycine (30.03 g/L) was used for the extraction of 0.1 g of soil (< 200 μm). The solution was regulated to pH 1.5 with concentrated HCl in 50-mL centrifuge tubes. Sealed bottles were placed into a water bath and rotated end-over-end at 37 °C ± 2 °C for 1 h. The extract was passed through a disposable 0.45-μm cellulose acetate filter into a clean 20-mL polyethylene vial. The samples were kept at 4 °C before elemental analysis by ICP-MS (Dodd et al. 2013; Li et al. 2016). The remaining solution was regulated to 7.0 with NaOH; then, intestinal phase extraction was performed by adding bile (1.75 g/L) and pancreatin (0.5 g/L) to the solutions. After shaking in a thermo bath at 37 °C, at 150 rpm for 4 h, the samples were centrifuged, filtered and moved to polyethylene tubes to keep at 4 °C, and then analysed by ICP-MS. The absolute bioaccessibility of PTEs was computed by dividing the extracted element (Cbioaccessible, mg/kg) by the total element content (Ctotal, mg/kg) in soils (Eq. (1)) (Li et al. 2015).
Data analysis
Pollution evaluation, risk assessment and statistical analysis
Quantification of the enrichment of PTEs can be done by several geochemical methods in soil samples. In the present study, enrichment factors (EFs) were calculated by the following equation based on Yuen et al. (2012) to evaluate the pollution level of PTEs:
where Ci is the concentration of the target element and Cref is the concentration of the reference element. Aluminum was considered as the reference element due to the low variation coefficient (VC) in the samples. The mean concentration of three background soils was compared with geochemical background of Mahshahr (Mehr et al. 2016), which is close to the Arvand Free Zone with similar soil types (Entisols and Aridisols) to ensure that three samples represent the geochemical background. The results were similar showing that the mean concentration was acceptable to be used as geochemical background (Table 1). Ranges of EF values corresponding to the levels of soil enrichment according to Birch and Olmos (2008) were defined as deficiency to minimal enrichment (< 2), moderate enrichment (2–5), significant enrichment (5–20), very high enrichment (20–40) and extremely high enrichment (> 40).
The modified pollution index (MPI) is a method to assess the degree of contamination of a soil using EF values which is thought to be more reliable than other indices due to normalisation versus the reference element (Brady et al. 2015), and was calculated as:
The following terminology was used for the MPI based on values: MPI < 1, unpolluted; 1 < MPI < 2, slightly polluted; 2 < MPI < 3, moderately polluted; 3 < MPI < 5, moderately–heavily polluted; 5 < MPI < 10, severely polluted; and MPI > 10, heavily polluted.
In order to determine human exposures to PTEs, a health risk evaluation model was used. Exposure to PTEs in soil can occur through soil consumption (ingestion), dermal contact, and inhalation. The chronic daily intake (CDI) of a contaminant via soil ingestion, dermal contact and inhalation was assessed using the three equations reported in the Electronic Supplementary Material (ESM) and in Table S2 according to RAIS (2020).
Based on standard EPA methods, the ratio of the dose obtained from exposure to site media compared to a specific reference dose (RfD) was indicated as the risk of non-carcinogenic effects. This ratio is known as the hazard quotient (HQ). The HQ was estimated by the following equation (USEPA 1989):
The maximum allowable risk through daily exposure via three-exposure route is expressed as RfD (RfDo, mg/kg/day) for ingestion, RfD for dermal contact (mg/kg/day) and RfC (mg/m3/day) for inhalation. The RfD threshold value is applied to reveal the bad health effect during a lifetime. The HQ can be used to express the non-cancer risk for humans. If the HQ exceeds 1, there might be adverse health effects (USEPA 1993). RfD values were taken from the risk assessment information system (RAIS 2020), Integrated Risk Information System (IRIS 2008) and Environmental Protection Agency (US EPA 2009). RfD for dermal soil contact was determined using Eq. (5) (RAIS 2020).
In this equation, RfDo is the oral RfD (mg/kg/day) and ABS (GI) is the fraction of ingested contaminant absorbed in the gastrointestinal tract. If the ABS (GI) is > 50%, it is set to 100% for the calculation of dermal toxicity values (RAIS 2020).
The overall potential for non-carcinogenic effects of multi-elements was evaluated by the hazard index (HI), which was formulised according to Guidelines for Health Risk Assessment of chemical mixture of USEPA (USEPA 1989).
where i refers to different PTEs. HI shows the addition of hazard quotient for more than one element and/or different exposure pathways (USEPA 1989). Therefore, a combination of non-cancer risks on humans across different exposure pathways can be estimated by summation of the HI of each exposure pathway (USEPA 1989).
where i refers to the different pathways.
To assess the relationship among data and hypothetical sources of PTEs in soil samples, the commercial statistics software package SPSS (version 23) for Windows was used to execute the Spearman correlation and PCA. PCA is often leveraged for source apportionment as it is a bilinear model to illustrate variance in investigated data by reduction of the number of orthogonal variables (Guo et al. 2004); therefore, it can provide a transparent view of the soil pollution profile. Prior to using the PCA method, Bartlett’s test of sphericity (p value) on the original experimental data and the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy were used to detect the presence of partial correlation and dependence among variables to exclude the potential non-independence of the original data, which can influence the PCA result. The KMO measure of sampling adequacy and Bartlett’s test of sphericity should reveal a value > 0.6 and p < 0.05, respectively, for applicable data (Gu et al. 2016). Concentrations of all the elements were autoscaled and normalised using z scores before PCA application.
After the determination of the number and specifications of feasible sources by PCA, quantitative source contributions of soil PTEs were then computed using multiple linear regression (PCA-MLR) (Cao et al. 2011; Thurston and Spengler 1985). It produces estimates of the kinds and relative significance of the sources using receptor modelling, based on a regression of the APCS. In PCA, factors with eigenvalues higher than 1 were retained. MLR was applied with the total concentration of PTEs as the dependent variable and the factor scores as independent variables (Pan et al. 2017; Shi et al. 2008). The MLR model is depicted by the basic equation:
where y is the total value of PTEs (∑ PTEs), Xi is the PCA factor score, mi is the regression coefficient and b is the residual error. The regression model is represented as follows, after normalisation of the variables of Eq. (8):
where z displays the standardised normal deviates of ∑ PTEs, Xi is the PCA factor score and Bi is the regression coefficient. MLR was carried out stepwise by SPSS software, and a default level of significance of 0.05 was applied.
