Abstract
There is evidence that water-soluble fraction (WSF) from fuel oil/diesel mixture affects marine microbiota. In order to establish a sequence of WSF effects during microalgal growth, this work aimed to monitor Dunaliella tertiolecta exposed to WSF during 15 days. Three different pigments (chlorophyll a, lutein, and β-carotene) and four metabolites (protein, lipids, fatty acids, and phenols) were studied, and FTIR spectroscopy was used to determine the biomolecular transitions of lipids and their accumulation. The results show that D. tertiolecta triggered a physiological and biochemical response with changes in growth rate, pigments, phenols, lipids, and proteins of the microalga, although fatty acid profile was unaltered. For all the biochemical parameters altered, there were significant differences with the controls. At the end of the assay, exposed D. tertiolecta showed similar values with the control on all the compounds analyzed, except lipids. FTIR absorbance showed an increase in unsaturated acyl chains within the exposed microalgae, giving support for a possible uptake of hydrocarbons from WSF. Variation in pigments and phenol contents is presented as an integrated antioxidant response to the stress imposed by WSF. Overall, this research provides information about the effects of WSF on D. tertiolecta, and the ability of this microalga to recover after long-term exposure to the water-soluble fraction of fuel oil/diesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the main problems faced by coastal ecosystems is hydrocarbon pollution (Parab et al. 2008). Some oil components are moderately water-soluble, and they may leach into seawater; this affects marine organisms, especially planktonic microorganisms, such as microalgae, since their growth and development are strictly dependent on water quality (Morales-Loo and Goutx 1990; Parab et al. 2008). Microalgae respond differently to hydrocarbon pollution. This response depends on the microalgae species, the type, and the concentration of the fuel oil (Kadiri and Eboigbodin 2012). For ocean service ships, fuel oil must be combined with other components, as diesel, to meet an acceptable specification to be suitable for larger engines (Draffin 2008). This mixture of hydrocarbons can be a source of contamination in the case of port accidents and accidental spills. Furthermore, diesel water-soluble fraction (WSF), also known as WAF (water-accommodated fraction), is toxicity associated with the development of several microalgae, and this effect is related to the heavy aromatic hydrocarbons in the soluble fraction of diesel (Pereira et al. 2012).
Marine microalgae, the most abundant species in aquatic environments, can develop a variety of mechanisms to reduce the toxicity of oil hydrocarbons (Torres et al. 2008), but the traits that make them more resistant are not extensively studied. Dunaliella tertiolecta, a marine microalga, is known as a more tolerant species to petroleum compounds than other microalgae (Siron et al. 1991; Okumura et al. 2003). Searching for the response to crude oil derivate toxicity, several authors have observed some degree of oil resistance in this microalga, but mechanisms developed to face hydrocarbons are still not completely understood. Morales-Loo and Goutx (1990) observed retardation of growth on D. tertiolecta under WSF, but total inhibition was not observed at 100% WSF. Siron et al. (1991) noticed stimulation of the exponential growth phase on D. tertiolecta exposed to WSF, following the reduction of photosynthetic capacity during the lag phase. Carrera-Martinez et al. (2010) studied photosynthetic response and attributed resilience to genetic adaptation, an idea also supported by others. Romero-Lopez et al. (2012) observed that D. tertiolecta exposed to petroleum or diesel (3%) responds through physiological acclimatization and becomes petroleum-resistant by rare mutations under higher hydrocarbon levels (9%). Bretherton et al. (2018) examined physiological responses during exposure to oil water-soluble components. Among ten marine species from marine microbiota, they identified D. tertiolecta as a “robust” species, with mostly unaffected growth rates or time spent in lag phase by water-accommodated fractions. In contrast, Shiu et al. (2020), who exposed nine marine microbial cells to water-accommodated fraction, suggest D. tertiolecta as a sensitive species based on the decrease of DNA in D. tertiolecta cells after a 24-h exposition to WSF. Recently, Bretherton et al. (2020) studied the response of 15 phytoplanktonic species to oil and oil dispersants and found that cell size and motility are essential determinants of the resistance of organisms to oil. However, their findings only account for a third of the variance observed, so further work is needed to identify other factors contributing to this resistance.
Besides physiological traits, biochemical and physiological variations are useful tools to understand and monitor biological phenomena under hydrocarbon exposition. In their work, Shiu et al. (2020) examined the effect of WSF on D. tertiolecta and pointed the triggering effect of oil not only on the release of extracellular polymeric substances but also in the induction of changes in their composition. Biochemical variations inside the cells under exposure to oil derivates might be an adequate means to understand the complete response. Oil WSF causes oxidative stress in some microalgae (Jiang et al. 2010; Ramadass et al. 2015), affecting cell membranes and the synthesis of proteins, and generates a decrease in the chlorophyll a content, among other noxious effects (Jiang et al. 2010). Microalgae have shown varied responses to oil (Bretherton et al. 2018), and it has been reported that microalgae under environmental stress conditions modify the concentrations of multiple bioactive compounds inside the cell, such as pigments, phenols, and lipids as a physiological response (Fazeli et al. 2006; Wang and Jia 2020). Carotenoids are pigments that protect the photosynthetic apparatus in plants by dissipating excess energy and acting as antioxidants (Ahmed et al. 2014). Under stress, the microalgae of the genus Dunaliella can accumulate these pigments as a physiological response (Fazeli et al. 2006). Studies demonstrated that phenolic compounds in plants act as a protective mechanism against a wide range of stresses, giving a variation in phenols per cell (López et al. 2015). This behavior has been reported for D. tertiolecta, which presented a significant variation in the content of phenolic compounds under metallic stress and different light intensity conditions (López et al. 2015; Gomez et al. 2016). For many microalgal species, protein and lipid production is modified in response to various stress conditions such as salinity change (Tammam et al. 2013), nutrient starvation (Rukminasari 2013; Poh et al. 2020), hydrocarbon exposure (Morales-Loo and Goutx 1990; El-Sheekh et al. 2000), or induced oxidative stress (Chu et al. 2020). Parab et al. (2008) stated that, at high concentrations of oil WSF, growth and the protein biosynthesis content of Thalassiosira sp. were affected, indicating that the biosynthesis of proteins might be considered a possible target of oil toxicity. For lipids, the crude oil WSF caused variations in the content of the different lipid classes on D. tertiolecta, showing an accumulation of hydrocarbons and sterols at the logarithmic phase (Morales-Loo and Goutx 1990). Besides, Shishlyannikov et al. (2017) stated that hydrocarbons, being nonpolar compounds, can be incorporated into the membrane and then inside the cell by passive diffusion, leading to an accumulation of hydrocarbons in the lipid bodies of microalgae.
