Abstract
The present study aims to investigate the impact of a metal-tolerant bacterium on metal detoxification and rape seedling growth promotion under Cd stress. The results showed that the isolated bacterium Enterobacter sp. Zm-123 has capability to resist Cd (200 mg/L), produce IAA (26.67 mg/L) and siderophores (82.34%), and solubilize phosphate (137.5 mg/L), etc. Zm-123 inoculation significantly enhanced the fresh weight of rape seedlings from 9.47 to 19.98% and the root length from 10.42 to 57.05% compared with non-inoculation group under different concentrations of Cd (0, 0.5, 1, 3, 5 mg/L) (p < 0.05). It also significantly increased the content of chlorophyll, soluble sugar, soluble protein, and proline (p < 0.05) in rape seedlings. Moreover, a significant elevation in catalase (CAT) and peroxidase (POD) activities and a significant reduction in malondialdehyde (MDA), electrolyte leakage (EL), and Cd content in rape seedlings were detected owing to Zm-123 inoculation (p < 0.05). The combined results imply that strain Zm-123 can alleviate the Cd phytotoxicity and promote the rape seedling growth by improving the physiological activity and antioxidant level, which can be potentially applied to protect plants from Cd toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along with the phosphate fertilizer application, sewage irrigation, and mining waste emission, a large amount of cadmium (Cd) has been introduced into soil, endangering food safety and human health (Shahid et al. 2016). Cd can be readily absorbed by the roots and transported to the shoots, leading to Cd accumulation in the edible parts of plants and forming a primary source of dietary Cd intake (Podazza et al. 2012). It has been reported that 72% of Cd in the daily diet of human comes from vegetables (Baldantoni et al. 2016). Cd may also deteriorate plant quality, such as slowing down the seed germination, inhibiting the root elongation, etiolating the leaves, and reducing the plant biomass (Tran and Popova 2013). These adverse impacts have been attributed to interference on plant growth and physiology as well as oxidative stress of excess Cd, represented by the reduced content of soluble sugar and protein, the unspecific oxidation of proteins and membrane lipids, extracellular electrolyte leakage, and proline accumulation in plants (Andresen and Küpper 2013; Scandalios 2005; Xu et al. 2012). Thus, it is urgent to take effective measures for alleviating the Cd uptake and eliminating Cd toxicity in vegetable plants.
Microbe-assisted phytostabilization has been considered as an efficient strategy in eliminating metal toxicity to plants. Plant growth–promoting bacteria (PGPB), such as Bacillus, Pseudomonas, Bradyrhizobium, Serratia, and Klebsiella, have been reported to enhance plant growth and reduce metal toxicity through providing indoleacetic acid (IAA), siderophores, phosphate solubilization activity, etc. (Pramanik et al. 2017; Yu et al. 2018; Pandey et al. 2013). Bacterial IAA interferes with the physiological process of plants by changing the plant auxin pool, enlarging root surface area and length, and subsequently increasing the nutrient uptake Patten and Glick (2002), besides, it can detoxify Cd via enhancing the antioxidant capability of plants (Sinha and Mukherjee 2008; Rizvi et al. 2019; Ahmad et al. 2016). The phosphate-solubilizing ability of PGPB can assist host plants to assimilate more phosphate (Yu and Zhou 2009), while microbial siderophores can help plants to sequester irons and complex toxic metals (Glick 2012). Therefore, it is reasonable to speculate that PGPB, especially those with metal resistance, may promote plant growth and alleviate Cd toxicity under Cd stress.
Enterobacter spp. have been known to promote plant growth under drought, salinity, and metal stress by providing IAA, siderophore, ACC deaminase, and phosphate solubilization activity in wheat, rice, and maize, whereas there is no relevant report in rape. China has the longest history of rape production in the world, accounting for one-third of the world’s rape planting area and total output (Liu et al. 2017; Yuan et al. 2020), while soil Cd pollution often causes Cd content in rapes exceeding the standard. Thus, the objectives of this study were (a) to evaluate the potential PGP characteristics and Cd tolerance ability of an isolated Enterobacter sp. and (b) to investigate the effects of these PGP characteristics on rape seedlings growth, physiological parameters, antioxidant capacity, and Cd uptake in edible parts under Cd stress using hydroponic culture system.
Materials and methods
Reagents
Cadmium chloride hemipentahydrate, copper sulfate pentahydrate, zinc nitrate hexahydrate, lead chloride, potassium dichromate, and other chemicals in analytical grade were purchased from Kermel Chemical Reagent Co., Ltd. (Tianjin, China).
Isolation and identification of Cd-tolerant bacteria
The rhizospheric soil for Cd-resistant bacteria isolation was taken from a mining area in the northeastern region of China. Ten grams of soil was transferred into a conical flask with 90-mL sterile distilled water and shaken in a rotator at 160 rpm and 28 °C for 30 min, then the obtained soil suspension was serially diluted (10−3–10−5) and an aliquot of 0.1 mL diluent was spread on Luria-Bertani agar medium supplied with 50 mg/L of Cd, and incubated at 28 °C for 3 days. The colonies on the medium plates were picked up and transferred several times to gain pure isolate. The physiological-biochemical characteristics of the isolate were determined according to “Bergey’s Manual of Systematic Bacteriology.” Besides, 16S rRNA gene was amplified using universal primer set f27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and r1492 (5′-TACGGTTACCTTGTTACGACTT-3′) (Yu et al. 2018). The PCR products were sequenced by Meiji Biotech (Shanghai, China), and the acquired sequences were compared with those homologous sequences in GenBank. Phylogenetic tree of the isolate was obtained using neighbor-joining method of software packages Mega version 6.0 at a bootstrap value of n = 1000. To visualize the Cd cytotoxic damage on bacterial morphology, bacterium and metal-treated bacterium were subjected to TEM (model JSM 6510 LV; JEOL, Japan) analysis.