The average percent contribution of each factor was computed as:
Results and discussion
The physicochemical properties of soil samples are presented in Table 1. Soil samples predominantly showed loamy texture (Fig. S1), with a great variation of clay fraction between 7 and 61%. The pH of soils was in the range of 6.3 to 8.6, which is considered to be neutral to slightly alkaline based on USDA (1998). The CEC exhibited a mean value of 14.07 cmol/kg and is considered to be low to moderate for most samples (Dehghani et al. 2017). The OM content and EC ranged between 8.02 and 28.17% and between 10.61 and 163.30 mS/cm, respectively. The fate of PTEs and their concentration in the environment can be influenced by soil physicochemical properties considerably; hence, physicochemical properties are very important for source recognition of soil pollutants (Zhang et al. 2008). Soil pH affects the sorption of PTEs as the solubility of metal hydroxides, metal carbonates and phosphates are directly controlled by the pH. It also influences metal hydrolysis, ion pair formation, solubility of organic matter as well as surface charge of iron and aluminum oxides, organic matter and clay edge (Appel and Ma 2002). Soil organic matter content can affect the soil concentration of PTEs due to the high affinity of some PTEs to organic matter (Zhang et al. 2008). Moreover, the CEC mineral content in a clay fraction of soil plays a major role in sorption and desorption of PTEs (Capra et al. 2014).
The summary statistics of the concentration of PTEs in Arvand Free Zone samples and in background areas are shown in Table 1. The comparison of the mean concentration of each element with the concentration in the local background and with world average showed that Cd, Co, Cr, Cu, Hg, Mo, Ni, Pb and Zn were enriched in the Arvand area.
The analysed elements were categorised into two groups based on VC values: Al, Co, Cr, Fe, Mn, Ni and Sc showed lower VCs, while As, Cd, Cu, Hg, Mo, Pb, Sb and Zn had higher values, probably affected by anthropogenic factors.
Concentrations of PTEs in soils were compared with those in other urban districts as shown in Table 2. Elemental concentrations in different cities and within cities are extremely variable due to the different traffic volumes, industrial activities and meteorological profiles, among other causes (Biasioli and Ajmone-Marsan 2007; Soltani et al. 2015). Generally, the average concentrations of Pb and Zn in urban soils of the Arvand Free Zone were higher than those in other cities, such as Xi’an, Xiangjiang, Beijing, Siena and Kragujevac. In addition, Cu had values higher than those in other selected cities, except Erbil.
Enrichment factors in soil samples for each element are presented in Fig. 2, while spatial variation is evidenced in Fig. 3 using GIS maps calculated by inverse distance weighting (IDW) interpolation. The results revealed that EF values were generally low for Al, As, Co, Cr, Fe, Ni and Sc. Nickel and Cr showed concentrations higher than world soil average, but no enrichment at any site, indicating that the major source was geogenic. Molybdenum, Pb, Cu, Zn, Cd, Sb and Hg showed low to severe enrichment in the order Hg > Sb > Pb > Mo > Cu > Zn > Cd. This can be representative of sources due to human activities for these elements.
The spatial variation of the EF for Al, Cr, Co, Fe, Ni and Sc was very low between all stations. Conversely, Cu and Sb showed a larger variation, with highest levels occurring in a field close to the Abadan oil refinery, used in past for oil waste burial (S29) and inside the Abadan oil refinery (S35). Also, the Khorramshahr landfill (S8) showed severe enrichment for Sb. Molybdenum revealed a high enrichment in the Arvandan shipyard factory (S24) and Abadan oil refinery (S35) stations. Moreover, Hg showed a different spatial distribution pattern, with high EF level in the Abadan industrial district (S31), in the Abadan petrochemical complex (S33) and in the field close to Abadan oil refinery station (S29).
Figure 4 shows the MPI results of the assessed elements; calculations showed that the 32% of the samples were severely polluted. Particularly, the soils were slightly polluted in Shalamcheh (S1), Minushahr (S28) and the road between Abadan and Arvandkenar (S44); moderately polluted in the Khorramshahr entrance from Shalamcheh (S3), Khorramshahr Power Plant (S22), the road between Abadan and Khorramshahr (S25), Shaneh village (S26), Islamic Azad University (S27), Tank Farm (S41) and Zolfaghar Square (S42); and moderately–heavily polluted at the area between the port and the customs of Khorramshahr (S5), chemical martyrs of Behbahan (S9), old bridge of Khorramshahr (S16), Ashayer Square (S18) and Abadan oil refinery (S35). The maximum values of MPI occurred at the Abadan industrial district (1091) followed by the Abadan petrochemical complex (1068). These stations were extremely enriched with Hg (1539 and 1506, respectively).
Source identification of PTEs
The normality of the elemental concentrations and the differences between the concentration of urban and industrial soils were checked using Shapiro–Wilk and Mann–Whitney (U) tests, respectively (non-parametric statistical procedures, Table S3). The results indicated that more than half of the analysed elements were non-normally distributed and only Cu, Mo and Zn displayed a significant difference between the urban and industrial soils (p < 0.05) (Table S3).
To establish inter-element relationships in soil samples, the Spearman correlation coefficients of PTEs were computed and the results are shown in Table S4. A very strong positive correlation was observed between Al, Co, Cr, Fe, Mn, Ni and Sc at p < 0.01, which can be indicative of their similar characteristics, fate and common origin. These elements are expected to be mostly of natural origin (Balabanova et al. 2011). Furthermore, significant correlations between Pb, Cu, Sb and Zn at the level of p < 0.01 suggest that these PTEs can be released from the same contamination sources. Mercury did not show a significant correlation with any other PTEs. This might be due to the different origins of Hg in the study area.
To this end, PCA was used to find out the origin of pollutants. Prior the use of PCA method, the KMO measure of sampling adequacy and Bartlett’s test of sphericity were applied. The KMO measure of sampling adequacy result was 0.69≈0.7 and Bartlett’s p = 0 < 0.05 for data, which meant the suitability of the data for PCA (Joliffe 2002). The source apportionment results of PCA using varimax rotation with Kaiser normalisation for PTE concentrations are presented in Table 3.
Based on the results of initial eigenvalues, PCA extracted four components explaining about the 87% of the cumulative variance (Table 3). Principal component 1 (PC1) presented high loadings of Ni, Co, Mn, Fe, Cr, Sc and Al. All these elements have been described in previous studies having predominantly lithogenic sources (Abbasi et al. 2018; Keshavarzi et al. 2018; Soltani et al. 2015); thus, this factor probably represent the natural sources of PTEs. The second rotated principal component (PC2) was strongly correlated to Mo, As and Cu. Arsenic and Mo are released during fossil fuel combustion (Tsukuda et al. 2005), which might be released from the industries such as Abadan oil refinery, while Cu had also other sources, as denoted from its equal value of loading on both the PC2 and the PC3. Principal component 3 (PC3) was linked to Pb, Zn, Cd, Sb and Cu contents of soil samples. These PTEs are released from many traffic-related sources, as the abrasion of tires and brake pads, corrosion, lubricating oils and fuel additives (Tomasevic and Anicic 2010). Pb, in particular, is a well-known vehicle-related metal in urban areas due to its use for several years as an anti-knocking agent in gasoline (Ajmone-Marsan and Biasioli 2010; Dehghani et al. 2017; Laidlaw and Filippelli 2008). However, the sources of Zn could also be linked to the industrial emissions and paints from vehicles, as well as wearing down of batteries. This factor may be related to the urban sampling points with high traffic density, such as S14, S20, S38 and S39. Principal component 4 (PC4) only presents loading from Hg. The maximum concentration of Hg occurred in Pasargad Arvand unit (32,238 μg/kg) in the Abadan industrial district, which produces petrochemical products. That site was followed by the Abadan petrochemical complex (30,845 μg/kg), and from a site close to the Abadan oil refinery (8560 μg/kg), used for burring oil wastes up to 50 years ago and nowadays used for construction. Therefore, PC4 is likely related to oil, its derivatives and petroleum wastes.