A novel tool to evaluate lipids in microalgae is Fourier transform infrared spectroscopy (FTIR). This method allows the monitoring of lipid accumulation while using a small amount of biomass and without destroying the samples. Grace et al. (2020) indicated that using the second-order derivative of FTIR absorbance of the spectra improves the identification of signals by enhancing the resolution of the absorption peaks of interest components.
Even though there is information on pigments, lipids, proteins, and phenolic content in D. tertiolecta, little information is available on the change of these compounds under stress conditions such as the exposure to soluble hydrocarbons of the fuel oil/diesel mix. Investigation of the effect of water-soluble oil components on these compounds in growing cells would provide an excellent basis for a better picture of the events preceding the death/survival/resilience of some marine microbiota species. The present work aimed to investigate the effects of the WSF on the microalgae D. tertiolecta on growth, pigments, and biochemical components. In this approach, we selected three different pigments (chlorophyll a, lutein, and β-carotene) and four metabolites (protein, lipids, fatty acids, and phenols), all of which are related to defense mechanisms in microalgae.
Materials and methods
Preparation of stock solution of the water-soluble fraction of fuel oil/diesel
The fuel oil was provided by Captain Norma Hernández of the Second Naval Region, Ensenada, Baja California, México. The diesel was purchased directly from a PEMEX gas station in the same city. The fuel oil was mixed with diesel (85:15). A stock solution of WSF was prepared with seawater and the hydrocarbon mixture (9:1) (Siron et al. 1991). The recipient was gently shaken without disturbing the oil/water interface. The natural seawater was previously filtered (0.45 μm), treated with UV light, and sterilized.
Microalgae culture conditions
Dunaliella tertiolecta was obtained from the culture collection of the Aquaculture Department of the Center of Scientific Research and Higher Education of Ensenada (CICESE), Baja California, México. The microalgae were grown in an initial culture batch in a 1-L bottle, with f/2 medium (Guillard and Ryther 1962), temperature of 19 ± 1 °C, and the salinity of 34 PSU, provided with continuous light (67.5 μmol m−2 s−1).
Toxicity test
Microalgal cells in the logarithmic phase were used for all the toxicity experiments. After preliminary testing (data not shown), a WSF 50% concentration was selected for the definitive toxicity test for a concentration high enough to cause an effect but not too high to kill microalgae. The definitive toxicity test was performed with a 105-mL aliquot of microalgae culture mixed in a 1-L glass bottle with WSF and seawater to a 50% WSF final. As a control group, a 105-mL aliquot of culture was placed in a 1-L bottle and mixed with seawater. The final volume for both groups was 900 mL, and the initial cell density was 100,000 cell mL−1. Control and test bottles were triplicated and placed in a temperature of 19 ± 1 °C, provided with continuous light (67.5 μmol m−2 s−1), in f/2 medium for 15 days. Samples from all six bottles were collected on days 3, 6, 9, 12, and 15 to monitor growth and biochemical response.
Monitoring of microalgal cultures
The cell density was estimated by counting cells using a Neubauer chamber with a microscope Primo Star, Carl Zeiss (n = 3). The growth rate (μ) was calculated according to Vonshak and Maske (1982). The biomass produced was quantified based on the total dry weight (DW). Fifty-milliliter samples were collected from the cultures and centrifuged. After centrifugation, the pellet was rinsed with 3% ammonium formate, and then freeze-dried. The DW of the biomass was determined using an analytical scale (Explorer Pro EP114C, OHAUS, Switzerland).
Determination of pigments by HPLC
Microalgae samples (15 mL) of the culture were harvested by centrifugation on days 3, 6, 9, 12, and 15. The supernatants were carefully removed, and the cell pellets were stored at − 20 °C until further analysis. For the extraction procedure, the cell pellets were defrosted, suspended in 1.5 mL of 100% methanol, and sonicated for 5 min. The mixtures were stored in the dark for 1 h at 4 °C and then centrifuged. The supernatants were recovered and filtered to be analyzed by HPLC, Agilent Technologies® chromatograph, model 1260 INFINITY, equipped with a Zorbax C8 column of 4.36 × 150 mm and particle size of 5 μm.
Standard pigments
Chlorophyll a (Sigma-Aldrich), β-carotene, and lutein (DHI, Denmark) were used to calculate the concentrations of pigments. The identification and quantification of the pigments were performed by triplicate, as described by Orosa et al. (2005).
Total phenolic determination
Microalgae were harvested by centrifugation at 4800 rpm for 15 min in a refrigerated centrifuge (Allegra 64R Centrifuge, Beckman Coulter), and freeze-dried. Total phenolic compounds in D. tertiolecta were extracted using a modification of the method described by Goiris et al. (2012). Briefly, freeze-dried biomass was extracted with ethanol/water 75% (v/v). The mixture was centrifuged at 4500 rpm for 15 min, and the supernatants obtained were stored at − 20 °C before analysis. Extractions were performed in the dark at room temperature. The phenolic content of D. tertiolecta in the extracts was determined by the Folin–Ciocalteu method modified by Goiris et al. (2012). An aliquot of the extract was mixed with the Folin–Ciocalteu reagent diluted tenfold. Afterward, a sodium bicarbonate solution was added to the mixture and then incubated. The absorbance was measured at 765 nm. Phenols and polyphenols were quantified (n = 3) using gallic acid as standard, and the results are expressed as milligrams of gallic acid equivalents (GAE) per gram of biomass.
Protein content
Protein content was determined by the method of Lowry et al. (1951). D. tertiolecta was harvested by centrifugation at 4500 rpm for 15 min, at 4 °C and conserved at − 80 °C in a nitrogen atmosphere. The pellet was resuspended in 1 mL of distilled water, and an aliquot was quantified following the Lowry method (n = 3). A calibration curve in the range of 25–500 μg mL−1 was generated by using bovine serum albumin (BSA) as standard.
Total lipid quantification
The sulfo-phospho-vanillin (SPV) assay for lipid estimation, described by Mishra et al. (2014), was used for lipid quantification of D. tertiolecta culture. The algal biomass was harvested via centrifugation at 4000 rpm for 10 min. The phospho-vanillin reagent was prepared with 12% (w/v) of vanillin, dissolved in ethanol:water:phosphoric acid (1:9:40), and then proceeded to the quantification following the sequence described in the methods cited above (n = 3). The absorbance at 530 nm was measured against a blank, using 100 μL of distilled water. Canola oil (Sigma-Aldrich) was used as the standard for the calibration curve.
