Abstract
A biosurfactant (BS) is a surface-active metabolite that is secreted by microbial metabolism, and can be used as a substitute for chemically synthesized surfactants. The first and most critical step to the successful application of BSs is to isolate bacterial strains with strong BS-producing capabilities. In this study, a BS-producing Serratia marcescens ZCF25 was isolated from the sludge of an oil tanker. Through polyphasic characterization using Fourier-transform infrared spectroscopy, thin layer chromatography, and gas chromatography-mass spectrometry, the produced BS was classified as a lipopeptide; it can decrease the water surface tension from 72.0 to 29.50 mN m−1 and has a critical micelle concentration of 220 mg/L. The BS showed a high tolerance over a wide range of pH (2–12), temperature (50–100 °C), and salinity (10–100 g/L). Furthermore, the inoculation of S. marcescens ZCF25 with fracturing flowback fluids could significantly (P < 0.05) reduce the chemical oxygen demand, concentration of alkanes, and concentration of polycyclic aromatic hydrocarbons, with removal efficiencies of 48.9%, 65.57%, and 64%, respectively. This is the first study on the application of BS-producing S. marcescens to treat fracturing flowback fluids. S. marcescens ZCF25 is a promising candidate for use in various industrial and bioremediation applications.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants have emulsification, foaming, dispersion, and anti-adhesive properties, which allow for their use in the chemical, food processing, and cosmetic industries (Sar et al. 2019). They have a broad range of applications, and are found in petroleum, textiles, food, agriculture, and new materials (Dollinger et al. 2018; Heinz et al. 2017). For instance, surfactant flooding is one of the most commonly utilized methods to enhanced oil recovery (Mpelwa et al. 2019); surfactant-enhanced remediation is a popular soil remediation technology (Pei et al. 2017). The global demand for surfactants is huge and growing rapidly, and according to market research, there will be more than 520 billion tons of surfactants in the global market by 2022 (Singh et al. 2019). However, as the earth’s environment becomes increasingly fragile, and natural resources continue to be limited, it is imperative to explore cost-effective and environmentally friendly BSs to replace chemically synthesized surfactants.
BSs are a group of active compounds produced by microorganisms, which primarily consist of hydrophilic and hydrophobic groups. In general, BSs are classified by their microbial origins, chemical compositions, and chemical structures. BSs comprise five primary types of chemical compositions: glycolipids, lipopeptides, polysaccharide-protein complexes, phospholipids, and fatty acids (or neutral lipids) (Santos et al. 2016). Compared to chemically synthesized surfactants, BSs have the advantages of being non-toxic, biodegradable, and ecologically safe (Saravanan and Vijayakuma 2015). In addition, BSs have antibacterial, antifungal, and antitumor properties, making them potential replacements for conventional therapies in many biomedical applications (Lydon et al. 2017; Satpute et al. 2016; Spano et al. 2016). Nevertheless, the relatively high production cost of BSs at a large-scale, as compared to synthetic surfactants, restricts their widespread application (Singh et al. 2019). Therefore, different strategies should be considered in order to fully explore the efficient utilization of BSs. For instance, the direct flooding of fermentation liquid has been proven as a cost-effective method to enhance oil recovery efficiency.
Fracturing flowback fluids (FFFs) are one of the main pollutants produced in the oil and gas extraction industry (Estrada and Bhamidimarri 2016; Shrestha et al. 2017). Based on the geology of the formation and the number of frac stages in the well, tens of thousands of liters of chemicals are injected into wells every year (Kahrilas et al. 2016; McLaughlin et al. 2016). The composition of the fluid is complex, containing various chemical treatment agents, saturated hydrocarbons, and polycyclic aromatic hydrocarbons (PAHs), among others. Furthermore, the random discharge of FFFs may cause significant harm to the environment (Elsner and Hoelzer 2016). Among the remediation techniques for FFFs, biological treatment is considered as a promising approach. However, research focusing on the bioremediation of FFFs is scarce.
Serratia marcescens are facultative bacteria belonging to the Enterobacteriaceae family, which is widely distributed in the natural environment. To date, studies have reported the production of BSs by S. marcescens using different petroleum hydrocarbon compounds as substrates. The BSs produced by S. marcescens primarily include lipopeptides and glycolipids. However, the BS-producing Serratia sp. strain has rarely been used to treat wastewater, such as FFFs. Moreover, BS-producing microbes generally exhibit hydrocarbon-degradation abilities due to their capacity to emulsify hydrocarbons through BS production, which further facilitates degradation by microbes (Morales-Guzmán et al. 2017; Patel et al. 2019). Therefore, bioaugmentation through the inoculation of BS-producing microorganisms into FFFs is a promising approach.