Heavy metal tolerance
Minimal inhibitory concentration (MIC) is the lowest metal concentration that can completely prevent bacterial growth and the maximum tolerant concentration (MTC) is the highest concentration of metal ion beyond which no visible bacteria growth occurred (Piotrowska-Seget et al. 2005). In this study, the MICs and MTCs of Cd(II), Pb(II), Zn(II), Cu(II), and Cr(VII) for the isolate were tested at a series of concentrations (100, 200, 300, 400, 600, 800, 1000 mg/L) on Luria-Bertani agar medium incubated at 30 °C for 3 days (Pramanik et al. 2016; Kumar et al. 2017).
Determination of growth curve
Growth curve of the isolate was determined with 100 mL of bacterial cultures (1 × 108 cells/mL in Luria-Bertani medium) supplemented with varying concentrations of Cd (0, 10, 20, 30, 40 and 50 mg/L) in 250-mL conical flasks. Flasks were kept in shaking condition at 30 °C for 28 h, and the optical density value was recorded at 600 nm every 2 h (Andrews 2001).
Selected PGP traits of the isolate
Indole acetic acid–producing ability
Production of indole acetic acid (IAA) by the isolate was quantified by spectrophotometric method as described by Clickmann et al. (Glickmann and Dessaux 1995). Briefly, 2 mL of the isolate suspension (108 cells/mL) was inoculated into 98 mL of Luria-Bertani medium with l-tryptophan (100 mg/L) and varying concentrations of Cd (0, 10, 20, 30, 40, and 50 mg/L). After 3 days, the amount of IAA in the filtrate was measured with a UV-Visible spectrophotometer (T6, Persee, China) at 540 nm and expressed as micrograms per milliliter of culture filtrate. Standard curve was plotted with chemical grade indole-3-acetic acid (Beijing Solarbio Science & Technology Co., Ltd., China).
Phosphate solubilization ability
Phosphate solubilization ability of the isolate was evaluated by cultivating the bacteria on agar-solidified Pikovskaya (PKO) medium at 30 °C for 5 days and measuring the diameter of the clear zone around the colony. Phosphate solubilization ability was also quantified after 2 mL of the isolate (1 × 108 cells/mL) was incubated in 98 mL PKO liquid medium containing varying concentrations of Cd (0, 10, 20, 30, 40, and 50 mg/L) at 180 rpm and 30 °C for 5 days (Chatterjee et al. 2012). The amount of soluble phosphate was measured colorimetrically after centrifugation of liquid cultures at 8000 rpm for 10 min (Ullah et al. 2013).
Siderophore-producing ability
Siderophore-producing ability of the isolate was detected using Chrome Azurol Sulphonate (CAS) medium at Cd concentrations of 0, 10, 20, 30, 40, and 50 mg/L referring to the method of Schwyn and Neilands (1987). Siderophore-producing ability was also quantified after 2 mL of the isolate (1 × 108 cells/mL) in 98 mL of LB liquid medium was incubated at 30 °C for 72 h. The supernatant was collected by centrifugation at 6000 rpm for 10 min, and then mixed with CAS solution at a ratio of 1:1. The siderophore content was calculated using the following formula: \( \mathrm{SU}\%=\frac{\mathrm{Ar}-\mathrm{As}}{\mathrm{Ar}}\kern0.5em \times \kern0.5em 100\% \), where Ar is the absorbance of the non-bacterial group as reference and As is the absorbance of the sample at 630 nm.
Germination experiment
Seeds of rape (purchased from North Seed Industry Co., Ltd., China) were first rinsed with 75% (v/v) ethanol and sodium hypochlorite (3% v/v) for 3 min and then rinsed with sterile distilled water for three times. Twenty aseptic seeds were distributed at equal distances from each other on sterile filter papers in a sterilized Petri dish. A total of 10 mL of CdCl2 solution (0, 10, 20, 30, 40, 50 mg/L) with 2 mL of bacterial suspension (1 × 108 cells/mL) was injected to per dish. The germination rate was calculated after 7 days’ incubation at 28 °C according to formula (Vijayaragavan et al. 2011): \( \mathrm{Germination}\ \mathrm{rate}\ \left(\%\right)=\frac{\mathrm{The}\ \mathrm{number}\ \mathrm{of}\ \mathrm{seeds}\ \mathrm{germinated}}{\mathrm{The}\ \mathrm{total}\ \mathrm{number}\ \mathrm{of}\ \mathrm{seeds}\ \mathrm{tested}.}\times 100\% \).
Hydroponic experiment
The hydroponic nutrient solution was filter-sterilized with a 0.45-μm membrane filter (Sartorius Stedim Biotech, Germany) before use. A 4 weeks’ hydroponic experiment was conducted to determine the effect of isolate on plant growth in a greenhouse (natural illumination, temperature between 24 and 30 °C, and the humidity remained at 65% ± 10%). After germination, the rape seedlings with the same growth rate were transplanted into a 1-L beaker containing Hoagland medium at Cd concentrations of 0, 0.5, 1, 3, and 5 mg/L. During the experiment, the Hoagland medium was replaced every 7 days accompanied with the addition of bacterial suspension (2 mL, 1 × 108 cells/mL) (Zhou et al. 2017; El-Nahrawy et al. 2019), and in the control group, bacterial suspension was replaced by an equivalent volume of sterile water. After 4 weeks of cultivation, the seedlings were harvested for physiochemical analysis and Cd content determination.