Fatty acid composition
Lyophilized algal cells harvested on day 15 were extracted three times with dichloromethane: methanol (2:1 v/v) using the Folch extraction method as modified by Badillo Zapata et al. (2016). The extract was preserved with 0.01% BHT to avoid oxidation.
The lipids obtained were saponified and esterified to form methyl esters of fatty acids (FAME). The total FAME was weighed and resuspended in hexane to obtain a final concentration of 100 mg mL−1 (Badillo Zapata et al. 2016). The samples were analyzed in an Agilent GC 6850 gas chromatograph, equipped with an injector split/splitless, a flame ionization detector (FID), and a capillary column (Agilent, DB-FFAP; (Agilent J&W, GC columns) 30 m × 0.320 mm, film thickness 0.25 μm). The carrier gas used was nitrogen. FAME peaks were identified and quantified using as standard a 37 component FAME standard mix (Supelco/Sigma-Aldrich) (n = 3). The total lipid content was calculated by summing up all peaks except for the solvent peak (Mishra et al. 2014).
Lipid characterization by Fourier transform infrared spectrometry
D. tertiolecta was harvested every third day by centrifugation for 15 min at a speed of 4000 rpm at 4 °C. Then, the pellet was transferred to an Eppendorf tube and frozen at − 20 °C with a nitrogen atmosphere. The pellets were lyophilized for 24 h and stored at − 20 °C. Functional groups in biomass were identified using a Perkin-Elmer FTIR Spectrum Two. Spectra were normalized by the Amide I band at ~ 1650 cm−1, and then smoothed by the Savitzky–Golay method using Origin Pro 8.0 software (Origin Lab Corporation, Northampton, MA, USA). Absorbances of the FTIR spectra were used to calculate the second-order derivative, and a negative peak was obtained for each band and shoulder in the raw absorption spectra (Stuart 2004). Second-order derivatives of absorbance were expressed as SDA. Then, the area of the negative peaks of the lipid bands was calculated to obtain the total lipid content of microalgae, using Eq. (1):
where:
(AUFA) = total sum of areas of the unsaturated fatty acid bands = (\( {A}_{1290\ {\mathrm{cm}}^{-1}} \)) + (\( {A}_{3010\ {\mathrm{cm}}^{-1}} \))
(\( {A}_{1290\ {\mathrm{cm}}^{-1}} \)) = area of the negative peak due to = C-H vibration
(\( {A}_{3010\ {\mathrm{cm}}^{-1}} \)) = area of the negative peak due to the vibration in CH=CH bonds
(ATAG) = total area of the triacylglycerols = (\( {A}_{1740\ {\mathrm{cm}}^{-1}} \))
(\( {A}_{1740\ {\mathrm{cm}}^{-1}} \)) = area of the negative peak due to the carbonyl stretches νC=O
From the results in Eq. (1), the biomolecular transitions of lipids were evaluated and monitored. ATAG and AUFA were calculated as a percentage of the total lipids.
Data analysis
Two-way analysis of variance (ANOVA) was performed to assess the normality of distribution of all continuous variables. Differences in biochemical responses between control and WSF exposed groups were assessed by Tukey’s method of pairwise comparisons. The statistical significance was established at 95% (p < 0.05). Pearson correlation analysis was performed to find possible relations among pigment variations with the exposure to WSF. The statistical analysis was carried out using Minitab Statistical Software (version 17.3.1). Results are presented as average ± standard deviation (n = 3).
Results
Effect of WSF on the growth of Dunaliella tertiolecta
The growth curves of Dunaliella tertiolecta in the medium with 50% WSF and control did not show a lag phase (Fig. 1a). The logarithmic phase (LP) in all culture media lasted 5 days, followed by a slow growth phase (SGP) that lasted 7 days. Then, a stationary phase (SP) was observed until the end of cultivation on day 15. At this stage, the maximum microalgal density of the group exposed to WSF (4.54 × 106 cells mL−1) had no significant difference with the control group (4.67 × 106 cells mL−1). The maximum specific growth rates (μ) were 1.08 on day 3 and 1.21 on day 4 for the exposed and the control group, respectively (Fig. 1b). A decrease in the growth rate was found at day 4 in the exposed group, with a significant difference compared with the control group.
Pigments
In the control group, a maximum of chlorophyll a (9.83 mg g−1) was observed in the early SGP (day 6). Under the presence of WSF in culture media, D. tertiolecta presented an increase in the chlorophyll a content between day 3 (1.91 mg g−1) and day 6 (5.71 mg g−1), reaching a level that was maintained until the end of the test (Fig. 2a). On day 6, chlorophyll a in the exposed group was 42% lower than the chlorophyll a in control (p < 0.05).
Pigments in Dunaliella tertiolecta exposed to 50% WSF and control group, in f/2 media. a Chlorophyll a, b lutein, and c β-carotene expressed in milligrams per gram of dry weight (mg g−1). Error bars represent standard error, n = 3. Asterisk indicates a significant difference between groups (p < 0.05)
In lutein productivity, there was no difference between groups. The maximum lutein content was found on day 6 for the control with 3.65 mg g−1 (Fig. 2b). After 15 days of incubation in WSF, the amount of lutein detected was 2.31 mg g−1, comparable value with the concentration found in the control group (2.02 mg g−1). The concentration of lutein presented significant differences between the phases of growth in the control (p < 0.05). At the SGP, the lutein content was higher than in the LP (day 3) and SP, with a tendency to decrease when the culture grew older. This behavior was not observed in the exposed group of D. tertiolecta, which did not show significant differences between phases.
The lutein content in the control group showed a high correlation (Pearson coefficient, R = 0.915) with the level of chlorophyll a in D. tertiolecta (Fig. 3); on the contrary, for WSF group this correlation was low (R = 0.561). For the exposed group, the ratio of a gram of lutein per gram of chlorophyll a was similar in the LP and SGP compared with the ratio calculated for the control group. At the SP (day 15), the ratio of lutein to chlorophyll a was higher for the exposed group (0.73 g g−1) when compared with the control at the same phase (0.5 g g−1) (p < 0.05).
D. tertiolecta cultivated with 50% WSF presented variations on β-carotene content. On day 3, the β-carotene content in the microalga exposed to WSF was significantly lower than the value in the control group (Fig. 2c). On the other hand, on day 6, this pigment in the exposed group was almost four times the value for the control. An increase through the growth curve in the β-carotene content was observed in the exposed culture, reaching a maximum content at the SP (days 12 and 15). The ratio of a gram of β-carotene per gram of chlorophyll a for the exposed group (0.031 g g−1) was more than six times the ratio found in the control group (0.004 g g−1), at the early SGP. At the SP, there was no significant difference in the ratio β-carotene:chlorophyll a between both groups.