In this study, we aimed to (1) isolate bacteria with strong BS-producing capabilities, (2) characterize the physical and chemical properties of BSs produced by isolated strains, and (3) investigate the effect of BS-producing bacteria on the bioremediation of FFFs.
Materials and methods
Isolation of BS-producing bacteria
The oil sludge collected from the ship repair enterprise of Zhoushan port, China, was used for the isolation of BS-producing bacteria. Around 0.1 g of oil sludge was added into 100 mL of mineral salt medium (MSM), supplemented with 1% carbon source (i.e., crude oil, olive oil, n-hexadecane, and phenanthrene, respectively). The composition of the MSM was as follows (L−1): 0.6 g Na2HPO4, 0.2 g KH2PO4, 4.0 g NaNO3, 0.615 g MgSO4.7H2O, 0.01 g CaCl2, and 0.01 g FeSO4. In order to identify the isolated bacteria, the 16S rRNA gene of the isolated bacteria was sequenced by TsingKe Biotech Company (Beijing, China) and analyzed using the MEGA version 7.0.
Hemolytic activity
The pure culture of each bacterial isolate was streaked on the blood agar plate and incubated at 30 °C for 48–72 h. Results were recorded based on the size of the clear zone (i.e., the zone of inhibition) (Manaargadoo-Catin et al. 2016).
Drop-collapsing test
In order to investigate the ability of cell-free supernatants to deform droplets, the drop-collapsing test was performed (Nayarisseri et al. 2018). Specifically, 50 μL of distilled water with methylene blue was added to 96 well plates that had been equilibrated at 37 °C for 1 h. Then, culture supernatant (5 μL) was added to the surface of the water, while 5 μL of distilled water was added as the control. The plates were observed after 1 min, where the collapse of the droplets indicated that the supernatants contained surfactant.
Oil spreading ability
The oil spreading experiment was carried out according to the methods described by Zhou et al. (2019). In brief, 40 mL of distilled water was added to a 90-mm diameter petri dish, followed by 100 μL of crude oil being added to the water. Subsequently, 10 μL of cell-free supernatant was dropped onto the crude oil surface. The diameter of the clear zone was measured and compared to that supplemented with 10 μL of distilled water as a control.
Emulsification activity (E24)
In this test, 3 mL of n-hexane and 3 mL of cell-free supernatant were combined in a test tube and homogenized by vortex for 2 min. After 24 h, the E24 was calculated using the following formula:
Surface tension measurement
Each selected bacterial strain was enriched for 3 days in 30 mL of MSM supplemented with carbon source (1% v/v). Cell-free supernatants of each strain before and after cultivation were prepared separately, and the surface tension of each sample was measured at 25 ± 2 °C using a surface tensiometer (BZY-201, Fangrui, Shanghai, China). All surface tension readings were collected in triplicate.
Optimization of BS-producing conditions
Six carbon sources were selected for optimization exploration, namely olive oil, glycerol, glucose, paraffin, n-hexadecane, and octadecane, which was added as the sole carbon source (1% v/v) in MSM. Five nitrogen sources were selected for optimization, namely sodium nitrate, ammonium chloride, peptone, yeast extract, and urea. Estimates of the BS yields of all treatments were represented by changes in surface tension.
Extraction and characterization of BS
Extraction of BS
The BS extraction was carried out using acid precipitation, followed by liquid-liquid extraction (Sun et al. 2016). The culture broth was centrifuged at 6000 rpm for 20 min to obtain cell-free supernatant. The supernatant was acidified to pH 2.0 with 6 N HCl to form a precipitate, which was then allowed to settle at 4 °C overnight. Subsequently, 200 mL of ethyl acetate was added to the precipitate, and the organic phase was separated and placed in a rotary vacuum evaporator and concentrated by evaporation to yield the BS of S. marcescens ZCF25, which was designated as BS-ZCF25.
Critical micelle concentration and stability evaluation of BS
The critical micelle concentration (CMC) of the extracted BS was determined in aqueous solution, which was measured by plotting the relationship between the BS concentration and the corresponding surface tension. The stability of BS-ZCF25 at different pH, temperature, and salinity was evaluated at the CMC. For the stability analysis, 10 mL BS-ZCF25 aqueous solution was heated at different temperatures (i.e., 50, 60, 70, 80, 90, and 100 °C) for 1 h, and cooled to room temperature, after which the surface tension was measured. The effect of pH on surface tension was evaluated by maintaining the pH (i.e., 2, 4, 6, 8, 10, and 12) of the BS-ZCF25 aqueous solution at different values using 2 N HCl. Similarly, the effect of NaCl concentrations (i.e., 10, 20, 40, 60, 80, and 100 g/L) on the activity of BS-ZCF25 was also investigated.
