Abstract
Numerous mitigation techniques have been incorporated to capture or remove SO2 with flue gas desulfurization (FGD) being the most common method. Regenerative FGD method is advantageous over other methods due to high desulfurization efficiency, sorbent regenerability, and reduction in waste handling. The capital costs of regenerative methods are higher than those of commonly used once-through methods simply due to the inclusion of sorbent regeneration while operational and management costs depend on the operating hours and fuel composition. Regenerable sorbents like ionic liquids, deep eutectic solvents, ammonium halide solutions, alkyl-aniline solutions, amino acid solutions, activated carbons, mesoporous silica, zeolite, and metal-organic frameworks have been reported to successfully achieve high SO2 removal. The presence of other gases in flue gas, e.g., O2, CO2, NOx, and water vapor, and the reaction temperature critically affect the sorption capacity and sorbent regenerability. To obtain optimal SO2 removal performance, other parameters such as pH, inlet SO2 concentration, and additives need to be adequately governed. Due to its high removal capacity, easy preparation, non-toxicity, and low regeneration temperature, the use of deep eutectic solvents is highly feasible for upscale utilization. Metal-organic frameworks demonstrated highest reported SO2 removal capacity; however, it is not yet applicable at industrial level due to its high price, weak stability, and robust formulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuel combustion (e.g., coal, petroleum, natural gas) has been widely practiced in industry as a method of producing steam for the use of turbines in electrical generation, production of heat in concrete and paper industries, smelting of iron ores in steel industry, etc. The fossil fuel used typically contains 0.5–5% sulfur which implicates that the burning of such materials will release sulfur dioxide (SO2), an acidic gas, into the atmosphere (ECE 2015). The release of SO2 into the atmosphere has been confirmed to pose harmful effects not only to the environment but also to the living beings. Due to its acidic properties, SO2 becomes the main contributor in acidification, via the formation of sulfate and sulfuric acid in the atmosphere. SO2 is the main source of acid rain, acid smog formation, acidification of water bodies (lake, stream), agricultural product damage, and quickening of the corrosion of buildings (Tailor and Sayari 2016; Zhang et al. 2017a). SO2 is detrimental to living beings even for short exposure, especially to asthmatic people as they may experience difficulties in respiratory and pulmonary functions as short as 10 min of exposure to SO2 (WHO 2018). Due to this reason, countries like the USA enforces a very strict restriction of SO2 emission limit of 75 ppb in a 1-h period without further limits set on a longer period (US EPA 2018). Exposure to SO2 will also worsen existing cardiovascular disease of humans.
Various mitigation methods have been realized to reduce the severity of SO2 on the surroundings, where the choice of methods to be applied depends on several main factors. A majority of existing plants were constructed by obeying the environmental regulation at the time of construction. However, due to stricter emission restriction, modification of the existing plant needs to be made to comply with current regulations. Some of these plants have limited space, which means that incorporation of desulfurization units with large space requirements is ineffective and inapplicable. The operating and maintenance costs of a desulfurization unit are critical in determining the most suitable mitigation technique. The increment or reduction of the cost is mainly affected by the factors governing the efficiency of the desulfurization process such as cost and regenerability of sorbent, treatment or management of the byproducts, temperature and pressure of the reaction, concentration of inlet SO2, the presence of water and other chemicals, and requirement of additives. In this paper, various processes of existing and emerging reversible SO2 emission control focusing on flue gas desulfurization will be studied. The details of each process will be thoroughly reviewed, and the critical parameters affecting the removal efficiency of SO2 and regenerability of the sorbent will be discussed.
SO2 emission control
A variety of techniques for controlling SO2 emissions based on prevention of emission or treatment of flue gas have been studied, and some have been applied in industries emitting SO2. The emission of SO2 is proportional to the content of sulfur in fuel and the amount used in firing, implicating that reduction of sulfur content can achieve significant reduction in emissions. Some of the techniques to reduce sulfur emissions are summarized in Table 1, with the advantages and disadvantages of each. These techniques can be either applied independently or combined with each other, depending on emission target.
Using fuel with an ultra-low amount of sulfur is indeed the ideal, environmentally friendly option in lowering SO2 emissions; however, acquiring cleaner fuel incurs a high refining cost. A favorable option is to install a sulfur recovery unit that produces saleable sulfur in the form of sulfuric acid, provided that the gas stream contains a rich amount of sulfur, and the recovery unit must be able to withstand the corrosive environment associated with acids of sulfur. For industries dealing with coal as a source of fuel, sulfur in the form of pyrite (Fe2S) can be easily removed by physically washing coal with water, although this may be counter-weighed by operational cost and efficiency due to fuel properties alteration. High efficiency of sulfur removal can be achieved through several end-of-pipe treatment methods like wet flue gas desulfurization (FGD), biological technologies, and electronic technologies using electron beam irradiation, but several problems do persist with each, e.g., high space requirement, high dependency on water, high cost and energy consumption, and high safety protection requirement, respectively. On the other hand, pulse corona discharge is a relatively new and immature technology for SO2 removal, despite having the advantage of not requiring an electron accelerator and high safety protection.
FGD is the most prominent method used to mitigate the problem with SO2 at industrial level mainly due to the simplicity of the process and high desulfurization capacity achieved (> 99%, by wet sorbent). A wide range of sorbents can be selected to be used in this method which are easily synthesized by various chemical compounds, naturally occurring materials or wastes produced by various processes. This will result in reduction in overall capital cost incurred for sorbent preparation and elimination of wastes that may become problematic to the environment. Several FGD methods are independent from water usage, leading to lower operational cost and no production of wastewater. Various sorbents utilized in FGD can be regenerated and recycled for successive sorption processes while simultaneously releasing the absorbed/adsorbed SO2 in the form of sulfuric acid (H2SO4), elemental sulfur, or liquid SO2. The release of these compounds is very advantageous to the industry due to its marketability and can be easily sold or utilized in other various processes.
As illustrated in Fig. 1, FGD can be classified into wet, dry, or semi-dry method, depending on the sorbent condition. In wet FGD, the sorbents are in slurry or solution form and the removal efficiency of SO2 via wet FGD is typically higher compared with those in dry FGD (Flagiello et al. 2018; Ma et al. 2018). This method usually results in the formation of moisture-saturated flue gas and wastewater, which demands large-unit installation areas, often difficult to be complied by older plants. Dry process involves the use of sorbent in solid, powder, pellets, etc., and the resulting wastes or byproducts are in dry form while the flue gas is water deficient. Semi-dry FGD is implemented to combine the excellent features of both wet and dry methods, i.e., the high removal efficiency and the production of dry byproducts, respectively, which are beneficial from an industrial point of view. In this method, dry sorbents are utilized, and fluids are injected at a certain interval to enhance the interaction between SO2 adsorbate and the sorbent.
FGD methods can be further divided into two categories, depending on the life cycle of sorbent: once-through or regenerative. The once-through method which involves scrubbing with lime or limestone is the more commonly utilized FGD due to its high removal efficiency. However, the spent sorbent from this method is not recycled, but rather disposed of as waste or if marketable, utilized as a byproduct. Some byproducts like gypsum can be marketed due to its usability in various applications. On the other hand, the sorbent utilized in regenerative FGD can be regenerated by releasing the absorbed/adsorbed SO2 in the form of gaseous SO2, elemental sulfur, or sulfuric acid through various means, e.g., thermal or chemical treatment. Regeneration of spent sorbent is an attractive property of this method as the sorbent can be recycled for several sorption/desorption cycles until SO2 removal capacity substantially deteriorates. Although the addition of a regeneration unit will incur additional capital and operational costs, these can be moderated by the lesser amount of wastes and byproducts generated from dry FGD.
At industrial scale, the selection of FGD type to be implemented is location and process specific as these industrial plants possess different in-site conditions, accessible resources, and waste stream prerequisite. Reaction parameters such as concentration of SO2, reaction temperature, composition of flue gas, possible equipment damages, waste, and byproduct handling are essential properties that need to be considered in designing FGD treatment units. Once-through FGD unit is appropriate for lower SO2 concentration as in coal-fired plant or for lean-sulfur flue gas, while regenerative FGD is well suited for plants producing high SO2 content, e.g., flue gas prevailing from copper converting units (Roy and Sardar 2015).
Designing FGD treatment units hugely depends on the capital cost and operational and management (O&M) costs. Capital and O&M costs of FGD methods are in the order of dry < semi-dry < wet. The costs for wet and semi-dry methods are higher mainly due to high requirement of water during the process and wastewater management (Silas et al. 2018). In addition to lack of water usage, the costs for the dry method are significantly lower as some of the sorbents used like activated carbon, metal oxide, and zeolite are cheap as they originate from waste of other processes or can be found naturally (Meimand et al. 2019). Addition of a regeneration unit will lead to increment of the overall costs, independent of FGD type. The summary of capital and O&M costs for different FGD types is shown in Table 2 (Poullikkas 2015).
Even though the cost of regenerative method is higher than that of the once-through method, the implementation of regenerative method is more appealing and beneficial to the industry due to a lesser requirement of waste and byproduct management which in turn reduces the overall cost of sorbent acquisition and preparation. This review paper will be centered on discussing the existing and emerging regenerative wet, dry, and semi-dry FGD methods and the factors that affect desulfurization efficiency and regenerability of the sorbent so that these methods can be integrated at industrial level. Semi-dry FGD will be briefly discussed as the recent trend regarding this method is more focused to utilizing the waste produced in other applications rather than exploring new semi-dry FGD sorbent or improving available sorbents.
Wet regenerative FGD
Ionic liquid absorption
Ionic liquid (IL) is a type of solvent consisting of asymmetrical organic molecules as the cation combined with an anion with low coordination properties. The solvents appear in a liquid state at room temperature and possess unique properties such as low vapor pressure, low volatilities, high thermal and chemical stability, designable structure, excellent solvent power for organic and inorganic compounds, high solvation capacities, and high polarity that dissolves polar SO2 gas. In the context of SO2 absorption, the frequently used cations in synthesizing ILs are imidazolium, guanidinium, phosphonium, quaternary ammonium, and hydroxyl ammonium (as shown in Fig. 2), paired with a wide range of anions.
The first task-specific ILs to absorb SO2 were reported by Wu et al. (2004) in which a base-functionalized IL, 1,1,3,3-tetramethylguanidinium lactate ([TMG][lactate]), showed the ability to absorb 1.0 mol of SO2/mol at a pressure of 1 atm with 8% SO2 in the gas, while also demonstrating the ability to be regenerated for several absorption/desorption cycles without losing absorption capacity. Since the discovery, several different task-specific ILs used for the same purpose were made from different combinations of compounds. Table 3 shows some of the reported ILs used for SO2 absorption and the molar fraction of each IL’s removal capacity. The removal capacities of these ILs depend on several factors: temperature and pressure of reaction and partial pressure of SO2.
Unfortunately, the preparation of task-specific ILs requires some tedious and complex steps, and the high expense creates a huge limitation for its usage at a large scale. In addition, ILs can only be regenerated for a limited absorption/desorption cycle, and eventually will be discarded. The aforementioned thermal and chemical stability of ILs resulted in poor biodegradability, especially for the one made of imidazolium cation or cation having short chain with polar functioning group (Abramenko et al. 2020). Due to their low vapor pressure, these substances are commonly released in wastewater. In the case of huge release of non-degraded ILs into the environment, potential bioaccumulation of the ILs may occur which may remain for a long period and consequently alter the ecosystem they were released into (Kudłak et al. 2015). ILs’ residues are commonly treated via UV radiation, wet mineralization, the Fenton oxidation, or electrochemical decomposition, culminating in additional operational costs for this FGD method.