Total phenolic content
The phenolic content ranged from 4.50 to 7.50 mg GAE g−1 on D. tertiolecta cultured with 50% WSF. In this group, the maximum of phenols observed on day 3 was higher than the control value. However, at the SP (day 12), the amount of phenol for the exposed group was lower than the value of the control group (Fig. 4). Phenols in D. tertiolecta exposed to WSF presented a tendency to decrease with time, showing significant differences between phases of growth. This tendency was not observed in the control group.
Protein
A maximum of protein was observed in the early SGP (day 6) on the control group (Fig. 5a); on the contrary, protein content in the exposed group showed no significant difference through the growth curve. The maximum protein content of the control group, found in day 6, was 33% greater than the value observed in the microalgae exposed to 50% WSF.
Total lipids
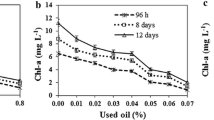
At the LP and SGP, there were no significant differences between the groups in the lipid content by sulfo-phospho-vanillin (SPV) analysis (Fig. 5b). In the total lipid content, there was a difference of more than 40% at the SP between the exposed group and the control, being the former higher than the latter. D. tertiolecta under stress showed an increase in the lipid content through growth. The total lipids for day 15 are shown in Table 1 as the percentage of microalgal lipid content based on the SPV quantification.
Fatty acid profile
There were no significant variations of FAME profile of extracted lipid from D. tertiolecta at day 15 between control and exposed group by GC analysis. For both, the major fatty acids of D. tertiolecta were a saturated fatty acid (SFA), 16:0 (18% of total fatty acids), and a polyunsaturated fatty acid (PUFA), 18:3 (37% of total fatty acids). Of the total fatty acids, 80% were identified in both groups. The total lipid amount based on the data obtained from the GC analysis showed no significant difference between the exposed and control groups. Data are shown as the percentage of microalgal lipid content based on the GC quantification in Table 1.
FTIR analysis
FTIR spectra of D. tertiolecta showed typical bands of absorptions for control and exposed groups, but differences in intensities were observed (range 4500–450 cm−1). In both groups, we identified the characteristic lipid bands, namely, the bands between 3050 and 2800 cm−1 (Fig. 6a, b), 1770–1710 cm−1 (Fig. 7a, b), and the peak ~ 1290 cm−1. Also, for lipid bands, we observed slightly overlapping peaks in the ranges studied. The bands were assigned to specific functional groups based on published works (Stuart 2004; Laurens and Wolfrum 2011; Grace et al. 2020). The FTIR data of day 6 and 12 are omitted for clarity.
FTIR analysis of the ester band carbonyl stretches (1760–1710 cm−1) in Dunaliella tertiolecta cells on days 3, 9, and 15 during the toxicity test. Absorption spectra of a exposed group and b control. Second-order derivative of absorbance (SDA) of the ester band (1760–1710 cm−1) of c exposed group and d control groups
SDA analysis of the C–H stretching vibration bands (3050 to 2800 cm−1) of lipids in D. tertiolecta showed differences among spectra from cells after 3, 9, and 15 days of exposition to WSF. On day 3, vibration in CH=CH bonds exhibits a small signal around 3010 cm−1, which splits on day 15 in two peaks at ~ 3013 cm−1 and ~ 3005 cm−1 (Fig. 6c). A weak peak was found at 2925 cm−1 on day 3 and 9. On day 15, a strong peak was observed in the same wavenumber. For the control group, the intensity of the CH2 band at 2925 cm−1 was similar for all the spectra (Fig. 6d).
For the range 1710–1770 cm−1, similar spectra were found for exposed and control groups during the toxicity test (Fig. 7). On days 3 and 9, the SDA analysis showed small intensity peaks around ~ 1740 cm−1 (carbonyl stretches, νC=O), for the two groups. However, on day 15, a stronger signal for this band was observed for both conditions, exposed and unexposed (Fig. 7c, d). SDA analysis also revealed a weak peak at 1290 cm−1 in all the spectra, with a small increase in intensity from day 3 to day 15 for the exposed group. For the control group, a small change in the opposite order given of time was found.
Based on Eq. (1) and the SDA analysis, the calculated percentage of triacylglycerols (TAG) on day 3 was almost 82% of the total lipids for the exposed group. A lower value of TAG was observed in the control group, being only 30% of the total lipids (Fig. 8a). In contrast, the percentage of unsaturated fatty acid (UFA) represented 18% and 70% for the exposed and control groups, respectively. On day 9, a minimum TAG percentage (36%) was observed for the exposed group, followed by an increase at the end of the experiment on day 15. For the control group, we observed a noticeable increase in the TAG on day 15. Correspondingly, the UFA percentage of the exposed group was 63% and 32% on days 9 and 15 (Fig. 8b). A noteworthy decrease in UFA percentages was from 54% (day 9) to 20% (day 15) for the control group.
The TAG:UFA ratio in the exposed group on day 3 was higher than the ratio found in the control group. For days 9 and 15, the TAG:UFA ratio in the exposed group was lower than the control group ratio. The TAG calculated by the SDA analysis comprised the saturated fatty acids (SFA) and the monounsaturated fatty acids (MUFA). The percentage of TAG calculated with SDA analysis was 25% (exposed group) and 40% (control) more than the percentage of SFA + MUFA by the GC analysis (Fig. 9).
Discussion
There is some evidence that crude oil, which is a complex mixture of hydrocarbons, is toxic for microalgae. There are also reports that WSF, a mixture of water-accommodated oil components, can affect cell growth in marine microbiota. In this study, we confirm previous findings by Morales-Loo and Goutx (1990), who describe Dunaliella tertiolecta with a diminution in the growth rate when cultured in 50% WSF. We found the same behavior for the group exposed to WSF at the logarithmic phase, suggesting that D. tertiolecta was affected by WSF. On the contrary, Bretherton et al. (2018) found no effect either on the lag phase retardation or growth rate. Further, Bretherton et al. (2020) classified D. tertiolecta as “resistant” among 15 phytoplanktonic species based on the growth rate, and photosynthetic parameter response to WSF. Recently, Shiu et al. (2020) showed a sensitive response from D. tertiolecta to WSF in the growing medium, in terms of DNA content inside the cells. Taken as a whole, the growth curve in our study reflects both facts previously stated: the responsiveness of D. tertiolecta to WSF in a first stage (decrease μ on day 4), and the ability to recover, reaching over 4 × 106 cells mL−1 at the stationary phase, a similar value found in the control group.