Results from the multiple regression analysis revealed that average model-predicted values and actual values were considerably close, with a ratio near unity, thus reliable. PCA-MLR analysis indicated that the natural sources contributed 32.3% of the total concentrations. The anthropogenic sources are largely predominant (67.7%), as expected, with the most important proportion being related to traffic, contributing for the 30.6%, followed by oil derivatives and petroleum waste with 20.1%, while fossil fuel combustion contributed for the 17%. Figure 5 displays the source apportionment results generated using MLR-APCS receptor modelling. Those results indicated that As (58%) and Mo (79%) concentrations in soils seem to be primarily due to fossil fuel combustion, while Cd (52%) and Pb (68%) can be primarily generated by traffic sources. Moreover, 45% and 36% of Cu, 43% and 50% of Sb and 39% and 49% of Zn appeared to be contributed by fossil fuel combustion and traffic, respectively. In addition, almost the totality of Hg (85%) was attributed by the model to the source may be related to oil products (Fig. 5).
BCR elemental partitioning
The fractionation pattern of PTEs in soil samples of the selected stations is displayed in Fig. 6. Results were considered to be acceptable for all elements apart Hg, due to its too low recovery, problem already evidenced on previous studies (Sladek and Gustin 2003).
Aluminium, Sc, Cr, Mn, Fe, Co and Ni were mainly present in the residual fraction (F4) at all sampling sites (with an average of 87%, 94%, 87%, 54%, 87%, 75% and 83%, respectively), which confirmed their probable prevailing natural source as found using EF on total concentrations. These elements in the residual phase are fixed in the crystal lattice of the samples and are not available for remobilisation or dissociation except under very harsh conditions (Dixon and Schulze 2004).
Conversely, the available fractions (F1 + F2 + F3) represented the majority for the elements having anthropic origin, as highlighted from PCA results. Zinc had more than the 50% of the total concentration in the fraction (F1 + F2 + F3), to a maximum of 69% in sample S33. Copper showed a similar availability to Zn, with both partly present in the F2 fraction, related to iron and manganese oxides (15% and 21%, on average); these are released when the matrix is subjected to reducing conditions, as in case of flooding (Ajmone Marsan et al. 2019). The fraction associated to organic matter (F3) accounted for the 25% of Cu, confirming its high affinity to organic matter. In fact, due to the high stability of organic Cu complexes, it could easily make complexes with organic matter (Li et al. 2001; Morillo et al. 2004; Ramirez et al. 2005). The association between high concentrations of Cu and organic matter has also been reported in several studies (Arias et al. 2008; Fytianos and Lourantou 2004; Ramirez et al. 2005; Wong et al. 2007). Arsenic was mainly extracted in the residual fraction (58%) followed by F2 (27%) and then F3 (11%). These findings are compatible with the results obtained from prior reports in which As was associated with reducible phases, indicating that Fe(III) oxyhydroxides and crystalline Fe(III) oxides have a great effect in his accumulation (Asta et al. 2010; Fukushi et al. 2003; Torres and Auleda 2013). Thus, if these minerals, especially the low crystalline ones, are reduced, As is released to the pore water (Torres and Auleda 2013). Moreover, the sites with the highest as availability (S8 (56%), S24 (56%) and S35 (60%)) were also the most enriched in As. Molybdenum was predominantly extracted in the F4, with an average of 63%, followed by the exchangeable fraction (20%). However, in S24 soil, the 76% of Mo was in an available form, mostly present in the reducible fraction.
Cadmium was mostly related to the Fe and Mn oxides, with an average of 31% extracted in F2. In S11, the more mobile fractions (F1 + F2, 70%) comprised the largest portion of Cd, compatible with the high EF obtained in this site. Antimony, with an average value of 57%, was largely distributed in the residual fraction. Results showed that the highest percentage of Sb associated with the F4 (99.7%) was occured in S25. The average percentage of Pb related to different fractions was in the following order: residual (40%), oxidisable (38%), reducible (21%) and soluble (1%).
Gastric and intestinal bioaccessibility of PTEs in soil samples
The results of the SBRC in vitro tests showed high values of bioaccessible concentrations for most of the elements apart Mo and Sb, which were below the detection limit in most of the soil samples. The maximum bioaccessible concentrations of Co, Ni, Cu, Zn and Pb were found in the sites close to the oil refinery station.
Bioaccessible fractions better illustrate the differences between PTEs. The average oral bioaccessibility (OBA) fraction of PTEs (as percentage of the total concentration) for gastric and intestinal phase is shown in Fig. 7.
The mean bioaccessibility of selected PTEs in the gastric phase ranged from 1.4% in the case of Mo to 77% for Cd and from 0.1% for Pb to 78% for Mo in the intestinal phase. The more bioaccessible PTEs in the gastric phase were Cd, As (51%) and Pb (45%), while the maximum average of PTE bioaccessibility for intestinal phase was determined for Mo, followed by Sb (26%). Bioaccessibility values were always low for Al, Fe, Cr and Ni, the metals mostly present in the crystalline phases of the soils, as observed using PCA and BCR results and in previous studies (Padoan et al. 2017). It is well understood that chemical forms and structure of the metals determine their bioaccessibility (Dieter et al. 1993; Freeman et al. 1992). Beside to the speciation, the differences between several studies could also be due to the diversity of soil properties. Therefore, understanding the controlling factors for metal bioaccessibility in soils needs further investigation.
With regard to the two exposure stages, the bioaccessibility of PTEs decreased from the gastric to intestinal phase except for Mo and Sb, as already observed in previous studies (Madrid et al. 2008; Pelfrêne et al. 2012). The pH increment (1.5 to 7) and the addition of pancreatin and bile salts in the intestinal phase can lead to the precipitation of some elements (Ruby 2004). In addition, in the carbonate-rich environment of the intestine, PTEs may be fixed in solution through complexation, undergo readsorption to preexistent or altered sites at the particle surface or precipitate as relatively insoluble compounds (Ren et al. 2015).
Conversely, Mo exhibits unusual geochemical behaviour since in most environmental media, it is likely to form oxyanions (Kabata-Pendias and Mukherjee 2007). As the soil solution becomes more alkaline, MoO42− availability is often found to increase as its adsorption on soil particles such as oxides and clay minerals raises up to a peak near pH 3, and then every unit increase above pH 3, MoO42− solubility enhances approximately 100-fold, primarily through reduced adsorption of metal oxides (Goldberg et al. 1996; Kaiser et al. 2005). Acidic soils may contain high Fe and Al oxides and hydroxides. It is reported that Mo adsorbs on Al and Fe oxides via ligand exchange with surface hydroxyl ions (Ferreiro et al. 1985; Jones 1957). Another type of MoO42− adsorption is the reaction between MoO42− and aluminum silicate minerals. The MoO42− ions react with octahedral Al by replacing the OH groups located on the surface plane of the mineral. This type of reaction is also prevalent under acidic condition in the gastric phase (Gupta 1997).