Deep eutectic solvent absorption
Deep eutectic solvent (DES), which possesses similar properties and characteristics to ILs, was introduced to overcome ILs’ flaws. Unlike ILs, the preparation of DES is much simpler, only by mixing two or more compounds until a homogenous liquid is formed, without addition of any solvent. A typical DES is made of a mixture of hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD), and the melting point of the resulting DES is lower than both HBA and HBD due to the formation of intermolecular hydrogen bonds between the compounds. Chemical compounds commonly utilized as HBA are organic quaternary ammonium salts like choline chloride or acetyl choline chloride while the compounds used for HBD are carboxylic acids, amides, alcohols, and azoles. Table 4 shows the possible combination of compounds in synthesizing DES (Kudłak et al. 2015), while Figs. 3 and 4 show the type of HBAs and HBDs commonly used for making DES reported for SO2 absorption.
Common DES is much cheaper compared with ILs as they are made of naturally occurring compounds and are safer to the environment due to their low toxicity and volatility. However, it should always be considered that the toxicity of DES mixture may be higher than the toxicity of the original HBA and HBD compounds separately. The density of DES is higher than that of water and also typically higher than that of HBD compounds. Majority of them also showed high thermal stability for temperature over 200 °C except for DES with sugar in their structure (Kudłak et al. 2015). Various types of DES made from combinations of different HBAs and HBDs have been reported for SO2 absorption, and some of them are listed in Table 5.
SO2 absorption by DES is a very rapid process and normally completed after 10–15 min via physical absorption. The removal of SO2 is governed by several important parameters: temperature of reaction, partial pressure, basicity of DES, and molar ratio of HBA:HBD. Like ILs, the spent DES can be regenerated by applying heat and the regenerated DES removal capacity can be maintained for several absorption/desorption cycles. In general, as the SO2 removal by DES is technically higher than that by ILs, added with its low toxicity, cheaper price, and easier synthesis, the usage of DES as an absorbent for removing SO2 from flue gas is more effective to be applied at industrial level.
Ammonium halide absorption
Aqueous ammonium halide is a solution consisting of quaternary ammonium cation and an anion from halogenic compounds: fluoride, chloride, bromide, or iodide. Ammonium halide solutions are commonly used in the syntheses of ionic liquids, e.g., caprolactam tetrabutylammonium chloride (CTAB), or as a hydrogen bond acceptor in DES production as seen in “Deep eutectic solvent absorption.” Several studies have been reported showing that aqueous ammonium halide solutions are able to remove SO2 from flue gas independently. Duan et al. (2016) studied the ability of different tetraalkylammonium halide solutions (fluoride, chloride, and bromide) with different alkyl lengths (methyl, ethyl, propyl, and butyl) to remove SO2. The results demonstrated that the solubility of SO2 increased with the length of the alkyl chain but decreased with the increment in sorbent concentration. SO2 removal is also temperature dependent as the SO2 absorption trend changes depending on temperature as shown in Table 6.
A similar result was obtained by Kumar et al. (2012) using tetraethylammonium halide, in which SO2 removal using bromide solution was found to be higher than that using chloride solution in the following order: I > Br > Cl. Ammonium chloride and ammonium bromide solutions have also been impregnated on pyrolyzed rice husk to remove mercury, SO2, and NO simultaneously (Zhu et al. 2016). However, weak removal of SO2 (80%) was observed, thus concluding that this sorbent combination is ineffective in treating SO2-containing flue gas. As the SO2 removal by independent ammonium halide is inferior to the others, ammonium halide solution is better utilized as a precursor of ILs or DES. However, it should be noted that ILs with N and F atoms are considered hazardous and have poor biodegradability and thus, their use should be prevented, if possible (Abramenko et al. 2020).
The Bunsen reaction
The feasibility of reversible SO2 removal using the Bunsen reaction has been recently reported by Zhu et al. (2017). The Bunsen reaction is the first step in the sulfur-iodine thermochemical cycle for the production of hydrogen as shown in Fig. 5. In a Bunsen reaction, water, SO2, and iodine chemically react to produce sulfuric acid (H2SO4) and hydrogen iodide (HI), which form two immiscible aqueous layers with sulfuric acid on top and hydrogen iodide at the bottom, together with unreacted iodine. As the boiling points of these two products are different, they can be easily separated by distillation and then further decomposed to complete the sulfur-iodine cycle: HI is decomposed into I2 and H2 while H2SO4 is decomposed into H2O, SO2, and O2. The SO2 removal efficiency of this I2/HI absorption system is over 98.8% which is close to the typical traditional FGD process using limestone. The removal efficiency of SO2 can be affected by the concentration of I2 and reaction temperature. Although the products are considered to be immiscible, a small portion of H2SO4 may still be mixed with the HI bottom layer and vice versa. This may lead to a possible undesired reaction, producing elemental sulfur which could block the reaction equipment.
It should be considered that the industrial flue gas typically contains other gases like NOx and O2 which may react with each other, producing nitric acid (HNO3). The presence of HNO3 will lower the pH value, resulting in inhibition of SO2 absorption. Nonetheless, SO2 removal using the Bunsen reaction is more advantageous as the market for H2SO4 is much better than the typical FGD wet system byproducts such as gypsum, ammonium sulfate, and magnesium sulfate. In addition, not only I2 produced at the end of the cycle can be recycled to absorb SO2 from incoming flue gas stream, but also a clean energy, H2, is being simultaneously harvested from the process.
Alkyl-aniline absorption
Aqueous solution of amine, which is basic in nature, is generally used in wet FGD by chemically reacting the solution with acidic SO2, which will be trapped as sulfites or sulfates. This method is highly efficient in removing SO2 from flue gas; however, the use of amine in large-scale SO2 capture is inefficient mainly due to its limited operation-temperature range. The solutions may evaporate into the gas stream due to their volatility which consequently led to inevitable corrosion of ducts and equipment in the flue gas system. Additionally, the regeneration of the amine sorbent requires high energy consumption. In terms of regenerability of the sorbent, only tertiary amine possesses the ability to be regenerated completely (Kim et al. 2019a). Recently, aniline, an aromatic amine, is widely employed in fossil-fueled power plants for SO2 absorption. Alkyl-aniline is an example of tertiary amine in which the N atom is attached to a phenyl group and alkyl groups and has been reported to be fully reversible upon desorption of SO2.
Figure 6 shows the structure of an amine and N,N-dibutylamine (DBA), a type of alkyl-aniline. The interaction between alkyl-aniline and acidic SO2 is neither too strong nor too weak, implicating that the absorption and desorption of SO2 can happen at a moderate temperature. Vo et al. (2019) tested the absorption of SO2 using 4 different alkyl-anilines: N,N-dimethylaniline (DMA), N,N-diethylaniline (DEA), N,N-dibutylaniline (DBA), and N-methyldiphenylaniline (MDPA), in the absence and presence of water. The basicity of these alkyl-anilines was found to be in the order of DBA > DEA > DMA > MDPA. The study in wet conditions showed that the SO2 removal by alkyl-aniline precisely followed the basicity of the sorbent. On the other hand, SO2 removal in dry conditions favored the sorbent with a shorter length of alkyl chain in the order of DEA > DMA > DBA > MDPA. The reduction in SO2 removal capacity by DBA is ascribed to the restriction on SO2 molecules to approach the basic center due to the presence of two large butyl groups.
Table 7 shows the removal of SO2 using various alkyl-aniline solutions, in both dry and wet conditions. In general, alkyl-aniline produces better SO2 removal in wet conditions as the presence of moisture will aid the interaction between the gaseous SO2 molecules with the sorbents. Although the interaction between SO2 and aniline differs depending on the structure of each aniline, all of them demonstrated highly reversible properties in SO2 absorption. Nonetheless, SO2 removal capacities of alkyl-aniline (in mol SO2/mol) are much lower compared with those of ILs or DES. DBA tested in wet conditions can be regenerated with the aid of N2 flow at 80 °C and is able to maintain the removal capacity for 6 consecutive adsorption/desorption cycles. The regeneration temperature is much lower than required by typical aqueous amine solution (120–130 °C), thus lowering the energy consumption of this process.
Amino acid solution absorption
Aqueous solution of amino acid is another example of a chemical compound utilized as an amine substitute for absorption of SO2 from flue gas. Amino acid demonstrates an attractive performance in SO2 removal due to having favorable characteristics such as low volatility, low ecotoxicity, high biodegradability, and environmentally friendly, attributed to the presence of amino groups (Deng and Jia 2012). Amino acid in aqueous solution typically exists in zwitterion form which may react with H+ and OH− present.
SO2 is absorbed into the amino acid solution mainly via physical absorption and weak chemical bonding (hydrogen bonding) which can be easily desorbed by heating at temperature range between 120 and 150 °C. Several studies on the performance of various amino acid solutions in SO2 removal are listed in Table 8.
Taking β-alanine as an example, its SO2 absorption follows a 2-step mechanism showed in Eqs. 3 and 4 (Deng et al. 2012; Rahmani et al. 2015):
-
(1)
Dissolution of SO2 in water and generation of hydrogen and bisulfite ions:
-
(2)
Hydrogen sulfite ions interact with carboxylic group of amino acid and form hydrogen bonding.
The removal of SO2 depends greatly on several different factors such as temperature of reaction, concentration of amino acid solution and SO2 gas, pH of absorbent, and liquid-gas ratio. Amino acid shows better SO2 removal compared with other solutions tested (amine and buffer solution) mainly due to the existence of both amino groups and carboxylic functional groups in their molecular structure (Deng et al. 2012; Rahmani et al. 2015). Comparing the performance of amino acid solution with that of other sorbents previously discussed, the SO2 removal capacity of amino acid is considered significantly weaker especially against DES.
Calcium-based sorbent absorption
The traditional wet FGD using calcium-based lime and limestone sorbent is classified as non-regenerative as the sorbent cannot be recycled for further use. However, Tian et al. (2015) recently reported a reversible calcium-based sorbent for removing SO2 from flue gas using aqueous calcium lactate (CaL2) solution produced from a mixture of calcium hydroxide, lactic acid, and water (Fig. 7). In the presence of water, CaL2 solution could achieve a removal capacity of 24.8 mg SO2/g, at temperature and pressure of 40 °C and 1 bar, respectively. Depending on the mole of H2SO3 reacting with calcium lactate sorbent, the products formed by the reactions are lactic acid and calcium sulfite (CaSO3) or calcium bisulfite Ca (HSO3)2. The removal of SO2 using aqueous calcium lactate solution is mainly affected by the temperature of reaction, concentration of the calcium lactate solution, and lactic acid quantity in the absorbent. Excess lactic acid is detrimental towards the removal of SO2 as the reactions would shift to the left favoring more product formation. This problem can be overcome simply by addition of any calcium-based compounds such as Ca (OH)2 or CaO which will eventually form CaL2.
As shown in Fig. 7, the spent absorbent can be regenerated by subjecting the generated CaSO3 and Ca (HSO3)2 to heat and removing water from the solution produced. No structural change in absorbent and no obvious loss of SO2 absorption capacity are demonstrated by the regenerated calcium lactate solution. It should be noted that low quantities of calcium sulfate (CaSO4) may also be formed during the main reaction due to the oxidation of CaSO3 by O2, which would reduce the amount of sorbent regenerated and require an additional byproduct management. Nonetheless, CaSO4 can be easily separated from calcium lactate solution due to the latter being water soluble.
Aluminum sulfate absorption and magnesia scrubbing
Wet FGD using basic aluminum sulfate (BAS) shown in Fig. 8 can be classified based on the type of byproduct produced: (i) BAS-gypsum and (ii) BAS-desorption regeneration. The first BAS method, which is associated with generation of gypsum as byproduct, is commonly used due to its simplicity, broad window of SO2 concentration, and high sorption capacity. However, this method always results in large secondary pollution of gypsum, thus increasing the cost of byproduct management. On the other hand, the latter method which demonstrated high desulfurization capacity can be regenerated via heat treatment, recovering the absorbed SO2 in pure form or as sulfuric acid (H2SO4), but is only feasible for a plant producing flue gas with high concentration of SO2 as mentioned earlier (Chen et al. 2016). The use of BAS as SO2 sorbent is highly dependent on the pH of the solution as high basicity will lead to precipitation of the sorbent itself. Other factors which critically affect the absorption capacity of BAS are Al content in BAS, reaction temperature, inlet SO2 concentration, and gas flow rate (Chen et al. 2016; Zhang et al. 2018).