There is a consensus that chlorophyll drops and impaired photosynthetic performance are two of the deleterious effects of oil and derivates in several microalgae (Morales-Loo and Goutx 1990; Romero-Lopez et al. 2012; Bretherton et al. 2018). In agreement with others, we found a decrease of chlorophyll a in D. tertiolecta under WSF compared with control on day 6. In the control group, from day 3 to day 6, a sharp 282% increase was observed, whereas exposed cells only increased their chlorophyll a by 198% in this period. From then on, D. tertiolecta under WSF kept chlorophyll levels with no significant change. In the same way as for growth rate, the variation of chlorophyll through time indicates an initial adverse response that evolves into a recovery phase. Several authors have depicted a sensitive-resistant D. tertiolecta in works similar to the present.
Bretherton et al. (2018) report D. tertiolecta as oil-resistant based on lag time, chlorophyll-specific growth rates, and photophysiological parameters as photosynthetic efficiency and PSII antenna size, all of them unaltered under WSF. In a recent report (Bretherton et al. 2020), they attribute cell size 30% of the variation in microalgae response to WSF. However, there are previous reports of retarded growth and hypothesis on the disruption of biosynthetic mechanisms required for a functional photosynthetic apparatus (Morales-Loo and Goutx 1990; Siron et al. 1991). On the other hand, Carrera-Martinez et al. (2010) documented the decrease of optimal quantum yield, relative electron transport rate, and photosynthetic efficiency in D. tertiolecta after 1 h of exposure to oil dilutions. They observed the presence of rare spontaneous mutations of the microalga after 30 days of oil exposure (Carrera-Martinez et al. 2010). Recently, Shiu et al. (2020) subjected D. tertiolecta to a 24-h exposure to WSF and found lower levels of DNA in survivors related to control. Our study, through growth, let us visualize the response of microalgae as a continuum of facts aiming to face WSF, rather than a series of deleterious effects. In this sense, one of the beneficial effects of diminishing chlorophyll would be to slow down photosynthesis and minimize oxidative stress.
Regarding accessory pigments, the drop of chlorophyll in the microalgae on day 6 was accompanied by a β-carotene increase. On day 3, exposed D. tertiolecta shows a low β-carotene content with respect to the control group. In contrast, on day 6, the exposed group overpassed the control group. After day 9, both growth curves look very much alike with no significant differences between control and exposed group, both groups rising β-carotene content toward the last day of the assay (day 15). Using the ratio β-carotene:chlorophyll for comparison on the 6th day, we see that the exposed group (ratio = 0.031) outpassed the control group (ratio 0.004) by 553%. This increase gives the exposed group advantages over the control group in quenching singlet oxygen generated by photosynthesis (Tammam et al. 2013), and also a better preparation to get rid of excess light absorbed from the environment. Concerning lutein, we did not find significant differences between both groups during the days when we took culture samples. This similarity suggests a parallel variation of lutein over time in both groups. However, when using the ratio lutein:chlorophyll, we found a significant difference between the control and exposed group on day 15. Correlation analysis to evaluate the strength of the relationship between lutein and chlorophyll indicated a strong correlation in the control group, showing that in D. tertiolecta under normal conditions, lutein changes in the same manner as chlorophyll a. Contrarily, week correlation was found for the microalga under WSF, suggesting there is a discrepancy between the production of chlorophyll and lutein. Lutein:chlorophyll ratio in exposed cells (0.73), higher than in control cells (0.5), shows an increase of 43%, suggesting a second defense mechanism is triggered and shows on day 15. The role of lutein in Dunaliella is not entirely unraveled, but it is worth to recall the functions of this pigment in higher plants, namely, structural stabilization of antenna proteins, light-harvesting (by the transference of excitation energy to Chl), and quenching of 3Chl states (Jahns and Holzwarth 2012). Noteworthy is the limited increase of lutein compared with β-carotene in exposed cells. Lutein and β-carotene have lycopene as a common precursor molecule, found in low levels, which gives rise to α- and β-carotene; then, lutein is produced by oxidation of α-carotene (Takaichi 2011). On this basis, the small increase of lutein on WSF exposed D. tertiolecta could be explained by the low level of lycopene, channeled toward increased production of β-carotene.
The maximum content of phenols in D. tertiolecta exposed to WSF was on day 3 and reached a level of 29% higher than the control group. On day 6 and later, phenols steadily decreased as growing. On the other hand, cells in the control group presented the highest value on day 12 and, compared with the exposed group, was significantly higher. At this point, it is useful to highlight that maximum phenol content in D. tertiolecta under WSF was before both β-carotene increment and chlorophyll drop on day 6. Also, it is essential to mention that the β-carotene increase in exposed D. tertiolecta coincided with chlorophyll drop (day 6) and outpassed the control level. Based on our results, we may point phenol increment as a fast response that initiates either simultaneous or sequentially with β-carotene. Together, both non-enzymatic antioxidants would be a deployment of an efficient and coordinated response of D. tertiolecta to protect itself from oxidative stress since the beginning of the immersion in WSF. In this way, phenols would be in a first defense line against ROS, followed by β-carotene, which contributes to dissipating the excess of energy generated by photosynthesis. Otherwise, the excess of free radicals generated by the decrease in chlorophyll with a constant illumination would cause irreversible damage to the structural components. Noteworthy, the decrease in phenols in the exposed group from the third day to the twelfth suggests that D. tertiolecta was excreting phenols to the medium. It is documented the ability of D. tertiolecta to exudate phenols when exposed to metals as a tool to either chelate some components or slow down the uptake by the cell (López et al. 2015). Summing up, the biochemical changes in the cell at 50% WSF on the first 12 days suggest a potential self-protective strategy to this toxic, with defensive lines before crossing the physiological threshold. Otherwise, D. tertiolecta would have to undergo genomic transformations to fit the contaminated environment, as observed by Carrera-Martinez et al. (2010) and Romero-Lopez et al. (2012).
The impact of the presence of WSF on the protein content of microalga was noted early (day 6). On this day, coincident with the significant drop in chlorophyll, protein content in exposed cells was low compared with the value of the control. On day 9 and after that, there was no significant difference between the control and the exposed group. Protein hydrolysis has been associated with antioxidative action against the peroxidation of lipids or fatty acids, and scavenging activity of protein hydrolysates from microalgal cells has been demonstrated (Kang et al. 2011). In agreement with Kang et al. (2011), the difference in protein between the groups may be explained as the replenishment or reinforcing of the antioxidant activity needed by D. tertiolecta to cope with WSF. Based on Kang et al. (2011), the products of the protein hydrolysis, amino acids, and peptides could be acting as scavengers against free radicals generated by WSF components. Up to this point, D. tertiolecta would be protected from oxidative stress by phenolic compounds, β-carotene, and products from hydrolyzed protein.