Also, the bioaccessibility of Sb increased in the intestinal phase rather than in the gastric phase. It is also easily adsorbed when it occurs as oxyanions, especially at low pH values (Kabata-Pendias and Mukherjee 2007). Iron (hydr)oxides (Spuller et al. 2007) and Al oxides (Brannon and Patrick 1985; Lintschinger et al. 1998; Tighe et al. 2005) are suggested to be important sorbents for Sb in soil. Amorphous Fe oxide and hematite play an important role in Sb sorption at pH 3 to 6.5 and pH < 7, respectively (Tighe et al. 2005).
Human health risk assessment
Table S5 summarises the non-carcinogenic health risks for adults and children connected to the exposure to elements in urban soils via different routes (ingestion, dermal and inhalation) in the study area. No exposure pathway showed health risks for adults and children, separately (HQ < 1). The ingestion HQ was higher than the other pathways and showed the highest contribution to HI (the total risk), indicating that ingestion is the most important pathway for PTEs, as seen in other studies (Du et al. 2013; Yuswir et al. 2015; Zhang et al. 2013; Zheng et al. 2010). For children, the HI of Al, As, Co, Cr, Cu, Fe, Hg, Mn, Ni and Pb was higher than 1, showing a potential non-carcinogenic effect in the Arvand Free Zone. However, when the bioaccessible concentrations were considered in the formulas, these risks decreased. The calculated HI based on total concentration was estimated to be approximately 2-fold higher for As, Cd, Mn, Pb and Sc; 3-fold for Cr; 4-fold for Co; 5-fold for Cu; 7-fold for Al and Ni; and more than 10-fold for Fe, Mo and Sb compared to that of bioaccessible concentration in the gastric phase for adults (fig. 8). The comparison of the HI levels for children showed similar results ranging from a 1.27-fold increase for Zn to 32.8-fold for Fe. Figure 8 shows that estimated HI based on total concentration was mostly greater than HI based on bioaccessible concentration in the intestinal phase for adults (Fig. 8). Moreover, the ratio of HI based on total concentration to HI based on bioaccessible concentration in the intestinal phase for children ranged between 1.14 for Mo and 217 for Fe.
According to bioaccessibility in the gastric phase, only the risk (HI) for As, Co, Cr, Mn, Mo and Pb exceeded the safety level for children and none of the PTEs showed health risks for adults except Pb. If the HI was based on the bioaccessibility of intestinal phase, As, Cr, Mn, Mo and Pb showed values higher than 1 for children and the HI of all analysed PTEs was lower than the safety level for adults. The HI of multiple PTEs from ingestion, dermal contact and inhalation exposures was found to be higher than 1 for children in all sampling sites. The maximum HI was 11, at site S29, implying that children would suffer high health risks (Table 4). For adults, the calculated HI was lower than 1 at all sampling points except S29 (ground close to Abadan oil refinery), S31 (Abadan industrial district) and S33 (Abadan petrochemical complex). However, when bioaccessible concentrations in gastric and intestinal phases were considered, S29, S31 and S33 revealed a non-carcinogenic health risk for children. Children are more sensitive to a certain dose of a toxin and are likely to accidentally ingest considerable quantities of soil or dust because of hand or finger sucking, which has been widely considered as one of the key element exposure pathways for children (Rasmussen et al. 2001). Since children are more susceptible to pollutants through hand-to-mouth activity and inhalation, PTE contamination should be controlled to prevent further health problems for this vulnerable age group.
Conclusion
Potentially toxic elements are widely distributed in many urban and industrial soils. This study showed that the anthropogenic sources contributed for the major part of the analysed concentrations of most of the selected elements. The identified sources were, in descending order, traffic, oil derivatives and petroleum waste. The chemometric analyses revealed, also, that Ni, Co, Mn, Fe, Cr, Sc and Al mostly originated from natural sources and were affected, to a lesser degree, by anthropogenic sources, as compared to the other metals. The industrial district of Abadan and petrochemical complex sites, which were severely enriched in Hg, showed the highest pollution in the Arvand Free Zone, while the ground close to Abadan oil refinery revealed high enrichment of Cu, Pb, Sb and Hg. The dominant source of Hg was oil products. Among the extracted PTEs in the SBRC test, Co, Ni, Cu, Zn and Pb revealed the highest bioaccessible concentration for the gastric phase and Mn, Ni, Cu and Pb for the intestinal phase at the ground close to Abadan oil refinery. Since the soil system is a major reservoir and sink for urban and industrial pollutants, it is consequently a good indicator for environmental pollution and an environment risk for human exposure. The health risk based upon total concentration was overestimated for all PTEs compared with those based on bioaccessible concentrations. Significant changes were found for Al, Cu, Fe, Mn, Ni, Mo and Sb for both children and adults. Although the HI values for all stations exceeded the safety level for children, taking into account the bioaccessible concentrations might give more realistic estimations of the health risks posed by PTE contamination. Overall, the health risk values and occurring adverse effects of PTEs were higher for children rather than the adults in all calculated methods.