BAS sorbent can be easily regenerated by heating sulfite, the byproduct obtained in the reaction. Unfortunately, the presence of O2 in the flue gas will induce difficulties in sorbent recovery as it helps in oxidization of sulfites into sulfates. This oxidization phase can be reduced by adding an inhibitor such as ethylene glycol (Chen et al. 2016, 2019). The introduction of ethylene glycol which is non-toxic in nature showed little effect towards sorption capacity of SO2, while increasing the concentration of ethylene glycol hindered the oxidation reaction from taking place. Hydroquinone is another oxidation inhibitor with a higher inhibition performance than ethylene glycol; however, its toxicity is making it less applicable in upscale treatment (Chen et al. 2019). Another problem commonly faced in regenerating BAS solution is the low desorption efficiency, using water bath 70%, microwave 75%, ultrasonic waves 82%, and vacuum 95% (Huang et al. 2017). The usage of falling film evaporator using converging-diverging tube reported by Huang et al. (2018) demonstrated desorption efficiency of 94.1%.
Another regenerable wet FGD sorbent commonly suffered from oxidation of sulfites is magnesia (MgO). Magnesia FGD is an appealing process especially for small and medium industrial boilers due to its high desulfurization capacity, process simplicity, small cost, and low energy consumption. Magnesium sulfite produced from this method can be easily regenerated by heat decomposition between 900 and 1000 °C, similar to the regenerative BAS method. Higher decomposition temperature may lead to lower desorption efficiency due to sintering of the sorbent (Yan et al. 2014). However, as the contact between the sulfites and oxygen is imminent during the process, the oxidation process will result in the formation of magnesium sulfate which will be discarded due to its poor utility. Oxidation inhibitors such as phenol, ethanol, and ascorbic acid were reported to successfully inhibit oxidation of sulfite, where the concentration of inhibitor, oxygen partial pressure, pH, and reaction temperature effectively affect this oxidation process (Lidong et al. 2013).
Dry regenerative FGD
Activated carbon adsorption
Utilization of sorbents originated from cheap and feasible sources in SO2 removal is deemed attractive from the economic point of view. In dry regenerative FGD method, activated carbon (AC) has been extensively applied as sorbents attributed to its favorable properties, namely large surface area, good distribution of porosity, and high extent of surface reactivity. These ACs are commonly obtained from various carbonaceous precursors originating from distinct sources such as wastes from agricultural industry, e.g., rice husk, oil palm, and coconut shell, and waste from industrial activities, e.g., cork powder, tires, and fly ash. Unfortunately, the use of AC in upscale treatment of SO2 often suffers from high flue gas temperature and small composition of acidic gas. These bottlenecks lead to low SO2 adsorption capacity, shorter breakthrough time, and weak selectivity. These drawbacks can be overcome by modifying AC surface to generate a charged surface of functional groups with high affinity towards SO2, while at the same time removing any functional groups that constrain the adsorption to take place (Abdulrasheed et al. 2018). Modification of AC surface will cause the adsorption to occur by two forces: (i) mass transfer of the SO2 adsorbate towards AC pore and surface, and (ii) chemical reaction between the added chemical groups and SO2. Modifying surface basicity of the AC by introducing basic additives like amine group and basic/amphoteric metallic oxides has been reported to improve the removal capacity of SO2 by the AC support. The incorporation of an amine functional group on the AC will inhibit the adsorption of CO2 in a simultaneous removal with SO2 as both gases are acidic. Due to this reason, amine-modified ACs are technically preferable to be used in a sulfur-lean flue gas e.g. from power plants (Abdulrasheed et al. 2018).
AC can be modified via several methods: metal loading, oxidation, and reduction. For metal-modified AC, basic metals are commonly added which will lead to the increase in surface basicity. The enhancement of removal capacity is attributed to strong binding ability of the metals towards the adsorbate. Modification of AC with liquid oxidants such as HNO3, H2SO4, and H3PO4 resulted in the increment of oxygen-containing radical groups attached on the AC surface, which increased the surface acidity. This will lead to enhanced adsorption ability of AC towards a polar substance, such as SO2. The introduction of reducing agents such as NaOH and KOH will reduce the surface functional groups present while simultaneously increasing the quantity of alkaline functional groups on the AC surface. This resulted in improvement of sorption capacity towards non-polar substances (Deng et al. 2016). SO2 removal using AC is mainly governed by reaction temperature, inlet SO2 concentration, and the type and concentration of additives. In Tables 9 and 10, the SO2 adsorption capacities of ACs in several recent studies are listed and categorized into two sections: metal and surface modified, respectively.
The improvement in SO2 removal capacity by basic additive-modified ACs such as copper, magnesium, calcium, and melamine can be ascribed to their ability to counter the increase of acidity brought about by acidic SO2. Additionally, the introduction of acidic groups by H3PO4, etc. on the AC surface may also improve SO2 capture as the acidic group formed on AC surface possesses high binding attraction towards polar molecules like SO2 (Deng et al. 2016). From Table 10, it can be seen that SO2 removal capacity of KOH-modified ACs is considered weak which is mainly attributed to higher binding preference of the alkaline group formed towards non-polar substances. The spent AC can be easily regenerated via thermal treatment where the equilibrium of the AC will be changed, releasing the adsorbed SO2. However, some of the adsorbed SO2 may not be released due to chemisorption, especially in the presence of O2 and H2O.
Mesoporous silica and carbon-silica composite adsorption
Other than activated carbon, silica-based sorbent is another type of sorbents commonly employed in SO2 removal from flue gas. Porous silica with a pore size between 2 and 50 nm is classified as mesoporous silica (MS). MS has a unique naming system of three letters followed by a number, e.g., MCM-41 (Mobil Composition of Matter-41), SBA-15 (Santa Barbara Amorphous material-15), and KIT-6 (Korea Institute of Science and Technology-6). Mesoporous silica is synthesized by reacting the silica template, tetraethyl orthosilicate (Si(OC2H5)4), and different directing template compounds depending on the desired MS. Recent studies on the synthesis of MS show that commonly found siliceous materials, e.g., rice husk ash, oil palm ash, and beach sand, can be used to substitute TEOS as the silica source (Razak et al. 2019; Salazar Hoyos et al. 2020; Sales et al. 2019). A general flow of MS preparation is shown in Fig. 9; however, additional substances may be added depending on desired properties of MS.
This kind of material has very high specific surface areas, ordered pore structures, and possibilities to be synthesized in varying morphologies. MS has been employed in a wide range of applications including drug delivery system, indoor air cleaning, catalysis, wastewater treatment, and flue gas removal. The non-modified MS was reported to demonstrate low sorption capacity of SO2 which can be attributed to the weak interaction between MS and SO2 adsorbate (Li et al. 2015). However, MS which possesses good thermal, mechanical, and hydrothermal stability is a good candidate for catalyst support. The ordered structure of MS provides ideal space for loading additives, with metal salts and amine being typically utilized. MCM-41, SBA-15, and KIT-6 have been modified with various additives, and their performance in SO2 removal is summarized in Table 11.
The SO2 removal performance of amine-modified MS is significantly higher compared with that of the metal-modified MS which may be related to the strength of amine to counter SO2 acidity. However, it should be noted that all studies on metal-modified MS were conducted at 673 K, while the adsorption of SO2 is much preferable to be done at a lower temperature. SO2 adsorption is a thermodynamically controlled process where low heat of adsorption occurs at high temperature and high SO2 uptake (Tailor et al. 2014b; Tailor and Sayari 2016). Increasing the amount of amine additives (up to an optimal value) has also been reported to give a positive effect on the sorbent performance (Li et al. 2015; Zhi et al. 2011). The interaction between amine-based MS and SO2 is considerably weak as the sorbent can be easily regenerated at 120–130 °C. Even though amine-based MS demonstrates better performance, its utilization suffers from a small range of temperature as mentioned earlier with amine sorbent. Due to its high volatility, a large amount of amine may be lost to evaporation and possibly causing corrosion to gas treatment equipment.
Carbon-silica composite (CSC) is a type of sorbent with large surface area and two different phases for adsorption, which means that acidic gas like CO2 or SO2 and basic gas like NH3 can be adsorbed at the same time. Typically, CSC is made by modification of mesoporous silica (MCM-41) by grafting of carbonaceous phase such as furfuryl alcohol or sucrose, via co-condensation or post-synthetic grafting (Furtado et al. 2012). Like ordinary MS, addition of metal additives may increase the removal efficiency of CSC. Nonetheless, the regenerability of CSC is not as efficient as MS, as the amount of SO2 removed in the subsequent purge is significantly reduced as some portions of gas from initial purge are adsorbed via chemisorption (Furtado et al. 2012). Additionally, typical industrial flue gas rarely contains a basic gas, which means that the use of CSC is very specific and impractical for common coal-fired plants.
Metal oxide and zeolite adsorption
Employment of metal-based catalysts as sorbent for SO2 capture is widely acceptable due to its high desulfurization efficiency. Typical metal catalysts used are metal oxides or mixed metal oxides, predominantly of transition metals attributed to low production cost, easy regeneration, and selective action (Gawande et al. 2012). These metal oxides are obtained mainly from two methods: (i) laboratory syntheses from metallic salt precursors and (ii) naturally occurring metal oxides. The different methods of synthesis such as precipitation, co-precipitation, hydrothermal, and urea hydrolysis lead to different abilities of SO2 removal due to the morphology of the sorbents produced (Zhao et al. 2011, Zhao et al. 2016b).
Natural carbonates such as limestone (Ogenga et al. 2010) and magnesite (Zhang et al. 2017b) can be employed as SO2 sorbent after being subjected to calcination process, producing metal oxides such as calcium oxide (CaO), magnesium oxide (MgO), and both CaO and MgO, respectively. The metal oxides obtained can achieve very high SO2 removal efficiency, up to 100%. However, as the calcination temperature is very high in the range of 600–900 °C, energetic requirement is high incurring high operating cost while simultaneously, sintering of catalyst may occur which will restrain the adsorption process. Similar to other sorbents, adsorbed SO2 can react with metal oxide sorbent to produce sulfites which oxidize into sulfates in the presence of oxygen.
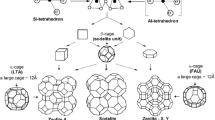
Zeolite is another adsorbent commonly utilized in removing SO2 from flue gas and can be obtained by laboratory syntheses or in the environment, similar to metal oxides. Highly ordered zeolites possess appealing properties such as high surface area and good thermal stability resulting in favorable materials for gas sequestration and removal. Clinoptilolite is a naturally occurring zeolite consisting of tetrahedral arrangement of aluminum and silica. This compound is widely used as a sorbent in various gas treatment processes attributed to their abundance in nature and low cost (Meimand et al. 2019). The structure and framework of a zeolite are shown in Fig. 10.
Synthesized zeolites are made from fly ash, a solid residue obtained from the combustion of fossil fuels collected by electrostatic precipitators or fabric filters. In total, 80% of ash produced from fossil fuel burning is categorized as fly ash which creates waste disposal problems (Pedrolo et al. 2017). Utilizing waste produced by coal combustion to overcome another problem like SO2 emission is deemed very attractive from the industrial point of view. Fly ash zeolites can be synthesized via fusion synthesis, molten salt synthesis, combination of microwave and ultrasound energies, etc. with hydrothermal being the most used method due to its simplicity, low energy usage, and broad range of zeolite topology produced (Czuma et al. 2016, 2019). SO2 removal capacities of various metal oxides and zeolites are summarized in Table 12. Unfortunately, compared with other dry FGD sorbents, SO2 removal capacities of metal oxides and zeolites can be considered weak. As discussed before, the regeneration of these sorbents also suffered from the production of sulfates during adsorption reaction, which will lead to reduction in the overall regeneration capacity of the sorbents. In addition, the hydrothermal method commonly used in the syntheses of these sorbents will result in production of alkaline solution waste of high pH, containing trace heavy metals which possess adverse effects towards the environment (Czuma et al. 2019).