Measurement of physiological indexes of rape seedlings
Fresh weight and root length were measured at the end of the hydroponic experiment. The chlorophyll in the seedlings was extracted by ethanol and its total content was measured according to the method of Xu et al. (2018). MDA content was assayed based on the TBA-based colorimetric method described by Demiral and Ismail (2005). Electrolyte leakage (EL) was measured according to the method reported by Wang et al. (2008). Ten rinsed leaf discs were submerged in 10 mL of distilled water and then vacuumed for 10 min. The initial electrical conductivity was recorded as the EC1. The leaf tissues were heated at 100 °C for 20 min and cooled to room temperature for measuring the final electrical conductance (EC2). The percent EL was recorded using the following formula: \( \mathrm{EL}\;\left(\%\right)=\frac{\mathrm{EC}1}{\mathrm{EC}2}\kern0.5em \times \kern0.5em 100\% \). The catalase (CAT) activity was determined as described by Aebi (1984). The guaiacol peroxidase (POD) activity was determined as described by Khan et al. (2014). Proline content was determined according to the protocol of Bates et al. (1973). The extraction was done by sulfosalicylic acid and the absorbance values were recorded at 520 nm. The soluble protein and sugar content of the seedlings were measured following the method of Bradford (1976) and Jin et al. (2013), respectively. Cd content in plants was measured after 4 weeks according to the method of Fang et al. (2016). Briefly, edible parts were separated, weighed, and dried at 80 °C in an oven overnight. 0.1 g of dried samples was subjected to hotblock (180 °C) wet digestion by HNO3 and HCl (v/v, 3/1). After digestion, the samples were cooled down and ultrapure water was added to get a final volume of 10.0 mL, then the Cd content in rape seedlings was measured by an atomic absorption spectrophotometer (AAS) (AA-6800, Shimadzu-GL, Japan).
Statistical analysis
All experiments were conducted in triplicate. Data were analyzed using SPSS 19.0 (SPSS Inc., USA) and expressed as means with their standard derivation (SD). The significant differences among the tested treatments were compared with Tukey’s multiple comparisons test with p < 0.05 as a significant level.
Results
Identification of Zm-123 isolate
The isolated strain, designated as Zm-123, is an aerobic, motile, rod-shaped, flagellum-forming gram-negative bacterium (Fig. 1(a)). The morphology of Zm-123 treated with 30 mg/L Cd showed shrunken cell and distorted surface possibly with Cd depositions (Fig. 1(b)). The colony of Zm-123 on a solid medium plate is milky white, spherical, shiny, smooth, and raised (Fig. 1(c)). Physiology and biochemistry characteristics of the strain are shown in Fig. 1(d). The phylogenetic analysis of 16S rRNA gene sequence indicated that Zm-123 (GenBank accession number MN493849.1) belongs to the genus Enterobacter (Fig. 1(e)).
Heavy metal resistant ability
Lead, cadmium, zinc, chromium, and copper are the main heavy metals caused soil and water pollution in China. In this paper, the MICs of Pb(II), Cd(II), Zn(II), Cr(VII), and Cu(II) on Zm-123 were 600, 200, 200, 400, and 100 mg/L, respectively. The maximum tolerant concentrations of Pb(II), Cd(II), Zn(II), Cr(VII),and Cu(II) on Zm-123 were 1000, 600, 400, 800, and 300 mg/L (Table S1) indicating that Zm-123 has a high tolerance to multiple heavy metals.
Growth characteristics
From the growth curves shown in Fig. 2, it was noticed that Zm-123 experienced a rapid growth within 20 h and a slow growth from 20 to 30 h in the absence and presence of Cd. The maximum viable counts of bacteria decreased from 4.23 × 109 to 1.46 × 109 cfu/mL (the OD600 ranged from 1.94 to 0.98), when Cd concentration increased from 0 to 50 mg/L. The threshold of Cd2+ concentration was 20 mg/L, below which the growth of Zm-123 was similar to that of in the absence of Cd and above which significant growth inhibition was observed. Although the resistant strain had tolerance to heavy metals, higher concentrations of Cd inhibited the metabolism of bacterium and slowed down the propagation of Zm-123.
Growth-promoting activity
Low concentration of Cd could stimulate IAA production as seen in Fig. 3(a); the maximum IAA of 28.30 mg/L was detected at 5 mg/L of Cd, which was higher than that no Cd addition (26.67 mg/L). As the concentration exceeded 5 mg/L, the IAA production was constantly decreased to 25.4–20.1 mg/L. Zm-123 developed a clear halo zone around its colony after 72 h’s incubation on PKO solid medium (Fig. S1a). As shown in Fig. S1b, the appearance of an orange halo around the colony on CAS agar plate indicated that Zm-123 produced siderophores. To further determine how Cd influences the selected important PGP traits, a quantitative test was conducted.
As shown in Fig. 3(b), phosphate solubilization was negatively affected by Cd concentration (Fig. 3(b)) and maximum phosphate of 137.50 mg/L was produced by strain Zm-123 in no Cd medium. Siderophore production of Zm-123 was decreased in a similar manner as seen in Fig. 3(c) and the maximum siderophore production (82.34% SU) was obtained in the absence of Cd. With the increment of Cd concentration, the siderophore production decreased significantly (p < 0.05), and it declined to a minimum of 10.78% SU at Cd concentration of 25 mg/L. The above results indicate that Zm-123 has plant growth–promoting ability.