References
Abbasi S, Keshavarzi B, Moore F, Mahmoudi MR (2018) Fractionation, source identification and risk assessment of potentially toxic elements in street dust of the most important center for petrochemical products, Asaluyeh County, Iran. Environ Earth Sci 77:673. https://doi.org/10.1007/s12665-018-7854-z
Abbasi S, Keshavarzi B, Moore F, Hopke PK, Kelly FJ, Dominguez AO (2020) Elemental and magnetic analyses, source identification, and oxidative potential of airborne, passive, and street dust particles in Asaluyeh County, Iran. Sci Total Environ 707:136132. https://doi.org/10.1016/j.scitotenv.2019.136132
Ajmone Marsan F, Padoan E, Madrid F, Vrščaj B, Biasioli M, Davidson CM (2019) Metal release under anaerobic conditions of urban soils of four European cities. Water Air Soil Pollut 230:53. https://doi.org/10.1007/s11270-019-4101-5
Ajmone-Marsan F, Biasioli M (2010) Trace elements in soils of urban areas. Water Air Soil Pollut 213:121–143. https://doi.org/10.1007/s11270-010-0372-6
Ali N, Malik RN, Mehdi T, Eqani SAMAS, Javeed A, Neels H, Covaci A (2013) Organohalogenated contaminants (OHCs) in the serum and hair of pet cats and dogs: biosentinels of indoor pollution. Sci Total Environ 449:29–36. https://doi.org/10.1016/j.scitotenv.2013.01.037
Amjadian K, Sacchi E (2016) Heavy metals (HMs) and polycyclic aromatic hydrocarbons (PAHs) in soils of different land uses in Erbil metropolis, Kurdistan Region, Iraq. Environ Monit Assess 188:605. https://doi.org/10.1007/s10661-016-5623-6
Appel C, Ma L (2002) Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J Environ Qual 31:581–589
Aquisman AE, Assim ZB, Wahi RB, Kwabena DE, Festus W (2019) Validation of the atomic absorption spectroscopy (AAS) for heavy metal analysis and geochemical exploration of sediment samples from the Sebangan River. Adv Anal Chem 9:23–33. https://doi.org/10.5923/j.aac.20190902.01
Arias R, Barona A, Ibarra-Berastegi G, Aranguiz I, Elías A (2008) Assessment of metal contamination in dregded sediments using fractionation and self-organizing maps. J Hazard Mater 151:78–85. https://doi.org/10.1016/j.jhazmat.2007.05.048
Ashayeri NY, Keshavarzi B (2019) Geochemical characteristics, partitioning, quantitative source apportionment, and ecological and health risk of heavy metals in sediments and water: a case study in Shadegan Wetland, Iran. Mar Pollut Bull 149:110495. https://doi.org/10.1016/j.marpolbul.2019.110495
Asta MP, Ayora C, Román-Ross G, Cama J, Acero P, Gault AG, Charnock JM, Bardelli F (2010) Natural attenuation of arsenic in the Tinto Santa Rosa acid stream (Iberian Pyritic Belt, SW Spain): the role of iron precipitates. Chem Geol 271:1–12. https://doi.org/10.1016/j.chemgeo.2009.12.005
Balabanova B, Stafilov T, Šajn R, Bačeva K (2011) Distribution of chemical elements in attic dust as reflection of their geogenic and anthropogenic sources in the vicinity of the copper mine and flotation plant. Arch Environ Contam Toxicol 61:173–184. https://doi.org/10.1007/s00244-010-9603-5
Biasioli M, Ajmone-Marsan F (2007) Organic and inorganic diffuse contamination in urban soils: the case of Torino (Italy). J Environ Monit 9:862–868. https://doi.org/10.1039/B705285E
Birch GF, Olmos MA (2008) Sediment-bound heavy metals as indicators of human influence and biological risk in coastal water bodies. ICES J Mar Sci 65:1407–1413. https://doi.org/10.1093/icesjms/fsn139
Brady JP, Ayoko GA, Martens WN, Goonetilleke A (2015) Development of a hybrid pollution index for heavy metals in marine and estuarine sediments. Environ Monit Assess 187. https://doi.org/10.1007/s10661-015-4563-x
Brannon JM, Patrick WH (1985) Fixation and mobilization of antimony in sediments. Environ Pollut B 9:107–126. https://doi.org/10.1016/0143-148X(85)90027-8
Burges A, Epelde L, Garbisu C (2015) Impact of repeated single-metal and multi-metal pollution events on soil quality. Chemosphere 120:8–15. https://doi.org/10.1016/j.chemosphere.2014.05.037
Cao Q, Wang H, Chen G (2011) Source apportionment of PAHs using two mathematical models for mangrove sediments in Shantou coastal zone, China. Estuar Coasts 34:950–960. https://doi.org/10.1007/s12237-011-9397-3
Capra GF, Coppola E, Odierna P, Grilli E, Vacca S, Buondonno A (2014) Occurrence and distribution of key potentially toxic elements (PTEs) in agricultural soils: a paradigmatic case study in an area affected by illegal landfills. J Geochem Explor 145:169–180. https://doi.org/10.1016/j.gexplo.2014.06.007
Chen X, Lu X (2018) Contamination characteristics and source apportionment of heavy metals in topsoil from an area in Xi’an City, China. Ecotoxicol Environ Saf 151:153–160. https://doi.org/10.1016/j.ecoenv.2018.01.010
Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21:1335–1346. https://doi.org/10.1016/j.apgeochem.2006.05.001
Dehghani S, Moore F, Keshavarzi B, Hale BA (2017) Health risk implications of potentially toxic metals in street dust and surface soil of Tehran, Iran. Ecotoxicol Environ Saf 136:92–103. https://doi.org/10.1016/j.ecoenv.2016.10.037
Dieter MP, Matthews HB, Jeffcoat RA, Moseman RF (1993) Comparison of lead bioavailability in F344 rats fed lead acetate, lead oxide, lead sulfide, or lead ore concentrate from Skagway, Alaska. J Toxicol Environ Health 39:79–93. https://doi.org/10.1080/15287399309531737
Dixon J, Schulze D (2004) Soil mineralogy with environmental applications. Soil Sci. Soc. Am. Inc, Fitchbg
Dodd M, Rasmussen PE, Chénier M (2013) Human and ecological risk assessment: comparison of two in vitro extraction protocols for assessing metals’ bioaccessibility using dust and soil reference materials. Hum Ecol Risk Assess 19:37–41. https://doi.org/10.1080/10807039.2012.719381
Du Y, Gao B, Zhou H, Ju X, Hao H, Yin S (2013) Health risk assessment of heavy metals in road dusts in urban parks of Beijing, China. Procedia Environ Sci 18:299–309. https://doi.org/10.1016/j.proenv.2013.04.039
Farsani MH, Shirmardi M, Alavi N, Maleki H, Sorooshian A, Babaei A, Asgharnia H, Marzouni MB, Goudarzi G (2018) Evaluation of the relationship between PM10 concentrations and heavy metals during normal and dusty days in Ahvaz, Iran. Aeolian Res 33:12–22. https://doi.org/10.1016/j.aeolia.2018.04.001
Fei X, Xiao R, Christakos G, Langousis A, Ren Z, Tian Y, Lv X (2019) Comprehensive assessment and source apportionment of heavy metals in Shanghai agricultural soils with different fertility levels. Ecol Indic 106:105508. https://doi.org/10.1016/j.ecolind.2019.105508
Ferreiro EA, Helmy AK, de Bussetti SG (1985) Molybdate sorption by oxides of aluminium and iron. Z Pflanzenernähr Bodenkd 148:559–566. https://doi.org/10.1002/jpln.19851480513