Thin layer chromatography
The extracted BS was characterized by thin layer chromatography (TLC) using a silica gel plate (Silica gel 254) and Methanol–n-hexane–acetic acid (65:25:2, v/v/v) as the solvent system. The colors of separated spots were visualized by spraying phosphomolybdic acid and a 0.25% ninhydrin solution.
Fourier-transform infrared spectroscopy
The BS was subjected to Fourier-transform infrared (FT-IR) spectroscopy analysis using a Nicolet iS10 infrared spectrometer (Thermo Fisher Scientific, Wisconsin, USA). The FT-IR spectra, with a resolution of 4 cm−1, were collected from 400 to 4000 cm−1.
Gas chromatography-mass spectrometry of fatty acids
The BS-ZCF25 was derivatized using 1 M HCl in methanol at 100 °C for 4 h and subjected to analysis using the gas chromatography-mass spectrometry (GC–MS) QP2020 (Shimadzu, Kyoto, Japan) equipped with an SH Rxi-5Sil MS column (30 m × 0.25 μm × 0.25 mm, Shimadzu). The GC–MS parameters were set according to the modified protocol of Joy et al. (2017).
Antibacterial activity of BS
The antibacterial activity of BSs was evaluated using a panel of pathogenic microorganisms including Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, and Candida albicans. Each strain was separately cultured in a nutrient broth at 37 °C overnight, and then the cell density of each strain was adjusted to 108 cfu/mL (equal to the 0.5 McFarland standard). Subsequently, the antibacterial activity of BSs on these pathogens was examined using the disk diffusion test (i.e., KB test) (Bhargav et al. 2016).
Evaluation of the petroleum hydrocarbon utilization spectra by Serratia marcescens ZCF25
A method based on the redox indicator 2,6-dichlorophenolindophenol (DCPIP) was used to evaluate the petroleum hydrocarbon utilization spectra by S. marcescens ZCF25 (Shuai et al. 2018). First, octacosane and phenanthrene were dissolved in petroleum ether in order to prepare the 10 mg/mL of stock solution. The cells were washed with 0.9% saline solution and resuspended by MSM. Subsequently, a bacterial suspension (200 μL, OD 600 = 1.0) was added to 800 μL of sterile mixture which comprised of 786 μL of MSM, 10 μL of hydrocarbon substrate (i.e., benzene, phenanthrene stock solution, n-decane, n-hexadecane, octacosane stock solution, crude oil, and atolin), and 4 μL 2,6-DCPIP solution (1.6 mg/mL). All the cultures were prepared in triplicate and incubated at 30 °C under 180 rpm. The colors of the cultures were then observed at 48, 72, and 96 h. A faded color indicated the positive degradation ability of S. marcescens ZCF25 towards the corresponding substrate, while a constant blue color suggested the negative microbial degradation ability.
Application of Serratia marcescens ZCF25 in the bioremediation of fracturing flowback fluids
The bioremediation experiment was performed by inoculating enriched cultures (5%, v/v) of S. marcescens ZCF25, pseudomonas sp. ZCF53, and a mixed culture of strains ZCF25 and ZCF53 (co-bioaugmentation), in an Erlenmeyer flask containing 100 mL of FFFs and incubated at 30 °C under 180 rpm. The samples were prepared in triplicate, and distilled water was used as a control. After 7 days of incubation, the culture was centrifuged at 8000g for 10 min to remove bacterial biomass. The chemical oxygen demand (COD) was determined using the standard potassium dichromate method (Shen et al. 2019). Petroleum hydrocarbon fractions were extracted using the liquid-liquid extraction, and analyzed to determine the removal efficiencies of different treatments (Robles-Molina et al. 2013). Petroleum hydrocarbon was then recovered using n-hexane, and the extraction was repeated three times. The extracts were combined and dehydrated with anhydrous Na2SO4. Finally, the extract was diluted 20 times, and the n-alkanes with different chain lengths (i.e., C8–C10, C11–C20, and C21–C40) and PAHs were analyzed using a GC–MS (QP2020, Shimadzu) equipped with an SH Rxi-5Sil MS column (30 m × 0.25 μm × 0.25 mm, Shimadzu) operating in selected ion monitoring mode. The sample extracts obtained were quantified according to previously described protocols (Sun et al. 2016). The temperature of the ion source and interface were set to 230 °C and 300 °C, respectively. The parameters of the column oven temperature were set as follows: an initial temperature of 50 °C with a hold time of 2 min, and was subsequently increased to 300 °C at 6 °C/min and held for 25 min. Helium was used as a carrier gas with a flow rate of 1.2 mL/min. The concentrations of n-alkanes (C8-C40) and PAHs were determined through comparisons with the standards.