Germination experiment
The effect of Zm-123 on the seed germination under Cd stress is shown in Table 1. Zm-123 treatment significantly improved the germination rate of rape seeds (p < 0.05) by 1.57% compared with that of the control group (93.66%). When the concentration of Cd was 10 mg/L, the germination rate of the Zm-123 treatment group was 2.01% higher than that of the non-treated group. As the concentration of Cd continued to increase, the germination rate decreased from 85.64 to 63.67% in the control group, while decreased from 89.42 to 78.78% in the Zm-123 group.
Hydroponics experiment
Measurement of growth and photosynthesis characteristics
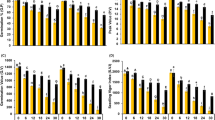
Previous research showed that Cd might cause the leaf chlorosis and the chlorophyll damage, which resulted in growth retardation (Najeeb et al. 2011). In our hydroponics experiment, the presence of Cd in the nutrient solution caused decrement in fresh weight compared with that in the absence of Cd (Fig. 4(a)). However, the Zm-123-inoculated group increased the fresh weight by 12.18%,17.10%, 9.47%, 18.40%, and 19.98% and facilitated the root elongation by 37.5%, 10.83%, 18.45%, 15.48%, and 57.12% compared with the non-inoculated group at Cd concentrations of 0, 0.5, 1, 3, and 5 mg/L, respectively (Fig. 4(a, b)). Apparently, Cd played a dual role in enhancing or suppressing chlorophyll content; low level of Cd (0.5 mg/L) increased the total chlorophyll contents of seedlings, while high levels of Cd (1, 3 and 5 mg/L) decreased total chlorophyll contents. Zm-123 inoculation significantly (p < 0.05) increased total chlorophyll contents by 18.56%, 7.6%, 18.03%, and 96.67% at Cd concentrations of 0.5, 1, 3, and 5 mg/L, respectively (Fig. 4(c)).
MDA and EL levels in rape seedlings
Oxidative stress caused by Cd has been recognized as a key factor that inhibits plant growth (Andresen and Küpper 2013; Farooq et al. 2013). In the present study, it was found that the levels of MDA in rape seedlings were elevated by Cd. There was no significant difference in MDA levels between the Zm-123-inoculated and Zm-123-non-inoculated group without Cd stress, whereas inoculation triggered a significant reduction (p < 0.05) compared with non-inoculation under Cd stress. In detail, Zm-123 decreased MDA level by 21.75%, 28.61%, 13.68%, and 11.80% at Cd concentrations of 0.5, 1, 3, and 5 mg/L compared with that in the corresponding non-inoculated group, respectively (Fig. 5(a)). Cd toxicity further led to a substantial increase in the EL level of rape seedlings (Fig. 5(b)). These results suggested that Cd contamination triggered strong oxidative stress, while Zm-123 application significantly (p < 0.05) lowered EL in rape seedlings in the presence of 0.5, 1, 3, and 5 mg/L Cd than that without the Zm-123 group.
Antioxidant enzyme activities (CAT and POD)
Antioxidative enzymes such as CAT and POD are important to Cd damage control in plants (Xu et al. 2012). The variation of CAT activity and POD activity with the increasing concentration of Cd is depicted in Fig. 5(c, d). Under Cd stress (0.5 mg/L and 1 mg/L of Cd), CAT activity and POD activity were significantly (p < 0.05) higher than that in unstressed. As Cd concentration increased from 0.5 to 5 mg/L, considerable decreases in the activities of CAT and POD were observed. The CAT activity in plants treated with Zm-123 was observed to be 10.53%, 12.12%, 22.34%, and 12.04% higher, respectively, at the 0.5, 1, 3, and 5 mg/L of Cd than that in the non-inoculated group. Similarly, POD activities in the Zm-123-inoculated group were higher than those in the non-inoculated group (Fig. 5(d)). Especially, a peak increase of 8.44% was obtained at 1 mg/L of Cd compared with that in non-inoculated.
Determination of proline, soluble sugar, and soluble protein contents in rape seedlings under Cd stress
Compared with the Cd-free group, proline contents were significantly elevated under Cd stress (p < 0.05). Inoculation of Zm-123 decreased proline contents compared with the non-inoculated groups under Cd stress. In detail, as Cd concentration increased from 0.5 to 5 mg/L, the proline content went up from 53.75 to 99.56 mg/g FW in non-inoculated and from 42.75 to 83.00 mg/g FW in the inoculated group, respectively. Especially, a great decrease (34.35%) was observed at 1 mg/L of Cd in contrast to the non-inoculated group. Soluble sugar, as energy and metabolic intermediates for plant growth and development and nutritional value for human beings, is greatly influenced by Cd (John et al. 2008). Without Zm-123 inoculation, the content of soluble sugar was enhanced from 3.01 to 10.63 mg/g FW as Cd concentration consistently increased from 0 to 1 mg/L, while decreased to 7.78 mg/g FW at Cd concentration of 5 mg/L. The addition of Zm-123 increased the content of soluble sugar by 40.00%, 19.57%, 11.21%, and 11.95% under the 0.5-, 1-, 3-, and 5-mg/L concentrations of Cd compared with the non-inoculated group, respectively (Fig. 6(b)). Considerable decreases in soluble protein contents were observed in the presence of Cd whereas Zm-123 inoculation significantly increased soluble protein contents in rape seedlings under Cd stress, and the maximum increment was obtained at Cd concentration of 1 mg/L. (Fig. 6(c)).