Metal organic framework adsorption
As the interest for new dry regenerable sorbents is constantly growing, porous metal-organic frameworks (MOFs) are recently introduced for SO2 removal. As illustrated in Fig. 11, MOFs are constructed of metal ions or clusters connected to organic ligands via coordination bonding resulting in infinite potential combinations. Metals typically used for the synthesis of MOFs are zinc, copper, magnesium, cobalt, cadmium, zirconium, titanium, lanthanide, etc., capable to adopt various geometries like tetrahedral, square, pyramidal, octahedral, trigonal, and bipyramidal geometries (Kumar et al. 2017). Hydrothermal, solvothermal, ultrasonic, and microwave methods are some of the techniques commonly used in synthesizing MOFs.
The use of MOFs in various applications is very appealing as these porous materials exhibit high surface areas, tailorable pores, tunable functional group composition, high chemical and thermal stability, and easy regenerability (Glomb et al. 2017; Savage et al. 2016; Wang et al. 2019). MOFs have been reported to be successfully utilized in a wide range of processes such as gas storage, catalysis, water treatment, and drug delivery. Many works have been done on CO2 and hydrocarbon sequestration, and H2 and CH4 storage using MOFs; however, their application on SO2 capture has been constrained by limitation in the stability of coordination compounds towards SO2 (Savage et al. 2016). MOFs suffer from the toxicity and corrosive properties of SO2 (Smith et al. 2019), which often resulted in structural damage of MOFs and irreversible SO2 uptake. To overcome this drawback which hinders the applications of MOF in removing SO2, several studies have been recently focused on synthesizing new MOFs with better stability and higher sorption capacities. These studies are summarized in Table 13.
Based on Table 13, SO2 sorption capacities of MOFs are much higher compared with those of other dry sorbents discussed in the previous sections. However, these studies are conducted at a laboratory level and up to this moment, MOFs are yet to be used in an upscale industrial level. Additionally, MOFs suffer from high cost for organic precursors, high toxicity of some metal cations, and robust formulation for application in reactors. In the case that these flaws can be solved, the use of MOFs is deemed very attractive and very beneficial for the industries.
Semi-dry FGD
Semi-dry FGD which is also referred to as spray-drying scrubbing is a method where SO2-containing flue gas is purified via the reaction with the sorbent, typically calcium hydroxide (Ca (OH)2), in a spray absorber. In brief, the slurry suspension mixture of Ca(OH)2 and water is injected as droplets onto the absorption tower via nozzles. The flue gas entering the tower will contact these alkaline droplets and be rapidly absorbed. Rapid evaporation of moisture will occur with the aid of hot flue gas while the alkaline droplets are heated into a dry powder. The presence of O2 will oxidize SO2 into SO3, which will further facilitate the removal process as the solubility of SO3 in the alkaline sorbent is higher. The reaction mechanism of semi-dry FGD is as follows (Hrdlička and Dlouhý 2019).
The resulting products, calcium sulfite (CaSO3) and calcium sulfate (CaSO4) will fall to the bottom of the absorption tower together with fly ash. These products are typically considered non-reusable which lead to ultimate disposal unless it undergoes further treatment or upgrade. Semi-dry FGD requires production of slurry droplets in adequate size and appropriate resident time to ensure complete drying of the sorbents (Roy and Sardar 2015). Other factors affecting the desulfurization process are Ca/S ratio and approach to adiabatic saturation temperature (ΔTa) (Zhang and Gui 2009). As the absorption process is controlled by gas-phase mass transfer, higher Ca/S molar ratio will enhance the desulfurization efficiency. ΔTa value should be low to prolong the lifetime of slurry droplets.
This method requires the utilization of efficient particulate control devices such as ESP or fabric filter, and expensive lime for the production of Ca(OH)2 sorbent which lead to higher capital and operational costs. However, the absorption towers are commonly fabricated using carbon steel which is much cheaper (Poullikkas 2015). In recent years, the studies on discovering new sorbents to be used in semi-dry FGD are definitely lacking; nonetheless, several studies were focused on improving the available technology by analyzing different types of reactors and incorporation of additives. Several recent studies reported various types of reactors for semi-dry FGD application such as powder-particle spouted bed (PPSB) reactor (Fakhari et al. 2015; Wu et al. 2020) and microwave irradiation reactor (Liu et al. 2020) in experimental studies and numerical simulation with promising results.
The effects of additive incorporation on semi-dry FGD were reported by various studies on simultaneous removal of SO2 with NO and/or Hg. Yi et al. (2020) analyzed the effect of three liquid-phase oxidants: K2S2O8, H2O2, and NaClO2, whereby the increase in oxidant concentrations resulted in the increment of desulfurization efficiency up to 98%. In a simultaneous removal of SO2, NO, and Hg, Zhao et al. (2015) obtained similar results with the addition of NaClO2 up to 2.5 mol/L. On the other hand, Du et al. (2020) investigated the effect of four different additives: NaOH, CaCl2, NaHCO3, and NaCl on a single gas desulfurization process. As with the two previous studies, SO2 removal was enhanced with the increment in additive concentration in the order of NaOH > CaCl2 > NaHCO3 > NaCl. Other than that, Zn-based and Na-based sorbent utilizations in semi-dry FGD could be more promising due to their regenerability (Zhang et al. 2015). In the former, traditional Ca(OH)2 was substituted with ZnO in the presence of water, leading to production of ZnSO3·2.5H2O. Thermal decomposition of the products resulted in the release of pure SO2 and solid ZnO. However, this method is unappealing due to high viscosity and cost of ZnO. Using ZnO together with the latter in the presence of water produced Na2SO3 (originated from Na2CO3) at the bottom of the absorption tower which is beneficial as it can be reutilized in the subsequent absorption cycle as shown in Fig. 12.
Factors affecting SO2 removal capacity and sorbent regenerability
Flue gas composition
The major bottleneck in SO2 desulfurization from flue gas is the constituents of the flue gas itself. A typical flue gas from coal-firing plant with medium to high sulfur content has 75–80 vol% N2, 12–15 vol% CO2, 1800 ppm SO2, 500 ppm NOx, 5–7 vol% H2O, 3–4 vol% O2, < 100 ppm CO, and 10–20 mg Nm−3 of particulates and small quantity of Hg/As (in ppb). Majority of the studies conducted in determining SO2 removal capacity of various sorbents did not reciprocate the actual composition of flue gas in their analysis. The presence of CO2, NOx, O2, and H2O significantly alters the removal capacity and the ability of the sorbent to be regenerated.
The existence of O2 in the flue gas technically did not affect SO2 sorption capacities attained by the sorbents. As discussed in previous sections, the presence of O2 which acts as an oxidizing agent will facilitate the oxidization of sulfites produced during the sorption process into sulfates (Tailor and Sayari 2016; Zhao and Hu 2013). This oxidation process is disadvantageous for the regeneration process as the sulfates produced will be discarded, which means that lesser sorbent quantity is regenerated. Additionally, O2 may also react with the target SO2 adsorbate, yielding SO3 gas which is typically undetected by gas analyzer during the study. This implies that a fraction of inlet SO2 gas will not be recovered during the regeneration process (Mathieu et al. 2012). The study by Zhao et al. (2015) on semi-dry FGD using Ca(OH)2 as sorbent showed that the presence of O2 can be neglected during the desulfurization process. However, O2 may oxidize SO2 into highly soluble SO3, which in turn facilitates its removal by the slurry (Yi et al. 2020).
Water exists in the flue gas in vapor form and their presence will aid SO2 removal especially in dry FGD. The enhancement of SO2 sorption capacity is ascribed to higher interaction between gaseous SO2 molecules with the sorbents in the presence of moisture. The adsorption of SO2 and the presence of water follow three steps: (1) dissolution of gas adsorbate in water, (2) diffusion into water film, and (3) adsorption onto the sorbent. SO2 which is acidic in nature and possesses high solubility in water, may easily react with hydroxyl ions on the moist basic surface of the sorbents (Rosas et al. 2017). Depending on the type of sorbents used, the presence of water in wet FGD may or may not have a significant effect on the SO2 removal efficiency. Vo et al. (2019) showed that the removal efficiency of several alkyl-anilines significantly improved in the presence of water while some others showed similar capacity as in dry condition. Similar results were obtained by Deng et al. (2015) in which the presence of water did not enhance SO2 removal capacity of DES.
In both wet and dry FGD, the sorption of SO2 in the absence of water occurs via physical sorption. In the presence of water, the sorption process is gradually dominated by weak chemical sorption (Deng and Jia 2012; Zhang et al. 2010). This created a problem in regenerating the sorbent as the chemically absorbed/adsorbed SO2 would have formed a stable compound, which may not be simply desorbed by heat treatment such as in the case of physisorbed SO2 (Fig. 13). Subsequent SO2 sorption of such sorbents will be less efficient due to incomplete desorption of the chemisorbed SO2 from the former cycle.
The effect of water on desulfurization efficiency is more prominent in the case of semi-dry FGD. One of the most important aspects of this method is having adequate resident time for complete drying of the sorbents. The increase in water content leads to the enhancement of sorbent dissolution which consequently provides more ions to be involved in the desulfurization reaction. However, higher water content also resulted in lower water temperature and slower rate of water vaporization, which means that complete sorbent drying may not be achieved in the case of excess water content (Wu et al. 2020). On the other hand, lack of water in the slurry is also disadvantageous to SO2 removal as the sorbent will be dried too soon; thus, the contact time between the SO2 and slurry is shorter resulting in lower desulfurization capacity.
The presence of CO2 in the flue gas also largely influences the sorption of SO2 especially in dry conditions. CO2 will create a hindrance effect on SO2 molecules especially on the surface of the sorbents. Increasing the concentration of CO2 will produce more collisions of CO2 particles per unit area of sorbent, further reducing the SO2 removal (Ozturk and Yildirim 2008). However, in the presence of water, the removal of SO2 will prevail over CO2 due to the former having higher solubility in water (Ozturk and Yildirim 2008). In contrast, the existence of CO2 in semi-dry FGD was reported to show virtually little effect on SO2 removal which can be attributed to stability and chemical inertness of CO2 (Liu et al. 2020; Zhao et al. 2015). Additionally, based on the results observed in two studies by Tailor et al. 2014b, Tailor and Sayari 2016) on mesoporous silica grafted with amine-based additives, the presence of CO2 did not affect desulfurization activity at all and SO2 broke through the sorption bed as soon as the gas stream entered the sorption column. This can be ascribed to the favorable incorporation of N-containing groups which hinder CO2 sorption over SO2. Therefore, FGD with sorbents modified with amine groups such as AC and MS is more suitable to be utilized in plants producing sulfur-lean flue gas, in which the presence of CO2 is evitable.
Nitrogen oxides (NOx) are used to describe gaseous pollutants consisting of nitric oxide (NO) and nitrogen dioxide (NO2). The existence of NOx in flue gas creates a more prominent effect on the desulfurization process due to the huge competition against SO2. In the presence of small concentration of NOx, the sorption of SO2 on the sorbent will prevail mainly due to the latter possessing higher diffusion coefficient in gas phase (Sumathi et al. 2010). Increasing the concentration of NOx will reduce SO2 removal capacity of the sorbent, and vice versa as larger surface areas and active sites for reduction and oxidation are occupied by the dominating gas (Sumathi et al. 2010). This phenomenon can also be observed in semi-dry FGD (Liu et al. 2020) in which the increment in NO concentration in flue gas inhibited the sorption of SO2 mainly due to competition reaction between these two gases. However, most of NOx in the atmosphere originates from transportation fuel while its average concentration in flue gas is 3.6 times lower than SO2, implying that higher SO2 removal efficiency can still be achieved.