Results and discussion
Isolation and identification of BS producers
In total, 30 strains with BS-producing ability were isolated, and the terminal restriction fragment length polymorphism technique was carried out using terminal fluorescent labeling in order to roughly classify isolates (results not shown). Based on the results, prospective isolates were narrowed down to four strains, among which two strains exhibited strong BS-producing capabilities (Table 1), with obvious oil displacement (5–6 cm in diameter) and high emulsification abilities. The two strains were identified as Serratia marcescens ZCF25 and Pseudomonas sp. ZCF53, whose phylogenetic trees were constructed and presented in Fig. 1 and Fig. S1, respectively. The 16S rRNA gene sequences of the strains ZCF25 and ZCF53 were submitted to GenBank of NCBI under the accession numbers MN435590 and MN795965, respectively. Based on various screening tests, S. marcescens ZCF25 exhibited the best performance, and was therefore selected for further study.
Optimization of BS-producing conditions for S. marcescens ZCF25
Among the six carbon substrates examined (i.e., olive oil, glycerol, glucose, paraffin, n-hexadecane, and octacosane), olive oil exhibited the best performance, as the corresponding cell-free supernatant showed the lowest surface tension (26.59 mN/m) (Fig. 2a). Furthermore, the highest OD600 was obtained when olive oil was used as the carbon source, suggesting that olive oil could enhance both bacterial growth and BS yields. Although glycerol oil and glucose are frequently used as carbon substrates for the production of BSs, reductions in the surface tension of the fermentation broth were relatively small when they were used as carbon substrates for S. marcescens ZCF25. The growth of S. marcescens ZCF25 was also slow when paraffin, n-hexadecane, and octacosane were used as the carbon sources, as indicated by the low OD600. In addition, there were small reductions in surface tension when paraffin, n-hexadecane, and octacosane were used. Moreover, the results suggested that reductions in surface tension were positively correlated with the biomass (Fig. 2a). Similar reductions in surface tension (ranging from 34.38 to 40.91 mN/m) were obtained when analyzing five different nitrogen sources (i.e., sodium nitrate, ammonium chloride, peptone, yeast extract, and urea) (Fig. 2b). A large amount of biomass was obtained when tryptone and sodium nitrate were used as the nitrogen sources. Considering the growth conditions and production costs, sodium nitrate was determined as the best nitrogen source for the production of BSs. A report (Saimmai et al. 2013) showed similar results, indicating that while the carbon source could affect the biomass yield and BS yield, using sodium nitrate as the nitrogen source could result in the largest reduction in surface tension.
Properties of BS produced by S. marcescens ZCF25
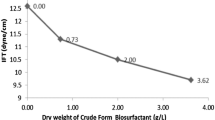
As shown in Fig. 3 a, the minimum surface tension reached was 29.50 mN/m at a BS-ZCF25 concentration of 220 mg/L. A recent study (Hu et al. 2018) reported that BSs produced by S. marcescens ZS6 exhibited a CMC of 19 g/L, which could reduce the surface tension to 35 mN/m, and Bezza and Nkhalambayausi Chirwa (2015) reported that the CMC of the lipopeptide BS produced by the Paenibacillus dendritiformis CN5 could decrease the surface tension of water to 34.6 ± 1 mN/m. Comparatively, the BS-ZCF25 in this work displayed outstanding interfacial activity with low CMC. Additionally, the CMC of BS-ZCF25 is comparably lower than synthetic surfactants such as sodium dodecyl sulfate (600 mg/l), sodium dodecyl benzene sulfonate (4000 mg/l), and Tween 80 (500 mg/l), which are commonly used in many industrial fields (Tian et al. 2016).