Franklin RL, Silva SA, Fa DIT (2016) Trace and some rare earth elements distribution in a sediment profile from Jurumirim Reservoir, São Paulo State, Brazil: total content and extracted phases. J Radioanal Nucl Chem 309:439–451. https://doi.org/10.1007/s10967-016-4785-6
Freeman GB, Johnson JD, Killinger JM, Liao SC, Feder PI, Davis AO, Ruby MV, Chaney RL, Lovre SC, Bergstrom PD (1992) Relative bioavailability of lead from mining waste soil in rats. Fundam Appl Toxicol 19:388–398. https://doi.org/10.1016/0272-0590(92)90178-K
Fukushi K, Sasaki M, Sato T, Yanase N, Amano H, Ikeda H (2003) A natural attenuation of arsenic in drainage from an abandoned arsenic mine dump. Appl Geochem 18:1267–1278. https://doi.org/10.1016/S0883-2927(03)00011-8
Fytianos K, Lourantou A (2004) Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece. Environ Int 30:11–17. https://doi.org/10.1016/S0160-4120(03)00143-0
Gee G, Bauder JW (1986) Particle-size analysis 1., Soil Science Society of America, American Society of Agronomy
Goldberg S, Forster HS, Godfrey CL (1996) Molybdenum adsorption on oxides, clay minerals, and soils 60
Gu F, Hall P, Miles NJ (2016) Performance evaluation for composites based on recycled polypropylene using principal component analysis and cluster analysis. J Clean Prod 115:343–353. https://doi.org/10.1016/j.jclepro.2015.12.062
Gulan L, Milenkovic B, Zeremski T, Milic G, Vuckovic B (2017) Persistent organic pollutants, heavy metals and radioactivity in the urban soil of Priština City, Kosovo and Metohija. Chemosphere 171:415–426. https://doi.org/10.1016/j.chemosphere.2016.12.064
Guney M, Zagury GJ, Dogan N, Onay TT (2010) Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater 182:656–664. https://doi.org/10.1016/j.jhazmat.2010.06.082
Guo H, Wang T, Louie PKK (2004) Source apportionment of ambient non-methane hydrocarbons in Hong Kong: application of a principal component analysis/absolute principal component scores (PCA/APCS) receptor model. Environ Pollut 129:489–498. https://doi.org/10.1016/j.envpol.2003.11.006
Gupta UC (1997) Molybdenum in agriculture. Cambridge University Press, Cambridge
Hu Y, Cheng H (2013) Application of stochastic models in identification and apportionment of heavy metal pollution sources in the surface soils of a large-scale region. Environ Sci Technol 47:3752–3760. https://doi.org/10.1021/es304310k
Huang Y, Deng M, Wu S, Japenga J, Li T, Yang X, He Z (2018) A modified receptor model for source apportionment of heavy metal pollution in soil. J Hazard Mater 354:161–169 https://doi.org/10.1016/j.jhazmat.2018.05.006
IRIS (2008) Integrated Risk Information System. Washington, DC: U.S. Environmental Protection Agency; 2008. Chromium
Joliffe IT (2002) Principal component analysis, 2nd edn. Springer, New York
Jones LHP (1957) The solubility of molybdenum in simplified systems and aqueous soil suspensions. J Soil Sci 8:313–327. https://doi.org/10.1111/j.1365-2389.1957.tb01891.x
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC Press, Boca Raton. https://doi.org/10.1201/b10158-25
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer Science & Business Media, Berlin
Kaiser BN, Gridley KL, Brady JN, Phillips T, Tyerman SD (2005) The role of molybdenum in agricultural plant production. Ann Bot 96:745–754. https://doi.org/10.1093/aob/mci226
Kelley ME, Brauning S, Schoof R, Ruby M (2002) Assessing oral bioavailability of metals in soil. Battelle Press
Keshavarzi B, Tazarvi Z, Rajabzadeh MA, Najmeddin A (2015) Chemical speciation, human health risk assessment and pollution level of selected heavy metals in urban street dust of Shiraz, Iran. Atmos Environ 119:1–10. https://doi.org/10.1016/j.atmosenv.2015.08.001
Keshavarzi B, Abbasi S, Moore F, Mehravar S, Sorooshian A, Soltani N, Najmeddin A (2018) Contamination level, source identification and risk assessment of potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in street dust of an important commercial center in Iran. Environ Manag 62:803–818. https://doi.org/10.1007/s00267-018-1079-5
Ko I, Chang Y, Lee C, Kim K (2005) Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J Hazard Mater 127:1–13. https://doi.org/10.1016/j.jhazmat.2005.06.041
Koch I, Sylvester S, Lai VW-M, Owen A, Reimer KJ, Cullen WR (2007) Bioaccessibility and excretion of arsenic in Niu Huang Jie Du Pian pills. Toxicol Appl Pharmacol 222:357–364. https://doi.org/10.1016/j.taap.2006.12.005
Laidlaw MAS, Filippelli GM (2008) Resuspension of urban soils as a persistent source of lead poisoning in children: a review and new directions. Appl Geochem 23:2021–2039. https://doi.org/10.1016/j.apgeochem.2008.05.009
Li X, Shen Z, Wai OWH, Li Y-S (2001) Chemical forms of Pb, Zn and Cu in the sediment profiles of the Pearl River Estuary. Mar Pollut Bull 42:215–223. https://doi.org/10.1016/S0025-326X(00)00145-4
Li J, Li K, Cave M, Li H-B, Ma LQ (2015) Lead bioaccessibility in 12 contaminated soils from China: correlation to lead relative bioavailability and lead in different fractions. J Hazard Mater 295:55–62. https://doi.org/10.1016/j.jhazmat.2015.03.061
Li H-B, Zhao D, Li J, Li S-W, Wang N, Juhasz AL, Zhu Y-G, Ma LQ (2016) Using the SBRC assay to predict lead relative bioavailability in urban soils: contaminant source and correlation model. Environ Sci Technol 50:4989–4996. https://doi.org/10.1021/acs.est.6b00480
Li F, Zhang J, Jiang W, Liu C, Zhang Z, Zhang C, Zeng G (2017) Spatial health risk assessment and hierarchical risk management for mercury in soils from a typical contaminated site, China. Environ Geochem Health 39:923–934. https://doi.org/10.1007/s10653-016-9864-7
Li F, Zhang J, Liu W, Liu J, Huang J, Zeng G (2018) An exploration of an integrated stochastic-fuzzy pollution assessment for heavy metals in urban topsoil based on metal enrichment and bioaccessibility. Sci Total Environ 644:649–660. https://doi.org/10.1016/j.scitotenv.2018.06.366
Liang Q, Grégoire DC (2000) Determination of trace elements in twenty six Chinese geochemistry reference materials by inductively coupled plasma-mass spectrometry. Geostand Newslett 24:51–63. https://doi.org/10.1111/j.1751-908X.2000.tb00586.x
Lintschinger J, Michalke B, Schulte-hostede S, Schramel P (1998) Studies on speciation of antimony in soil contaminated by industrial activity. Int J Environ Anal Chem 72:11–25. https://doi.org/10.1080/03067319808032641
Liu R, Wang M, Chen W, Peng C (2016) Spatial pattern of heavy metals accumulation risk in urban soils of Beijing and its influencing factors. Environ Pollut 210:174–181. https://doi.org/10.1016/j.envpol.2015.11.044
Luo X-S, Xue Y, Wang Y-L, Cang L, Xu B, Ding J (2015) Source identification and apportionment of heavy metals in urban soil profiles. Chemosphere 127:152–157. https://doi.org/10.1016/j.chemosphere.2015.01.048