Reaction temperature
Temperature plays a critical role in determining SO2 removal capacity in adsorption/absorption process. The removal rate of SO2 generally became faster with increment in reaction temperature. However, there is always a critical limit in the reaction temperature, as further increase in temperature beyond a maximum point can reduce the sorption capacity and removal efficiency due to the exothermic nature of SO2 sorption process. At high temperature, the sorption process suffers from low heat of adsorption at high SO2 uptake mainly due to surface heterogeneity and loss of enthalpy caused by diminishing free energy of sorption process and degree of freedom during the process (Li and Ma 2018; Tailor et al. 2014b; Tailor and Sayari 2016). The only exception is for recently reported SO2 removal via the Bunsen reaction, which is an endothermic reaction that favors high temperature (Zhu et al. 2017). In semi-dry FGD, the increment in reaction temperature is disadvantageous towards the removal capacity as the drying of the sorbent is quicker, reducing the contact time between SO2 and moisture which ultimately resulting in lower desulfurization efficiency (Fakhari et al. 2015).
Figure 14 summarizes the temperatures for SO2 regeneration by all sorbents discussed earlier. Majority of the studies were conducted at temperatures between 293 and 333 K with few exceptions of several activated carbons (353–473 K), mesoporous silica (373–673 K), and metal oxides and zeolites (373–498 K), supporting the observation that SO2 removal is favorable at lower temperatures. However, it should be taken into consideration that the flue gas temperature can be as high as 1200 °C. If the reaction is to be conducted at low temperature such as at room temperature, a plant needs to incorporate a cooling method in between the flue gas exhaust and treatment unit so that the target temperature can be attained. This will incur additional operating and maintenance cost, but due to reduction in energy requirement during the process and regenerability of sorbent, the incurred cost could be recovered in a short period of time.
Temperature also plays an important role in regeneration of sorbent as the majority of the sorbents can be easily regenerated via heat treatment and in some cases, with the assistance of inert gas like N2. The temperature used during the regeneration process is highly dependent on the type of sorbent used and the sorption path of SO2 (physisorption or chemisorption). For some sorbents, temperature must be strictly controlled as the temperature window between sorption/desorption is small; e.g., for alkyl-aniline, desorption occurs at a very low temperature of 353 K (Vo et al. 2019) and for amine-modified sorbent, absorbed/adsorbed SO2 can be released at 393–403 K (Tailor and Sayari 2016; Zhi et al. 2011). In addition, due to amine’s volatility, the desorption temperature must be controlled to prevent its evaporation together with the release of SO2. On the other hand, the regeneration temperature may go very high, up to 1273 K in the case of magnesia. Optimal desorption temperature should be determined to prevent incomplete desorption at inadequate temperature or sintering of sorbents which causes structural damage of sorbent due to excessive heating.
pH of sorbent
Due to the acidic properties of SO2, the pH of the sorbents needs to be regulated during the desulfurization process. Sufficient basicity needs to be provided by the sorbents for the goal of reducing the acidity caused by the adsorbed/absorbed SO2. In the context of wet methods, an alkaline environment is favorable for desulfurization as the removal efficiency will increase linearly when the pH is increased from slightly acidic towards alkaline pH (Liangliang et al. 2019). The pH of the sorbent will determine the existence of sulfur anion in the solution, where OH− and SO32− will be present at the pH range of 7–8. H2SO3 formed during the reaction will be neutralized and the reaction will shift to the right, facilitating the mass transfer of SO2 from the gas phase towards the sorbent (Liangliang et al. 2019). However, the pH value should not be too high as it may lead to the precipitation of sorbents.
In wet FGD, the basicity of the sorbent may be provided by natural basicity of the sorbent itself or by the incorporation of alkaline species like NaOH and KOH, where pH of the sorbent can be maintained due to buffer effect, promoting SO2 removal. In dry FGD, the basicity of the sorbents is improved by impregnation of sorbent with alkali metals or alkaline solution. In most cases, the removal efficiency increases linearly with the increase in pH and quickly decreases following reduction in pH values.
In semi-dry FGD, increasing the pH from 1.1 to 6 is beneficial towards SO2 removal as higher amounts of acidic SO2 gas can be absorbed, but further pH increase leads to reduction in removal capacity due to the presence of hydroxide ions (Liu et al. 2020). However, several studies have reported that instead of conducting desulfurization process at alkaline pH, SO2 sorption can also be operated at lower temperatures as some sorbents possess the ability to remove SO2 at neutral or slightly acidic pH as shown in Table 14.
Inlet SO2 concentration
The concentration of SO2 upon entering a desulfurization unit is important in the FGD process. The average concentration of SO2 in flue gas is 1800 ppm for medium to high sulfur-content coal which is well suited for regenerative FGD method. The increment in SO2 inlet concentration usually leads to enhancement in SO2 sorption rate, where in some cases, linear correlation can be observed. This can be attributed to the increase in the SO2 concentration gradient as more SO2 molecules are available which consequently enhance the diffusion driving force and sorption capacity (Chen et al. 2016). However, better driving force caused by increment of SO2 does not necessarily mean that the removal efficiency obtained by the sorbent is enhanced as this process is also critically influenced by the quantity of sorbent used. As shown in Fig. 15, in the case where the ratio of sorbent utilized to inlet SO2 concentration is too low, further increment of inlet SO2 concentration will have no further enhancement on the process as the removal capacity will remain constant while the removal efficiency is reduced (Deng and Jia 2012; Rahmani et al. 2015). This is attributed to the rapid increment in feed SO2 amount in comparison with the amount of SO2 that could be adsorbed/absorbed. In addition, limited space is available for high feed gas concentration where the sorbent will be saturated, consequently leading to shorter breakthrough time and reduction in SO2 removal efficiency (Deng and Jia 2012; Rahmani et al. 2015). On the other hand, when the ratio of sorbent utilized to the ratio of inlet SO2 concentration is too high, the amount of SO2 molecules to be absorbed/adsorbed may be insufficient; thus, a huge amount of sorbent is wasted and optimal removal capacity is not achieved (Severa et al. 2018).
Liquid/gas ratio
As the dry FGD method is conducted in the absence of liquid, variation of the liquid/gas ratio (RLG) only effectively affects the removal capacity of SO2 in the wet FGD method. RLG can be expressed as the ratio between liquid flow as a function of treated gas flow, at the same temperature and pressure. In the condition where invariable concentration of SO2 gas is used, higher RLG will provide greater liquid-gas mass transfer effective surface area as more liquid sorbents are in contact with SO2 gas, increasing the alkalinity and facilitating mass transfer which consequently enhances the removal efficiency of SO2 (Rahmani et al. 2015). Nonetheless, similar to other parameters governing desulfurization efficiency, further increase in RLG ratio beyond a certain critical point only induces small improvement in the overall SO2 removal efficiency. As the liquid flow is increased, the amount of droplets per unit volume will significantly increase which will collide with each other and form larger droplets, causing the effective mass transfer to be reduced (Zhu et al. 2015). Optimal RLG should always be used as RLG value beyond the critical point will also increase energy consumption and operating cost and cause wastage of sorbent (Rahmani et al. 2015; Zhu et al. 2015).
Incorporation of additives
In the dry FGD method, raw AC and MS sorbent typically suffers from low SO2 adsorption capacity, short breakthrough time, and weak interaction between the sorbents. Additive is widely incorporated into desulfurization sorbent due to its capability to alter the chemical and physical properties of the sorbent and provide active sites for the attachment of SO2. Modification of the sorbent surface could lead to generation of certain functional groups with high selectivity towards SO2, while at the same time destroying functional groups that hinder SO2 adsorption (Abdulrasheed et al. 2018). Additives which can provide sufficient basicity such as metal salt, amines, and hydroxide solutions are commonly added to counter the increase in acidity brought by adsorbed acidic SO2 molecules. Table 15 summarizes different types of additives previously reported in SO2 removal studies.
The incorporation of additives onto sorbents always results in improvement of the sorption capacities; however, other reaction parameters should also be regulated to obtain optimal adsorption conditions. Taking MS as an example, both metal-modified and amine-modified MS exhibit high sorption capacities, but all sorbents from the latter group outperformed the former as shown in Table 11. It should be noted that the metal-modified MS performance has been analyzed at a very high temperature (673 K) and very low inlet SO2 concentration (250 ppm) compared with the lower temperature of 298–373 K and higher SO2 concentration of 500–2000 ppm for amine-based sorbent. As discussed in “Reaction temperature” and “Inlet SO2 concentration,” lower reaction temperature and higher inlet concentration are highly beneficial towards the SO2 removal performance.
In semi-dry FGD, SO2 removal efficiency was enhanced with the increase of additive concentration. The incorporation of liquid-phase oxidizing additives such as K2S2O8, H2O8, and NaClO2 facilitates the respective oxidization of SO2, sulfites (SO32−), and hydrogen sulfites (HSO3−) into SO3, sulfates (SO42−), and hydrogen sulfites (HSO4−) as the contact between SO2 and alkaline droplets occurs (Yi et al. 2020). As SO3 and H2SO4 possess high solubility, the mass transfer resistance can be overcome easily, thus aiding the desulfurization reaction. The addition of alkaline additives with hygroscopic nature such as NaOH is highly beneficial for semi-dry FGD as it will prolong the lifetime of the slurry droplets (Du et al. 2020).
Conclusion
Various flue gas cleaning technologies for removing SO2 from the atmosphere have been widely adopted by the industry with the flue gas desulfurization method being the preferred method due to the ability of achieving high removal capacity, simplicity, and possibility of sorbent regeneration. Development of newer flue gas desulfurization methods is constantly progressing with the main objective of attaining high desulfurization efficiency commonly obtained via wet FGD method and reducing or removing the production of waste and byproducts typically acquired with the dry FGD method. The utilization of ionic liquids (ILs), deep eutectic solvents (DES), ammonium halide solution, the Bunsen reaction, alkyl-aniline solution, amino acid solution, calcium lactate solution, aluminum sulfate, magnesia, and wastewater as sorbents in wet FGD has successfully reduced problems commonly faced by wet FGD due to having great regenerability and production of useful byproducts. On the other hand, low removal efficiency frequently suffered by the dry FGD system can be overcome by substituting typical sorbent with metal- and surface-modified activated carbon, modified mesoporous silica catalyst, carbon silica composites, metal oxides, zeolites, and metal-organic frameworks. Numerous reaction parameters should be considered in these methods as high desulfurization capacity and efficiency are always desired. Factors such as flue gas composition, reaction temperature, pH, and inlet SO2 concentration are considered the main governing parameters for all desulfurization methods while other factors (L/G ratio, incorporation of additives) only affect several of the discussed processes. This review on the existing and emerging flue gas cleaning technologies accompanied by parameters affecting removal capacity is hoped to be helpful to facilitate and guide further and future development regarding this specific topic.