When the BS-ZCF25 solution was treated at a temperature range of 50–100 °C, a pH range of 2–12, and a NaCl concentration of 10–100 g/L, the surface tension was maintained at 33.16 ± 1.66 mN/m, 31.41 ± 1.77 mN/m, and 29.87 ± 0.59 mN/m, respectively (Fig. 3b). The results showed great stability of BS-ZCF25 under a wide range of pH, temperature, and salinity conditions. It was reported that the stability of the produced BS by S. marcescens strain UCP-1549 was influenced in reducing surface tension by acid pH, NaCl concentration up to 6% (Helvia et al. 2017). Thus, BS-ZCF25 can be applied for bioremediation purposes in various environments. In addition, the BS produced by Pseudomonas sp. ZCF53 showed a CMC of 128.82 mg/L, and had excellent tolerance against various temperature, salinity, and pH ranges (Fig. S2).
Chemical characterization of BS-ZCF25
The TLC characterization of BS produced by S. marcescens ZCF25 revealed a blue-violet spot and a purplish-red spot when sprayed with a phosphomolybdic acid reagent and a ninhydrin reagent, respectively. This indicated the presence of fatty acid and amino acids group (Fig. 4a). Taken together, it can be concluded that BS-ZCF25 has lipopeptide properties. The FT-IR spectra of BS-ZCF25 showed a strong absorption peak at 3391 cm−1 in the region of 3250–3500 cm−1, and a peak at 1077 cm−1, which were attributable to the stretching vibrations and bending of -NH and -OH groups (Fig. 4b). The absorption peaks at 2923 cm−1 and 2853 cm−1 indicated the presence of methyl and methylene groups, respectively, most likely due to the stretching vibration of C-H groups. The intense absorption peaks at 1544 cm−1 confirmed the presence of carbonyl (-C=O) group. Furthermore, the FT-IR spectrum revealed the presence of aliphatic group in combination with peptide groups, and showed similarities to the lipopeptide BS produced by S. marcescens UCP 1549 (Araujo et al. 2019) which also supported the finding.
a TLC, b FT-IR, and c GC–MS spectra of BS-ZCF25. a Lane I was developed with phosphomolybdic acid in order to detect lipids, with cyan coloring showing a positive correlation. Lane II was developed with a 0.25% ninhydrin solution to detect peptides, with erythrinus coloring showing a positive correlation
The GC–MS spectra of BS-ZCF25 revealed the presence of three peaks, which were identified as methyl hexadecanoate (C16:0), methyl 9-octadecenoate (C18:1), and methyl 10-octadecenoate (C18:1) (Fig. 4c). Combined with the spectro-chromatographic characteristics of BS-ZCF25, the result suggested that BS-ZCF25 could be categorized as a lipopeptide, composed by β-hydroxy fatty acids of hexadecenoic acid and octadecanoic acid (Rosas-Galván et al. 2018).
Antibacterial activity of BS-ZCF25
Highly hydrophobic peptides have been proven to be highly toxic to both eukaryotic and prokaryotic cells, while peptides with amphiphilic structures can target and bind to the hydrophobic nucleus of cell membranes (Chu-Kung et al. 2010). Therefore, the antimicrobial activity of BS-ZCF25 was examined using five pathogens, and the results showed that BS-ZCF25 had positive antimicrobial effects against Staphylococcus epidermidis and Escherichia coli. The zone of inhibition by BS-ZCF25 under a concentration of 2.0 mg/mL was approximately 1.73 cm for Escherichia coli (gram-negative bacteria), and 2.25 cm for Staphylococcus epidermidis (gram-positive bacteria), respectively. Similarly, the BS produced by a strain of S. marcescens, as reported by Kadouri and Shanks (2013), exhibited antibacterial activity against gram-positive bacteria such as methicillin-resistant Staphylococcus aureus. Another study reported that Serratia surfactantfaciens sp. YD25T could produce serrawettin W2, which exhibited positive antibacterial activity against gram-positive bacteria and some gram-negative bacteria, such as Pseudomonas sp. (Su et al. 2019). These results imply that BS-ZCF25 has potential applications as an antibacterial agent.
Application of S. marcescens ZCF25 in the bioremediation of FFFs
BS-producing microorganisms can produce BSs to reduce the surface tension and increase the solubility of many organic pollutants, thereby enhancing the metabolism and degradation of petroleum hydrocarbons (Santos et al. 2016). The petroleum hydrocarbon utilization performance of S. marcescens ZCF25, as examined by the 2,6-DCPIP test (Table 2), indicated that crude oil and atolin were preferably utilized over benzene and cyclooctane. This was evident as the reaction systems containing crude oil and atolin became colorless after 48 h in the presence of S. marcescens ZCF25. Meanwhile, S. marcescens ZCF25 was also able to utilize phenanthrene, n-hexadecane, and octacosane, with the color reaction systems fading after 72 h (Table 2). Considering the BS-producing capacity and the wide petroleum hydrocarbon utilization spectra of S. marcescens ZCF25, its application for the bioremediation of FFFs was carried out. In general, co-culture exhibits better degradation performance better than single bacterial strain. As Pseudomonas sp. ZCF53 also exhibited the ability to degrade a wide range of petroleum hydrocarbons, it would be interesting to examine whether the co-culture of S. marcescens ZCF25 and Pseudomonas sp. ZCF53 could further promote the bioremediation of FFFs.