Accumulation of Cd in rape seedlings
Apparently, Cd concentrations in edible parts of rape seedlings inoculated with Zm-123 were significantly lower (p < 0.05) as compared with those in non-inoculated (Fig. 6(d)). In addition, under different concentrations of Cd, the Cd contents ranged from 0.25 to 0.85 mg/g in the non-inoculated group and from 0.16 to 0.75 mg/g in the inoculated group. Inoculation decreased Cd contents by 35.87%, 19.38%, 18.57%, and 11.13% compared with those in the non-inoculated group at Cd concentrations of 0.5, 1, 3, and 5 mg/L, respectively. Particularly, Zm-123 inoculation had the best effect on Cd reduction at 0.5 mg/L of Cd.
Discussion
Several Enterobacter spp. have been reported to possess high Cd resistance and PGP properties that can be potentially used in microbe-assisted phytostabilization (Abd El-Azeem et al. 2012; Pramanik et al. 2018; Madhaiyan et al. 2010). In the present research, a novel Enterobacter sp. Zm-123 with multiple metal resistance and PGP traits including IAA production, siderophore production, and phosphate solubilization activity was isolated. Our study clearly indicated that strain Zm-123 tolerated the maximum limit of Cd2+ ions up to 600 mg/L, which was much higher than previously isolated Enterobacter sp. MH8b (225 mg/L) (Płociniczak et al. 2013) and Enterobacter sp. CIK-521 (325 mg/L) (Ahmad et al. 2016). Moreover, it possesses much higher capability in IAA production, siderophore production, and phosphate solubilization activity than other Enterobacter spp. (Liu et al. 2018; Singh et al. 2018). Many studies indicated that the actual PGP effects might be quite different in the interactions between different plants, bacteria, and metals, which would greatly influence the final remediation efficiency of microbe-associated phytostabilization. Therefore, this study devoted to investigating the actual effect and potential mechanism of Zm-123 on the growth of rape seedlings under Cd stress.
Effects of IAA production on physiological characteristics and antioxidant capacity of rape seedlings
Cd has an adverse impact on plant growth by interfering with the physiological process and decreasing the antioxidant capacity (Dell’Amico et al. 2008). Indole-3-acetic acid (IAA) is the most abundant and naturally occurring auxin, well known for its regulatory function in plant growth and stress resistance (Fässler et al. 2010). Agami and Mohamed (2013) reported that exogenous IAA has significantly increased root and shoot length, photosynthetic pigment content, many physiological parameters, and antioxidant enzyme activities in wheat (Triticum aestivum L.). Exogenous IAA also greatly increases the contents of soluble sugar and protein in plants under Cd stress (Zhang et al. 2020) and protects plants from Cd-induced oxidative damage by rapidly degrading MDA and proline accumulated in various parts of plants (Ran et al. 2020). In the present study, Zm-123 was also found to enhance root elongation regardless of the presence of Cd and increased the chlorophyll content by 96.67% at Cd concentration of 5 mg/L. In addition, application of Zm-123 increased soluble sugar and soluble protein in rape seedlings by 40.00% and 7.00% and by 11.96% and 10.73% at 0.5 mg/L and 5 mg/L Cd, respectively. Furthermore, Zm-123 significantly decreased MDA by 28.66% and proline by 25.57%, and increased CAT and POD in leaves by 12.12% and 8.44% at 1 mg/L Cd compared with that in non-inoculated treatment, respectively. In agreement with our results, a similar reduction rate in MDA and proline content was found in the Brassica juncea seedlings inoculated by Enterobacter sp. IU02 exposed to Cd (Ullaha et al. 2019), while a promotion in CAT and POD activity was found in the research of rice seedlings inoculated with Enterobacter sp. K6 under Cd stress (Pramanik et al. 2018). Combined with these results, it can be deduced that Zm-123 inoculation can significantly improve the physiological characteristics and antioxidant capacity of rape seedlings under Cd stress.
Effects of phosphate solubilization on plant growth, antioxidant capacity, and Cd uptake in rape seedlings
Phosphorus (P) as an essential macro-element is involved not only in plant growth and cell function (Dai et al. 2017) but also in the absorption and transportation of Cd (Dai et al. 2018). High concentrations of heavy metals in the rhizosphere inhibit the transportation of phosphate and other essential nutrients, while plant-associated bacteria can replenish these nutrients through solubilizing phosphate and reducing Cd uptake in the root (Jia et al. 2020). Previous reports showed that inoculation of the phosphate-solubilizing Serratia sp. significantly enhanced plant growth parameters including chlorophyll contents, root and shoot lengths, and biomass (Yu and Zhou 2009). Zm-123 can solubilize 13.58 to 137.5 mg/L of phosphate at different concentrations of Cd, which may help rape seedlings to acquire more P nutrients and increase the fresh weight, root length, and chlorophyll contents subsequently. P supply also can promote the antioxidant system and reduce lipid peroxidation in plants under Cd stress by increasing the related gene expression (Wang et al. 2015). In the present study, MDA content and antioxidant enzyme activities were greatly reduced by the inoculation of Zm-123, which might be attributed to phosphate solubilized by Zm-123. In addition, the previous experiment also demonstrated that P supplementation increased Cd accumulation in the roots in mangrove seedlings subjected to Cd stress, indicating that much of Cd was fixed in the root, rather than transported to shoot (Dai et al. 2017). In the present study, Cd contents in edible parts of rape seedlings also decreased after application of Zm-123 compared with those in non-inoculated treatment.