References
Abdulrasheed AA, Jalil AA, Triwahyono S, Zaini MAA, Gambo Y, Ibrahim M (2018) Surface modification of activated carbon for adsorption of SO2 and NOX: a review of existing and emerging technologies. Renew Sust Energ Rev 94:1067–1085. https://doi.org/10.1016/j.rser.2018.07.011
Abramenko N, Kustov L, Metelytsia L, Kovalishyn V, Tetko I, Peijnenburg W (2020) A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids. J Hazard Mater 384:121429. https://doi.org/10.1016/j.jhazmat.2019.121429
Atanes E, Nieto-Marquez A, Cambra A, Ruiz-Perez MC, Fernandez-Martinez F (2012) Adsorption of SO2 onto waste cork powder-derived activated carbons. Chem Eng J 211–212:60–67. https://doi.org/10.1016/j.cej.2012.09.043
Bai BC, Lee CW, Lee YS, Im JS (2016) Metal impregnate on activated carbon fiber for SO2 gas removal: assessment of pore structure, Cu supporter, breakthrough, and bed utilization. Colloids Surf A Physicochem Eng Asp 509:73–79. https://doi.org/10.1016/j.colsurfa.2016.08.038
Berger M, Fioux P, Dorge S, Nouali H, Habermacher D, Fiani E, Vierling M, Moliere M, Brilhac JF, Patarin J (2017) Structure-performance relationship in CuO/SBA-15-type SOx adsorbent: evolution of copper-based species under different regenerative treatments. Catal Sci Technol 7:4115–4128. https://doi.org/10.1039/c7cy01010a
Berger M, Brillard A, Dorge S, Habermacher D, Nouali H, Kerdoncuff P, Vierling M, Moliere M, Patarin J, Brilhac J (2020) Modeling SOx trapping on a copper-doped CuO/SBA-15 sorbent material. J Hazard Mater 385:121579. https://doi.org/10.1016/j.jhazmat.2019.121579
Boutillara Y, Tombeur JL, De Weireld G, Lodewyckx P (2019) In-situ copper impregnation by chemical activation with CuCl2 and its application to SO2 and H2S capture by activated carbons. Chem Eng J 372:631–637. https://doi.org/10.1016/j.cej.2019.04.183
Brandt P, Nuhnen A, Lange M, Möllmer J, Weingart O, Janiak C (2019) Metal-organic frameworks with potential application for SO2 separation and flue gas desulfurization. ACS Appl Mater Interfaces 11:17350–17358. https://doi.org/10.1021/acsami.9b00029
Carter JH, Han X, Moreau FY, Da Silva I, Nevin A, Godfrey HGW, Tang CC, Yang S, Schröder M (2018) Exceptional adsorption and binding of sulfur dioxide in a robust zirconium-based metal-organic framework. J Am Chem Soc 140:15564–15567. https://doi.org/10.1021/jacs.8b08433
Chen K, Lin W, Yu X, Luo X, Ding F, He X, Haoran L, Wang C (2015) Designing of anion-functionalized ionic liquids for efficient capture of SO2 from flue gas. AICHE J 00:1–7. https://doi.org/10.1002/aic
Chen M, Deng X, He F (2016) Removal of SO2 from flue gas using basic aluminum sulfate solution with the byproduct oxidation inhibition by ethylene glycol. Energy Fuel 30:1183–1191. https://doi.org/10.1021/acs.energyfuels.5b02411
Chen Y, Huang B, Huang M, Lu Q, Huang B (2018a) Sticky rice lime mortar-inspired in situ sustainable design of novel calcium-rich activated carbon monoliths for efficient SO2 capture. J Clean Prod 183:449–457. https://doi.org/10.1016/j.jclepro.2018.02.167
Chen Y, Jiang B, Dou H, Zhang L, Tantai X, Sun Y, Zhang H (2018b) Highly efficient and reversible capture of low partial pressure SO2 by functional deep eutectic solvents. Energy Fuel 32:10737–10744. https://doi.org/10.1021/acs.energyfuels.8b01794
Chen M, Xie B, He F, Deng X (2019) Efficient inhibition of S(IV) oxidation in a novel basic aluminum sulfate regenerative flue gas desulfurization process by ethylene glycol: kinetics and reaction mechanism. Energy Fuel 33:1383–1391. https://doi.org/10.1021/acs.energyfuels.8b03862
Chiu CH, Lin HP, Kuo TH, Chen SS, Chang TC, Su KH, Hsi HC (2015) Simultaneous control of elemental mercury/sulfur dioxide/nitrogen monoxide from coal-fired flue gases with metal oxide-impregnated activated carbon. Aerosol Air Qual Res 15:2094–2103. https://doi.org/10.4209/aaqr.2015.03.0176
Cui G, Zhang F, Zhou X, Li H, Wang J, Wang C (2015) Tuning the basicity of cyano-containing ionic liquids to improve SO2 capture through cyano-sulfur interactions. Chem - A Eur J 21:5632–5639. https://doi.org/10.1002/chem.201405683
Czuma N, Zarȩbska K, Baran P (2016) Analysis of the influence of fusion synthesis parameters on the SO2 sorption properties of zeolites produced out of fly ash. E3S Web Conf 10:23–26. https://doi.org/10.1051/e3sconf/20161000010
Czuma N, Baran P, Franus W, Zabierowski P, Zarębska K (2019) Synthesis of zeolites from fly ash with the use of modified two-step hydrothermal method and preliminary SO2 sorption tests. Adsorpt Sci Technol 37:61–76. https://doi.org/10.1177/0263617418810607
Czuma N, Franus W, Baran P, Ćwik A, Zareska K (2020a) SO2 sorption properties of fly ash zeolites. Turk J Chem 44:155–167. https://doi.org/10.3906/kim-1905-50
Czuma N, Panek R, Baran P, Zarębska K (2020b) The influence of binder for pelletization of fly ash zeolites on sorption properties in relation to SO2. Clay Miner:1–8. https://doi.org/10.1180/clm.2020.3
Deng R, Jia L (2012) Reversible removal of SO2 at low temperature by L-a-alanine supported on γ-Al2O3. Fuel 93:385–390. https://doi.org/10.1016/j.fuel.2011.11.024
Deng R, Jia L, Song Q, Su S, Tian Z (2012) Reversible absorption of SO2 by amino acid aqueous solutions. J Hazard Mater 229–230:398–403. https://doi.org/10.1016/j.jhazmat.2012.06.020
Deng H, Yi H, Tang X, Liu H, Zhou X (2013) Interactive effect for simultaneous removal of SO2, NO, and CO2 in flue gas on ion exchanged zeolites. Ind Eng Chem Res 52:6778–6784. https://doi.org/10.1021/ie303319f
Deng D, Han G, Jiang Y (2015) Investigation of a deep eutectic solvent formed by levulinic acid with quaternary ammonium salt as an efficient SO2 absorbent. New J Chem 39:32–34. https://doi.org/10.1039/C5NJ01629K
Deng ZL, Liang MN, Li HH, Zhu ZJ (2016) Advances in preparation of modified activated carbon and its applications in the removal of chromium (VI) from aqueous solutions. IOP Conf Ser Earth Environ Sci 39:012065. https://doi.org/10.1088/1755-1315/39/1/012065
Deng D, Liu X, Gao B (2017) Physicochemical properties and investigation of azole-based deep eutectic solvents as efficient and reversible SO2 absorbents. Ind Eng Chem Res 56:13850–13856. https://doi.org/10.1021/acs.iecr.7b02478
Du C, Yi H, Tang X, Zhao S, Gao F, Yu Q, Yang Z, Yang K, Xie X, Ma Y (2020) Desulfurization and denitrification experiments in SDA system: a new high-efficient semi-dry process by NaClO2. Sep Purif Technol 230:115873. https://doi.org/10.1016/j.seppur.2019.115873
Duan E, Zhang P, Yang K, Liang W, Yu M, Wang S, Niu J (2016) Effect of alkyl and halide moieties on the absorption and stratification of SO2 in tetrabutylammonium halide aqueous solutions. RSC Adv 6:55401–55405. https://doi.org/10.1039/c6ra05677f
ECE (2015) Guidance document on control techniques for emissions of sulphur, nitrogen oxides, volatile organic compounds and particulate matter (including PM10, PM2.5 and black carbon) from stationary sources. https://www.unece.org/fileadmin/DAM/env/documents/2012/EB/ECE.EB.AIR.117_AV.pdf. Accessed 14 February 2020
Fakhari MA, Rahimi A, Hatamipour MS, Fozooni A (2015) Non-isothermal modeling of simultaneous CO2 and SO2 removal in a semi-dry spouted bed reactor. Process Saf Environ Prot 98:342–353. https://doi.org/10.1016/j.psep.2015.09.001
Fan X, Zhang X (2013) Simultaneous removal of SO2 and NO with activated carbon from sewage sludge modified by chitosan. Appl Mech Mater 253–255:960–964. https://doi.org/10.4028/www.scientific.net/AMM.253-255.960
Flagiello D, Erto A, Lancia A, Di Natale F (2018) Experimental and modelling analysis of seawater scrubbers for sulphur dioxide removal from flue-gas. Fuel 214:254–263. https://doi.org/10.1016/j.fuel.2017.10.098
Fu Y, Wang Z, Li S, He X, Pan C, Yan J, Yu G (2018) Functionalized covalent triazine frameworks for effective CO2 and SO2 removal. ACS Appl Mater Interfaces 10:36002–36009. https://doi.org/10.1021/acsami.8b13417
Furtado AMB, Barpaga D, Mitchell LA, Wang Y, Decoste JB, Peterson GW, Levan MD (2012) Organoalkoxysilane-grafted silica composites for acidic and basic gas adsorption. Langmuir 28:17450–17456. https://doi.org/10.1021/la303203k
Gaudin P, Dorge S, Nouali H, Kehrli D, Michelin L, Josien L, Fioux P, Vidal L, Soulard M, Vierling M, Molière M, Brilhac JF, Patarin J (2015) Synthesis of Cu-Ce/KIT-6 materials for SOx removal. Appl Catal A Gen 504:110–118. https://doi.org/10.1016/j.apcata.2014.11.024
Gaudin P, Fioux P, Dorge S, Nouali H, Vierling M, Fiani E, Moliere M, Brilhac JF, Patarin J (2016) Formation and role of Cu+ species on highly dispersed CuO/SBA-15 mesoporous materials for SOx removal: an XPS study. Fuel Process Technol 153:129–136. https://doi.org/10.1016/j.fuproc.2016.07.015g
Gawande MB, Pandey K, Jayaram RV (2012) Role of mixed metal oxides in catalysis science — versatile applications in organic synthesis. Catal Sci Technol 2:1113–1125. https://doi.org/10.1039/c2cy00490a
Glomb S, Woschko D, Makhloufi G, Janiak C (2017) Metal-organic frameworks with internal urea-functionalized dicarboxylate linkers for SO2 and NH3 adsorption. ACS Appl Mater Interfaces 9:37419–37434. https://doi.org/10.1021/acsami.7b10884
Guo JX, Shu S, Liu XL, Wang XJ, Yin HQ, Chu YH (2017) Influence of Fe loadings on desulfurization performance of activated carbon treated by nitric acid. Environ Technol 38:266–276. https://doi.org/10.1080/09593330.2016.1189973
Hou Y, Zhang R, Han X, Huang Z, Cui Y (2017) The mechanism of CO regeneration on V2O5/AC catalyst and sulfur recovery. Chem Eng J 316:744–750. https://doi.org/10.1016/j.cej.2017.02.020
Hrdlička J, Dlouhý T (2019) Full-scale evaluation of SO2 capture increase for semi-dry FGD technology. J Energy Inst 92:1399–1405. https://doi.org/10.1016/j.joei.2018.09.002
Huang K, Deng X, Chen M (2017) Falling film evaporator for desorption of basic aluminum sulfate SO2-rich solution and enhancement of heat and mass transfer. Energy Fuel 31:13871–13882. https://doi.org/10.1021/acs.energyfuels.7b02206