On the other hand, the low content of protein in exposed cells can also be explained as a consequence of impaired protein biosynthesis. This metabolic pathway has been reported as a possible target of hydrocarbon toxicity at high concentrations of fuel oil and its derivatives (El-Sheekh et al. 2000; Parab et al. 2008), and Chen et al. (2008) described high sensitivity of protein synthesis in some marine species to oil components. According to these facts, oil WSF in our study could be destroying the membrane system of D. tertiolecta and slowing down protein synthesis. Marwood et al. (2001) found damage to the chlorophyll and proteins in the photosynthetic apparatus, caused by polycyclic aromatic hydrocarbon (PAH). Chlorophyll and protein simultaneous decrements in WSF exposed cells of our study add new evidence to support the hypothesis on the sensitivity of protein to this toxic. It is essential to point the recovery of D. tertiolecta from the toxic effects after 9 days, given the values of protein in both groups and the stabilization of chlorophyll content. Here, we may think that two goals are achieved when control-like levels of metabolites are restored: the acclimation of D. tertiolecta to WSF and the recovery from the adverse effect of the WSF.
Another response of microalgae originated by the presence of WSF was the accumulation of lipids. On days 12 and 15, total lipids in exposed cells were more than 40% higher than the control. Previous works have reported an increase in total lipids in D. salina under oxidative stress, suggesting that an elevation of ROS mediates lipid accumulation (Yilancioglu et al. 2014). Temporal order and causal links between metabolic events and photosynthesis imbalances remain to be entirely elucidated. However, our data on β-carotene, lutein, and phenol production suggest an ordered but insufficient antioxidant response from D. tertiolecta under WSF to balance ROS production. Instead, there might be an excess of free radicals leading to increasing lipids. The findings that support this hypothesis were recently reported by Chu et al. (2020), who revealed ROS contribution to intracellular signaling by shifting precursors from starch synthesis to fatty acid synthesis, thus increasing lipid accumulation. However, fatty acids by themselves do not account for the excess of lipids in the WSF group, which was 1.4 times the value in control. The percentage of fatty acids represented 89% of the total of lipids in the control group, while for the exposed D. tertiolecta fatty acid content was only 57% of the total. Other lipophilic compounds such as sterols, hydrocarbons, triacylglycerides, and neutral lipids account for the overage in D. tertiolecta exposed to WSF, functioning either as a reserve or involved in adaptation to harsh environments (Morales-Loo and Goutx 1990; Solovchenko 2012). Regarding hydrocarbons, several authors have documented the accumulation of oil WSF in the phytoplankton cells, and the interaction of n-alkanes and PAH with cell membranes (Jiang et al. 2010; Shishlyannikov et al. 2017). Moreover, recent research provides evidence that n-alkanes contribute to the transmembrane transport of hydrocarbons from the aquatic environment into the cell, a process considered a universal process for microalgae (Shishlyannikov et al. 2017).
Besides the total lipids, we examined the fatty acid profile in D. tertiolecta at the end of the assay (day 15). For both the control and the exposed groups, the major fatty acids were C18:3, C:16:0, C18:2, C17:0, and C18:1 (37, 18, 10.5, 6, and 3.5% of the total FA respectively). These figures are consistent with other reports on D. tertiolecta with the same predominant components (Volkman et al. 1989) and the same profile (Lee et al. 2014). Our data are also in agreement with an unaltered fatty acid profile of D. tertiolecta by petroleum compounds at high concentrations (Siron et al. 1986).
Differences in the types of lipids in the microalgae between the exposed group and the control can be disclosed with the spectral FTIR exploration. Using SDA analysis, we observed an increase in the lipid signals on day 15, in agreement with the results by the SPV method. The increase of the CH band intensity and the band between 3020 and 3000 cm−1 due to the vibrations of =C–H absorption in the exposed group showed changes concerning the UFA composition at day 15, and the ratio TAG:UFA exhibits the variations in lipids between our groups. For the exposed group, this ratio was higher than the control value on day 3, indicating an effect of the WSF. An opposite situation occurred at the end of the test, since the ratio TAG:UFA was half the ratio of the control at day 15. Also, on day 3, there was a noticeable difference between groups in TAG when the exposed group presented twice as much as the control. However, there was not a significant difference between groups on days 9 and 12. On day 15, the TAG level in the control group outpassed the exposed cells. The opposite situation was observed on UFA levels. On day 3, exposed cells show the lowest level, but a rise on days 6 and 9, outpassing the control.
From the preceding discussion, we may think there were some TAG and UFA content adjustments through growth. On day 3, exposed D. tertiolecta shows a high TAG content and low content of UFA as possible hydrolysis of TAG, to release UFA. This tendency, opposite to the control, is maintained until day 9. After that, UFA content lowers and TAG rises, keeping the same tendency and a difference by the end of the assay. The fatty acid profile was only performed on day 15; therefore, it was not possible to confirm UFA and TAG changes as part of the initial response to WSF.
Pick and Avidan (2017) reported D. tertiolecta under nutrient stress presented a decrease in the photosynthetic carbon assimilation rate, leading to an accumulation of TAG; they also stated that starch and polar lipids are the principal providers in TAG biosynthesis. On these bases, our data on days 3 through 6 may represent a late stage of TAG accumulation and the beginning of the adjustments to face WSF, but additional research must be done to clarify this response completely.
On the other hand, the increased =C–H signal revealed by the SDA analysis, and TAG:UFA for the exposed group at day 15, can be the basis to account for WSF effect on lipids in D. tertiolecta. Given the absence of variation on total FA upon WSF exposition and enhanced signal of UFA, we can infer there was a xenobiotic inside the cell. Since the enhanced signals can be attributed to the absorption of the acyl chains of oil hydrocarbons, we conclude that WSF is the contributor for this foreign agent. In this regard, our findings are in agreement with several authors, who have demonstrated that marine microalgae can accumulate hydrocarbons during exposure to crude oil and its derivatives (Morales-Loo and Goutx 1990; Wolfe et al. 1998; Wang et al. 2002). Even more, Shishlyannikov et al. (2017) stated that the microalga Synedra acus subsp. radians accumulated n-alkanes with a chain length similar to the acyl chains of its FA. Our FTIR data support the fact that oil can interact with the membrane leading to an accumulation of hydrocarbons in the microalgae.