Effects of siderophore production on plant growth and Cd content in rape seedlings
Cd can impede plants to assimilate essential elements, such as Mn and Fe, which inhibit chlorophyll synthesis, disturb the chloroplast ultrastructure, or cause chlorophyll degradation (Sinha and Mukherjee 2008). Siderophores as iron-chelating agents are generally produced by microorganisms under iron-limiting conditions or metal stress (Braud et al. 2009). PGPB with siderophores producing capability can supply unavailable form of Fe3+ to the plant and accelerate its mobility in the rhizosphere (Rajkumar et al. 2012). Inoculation of Zm-123 increased the chlorophyll contents in rape seedlings under Cd stress. Similar to our study, Dimkpa et al. (2009) reported that there was an increase in chlorophyll content after application of the siderophore-producing bacterium. Moreover, it has been reported that microbial siderophores have potential in metal uptake and mobilization or metal resistance development in the presence of toxic metals (Gaonkar and Bhosle 2013). Kumari et al. (2019) have found that siderophores from Aspergillus nidulans significantly recovered the plant growth and declined As accumulation in wheat by elevating the SOD, CAT, and POD activities under As stress. Siderophores originated from PGPB might also increase Fe acquisition and decrease Cd uptake in plants by inhibiting the expression of iron-regulated transporter (IRT) (Fan et al. 2014; He et al. 2017; Luo et al. 2012). Upon Zm-123 treatment, Cd accumulation in edible parts of rape seedlings was decreased considerably, supporting our view that siderophore could bind with the metal ions and interfere with their entry into the plants.
Conclusion
In conclusion, the isolated strain Enterobacter sp. Zm-123 is a Cd-resistant bacterium with PGR characteristics including IAA and siderophore production and phosphate solubilization ability. Inoculation of Zm-123 can improve root elongation and physiological status, alleviate oxidative stress, and reduce Cd uptake in rape seedlings under Cd. The newly isolated Enterobacter sp. Zm-123 may be a suitable candidate for microbe-assisted phytostabilization due to its high Cd resistance and PGP properties.
References
Abd El-Azeem SAM, Elwan MW, Sung JK, Ok YS (2012) Alleviation of salt stress in eggplant (Solanum melongena L.) by plant-growth-promoting rhizobacteria. Commun Soil Sci Plan 43(9):1303–1315. https://doi.org/10.1080/00103624.2012.666305
Aebi H (1984) Catalase in vitro. In Methods in enzymology 105:121-126 academic press. https://doi.org/10.1016/S0076-6879(84)05016-3
Agami RA, Mohamed GF (2013) Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol Environ Saf 94:164–171. https://doi.org/10.1016/j.ecoenv.2013.04.013
Ahmad I, Akhtar MJ, Asghar HN, Ghafoor U, Shahid M (2016) Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J Plant Growth Regul 35(2):303–315. https://doi.org/10.1007/s00344-015-9534-5
Andresen E, Küpper H (2013) Cadmium toxicity in plants. Met Ions Life Sci 11:395–413. https://doi.org/10.1007/978-94-007-5179-8_13
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48(suppl_1):5–16. https://doi.org/10.1093/jac/dkf083
Baldantoni D, Morra L, Zaccardelli M, Alfani A (2016) Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol Environ Saf 123:89–94. https://doi.org/10.1016/j.ecoenv.2015.05.019
Bates LS, Waldren RP, Teare ID, 1973. Rapid determination of free proline for water-stress studies. Plant soil, 39(1), 205-207. https://doi.org/10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Braud A, Jez’equel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74:280–286. https://doi.org/10.1016/j.chemosphere.2008.09.013
Chatterjee S, Mukherjee A, Sarkar A, Roy P (2012) Bioremediation of lead by lead-resistant microorganisms, isolated from industrial sample. Adv Biosci Biotechnol 3(03):290–295. https://doi.org/10.4236/abb.2012.33041
Dai M, Lu H, Liu W, Jia H, Hong H, Liu J, Yan C (2017) Phosphorus mediation of cadmium stress in two mangrove seedlings Avicennia marina and Kandelia obovata differing in cadmium accumulation. Ecotoxicol Environ Saf 139:272–279. https://doi.org/10.1016/j.ecoenv.2017.01.017
Dai M, Liu W, Hong H, Lu H, Liu J, Jia H, Yan C (2018) Exogenous phosphorus enhances cadmium tolerance by affecting cell wall polysaccharides in two mangrove seedlings Avicennia marina (Forsk.) Vierh and Kandelia obovata (S., L.) Yong differing in cadmium accumulation. Mar Pollut Bull 126:86–92. https://doi.org/10.1016/j.marpolbul.2017.10.083
Dell’Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem 40(1):74–84. https://doi.org/10.1016/j.soilbio.2007.06.024
Demiral T, Ismail T (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53(3):247–257. https://doi.org/10.1016/j.envexpbot.2004.03.017
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009) Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41(1):154–162. https://doi.org/10.1016/j.soilbio.2008.10.010
El-Nahrawy S, Elhawat N, Alshaal T (2019) Biochemical traits of Bacillus subtilis MF497446: its implications on the development of cowpea under cadmium stress and ensuring food safety. Ecotox Environ Safe 180:384–395. https://doi.org/10.1016/j.ecoenv.2019.04.088