The COD of FFFs decreased from 6533 mg/L in the original sample to 3342 mg/L (48.9% removal efficiency), 3880 mg/L (40.6% removal efficiency), 3557 mg/L (45.6% removal efficiency), and 5626 mg/L (13.9% removal efficiency) as a result of bioremediation using S. marcescens ZCF25, Pseudomonas sp. ZCF53, co-bioaugmentation, and a control treatment, respectively (Fig. 5a). The results showed that S. marcescens ZCF25 exhibited the best performance on COD removal, suggesting that it could utilize organic pollutants in the bioremediation of FFFs and effectively accelerate the removal of COD. The initial concentrations of alkanes and PAHs in FFFs were 1728.6 mg/L and 540.1 μg/L, respectively. As shown in Fig. 5 b, reductions of 119.5 mg/L (6.88% reduction), 1131.5 mg/L (65.57% reduction), 578.7 mg/L (33.53% reduction), and 1553.6 mg/L (89.88% reduction) for ∑n-alkanes were observed in the control, S. marcescens ZCF25, Pseudomonas sp. ZCF53, and co-bioaugmentation treatment, respectively. The addition of S. marcescens ZCF25 into FFFs achieved better degradation performance than that of Pseudomonas sp. ZCF53. Besides, it could be observed that the reduction of alkanes content was mainly attributable to the degradation of middle- (C11-C20) and long-chain (C21-C40) n-alkanes (> 89%). Furthermore, reductions of 94.5 μg/L (17.5% reduction), 345.57 μg/L (64% reduction), 300.6 μg/L (55.67% reduction), and 398.76 μg/L (73.8% reduction) for ∑PAHs were obtained in the control, S. marcescens ZCF25, Pseudomonas sp. ZCF53, and co-bioaugmentation treatment, respectively (Fig. 5b).
The GC–MS chromatograms of total petroleum hydrocarbon in the original FFFs, and that remediated with S. marcescens ZCF25, Pseudomonas sp. ZCF53, the mixed culture of S. marcescens ZCF25 and Pseudomonas sp. ZCF53, as well as the control sample, are shown in Fig. 6. The origin and control treatments exhibited low TPH removal, whereas remarkable reductions in TPH were achieved in the bioaugmented treatments. Although FFFs generally consist of complex and harmful petroleum hydrocarbons, we found that S. marcescens ZCF25 had a high capacity for the treatment of refractory petroleum hydrocarbons. In addition, the co-cultivation of S. marcescens ZCF25 with Pseudomonas sp. ZCF53 enhanced the degradation performance. A similar effect was also observed when Stenotrophomonas sp. N was co-cultivated with the P. aeruginosa strain WatG (Ueno et al. 2007) in the degradation of diesel oil, which is likely attributable to the rhamnolipids produced by WatG. Generally, higher degradation is achieved in a mixed consortium by the synergistic action between different bacteria. In this study, the BSs produced by BS-producing bacteria could increase the solubility and bioavailability of hydrophobic organic compounds, thereby facilitating degradation performance. Some studies have demonstrated that the S. marcescens strain has petroleum hydrocarbon-degradation capabilities (Borah et al. 2019; Smulek et al. 2020). Nevertheless, this study is the first report on the application of BS-producing S. marcescens for the treatment of FFFs.
a GC–MS spectra of total petroleum hydrocarbons in the fracturing fluid of the original sample, b without the inoculation of bacteria but incubated under the same conditions, c inoculated with Serratia marcescens ZCF25, d inoculated with Pseudomonas sp. ZCF53, e inoculated with the mixed culture of Serratia marcescens ZCF25 and Pseudomonas sp. ZCF53
Conclusions
In this study, a BS-producing bacterium, designated as S. marcescens ZCF25, was isolated from oil sludge. The corresponding BS that was produced and its potential for bioremediation purposes were investigated. The BS-ZCF25 decreased water surface tension drastically, with a CMC of 220 mg/mL. It also exhibited high stability over a wide range of pH, temperature, and NaCl concentrations. Chemical and spectro-chromatographic analysis suggested that BS-ZCF25 could be classified as a lipopeptide. Moreover, S. marcescens ZCF25 exhibited good performance in the bioremediation of FFFs. The findings in this study indicate that S. marcescens ZCF25 can readily adapt to complex and harsh environments, and can efficiently degrade organic pollutants, demonstrating the considerable potential of S. marcescens ZCF25 for in situ bioremediation.