In conclusion, our results indicate the uptake of hydrocarbons by D. tertiolecta is a physiological response to the stress imposed by soluble hydrocarbons of the mix fuel oil/diesel. Besides, this marine microalga induces a biochemical response as a strategy for protection and recovery from water-soluble oil components effects. On exposure to WSF, D. tertiolecta modified levels of pigments, phenols, protein, and lipids through growth, although, at the end of the toxicity test, the compounds under study reached control-like levels, except for lipids. Therefore, the microalgae showed the capacity to recover and adapt from long-term exposure to WSF.
References
Ahmed F, Fanning K, Netzel M, Turner W, Li Y, Schenk PM (2014) Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem 165:300–306. https://doi.org/10.1016/j.foodchem.2014.05.107
Badillo Zapata D, Lazo JP, Herzka SZ, Viana MT (2016) The effect of substituting fishmeal with poultry by-product meal in diets for Totoaba macdonaldi juveniles. Aquac Res 47:1778–1789. https://doi.org/10.1111/are.12636
Bretherton L, Williams A, Genzer J, Hillhouse J, Kamalanathan M, Finkel ZV, Quigg A (2018) Physiological response of 10 phytoplankton species exposed to macondo oil and the dispersant, Corexit. J Phycol 54:317–328. https://doi.org/10.1111/jpy.12625
Bretherton L, Hillhouse J, Kamalanathan M, Finkel ZV, Irwin AJ, Quigg A (2020) Trait-dependent variability of the response of marine phytoplankton to oil and dispersant exposure. Mar Pollut Bull 153:110906. https://doi.org/10.1016/j.marpolbul.2020.110906
Carrera-Martinez D, Mateos-Sanz A, Lopez-Rodas V, Costas E (2010) Microalgae response to petroleum spill: an experimental model analysing physiological and genetic response of Dunaliella tertiolecta (Chlorophyceae) to oil samples from the tanker Prestige. Aquat Toxicol 97:151–159. https://doi.org/10.1016/j.aquatox.2009.12.016
Chen G, Xiao H, Tang X-X (2008) Responses of three species of marine red-tide microalgae to pyrene stress in protein and nucleic acid synthesis. Mar Environ Sci 27:302–347
Chu H, Ren L, Yang L, Chen J, Zhou X, Zhang Y (2020) Metabolomics reveals a lipid accumulation mechanism involving carbon allocation in Scenedesmus obliquus under norfloxacin stress. Renew Energy 157:585–592. https://doi.org/10.1016/j.renene.2020.05.051
Draffin N (2008) An introduction to bunkering (Spanish), First Edit. Petrospot Limited
El-Sheekh MM, El-Naggar AH, Osman MEH, Haieder A (2000) Comparative studies on the green algae Chlorella homosphaera and Chlorella vulgaris with respect to oil pollution in the river Nile. Water Air Soil Pollut 124:187–204. https://doi.org/10.1023/A:1005268615405
Fazeli MR, Tofighi H, Samadi N et al (2006) Carotenoids accumulation by Dunaliella tertiolecta (lake urmia isolate) and <i>Dunaliella salina<i/> (ccap 19/18 & wt) under stress conditions. DARU J Pharm Sci 14:19–23
Goiris K, Muylaert K, Fraeye I, Foubert I, de Brabanter J, de Cooman L (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol 24:1477–1486. https://doi.org/10.1007/s10811-012-9804-6
Gomez AL, Lopez JA, Rodriguez A, Fortiz J, Martinez LR, Apolinar A, Enriquez LF (2016) Production of phenolic compounds by four species of marine microalgae under different light conditions (Spanish). Lat Am J Aquat Res 44:137–143. https://doi.org/10.3856/vol44-issue1-fulltext-14
Grace CEE, Lakshmi PK, Meenakshi S et al (2020) Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochim Acta Part A Mol Biomol Spectrosc 224:117382. https://doi.org/10.1016/j.saa.2019.117382
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8:229–239. https://doi.org/10.1139/m62-029
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta Bioenerg 1817:182–193. https://doi.org/10.1016/j.bbabio.2011.04.012
Jiang Z, Huang Y, Xu X, Liao Y, Shou L, Liu J, Chen Q, Zeng J (2010) Advance in the toxic effects of petroleum water accommodated fraction on marine plankton. Acta Ecol Sin 30:8–15. https://doi.org/10.1016/j.chnaes.2009.12.002
Kadiri M, Eboigbodin A (2012) Phytotoxicity assessment of water soluble fractions of refined petroleum products using microalgae. Acta Bot Hung 54:301–311. https://doi.org/10.1556/ABot.54.2012.3-4.9
Kang K-H, Qian Z-J, Ryu B, Kim S-K (2011) Characterization of growth and protein contents from microalgae Navicula incerta with the investigation of antioxidant activity of enzymatic hydrolysates. Food Sci Biotechnol 20:183–191. https://doi.org/10.1007/s10068-011-0025-6
Laurens LML, Wolfrum EJ (2011) Feasibility of spectroscopic characterization of algal lipids: chemometric correlation of NIR and FTIR spectra with exogenous lipids in algal biomass. Bioenergy Res 4:22–35. https://doi.org/10.1007/s12155-010-9098-y
Lee SY, Kim SH, Hyun SH, Suh HW, Hong SJ, Cho BK, Lee CG, Lee H, Choi HK (2014) Fatty acids and global metabolites profiling of Dunaliella tertiolecta by shifting culture conditions to nitrate deficiency and high light at different growth phases. Process Biochem 49:996–1004. https://doi.org/10.1016/j.procbio.2014.02.022
López A, Rico M, Santana-Casiano JM, González AG, González-Dávila M (2015) Phenolic profile of Dunaliella tertiolecta growing under high levels of copper and iron. Environ Sci Pollut Res 22:14820–14828. https://doi.org/10.1007/s11356-015-4717-y
Lowry OH, Rosebrough NJ, et al (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193:265–275.