Huang K, Deng X, He F (2018) SO2 enhanced desorption from basic aluminum sulfate desulphurization-regeneration solution by falling-film evaporation. RSC Adv 8:5550–5558. https://doi.org/10.1039/c7ra12963g
Jiang B, Zhang H, Zhang L, Zhang N, Huang Z, Chen Y, Sun Y, Tantai X (2019) Novel deep eutectic solvents for highly efficient and reversible absorption of SO2 by preorganization strategy. ACS Sustain Chem Eng 7:8347–8357. https://doi.org/10.1021/acssuschemeng.8b06822
Kim C, Choi W, Choi M (2019a) SO2 -resistant amine-containing CO2 adsorbent with a surface protection layer. ACS Appl Mater Interfaces 11:16586–16593. https://doi.org/10.1021/acsami.9b02831
Kim MI, Im JS, Seo SW, Cho JH, Lee YS, Kim S (2019b) Preparation of pitch-based activated carbon with surface-treated fly ash for SO2 gas removal. Carbon Lett:1–7. https://doi.org/10.1007/s42823-019-00107-y
Kudłak B, Owczarek K, Namieśnik J (2015) Selected issues related to the toxicity of ionic liquids and deep eutectic solvents-a review. Environ Sci Pollut Res 22:11975–11992. https://doi.org/10.1007/s11356-015-4794-y
Kumar A, McGrady GS, Passmore J, Grein F, Decken A (2012) Reversible SO2 uptake by tetraalkylammonium halides: energetics and structural aspects of adduct formation between SO2 and halide ions. Z Anorg Allg Chem 638:744–753. https://doi.org/10.1002/zaac.201100476
Kumar P, Pournara A, Kim KH, Bansal V, Rapti S, Manos MJ (2017) Metal-organic frameworks: challenges and opportunities for ion-exchange/sorption applications. Prog Mater Sci 86:25–74. https://doi.org/10.1016/j.pmatsci.2017.01.002
Li B, Ma C (2018) Study on the mechanism of SO2 removal by activated carbon. Energy Procedia 153:471–477. https://doi.org/10.1016/j.egypro.2018.10.063
Li X, Zhang L, Zheng Y, Zheng C (2015) SO2 absorption performance enhancement by ionic liquid supported on mesoporous molecular sieve. Energy Fuel 29:942–953. https://doi.org/10.1021/ef5022285
Li G, Wang Q, Jiang T, Luo J, Rao M, Peng Z (2017) Roll-up effect of sulfur dioxide adsorption on zeolites FAU 13X and LTA 5A. Adsorption 23:699–710. https://doi.org/10.1007/s10450-017-9887-0
Li L, Da Silva I, Kolokolov DI, Han X, Li J, Smith G et al (2019) Post-synthetic modulation of the charge distribution in a metal-organic framework for optimal binding of carbon dioxide and sulfur dioxide. Chem Sci 10:1472–1482. https://doi.org/10.1039/c8sc01959b
Liangliang Z, Shuying W, Yue G, Baochang S, Yong L, Haikui Z, Guangwen C, Jianfeng C (2019) Absorption of SO2 with calcium-based solution in a rotating packed bed. Sep Purif Technol 214:148–155. https://doi.org/10.1016/j.seppur.2018.03.065
Lidong W, Yongliang M, Wendi Z, Qiangwei L, Yi Z, Zhanchao Z (2013) Macrokinetics of magnesium sulfite oxidation inhibited by ascorbic acid. J Hazard Mater 258–259:61–69. https://doi.org/10.1016/j.jhazmat.2013.04.018
Liu Y, Shan Y, Wang Y (2020) Novel simultaneous removal technology of NO and SO2 using a semi-dry microwave activation persulfate system. Environ Sci Technol 54:2031–2042. https://doi.org/10.1021/acs.est.9b07221
Long G, Yang C, Yang X, Zhao T, Liu F, Cao J (2020) Bisazole-based deep eutectic solvents for efficient SO2 absorption and conversion without any additives. ACS Sustain Chem Eng 8:2608–2613. https://doi.org/10.1021/acssuschemeng.9b07735
Ma X, Li J, Rankin MA, Croll LM, Dahn JR (2017) Highly porous MnOx prepared from Mn(C2O4).3H2O as an adsorbent for the removal of SO2 and NH3. Microporous Mesoporous Mater 244:192–198. https://doi.org/10.1016/j.micromeso.2016.10.019
Ma Y, Yuan D, Mu B, Gao L, Zhang X, Zhang H (2018) Synthesis, properties and application of double salt (NH4)2.Mg(SO4)2·6H2O in wet magnesium-ammonia FGD process. Fuel 219:12–16. https://doi.org/10.1016/j.fuel.2018.01.055
Mathieu Y, Soulard M, Patarin J, Molière M (2012) Mesoporous materials for the removal of SO2 from gas streams. Fuel Process Technol 99:35–42. https://doi.org/10.1016/j.fuproc.2012.02.005
Meimand MM, Javid N, Malakootian M (2019) Adsorption of sulfur dioxide on clinoptilolite/nano iron oxide and natural clinoptilolite. Heal Scope 8:69158. https://doi.org/10.5812/jhealthscope.69158
Meng X, Wang J, Jiang H, Zhang X, Liu S, Hu Y (2016) Guanidinium-based dicarboxylic acid ionic liquids for SO2 capture. J Chem Technol Biotechnol 92:767–774. https://doi.org/10.1002/jctb.5052
Nieto-Márquez A, Atanes E, Morena J, Fernández-Martínez F, Valverde JL (2016) Upgrading waste tires by chemical activation for the capture of SO2. Fuel Process Technol 144:274–281. https://doi.org/10.1016/j.fuproc.2016.01.009
Ning-Jie F, Jia-Xiu G, Song S, Jian-Jun L, Ying-Hao C (2017) Influence of textures, oxygen-containing functional groups and metal species on SO2 and NO removal over Ce-Mn/NAC. Fuel 202:328–337. https://doi.org/10.1016/j.fuel.2017.04.035
Ogenga DO, Mbarawa MM, Lee KT, Mohamed AR, Dahlan I (2010) Sulphur dioxide removal using South African limestone/siliceous materials. Fuel 89:2549–2555. https://doi.org/10.1016/j.fuel.2010.04.029
Ozturk B, Yildirim Y (2008) Investigation of sorption capacity of pumice for SO2 capture. Process Saf Environ Prot 86:31–36. https://doi.org/10.1016/j.psep.2007.10.010
Pedrolo DRS, De Menezes Quines LK, De Souza G, Marcilio NR (2017) Synthesis of zeolites from Brazilian coal ash and its application in SO2 adsorption. J Environ Chem Eng 5:4788–4794. https://doi.org/10.1016/j.jece.2017.09.015
Pham XM, Pham DL, Hanh NT, Dang Thi TA, Thuy Giang LN, Phuong HT, Anh NT, Nhung HT, le GT, Hoang MH, Nguyen TV (2019) An initial evaluation on the adsorption of SO2 and NO2 over porous Fe3O4 nanoparticles synthesized by facile scalable method. J Chem 2019:9742826–9742827. https://doi.org/10.1155/2019/9742826
Poullikkas A (2015) Review of design, operating, and financial considerations in flue gas desulfurization systems. Energy Technol Policy 2:92–103. https://doi.org/10.1080/23317000.2015.1064794
Raghunath CV, Pandey P, Saini R, Mondal MK (2016) Absorption of SO2 and NO through an integrative process with a cost-effective aqueous oxidant. Perspect Sci 8:699–701. https://doi.org/10.1016/j.pisc.2016.06.063
Rahmani F, Mowla D, Karimi G, Golkhar A, Rahmatmand B (2015) SO2 removal from simulated flue gas using various aqueous solutions : absorption equilibria and operational data in a packed column. Sep Purif Technol 153:162–169. https://doi.org/10.1016/j.seppur.2014.10.028
Razak HA, Abdullah N, Setiabudi HD, Yee CS, Ainirazali N (2019) Influenced of Ni loading on SBA-15 synthesized from oil palm ash silica for syngas production. IOP Conf Ser Mater Sci Eng 702:012024. https://doi.org/10.1088/1757-899X/702/1/012024
Ren S, Hou Y, Tian S, Wu W, Liu W (2012) Deactivation and regeneration of an ionic liquid during desulfurization of simulated flue gas. Ind Eng Chem Res 51:3425–3429. https://doi.org/10.1021/ie202328c
Rodríguez-Albelo LM, López-Maya E, Hamad S, Ruiz-Salvador AR, Calero S, Navarro JAR (2017) Selective sulfur dioxide adsorption on crystal defect sites on an isoreticular metal organic framework series. Nat Commun 8:14457. https://doi.org/10.1038/ncomms14457
Rosas JM, Ruiz-Rosas R, Rodríguez-Mirasol J, Cordero T (2017) Kinetic study of SO2 removal over lignin-based activated carbon. Chem Eng J 307:707–721. https://doi.org/10.1016/j.cej.2016.08.111
Roy P, Sardar A (2015) SO2 emission control and finding a way out to produce sulphuric acid from industrial SO2 emission. J Chem Eng Process Technol 6:1–7. https://doi.org/10.4172/2157-7048.1000230
Salazar Hoyos LA, Faroldi BM, Cornaglia LM (2020) A coke-resistant catalyst for the dry reforming of methane based on Ni nanoparticles confined within rice husk-derived mesoporous materials. Catal Commun 135:105898. https://doi.org/10.1016/j.catcom.2019.105898
Sales RV, Moura HOMA, Câmara ABF, Rodríguez-Castellón E, Silva JAB, Pergher SBC et al (2019) Assessment of Ag nanoparticles interaction over low-cost mesoporous silica in deep desulfurization of diesel. Catalysts 9:651. https://doi.org/10.3390/catal9080651
Savage M, Cheng Y, Easun TL, Eyley JE, Argent SP, Warren MR, Lewis W, Murray C, Tang CC, Frogley MD, Cinque G, Sun J, Rudić S, Murden RT, Benham MJ, Fitch AN, Blake AJ, Ramirez-Cuesta AJ, Yang S, Schröder M (2016) Selective adsorption of sulfur dioxide in a robust metal–organic framework material. Adv Mater 28:8705–8711. https://doi.org/10.1002/adma.201602338
Severa G, Bethune K, Rocheleau R, Higgins S (2015) SO2 sorption by activated carbon supported ionic liquids under simulated atmospheric conditions. Chem Eng J 265:249–258. https://doi.org/10.1016/j.cej.2014.12.051
Severa G, Head J, Bethune K, Higgins S, Fujise A (2018) Comparative studies of low concentration SO2 and NO2 sorption by activated carbon supported [C2mim][Ac] and KOH sorbents. J Environ Chem Eng 6:718–727. https://doi.org/10.1016/j.jece.2017.12.020
Sfechiş S, Abrudean M, Sas DM, Ungureşan ML, Clitan I, Mureşan V (2015) Modeling and simulation of the sulfur dioxide adsorption process in natural zeolites. Appl Mech Mater 811:35–42. https://doi.org/10.4028/www.scientific.net/amm.811.35
Sheng K, Kang Y, Li J, Xu H, Li D (2020) High-efficiency absorption of SO2 by a new type of deep eutectic solvents. Energy Fuel 34:3440–3448. https://doi.org/10.1021/acs.energyfuels.9b03619
Silas K, Ghani WAWAK, Choong TSY, Rashid U (2018) Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NOx removal from flue gas. Fuel Process Technol 180:155–165. https://doi.org/10.1016/j.fuproc.2018.08.018
Smith GL, Eyley JE, Han X, Zhang X, Li J, Jacques NM, Godfrey HGW, Argent SP, McCormick McPherson LJ, Teat SJ, Cheng Y, Frogley MD, Cinque G, Day SJ, Tang CC, Easun TL, Rudić S, Ramirez-Cuesta AJ, Yang S, Schröder M (2019) Reversible coordinative binding and separation of sulfur dioxide in a robust metal–organic framework with open copper sites. Nat Mater 18:1358–1365. https://doi.org/10.1038/s41563-019-0495-0
Song X, Ma X, Ning G, Gao J (2017) Pitch-based nitrogen-doped mesoporous carbon for flue gas desulfurization. Ind Eng Chem Res 56:4743–4749. https://doi.org/10.1021/acs.iecr.7b00054