Fan SK, Fang XZ, Guan MY, Ye YQ, Lin XY, Du ST, Jin CW (2014) Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front Plant Sci 5:721. https://doi.org/10.3389/fpls.2014.00721
Fang XZ, Tian WH, Liu XX, Lin XY, Jin CW, Zheng SJ (2016) Alleviation of proton toxicity by nitrate uptake specifically depends on nitrate transporter 1.1 in Arabidopsis. New Phytol 211(1):149–158. https://doi.org/10.1111/nph.13892
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249. https://doi.org/10.1016/j.ecoenv.2013.07.006
Fässler E, Evangelou MW, Robinson BH, Schulin R (2010) Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 80:901–907. https://doi.org/10.1016/j.chemosphere.2010.04.077
Gaonkar T, Bhosle S (2013) Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils. Chemosphere 93(9):1835–1843. https://doi.org/10.1016/j.chemosphere.2013.06.036
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:1–15. https://doi.org/10.6064/2012/963401
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61(2):793–796. https://doi.org/10.0000/PMID16534942
He XL, Fan SK, Zhu J, Guan MY, Liu XX, Zhang YS, Jin CW (2017) Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 416(1–2):453–462. https://doi.org/10.1007/s11104-017-3232-y
Jia H, Hou DY, O’Connor D, Pan SZ, Zhu J, Bolan NS, Mulder J (2020) Exogenous phosphorus treatment facilitates chelation-mediated cadmiumdetoxification in perennial ryegrass (Lolium perenne L.). J Hazard Mater 389:121849. https://doi.org/10.1016/j.jhazmat.2019.121849
Jin CW, Liu Y, Mao QQ, Wang Q, Du ST (2013) Mild Fe-deficiency improves biomass production and quality of hydroponic-cultivated spinach plants (Spinacia oleracea L.). Food Chem 138(4):2188–2194. https://doi.org/10.1016/j.foodchem.2012.12.025
John R, Ahmad P, Gadgil K, Sharma S (2008) Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil Environ 54:262–270. https://doi.org/10.17221/2787-PSE
Khan AR, Ullah I, Khan AL, Hong SJ, Waqas M, Park GS, Kwak Y, Choi J, Jung BK, Park M, Shin JH (2014) Phytostabilization and physicochemical responses of Korean ecotype Solanum nigrum L. to cadmium contamination. Water Air Soil Pollut 225:2147. https://doi.org/10.1007/s11270-014-2147-y
Kumar S, Tripathi VR, Vikram S, Kumar B, Garg SK (2017) Characterization of mar and heavy metal-tolerant E. coli o157:h7 in water sources: a suggestion for behavioral intervention. Environ Dev Sustain 20(5):1–15. https://doi.org/10.1007/s10668-017-9998-5
Kumari S, Khan A, Singh P, Dwivedi SK, Ojha KK, Srivastava A (2019) Mitigation of As toxicity in wheat by exogenous application of hydroxamate siderophore of Aspergillus origin 41(7):107 https://doi.org/10.1007/s11738-019-2902-1
Liu C, Huang J, Leng BF, Feng ZC, Li JP (2017) Current situation,development difficulties and suggestions of Chinese rape industry. Journal of China Agricultural University 12:203–210. (in Chinese). https://doi.org/10.11841/j.issn.1007-4333.2017.12.27
Liu JL, Tang L, Gao H, Zhang M, Guo CH (2018) Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. Journal of the Science of Food & Agriculture 99(1):281–289. https://doi.org/10.1002/jsfa.9185
Luo BF, Du ST, Lu KX, Liu WJ, Lin XY, Jin CW (2012) Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J Exp Bot 63(8):3127–3136. https://doi.org/10.1093/jxb/ers036
Madhaiyan M, Poonguzhali S, Lee J, Saravanan V, Lee K, Santhanakrishnan P (2010) Enterobacter arachidis sp. nov., a plant growth-promoting diazotrophic bacterium isolated from rhizosphere soil of groundnut. Int J Syst Evol Microbiol 60:1559–1564. https://doi.org/10.1099/ijs.0.013664-0
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574. https://doi.org/10.1016/j.jhazmat.2010.11.037
Pandey S, Ghosh PK, Ghosh S, De TK, Maiti TK (2013) Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J Microbiol 51:11–17. https://doi.org/10.1007/s12275-013-2330-7
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68(8):3795–3801. https://doi.org/10.1128/AEM.68.8.3795-3801.2002
Piotrowska-Seget Z, Cycon M, Kozdroj J (2005) Metal tolerant bacteria occurring in heavily polluted soil and mine spoil. Appl Soil Ecol 28:237–246. https://doi.org/10.1016/j.apsoil.2004.08.001
Płociniczak T, Sinkkonen A, Romantschuk M, Piotrowska-Seget Z (2013) Characterization of Enterobacter intermedius MH8b and its use for the enhancement of heavy metals uptake by Sinapis alba L. Appl Soil Ecol 63:1–7. https://doi.org/10.1016/j.apsoil.2012.09.009
Podazza G, Arias M, Prado FE (2012) Cadmium accumulation and strategies to avoid its toxicity in roots of the citrus rootstock Citrumelo. J Hazard Mater 215–216:83–89. https://doi.org/10.1016/j.jhazmat.2012.02.031
Pramanik K, Ghosh PK, Ghosh A, Sarkar A, Maiti TK (2016) Characterization of PGP traits of a hexavalent chromium resistant Raoultella sp. isolated from the rice field near industrial sewage of Burdwan District, WB, India. Soil. Sediment. Contam 25(3):313–331. https://doi.org/10.1080/15320383.2016.1137861