Marwood CA, Solomon KR, Greenberg BM (2001) Chlorophyll fluorescence as a bioindicator of effects on growth in aquatic macrophytes from mixtures of polycyclic aromatic hydrocarbons. Environ Toxicol Chem 20:890–898. https://doi.org/10.1002/etc.5620200425
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang JW (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333. https://doi.org/10.1016/j.biortech.2013.12.077
Morales-Loo MR, Goutx M (1990) Effects of water-soluble fraction of the Mexican crude oil “Isthmus Cactus” on growth, cellular content of chlorophyll a, and lipid composition of planktonic microalgae. Mar Biol 104:503–509. https://doi.org/10.1007/BF01314357
Okumura Y, Koayama J, Uno S (2003) The relationship between logPow and molecular weight of polycyclic aromatic hydrocarbons and EC50 values of marine microalgae. La Mer 41:182–191
Orosa M, Franqueira D, Cid A, Abalde J (2005) Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour Technol 96:373–378. https://doi.org/10.1016/j.biortech.2004.04.006
Parab SR, Pandit RA, Kadam AN, Indap MM (2008) Effect of Bombay high crude oil and its water-soluble fraction on growth and metabolism of diatom Thalassiosira sp. Indian J Mar Sci 37:251–255
Pereira SA, Araújo VQ, Reboucas MV, Vieira FSV, de Almeida MVA, Chinalia FA, Nascimento IA (2012) Toxicity of biodiesel, diesel and biodiesel/diesel blends: comparative sub-lethal effects of water-soluble fractions to microalgae species. Bull Environ Contam Toxicol 88:234–238. https://doi.org/10.1007/s00128-011-0430-9
Pick U, Avidan O (2017) Triacylglycerol is produced from starch and polar lipids in the green alga Dunaliella tertiolecta. J Exp Bot 68:4939–4950. https://doi.org/10.1093/jxb/erx280
Poh ZL, Amalina Kadir WN, Lam MK, Uemura Y, Suparmaniam U, Lim JW, Show PL, Lee KT (2020) The effect of stress environment towards lipid accumulation in microalgae after harvesting. Renew Energy 154:1083–1091. https://doi.org/10.1016/j.renene.2020.03.081
Ramadass K, Megharaj M, Venkateswarlu K, Naidu R (2015) Toxicity and oxidative stress induced by used and unused motor oil on freshwater microalga, Pseudokirchneriella subcapitata. Environ Sci Pollut Res 22:8890–8901. https://doi.org/10.1007/s11356-014-3403-9
Romero-Lopez J, Lopez-Rodas V, Costas E (2012) Estimating the capability of microalgae to physiological acclimatization and genetic adaptation to petroleum and diesel oil contamination. Aquat Toxicol 124–125:227–237. https://doi.org/10.1016/j.aquatox.2012.08.001
Rukminasari N (2013) Effect of temperature and nutrient limitation on the growth and lipid content of three selected microalgae (Dunaliella tertiolecta, Nannochloropsis sp. and Scenedesmus sp.) for biodiesel production. Int J Mar Sci. https://doi.org/10.5376/ijms.2013.03.0017
Shishlyannikov SM, Nikonova AA, Klimenkov IV, Gorshkov AG (2017) Accumulation of petroleum hydrocarbons in intracellular lipid bodies of the freshwater diatom Synedra acus subsp. radians. Environ Sci Pollut Res 24:275–283. https://doi.org/10.1007/s11356-016-7782-y
Shiu R-F, Chiu M-H, Vazquez CI, Tsai YY, le A, Kagiri A, Xu C, Kamalanathan M, Bacosa HP, Doyle SM, Sylvan JB, Santschi PH, Quigg A, Chin WC (2020) Protein to carbohydrate (P/C) ratio changes in microbial extracellular polymeric substances induced by oil and Corexit. Mar Chem 223:103789. https://doi.org/10.1016/j.marchem.2020.103789
Siron R, Carles D, Rontani J-F, Morales R, Berland B, Giusti G (1986) Effects of water soluble petroleum compounds on the fatty acids composition of phytoplankton. In: Strategies and advanced techniques for marine pollution studies. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 463–464
Siron R, Giusti G, Berland B, Morales-Loo R, Pelletier E (1991) Water-soluble petroleum compounds: chemical aspects and effects on the growth of microalgae. Sci Total Environ 104:211–227. https://doi.org/10.1016/0048-9697(91)90073-N
Solovchenko AE (2012) Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ J Plant Physiol 59:167–176. https://doi.org/10.1134/s1021443712020161
Stuart B (2004) Infrared spectroscopy: fundamentals and applications. Wiley, Ltd
Takaichi S (2011) Carotenoids in algae: distributions, biosyntheses and functions. Mar Drugs 9:1101–1118. https://doi.org/10.3390/md9061101
Tammam A, Fakhry E, El-Sheekh M (2013) Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliella salina and Dunaliella tertiolecta. African J Biotechnol 10:3795–3808. https://doi.org/10.5897/AJB10.2392
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol Environ Saf 71:1–15. https://doi.org/10.1016/j.ecoenv.2008.05.009
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Bio Ecol 128:219–240. https://doi.org/10.1016/0022-0981(89)90029-4
Vonshak A, Maske H (1982) Algae: growth techniques and biomass production. Tech Bioprod Photosynth
Wang B, Jia J (2020) Photoprotection mechanisms of Nannochloropsis oceanica in response to light stress. Algal Res 46:101784. https://doi.org/10.1016/j.algal.2019.101784
Wang X, Zhang J, Shi X et al (2002) Determination of toxicokinetic parameters for bioconcentration of water-soluble fraction of petroleum hydrocarbon associated with No. 0 Diesel in Changjiang estuary and Jiaozhou Bay: model versus mesocosm experiments. Arch Environ Contam Toxicol 42:272–279. https://doi.org/10.1007/s00244-001-0025-2
Wolfe MF, Schlosser JA, Singaram S et al (1998) Influence of dispersants on the bioavailability and trophic transfer of petroleum hydrocarbons to primary levels of a marine food chain. Aquat Toxicol 42:211–227. https://doi.org/10.1016/s0166-445x(97)00096-9
Yilancioglu K, Cokol M, Pastirmaci I, Erman B, Cetiner S (2014) Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS One 9:e91957. https://doi.org/10.1371/journal.pone.0091957
Acknowledgments
We thank Dr. Juan Pablo Lazo and MS. Abelardo Campos for their technical support.
Funding
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT-México), Laboratorio de Microalgas, Departamento de Acuicultura, CICESE and Biociencias y Tecnologías, S. A. P. I. de C. V. (BIOCYT), México. The authors appreciate the support of the CONACYT 4940 and 295075 projects. M.S. Salinas-Whittaker is grateful for the Ph.D. studentship funding (number CVU 551659, CONACyT-México).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salinas-Whittaker, S., Gómez-Gutiérrez, C.M., Cordero-Esquivel, B. et al. Effects of the water-soluble fraction of the mixture fuel oil/diesel on the microalgae Dunaliella tertiolecta through growth. Environ Sci Pollut Res 27, 35148–35160 (2020). https://doi.org/10.1007/s11356-020-09796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09796-9