Sumathi S, Bhatia S, Lee KT, Mohamed AR (2010) SO2 and NO simultaneous removal from simulated flue gas over cerium-supported palm shell activated at lower temperatures–role of cerium on NO removal. Energy Fuel 24:427–431. https://doi.org/10.1021/ef900843g
Sun S, Niu Y, Xu Q, Sun Z, Wei X (2015) Efficient SO2 absorptions by four kinds of deep eutectic solvents based on choline chloride. Ind Eng Chem Res 54:8019–8024. https://doi.org/10.1021/acs.iecr.5b01789
Sun Y, Sun G, Sage V, Sun Z, Zhang J, Zhang L (2017) Preparation of hybrid porous carbon using black liquor lignin impregnated with steelmaking slag and its performance in SO2 removal. Environ Prog Sustain Energy Environ Prog Sustai Energy 00:1–11. https://doi.org/10.1002/ep.10350
Sun Y, Yang G, Zhang L (2018) Hybrid adsorbent prepared from renewable lignin and waste egg shell for SO2 removal: characterization and process optimization. Ecol Eng 115:139–148. https://doi.org/10.1016/j.ecoleng.2018.02.013
Tailor R, Sayari A (2016) Grafted propyldiethanolamine for selective removal of SO2 in the presence of CO2. Chem Eng J 289:142–149. https://doi.org/10.1016/j.cej.2015.12.084
Tailor R, Abboud M, Sayari A (2014a) Supported polytertiary amines: highly efficient and selective SO2 adsorbents. Environ Sci Technol 48:2025–2034
Tailor R, Ahmadalinezhad A, Sayari A (2014b) Selective removal of SO2 over tertiary amine-containing materials. Chem Eng J 240:462–468. https://doi.org/10.1016/j.cej.2013.11.002
Tian S, Hou Y, Wu W, Ren S, Wang C, Qian J (2015) Reversible absorption of SO2 from simulated flue gas by aqueous calcium lactate solution. J Taiwan Inst Chem Eng 54:71–75. https://doi.org/10.1016/j.jtice.2015.03.026
US EPA (2018) Fact sheet proposed decision primary National Ambient Air Quality Standard for sulfur oxides. Retrieved from https://www.epa.gov/sites/production/files/2018-05/documents/fact_sheet_primary_so2_naaqs_npr_final.pdf accessed 15 march 2020
Vo HT, Cho SH, Lee U, Jae J, Kim H, Lee H (2019) Reversible absorption of SO2 with alkyl-anilines: the effects of alkyl group on aniline and water. J Ind Eng Chem 69:338–344. https://doi.org/10.1016/j.jiec.2018.09.033
Wang P, Jiang X, Zhang C, Zhou Q, Li J, Jiang W (2017) Desulfurization and regeneration performance of titanium-ore-modified activated coke. Energy Fuel 31:5266–5274. https://doi.org/10.1021/acs.energyfuels.6b03153
Wang A, Fan R, Pi X, Zhou Y, Chen G, Chen W, Yang Y (2018) Nitrogen-doped microporous carbons derived from pyridine ligand-based metal-organic complexes as high-performance SO2 adsorption sorbents. ACS Appl Mater Interfaces 10:37407–37416. https://doi.org/10.1021/acsami.8b12739
Wang A, Fan R, Pi X, Hao S, Zheng X, Yang Y (2019) N-doped porous carbon derived by direct carbonization of metal-organic complexes crystal materials for SO2 adsorption. Cryst Growth Des 19:1973–1984. research-article. https://doi.org/10.1021/acs.cgd.8b01925
Wei L, Gao Z, Wang Y (2017) Integrated two-stage adsorption for selective removal of CO2 and SO2 by amine-functionalized SBA-15. Asia-Pac J Chem Eng 12:660–670. https://doi.org/10.1002/apj.2108
World Health Organisation (WHO) (2018) Ambient (outdoor) air pollution. Retrieved from https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health Accessed 31 March 2020
Wu W, Han B, Gao H, Liu Z, Jiang T, Huang J (2004) Desulfurization of flue gas: SO2 absorption by an ionic liquid. Angew Chem 116:2469–2471. https://doi.org/10.1002/ange.200353437
Wu F, Yue K, Gao W, Gong M, Ma X, Zhou W (2020) Numerical simulation of semi-dry flue gas desulfurization process in the powder-particle spouted bed. Adv Powder Technol 31:323–331. https://doi.org/10.1016/j.apt.2019.10.024
Yan Z, Liu L, Zhang Y, Liang J, Wang J, Zhang Z, Wang X (2013) Activated semi-coke in SO2 removal from flue gas: selection of activation methodology and desulfurization mechanism study. Energy Fuel 27:3080–3089. https://doi.org/10.1021/ef400351a
Yan L, Lu X, Wang Q, Guo Q (2014) Recovery of SO2 and MgO from by-products of MgO wet flue gas desulfurization. Environ Eng Sci 31:621–630. https://doi.org/10.1089/ees.2014.0004
Yang D, Hou M, Ning H, Zhang J, Ma J, Yang G, Han B (2013) Efficient SO2 absorption by renewable choline chloride-glycerol deep eutectic solvents. Green Chem 15:2261–2265. https://doi.org/10.1039/C3GC40815A
Yang L, Jiang X, Yang ZS, Jiang WJ (2015) Effect of MnSO4 on the removal of SO2 by manganese-modified activated coke. Ind Eng Chem Res 54:1689–1696. https://doi.org/10.1021/ie503729a
Yang D, Han Y, Qi H, Wang Y, Dai S (2017a) Efficient absorption of SO2 by EmimCl-EG deep eutectic solvents. ACS Sustain Chem Eng 5:6382–6386. https://doi.org/10.1021/acssuschemeng.7b01554
Yang L, Jiang X, Jiang W, Wang P, Jin Y (2017b) Cyclic regeneration of pyrolusite-modified activated coke by blending method for flue gas desulfurization. Energy Fuel 31:4556–4564. https://doi.org/10.1021/acs.energyfuels.7b00125
Yang W, Zhang J, Ma Q, Zhao Y, Liu Y, He H (2017c) Heterogeneous reaction of SO2 on manganese oxides: the effect of crystal structure and relative humidity. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-04551-6
Yavuz R, Akyildiz H, Karatepe N, Çetinkaya E (2010) Influence of preparation conditions on porous structures of olive stone activated. Fuel Process Technol 91:80–87. https://doi.org/10.1016/j.fuproc.2009.08.018
Yi H, Du C, Ma Y, Tang X, Zhao S, Gao F, Yang Z, Huang Y, Yang K, Xie X (2020) A novel semi-dry method for the simultaneous removal of Hg and SO2 using spray drying absorption method. J Chem Technol Biotechnol 95:1–10. https://doi.org/10.1002/jctb.6328
Zhang Q, Gui K (2009) A novel semidry flue gas desulfurization process with the magnetically fluidized bed reactor. J Hazard Mater 168:1341–1345. https://doi.org/10.1016/j.jhazmat.2009.03.019
Zhang L, Cui L, Li B, Wang W, Ma C (2010). Experimental Study of SO2 Removal by Powder Activated Carbon in Fluidized Bed Reactor. 2010 Asia-Pacific Power Energy Eng. Conf. 1–4. https://doi.org/10.1109/APPEEC.2010.5448224
Zhang Y, Wang T, Yang H, Zhang H, Zhang X (2015) Experimental study on SO2 recovery using a sodium-zinc sorbent based flue gas desulfurization technology. Chin J Chem Eng 23:241–246. https://doi.org/10.1016/j.cjche.2014.10.007
Zhang F, Cui G, Zhao N, Huang Y, Zhao Y, Wang J (2016a) Improving SO2 capture by basic ionic liquids in an acid gas mixture (10% vol SO2) through tethering a formyl group to the anions. RSC Adv 6:86082–86088. https://doi.org/10.1039/c6ra18589d
Zhang X, Feng X, Li H, Peng J, Wu Y, Hu X (2016b) Cyano-containing protic ionic liquids for highly selective absorption of SO2 from CO2: experimental study and theoretical analysis. Ind Eng Chem Res 55:11012–11021. https://doi.org/10.1021/acs.iecr.6b02588
Zhang Y, Lu D, Zhang JJ, Wu C (2016c) Synthesis and characterization of imidazolium poly (azolyl) borate ionic liquids and their potential application in SO2 absorption. RSC Adv 6:66078–66086. https://doi.org/10.1039/c6ra10356a
Zhang K, Ren S, Hou Y, Wu W (2017a) Efficient absorption of SO2 with low-partial pressures by environmentally benign functional deep eutectic solvents. J Hazard Mater 324:457–463. https://doi.org/10.1016/j.jhazmat.2016.11.012
Zhang Q, Tao Q, He H, Liu H, Komarneni S (2017b) An efficient SO2-adsorbent from calcination of natural magnesite. Ceram Int 43:12557–12562. https://doi.org/10.1016/j.ceramint.2017.06.130
Zhang Z, Wang J, Lang L (2018) Influence of key factors on the characteristics of flue gas desulfurization of basic aluminum sulfate by bubbles. ACS Omega 3:16369–16376. https://doi.org/10.1021/acsomega.8b01855
Zhao Y, Hu G (2013) Removal of sulfur dioxide from flue gas using the sludge sodium humate. Sci World J 2013:573051–573058. https://doi.org/10.1155/2013/573051
Zhao L, Li X, Qu Z, Zhao Q, Liu S, Hu X (2011) The NiAl mixed oxides: the relation between basicity and SO2 removal capacity. Sep Purif Technol 80:345–350. https://doi.org/10.1016/j.seppur.2011.04.035
Zhao Y, Hao R, Qi M (2015) Integrative process of preoxidation and absorption for simultaneous removal of SO2, NO and Hg0. Chem Eng J 269:159–167. https://doi.org/10.1016/j.cej.2015.01.064
Zhao J, Ren S, Hou Y, Zhang K, Wu W (2016a) SO2 absorption by carboxylate anion-based task-specific ionic liquids: effect of solvents and mechanism. Ind Eng Chem Res 55:12919–12928. https://doi.org/10.1021/acs.iecr.6b02801
Zhao L, Bi S, Pei J, Li X, Yu R, Zhao J, Martyniuk CJ (2016b) Adsorption performance of SO2 over ZnAl2O4 nanospheres. J Ind Eng Chem 41:151–157. https://doi.org/10.1016/j.jiec.2016.07.019
Zhi Y, Zhou Y, Su W, Sun Y, Zhou L (2011) Selective adsorption of SO2 from flue gas on triethanolamine-modified large pore SBA-15. Ind Eng Chem Res 50:8698–8702. https://doi.org/10.1021/ie2004658
Zhu F, Gao J, Chen X, Tong M, Zhou Y, Lu J (2015) Hydrolysis of urea for ammonia-based wet flue gas desulfurization. Ind Eng Chem Res 54:9072–9080. https://doi.org/10.1021/acs.iecr.5b02041
Zhu C, Duan Y, Wu CY, Zhou Q, She M, Yao T, Zhang J (2016) Mercury removal and synergistic capture of SO2/NO by ammonium halides modified rice husk char. Fuel 172:160–169. https://doi.org/10.1016/j.fuel.2015.12.061
Zhu Z, Ma Y, Qu Z, Fang L, Zhang W, Yan N (2017) Study on a new wet flue gas desulfurization method based on the Bunsen reaction of sulfur-iodine thermochemical cycle. Fuel 19:33–37. https://doi.org/10.1016/j.fuel.2017.01.045
Funding
This study was supported by Collaborative Research Grant (9023-00001) between the Universiti Malaysia Perlis and Universiti Teknologi Malaysia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hanif, M.A., Ibrahim, N. & Abdul Jalil, A. Sulfur dioxide removal: An overview of regenerative flue gas desulfurization and factors affecting desulfurization capacity and sorbent regeneration. Environ Sci Pollut Res 27, 27515–27540 (2020). https://doi.org/10.1007/s11356-020-09191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09191-4