References
Araujo HWC, Andrade RFS, Montero-Rodriguez D, Rubio-Ribeaux D, Alves da Silva CA, Campos-Takaki GM (2019) Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microb Cell Factories 18:1–13. https://doi.org/10.1186/s12934-018-1046-0
Bezza FA, Nkhalambayausi Chirwa EM (2015) Biosurfactant from paenibacillus dendritiformis and its application in assisting polycyclic aromatic hydrocarbon (PAH) and motor oil sludge removal from contaminated soil and sand media. Process Saf Environ 98:354–364. https://doi.org/10.1016/j.psep.2015.09.004
Bhargav HS, Shastri SD, Poornav SP, Darshan KM, Nayak MM (2016) Measurement of the zone of inhibition of an antibiotic. IEEE. 10.1109 / IACC.2016.82
Borah D, Agarwal K, Khataniar A, Konwar D, Gogoi SB, Kallel M (2019) A newly isolated strain of Serratia sp. from an oil spillage site of assam shows excellent bioremediation potential. 3 Biotech 9:1–12. https://doi.org/10.1007/s13205-019-1820-7
Chu-Kung AF, Nguyen R, Bozzelli KN, Tirrell M (2010) Chain length dependence of antimicrobial peptide–fatty acid conjugate activity. J Colloid Interf Sci 345:160–167. https://doi.org/10.1016/j.jcis.2009.11.057
Dollinger J, Schacht VJ, Gaus C, Grant S (2018) Effect of surfactant application practices on the vertical transport potential of hydrophobic pesticides in agrosystems. Chemosphere 209:78–87. https://doi.org/10.1016/j.chemosphere.2018.06.078
Elsner M, Hoelzer K (2016) Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ Sci Technol 50:3290–3314. https://doi.org/10.1021/acs.est.5b02818
Estrada JM, Bhamidimarri R (2016) A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 182:292–303. https://doi.org/10.1016/j.fuel.2016.05.051
Heinz H, Pramanik C, Heinz O, Ding Y, Mishra RK, Marchon D, FlattcIrin RJ, Estrela-Lopis I, Llop J, Moya S, Ziolo RF (2017) Nanoparticle decoration with surfactants: molecular interactions, assembly, and applications. Surf Sci Rep 72:1–58. https://doi.org/10.1016/j.surfrep.2017.02.001
Helvia WCA, Rosileide FSA, Montero-Rodríguez D, Santos VP, Patricia CVSM, Carlos FBCF, Carlos AAS, Campos-Takaki GM (2017) Biochemical and molecular identification of newly isolated pigmented bacterium and improved production of biosurfactant. Afr J Microbiol Res 11:945–954. https://doi.org/10.5897/ajmr2016.8340
Hu X, Cheng T, Liu J (2018) A novel Serratia sp. ZS6 isolate derived from petroleum sludge secretes biosurfactant and lipase in medium with olive oil as sole carbon source. AMB Express 8:1–12. https://doi.org/10.1186/s13568-018-0698-9
Joy S, Rahman PKSM, Sharma S (2017) Biosurfactant production and concomitant hydrocarbon degradation potentials of bacteria isolated from extreme and hydrocarbon contaminated environments. Chem Eng J 317:232–241. https://doi.org/10.1016/j.cej.2017.02.054
Kadouri DE, Shanks RM (2013) Identification of a methicillin-resistant Staphylococcus aureus inhibitory compound isolated from Serratia marcescens. Res Microbiol 164:821–826. https://doi.org/10.1016/j.resmic.2013.06.002
Kahrilas GA, Blotevogel J, Corrin ER, Borch T (2016) Downhole transformation of the hydraulic fracturing fluid biocide glutaraldehyde: implications for flowback and produced water quality. Environ Sci Technol 50:11414–11423. https://doi.org/10.1021/acs.est.6b02881
Lydon HL, Baccile N, Callaghan B, Marchant R, Mitchell CA, Banat IM (2017) Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob Agents Ch 61:1–9. https://doi.org/10.1128/AAC.02547-16
Manaargadoo-Catin M, Ali-Cherif A, Pougnas JL, Perrin C (2016) Hemolysis by surfactants—a review. Adv Colloid Interfac 228:1–16. https://doi.org/10.1016/j.cis.2015.10.011