Madrid F, Biasioli M, Ajmone-Marsan F (2008) Availability and bioaccessibility of metals in fine particles of some urban soils:21–32. https://doi.org/10.1007/s00244-007-9086-1
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300:229–243. https://doi.org/10.1016/S0048-9697(02)00273-5
Mehr MR, Keshavarzi B, Moore F, Sacchi E (2016) Contamination level and human health hazard assessment of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in street dust deposited in Mahshahr, southwest of Iran. Hum Ecol Risk Assess 22:1726–1748. https://doi.org/10.1080/10807039.2016.1219221
Mehr MR, Keshavarzi B, Moore F, Sharifi R, Lahijanzadeh A, Kermani M (2017) Distribution, source identification and health risk assessment of soil heavy metals in urban areas of Isfahan Province, Iran. J Afr Earth Sci 132:16–26. https://doi.org/10.1016/j.jafrearsci.2017.04.026
Mihailović A, Budinski-Petković L, Popov S, Ninkov J, Vasin J, Ralević NM, Vučinić Vasić M (2015) Spatial distribution of metals in urban soil of Novi Sad, Serbia: GIS based approach. J Geochem Explor 150:104–114. https://doi.org/10.1016/j.gexplo.2014.12.017
Moore F, Keshavarzi B (2014) Medical geology of Khuzestan Province (phase 1), Iran Environmental Protection Agency (EPA), Khuzestan Province: Internal Report
Morillo J, Usero J, Gracia I (2004) Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 55:431–442. https://doi.org/10.1016/j.chemosphere.2003.10.047
Nannoni F, Protano G (2016) Chemical and biological methods to evaluate the availability of heavy metals in soils of the Siena urban area (Italy). Sci Total Environ 568:1–10. https://doi.org/10.1016/j.scitotenv.2016.05.208
Padoan E, Romè C, Ajmone-Marsan F (2017) Bioaccessibility and size distribution of metals in road dust and roadside soils along a peri-urban transect. Sci Total Environ 601–602:89–98. https://doi.org/10.1016/j.scitotenv.2017.05.180
Pan H, Lu X, Lei K (2017) A comprehensive analysis of heavy metals in urban road dust of Xi’an, China: contamination, source apportionment and spatial distribution. Sci Total Environ 609:1361–1369. https://doi.org/10.1016/j.scitotenv.2017.08.004
Papa S, Bartoli G, Pellegrino A, Fioretto A (2010) Microbial activities and trace element contents in an urban soil. Environ Monit Assess 165:193–203. https://doi.org/10.1007/s10661-009-0938-1
Pelfrêne A, Waterlot C, Mazzuca M, Nisse C, Cuny D, Richard A, Denys S, Heyman C, Roussel H, Bidar G, Douay F (2012) Bioaccessibility of trace elements as affected by soil parameters in smelter-contaminated agricultural soils: a statistical modeling approach. Environ Pollut 160:130–138. https://doi.org/10.1016/j.envpol.2011.09.008
Peña-Fernández A, González-Muñoz MJ, Lobo-Bedmar MC (2014) Establishing the importance of human health risk assessment for metals and metalloids in urban environments. Environ Int 72:176–185. https://doi.org/10.1016/j.envint.2014.04.007
Prabhakar G, Sorooshian A, Toffol E, Arellano AF, Betterton EA (2014) Spatiotemporal distribution of airborne particulate metals and metalloids in a populated arid region. Atmos Environ 92:339–347. https://doi.org/10.1016/j.atmosenv.2014.04.044
Qing X, Yutong Z, Shenggao L (2015) Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol Environ Saf 120:377–385. https://doi.org/10.1016/j.ecoenv.2015.06.019
Qu M, Wang Y, Huang B, Zhao Y (2018) Source apportionment of soil heavy metals using robust absolute principal component scores-robust geographically weighted regression (RAPCS-RGWR) receptor model. Sci Total Environ 626:203–210. https://doi.org/10.1016/j.scitotenv.2018.01.070
RAIS (2020) Risk exposure models for chemicals user’s guide. The Risk Assessment Information System. https://rais.ornl.gov/tools/rais_chemical_risk_guide.html. Accessed 29 April 2020
Ramirez M, Massolo S, Frache R, Correa JA (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar Pollut Bull 50:62–72. https://doi.org/10.1016/j.marpolbul.2004.08.010
Rasmussen PE, Subramanian KS, Jessiman BJ (2001) A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci Total Environ 267:125–140. https://doi.org/10.1016/S0048-9697(00)00775-0
Rastegari M, Keshavarzi B, Moore F, Shari R, Lahijanzadeh A, Kermani M (2017) Distribution, source identification and health risk assessment of soil heavy metals in urban areas of Isfahan Province, Iran. J Afr Earth Sci 132:16–26. https://doi.org/10.1016/j.jafrearsci.2017.04.026
Rauret G, López-Sánchez J-F, Sahuquillo A, Barahona E, Lachica M, Ure AM, Davidson CM, Gomez A, Lück D, Bacon J, Yli-Halla M, Muntau H, Quevauviller P (2000) Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483){,} complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J Environ Monit 2:228–233. https://doi.org/10.1039/B001496F
Ren J, Williams P, Luo J (2015) Sediment metal bioavailability in Lake Taihu, China: evaluation of sequential extraction, DGT, and PBET techniques. Environ Sci Pollut Res 22:12919–12928
Ruby MV (2004) Bioavailability of soil-borne chemicals: abiotic assessment tools. Hum Ecol Risk Assess 10:647–656. https://doi.org/10.1080/10807030490484291
Ruby MV, Lowney YW (2012) Selective soil particle adherence to hands: implications for understanding oral exposure to soil contaminants. Environ Sci Technol 46:12759–12771. https://doi.org/10.1021/es302473q
Ryan J, Estefan G, Rashid A (2007) Soil and plant analysis laboratory manual. ICARDA. ICARDA
Schumacher B (2002) Methods for the determination of total organic carbon (TOC) in soils and sediments. US Environ. Prot. Agency, Off. Res. Dev
Shi G, Chen Z, Xu S, Zhang J, Wang L, Bi C, Teng J (2008) Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ Pollut 156:251–260. https://doi.org/10.1016/j.envpol.2008.02.027
Shi G, Chen Z, Bi C, Wang L, Teng J, Li Y, Xu S (2011) A comparative study of health risk of potentially toxic metals in urban and suburban road dust in the most populated city of China. Atmos Environ 45:764–771. https://doi.org/10.1016/j.atmosenv.2010.08.039
Shokrollahzadeh S, Azizmohseni F, Golmohammad F (2008) Biodegradation potential and bacterial diversity of a petrochemical wastewater treatment plant in Iran. Bioresour Technol 99:6127–6133. https://doi.org/10.1016/j.biortech.2007.12.034
Siciliano SD, James K, Zhang G, Schafer AN, Peak JD (2009) Adhesion and enrichment of metals on human hands from contaminated soil at an Arctic urban brownfield. Environ Sci Technol 43:6385–6390. https://doi.org/10.1021/es901090w
Sladek C, Gustin MS (2003) Evaluation of sequential and selective extraction methods for determination of mercury speciation and mobility in mine waste. Appl Geochem 18:567–576. https://doi.org/10.1016/S0883-2927(02)00115-4
Soltani N, Keshavarzi B, Moore F, Tavakol T, Lahijanzadeh AR, Jaafarzadeh N, Kermani M (2015) Ecological and human health hazards of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran. Sci Total Environ 505:712–723. https://doi.org/10.1016/j.scitotenv.2014.09.097