Pramanik K, Mitra S, Sarkar A, Soren T, Maiti TK (2017) Characterization of cadmium-resistant klebsiella pneumoniae mcc 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ Sci Pollut Res 24(31):24419–24437. https://doi.org/10.1007/s11356-017-0033-z
Pramanik K, Mitra S, Sarkar A, Maiti TK (2018) Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J Hazard Mater 351:317–329. https://doi.org/10.1016/j.jhazmat.2018.03.009
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574. https://doi.org/10.1016/j.biotechadv.2012.04.011
Ran JK, Zheng W, Wang HB, Wang HJ, Li QC (2020) Indole-3-acetic acid promotes cadmium (Cd) accumulation in a Cd hyperaccumulator and a non-hyperaccumulator by different physiological 191:110213 https://doi.org/10.1016/j.ecoenv.2020.110213
Rizvi A, Ahmed B, Zaidi A, Khan MS (2019) Heavy metal mediated phytotoxic impact on winter wheat: oxidative stress and microbial management of toxicity by Bacillus subtilis BM2. RSC advances 9. https://doi.org/10.1039/c9ra00333a
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 387:995–1014. https://doi.org/10.1590/S0100-879X2005000700003
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PMC (2016) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241. https://doi.org/10.1007/398_2016_8
Singh RP, Mishra S, Jha P, Raghuvanshi S, Jha PN (2018) Effect of inoculation of zinc-resistant bacterium, enterobacter ludwigii, cdp-14 on growth, biochemical parameters and zinc uptake in wheat ( triticum aestivum, l.) plant. Ecol Eng 116:163–173. https://doi.org/10.1016/j.ecoleng.2017.12.033
Sinha S, Mukherjee SK (2008) Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56(1):55–60. https://doi.org/10.1007/s00284-007-9038-z
Tran TA, Popova LP (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk J Bot 371:1–13. https://doi.org/10.3906/bot-1112-16
Ullah I, Khan AR, Park GS, Lim JH, Waqas M, Lee IJ, Shin JH (2013) Analysisof phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci Biotechnol 22:25–31. https://doi.org/10.1007/s10068-013-0044-6
Ullaha I, Al-Johnya BO, Al-Ghamdia KMS, Al-Zahranib HAA, Anwara Y, Firoza A, Al-Kenania N, Almatry MAA (2019) Endophytic bacteria isolated from Solanum nigrum L., alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesisby sodA gene. Ecotoxicol Environ Saf 174:197–207. https://doi.org/10.1016/j.ecoenv.2019.02.074
Vijayaragavan M, Prabhahar C, Sureshkumar J, Natarajan A, Vijayarengan P, Sharavanan S (2011) Toxic effect of cadmium on seed germination, growth and biochemical contents of cowpea (Vigna unguiculata L.) plants. International multidisciplinary research journal 1(5)
Wang Z, Zhang Y, Huang Z, Huang L (2008) Antioxidative response of metal-accumulator and non-accumulator plants under cadmium stress. Plant Soil 310(1–2):137–149. https://doi.org/10.1007/s11104-008-9641-1
Wang H, Wang T, Ahmad I (2015) Involvement of phosphate supplies in different transcriptional regulation pathway of Oryza sativa L.’s antioxidative system in response to arsenite and cadmium stress. Ecotoxicology 24:1259–1268. https://doi.org/10.1007/s10646-015-1496-7
Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, Liu X (2012) Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol 196:125–138. https://doi.org/10.1111/j.1469-8137.2012.04236.x
Xu Q, Pan W, Zhang R, Lu Q, Xue W, Wu C, Du S (2018) Inoculation with Bacillus subtilis and Azospirillum brasilense produces abscisic acid that reduces Irt1-mediated cadmium uptake of roots. J Agric Food Chem 66(20):5229–5236. https://doi.org/10.1021/acs.jafc.8b00598
Yu ZG, Zhou QX (2009) Growth responses and cadmium accumulation of Mirabilis jalapa L. under interaction between cadmium and phosphorus. J Hazard Mater 167:38–43. https://doi.org/10.1016/j.jhazmat.2008.12.082
Yu SM, Liang JS, Bai X, Dong LY, Liu XS, Wei YN, Qu JJ (2018) Inoculation of plant growth-promoting bacteria Bacillus sp. YM-1 alleviates the toxicity of Pb to pakchoi. Environ Sci Pollut Res 25(28):28216–28225. https://doi.org/10.1007/s11356-018-2802-8
Yuan T, Gu J, Zhou H, Huang F, Liao B (2020) Translocation and accumulation of cadmium and lead in the tissues of 39 rape cultivars grown in a polluted farmland. Environ Sci Pollut Res 3:15888–15900. https://doi.org/10.1007/s11356-020-07697-5
Zhang CY , He Q, Wang MH, Gao XZ, Chen JJ, Shen CW (2020) Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis) 190: 110090 https://doi.org/10.1016/j.ecoenv.2019.110090
Zhou C, Zhu L, Ma Z, Wang J (2017) Bacillus amyloliquefaciens SAY09 increases cadmium resistance in plants by activation of auxin-mediated signaling pathways. Genes 8(7):173. https://doi.org/10.3390/genes8070173
Funding
This study was supported by the National Key Research Project of China (grant number: 2017YFD0801104)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 5675 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Jin, Z., Zhang, X. et al. Alleviation of Cd phytotoxicity and enhancement of rape seedling growth by plant growth–promoting bacterium Enterobacter sp. Zm-123. Environ Sci Pollut Res 27, 33192–33203 (2020). https://doi.org/10.1007/s11356-020-09558-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09558-7