Soltani N, Keshavarzi B, Moore F, Sorooshian A, Ahmadi MR (2017) Distribution of potentially toxic elements (PTEs) in tailings, soils, and plants around Gol-E-Gohar iron mine, a case study in Iran. Environ Sci Pollut Res 24:18798–18816. https://doi.org/10.1007/s11356-017-9342-5
Song H, Hu K, An Y, Chen C, Li G (2018) Spatial distribution and source apportionment of the heavy metals in the agricultural soil in a regional scale. J Soils Sediments 18:852–862. https://doi.org/10.1007/s11368-017-1795-0
Spuller C, Weigand H, Marb C (2007) Trace metal stabilisation in a shooting range soil: mobility and phytotoxicity. J Hazard Mater 141:378–387. https://doi.org/10.1016/j.jhazmat.2006.05.082
Stajic JM, Milenkovic B, Pucarevic M, Stojic N, Vasiljevic I, Nikezic D (2016) Chemosphere exposure of school children to polycyclic aromatic hydrocarbons, heavy metals and radionuclides in the urban soil of Kragujevac City, Central Serbia. Chemosphere 146:68–74. https://doi.org/10.1016/j.chemosphere.2015.12.006
Sutherland RA, Tack FMG (2002) Determination of Al, Cu, Fe, Mn, Pb and Zn in certified reference materials using the optimized BCR sequential extraction procedure. Anal Chim Acta 454:249–257. https://doi.org/10.1016/S0003-2670(01)01553-7
Szolnoki Z, Farsang A, Puskás I (2013) Cumulative impacts of human activities on urban garden soils: origin and accumulation of metals. Environ Pollut 177:106–115. https://doi.org/10.1016/j.envpol.2013.02.007
Tepanosyan G, Sahakyan L, Belyaeva O, Maghakyan N, Saghatelyan A (2017) Human health risk assessment and riskiest heavy metal origin identification in urban soils of Yerevan, Armenia. Chemosphere 184:1230–1240. https://doi.org/10.1016/j.chemosphere.2017.06.108
Thurston GD, Spengler JD (1985) A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos Environ 19:9–25. https://doi.org/10.1016/0004-6981(85)90132-5
Tighe M, Lockwood P, Wilson S (2005) Adsorption of antimony(v) by floodplain soils{,} amorphous iron(iii) hydroxide and humic acid. J Environ Monit 7:1177–1185. https://doi.org/10.1039/B508302H
Tomasevic M, Anicic M (2010) Trace element content in urban tree leaves and SEM-EDAX characterization of deposited particles. FU Phys Chem Tech 8:1–13. https://doi.org/10.2298/FUPCT1001001T
Torres E, Auleda M (2013) A sequential extraction procedure for sediments affected by acid mine drainage. J Geochem Explor 128:35–41. https://doi.org/10.1016/j.gexplo.2013.01.012
Tsukuda S, Sugiyama M, Harita Y, Nishimura K (2005) Atmospheric bulk deposition of soluble phosphorus in Ashiu experimental Forest, Central Japan: source apportionment and sample contamination problem. Atmos Environ 39:823–836. https://doi.org/10.1016/j.atmosenv.2004.10.028
US EPA (2009) United States Environmental Protection Agency, risk-based concentration table. United States Environmental Protection Agency, Washington, DC, Philadelphia
USDA (1998) Soil quality indicators: pH. Eur J Soil Biol 38:2. https://doi.org/10.1016/S1164-5563(01)01111-6
USEPA (1989) Risk assessment guidance for superfund volume I human health evaluation manual (part A) I
USEPA (1993) Reference dose (RfD): description and use in health risk assessments. US Environ. Prot. Agency, Washington, DC
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107. https://doi.org/10.1016/j.microc.2009.09.014
Wong CSC, Wu SC, Duzgoren-Aydin NS, Aydin A, Wong MH (2007) Trace metal contamination of sediments in an e-waste processing village in China. Environ Pollut 145:434–442. https://doi.org/10.1016/j.envpol.2006.05.017
Wu S, Peng S, Zhang X, Wu D, Luo W, Zhang T, Zhou S, Yang G, Wan H, Wu L (2015) Levels and health risk assessments of heavy metals in urban soils in Dongguan, China. J Geochem Explor 148:71–78. https://doi.org/10.1016/j.gexplo.2014.08.009
Xie J-J, Yuan C-G, Shen Y-W, Xie J, He K-Q, Zhu H-T, Zhang K-G (2019) Bioavailability/speciation of arsenic in atmospheric PM2.5 and their seasonal variation: a case study in Baoding City, China. Ecotoxicol Environ Saf 169:487–495. https://doi.org/10.1016/j.ecoenv.2018.11.026
Xiong T, Dumat C, Pierart A, Shahid M, Kang Y, Li N, Bertoni G, Laplanche C (2016) Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: field study of soil--plant--atmosphere transfers in urban areas, South China. Environ Geochem Health 38:1283–1301. https://doi.org/10.1007/s10653-016-9796-2
Yang K, Cattle SR (2018) Contemporary sources and levels of heavy metal contamination in urban soil of Broken Hill, Australia after ad hoc land remediation. Int J Min Reclam Environ 32:18–34. https://doi.org/10.1080/17480930.2016.1208859
Yuen JQ, Olin PH, Lim HS, Benner SG, Sutherland RA, Ziegler AD (2012) Accumulation of potentially toxic elements in road deposited sediments in residential and light industrial neighborhoods of Singapore. J Environ Manag 101:151–163. https://doi.org/10.1016/j.jenvman.2011.11.017
Yuswir NS, Praveena SM, Aris AZ, Ismail SNS, Hashim Z (2015) Health risk assessment of heavy metal in urban surface soil (Klang District, Malaysia). Bull Environ Contam Toxicol 95:80–89. https://doi.org/10.1007/s00128-015-1544-2
Zhang C, Wu L, Luo Y, Zhang H, Christie P (2008) Identifying sources of soil inorganic pollutants on a regional scale using a multivariate statistical approach: role of pollutant migration and soil physicochemical properties. Environ Pollut 151:470–476. https://doi.org/10.1016/j.envpol.2007.04.017
Zhang J, Deng H, Wang D, Chen Z, Xu S (2013) Toxic heavy metal contamination and risk assessment of street dust in small towns of Shanghai suburban area, China. Environ Sci Pollut Res 20:323–332. https://doi.org/10.1007/s11356-012-0908-y
Zheng N, Liu J, Wang Q, Liang Z (2010) Heavy metals exposure of children from stairway and sidewalk dust in the smelting district, northeast of China. Atmos Environ 44:3239–3245. https://doi.org/10.1016/j.atmosenv.2010.06.002
Acknowledgements
The authors would like to acknowledge the help of the research committee of Shiraz University Medical Geology Research Centre, Shiraz University, for the logistic assistance and the Department of Agricultural, Forest and Food Sciences of University of Turin for the analytical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 349 kb).
Rights and permissions
About this article
Cite this article
Mokhtarzadeh, Z., Keshavarzi, B., Moore, F. et al. Potentially toxic elements in the Middle East oldest oil refinery zone soils: source apportionment, speciation, bioaccessibility and human health risk assessment. Environ Sci Pollut Res 27, 40573–40591 (2020). https://doi.org/10.1007/s11356-020-09895-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09895-7