McLaughlin MC, Borch T, Blotevogel J (2016) Spills of hydraulic fracturing chemicals on agricultural topsoil: biodegradation, sorption, and co-contaminant interactions. Environ Sci Technol 50:6071–6078. https://doi.org/10.1021/acs.est.6b00240
Morales-Guzmán G, Ferrera-Cerrato R, Rivera-Cruz MDC, Torres-Bustillos LG, Arteaga-Garibay RI, Mendoza-López MR, Esquivel-Cote R, Alarcón A (2017) Diesel degradation by emulsifying bacteria isolated from soils polluted with weathered petroleum hydrocarbons. Appl Soil Ecol 121:127–134. https://doi.org/10.1016/j.apsoil.2017.10.003
Mpelwa M, Tang S, Jin L, Hu R (2019) New sulfonate gemini surfactants: synthesis and evaluation for enhanced oil recovery applications. J Disper Sci Technol:1–9. https://doi.org/10.1080/01932691.2019.1652189
Nayarisseri A, Singh P, Singh SK (2018) Screening, isolation and characterization of biosurfactant-producing Bacillus tequilensis strain ANSKLAB04 from brackish river water. Int J Environ Sci Te 16:7103–7112. https://doi.org/10.1007/s13762-018-2089-9
Patel S, Homaei A, Patil S, Daverey A (2019) Microbial biosurfactants for oil spill remediation: pitfalls and potentials. Appl Microbiol Biotechnol 103:27–37. https://doi.org/10.1007/s00253-018-9434-2
Pei G, Zhu Y, Cai X, Shi W, Li H (2017) Surfactant flushing remediation of o-dichlorobenzene and p-dichlorobenzene contaminated soil. Chemosphere 185:1112–1121. https://doi.org/10.1016/j.chemosphere.2017.07.098
Robles-Molina J, Gilbert-Lopez B, Garcia-Reyes JF, Molina-Diaz A (2013) Comparative evaluation of liquid-liquid extraction, solid-phase extraction and solid-phase microextraction for the gas chromatography-mass spectrometry determination of multiclass priority organic contaminants in wastewater. Talanta 117:382–391. https://doi.org/10.1016/j.talanta.2013.09.040
Rosas-Galván SN, Martínez-Morales F, Marquina-Bahena S, Tinoco-Valencia R, Serrano-Carreón L, Bertrand B, León-Rodríguez R, Guzmán-Aparicio J, Alvaréz-Berber L, María R, Trejo-Hernández (2018) Improved production, purification, and characterization of biosurfactants produced by Serratia marcescens SM3 and its isogenic SMRG-5 strain. Biotechnol Appl Biochem 65:690–700. https://doi.org/10.1002/bab.1652
Saimmai A, Udomsilp S, Maneerat S (2013) Production and characterization of biosurfactant from marine bacterium Inquilinus limosus KB3 grown on low-cost raw materials. Ann Microbio 63:1327–1339. https://doi.org/10.1007/s13213-012-0592-7
Santos DK, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2016) Biosurfactants: multifunctional biomolecules of the 21st century international. Int J Mol Sci 17:401. https://doi.org/10.3390/ijms17030401
Sar P, Ghosh A, Scarso A, Saha B (2019) Surfactant for better tomorrow: applied aspect of surfactant aggregates from laboratory to industry. Res Chem Intermediat 45:6021–6041. https://doi.org/10.1007/s11164-019-04017-6
Satpute SK, Kulkarni GR, Banpurkar AG, Banat IM, Mone NS, Patil RH, Cameotra SS (2016) Biosurfactants from Lactobacilli species: properties, challenges and potential biomedical applications. J Basic Microb 56:1140–1158. https://doi.org/10.1002/jobm.201600143
Shen L, Wang W, Li T, Cui Y, Wang B, Yu G, Wang X, Wei D, Xiao J, Deng S (2019) Powdered activated coke for COD removal in the advanced treatment of mixed chemical wastewaters and regeneration by fenton oxidation. Chem Eng J 371:631–638. https://doi.org/10.1016/j.cej.2019.04.086
Shrestha N, Chilkoor G, Wilder J, Gadhamshetty V, Stone JJ (2017) Potential water resource impacts of hydraulic fracturing from unconventional oil production in the bakken shale. Water Res 108:1–24. https://doi.org/10.1016/j.watres.2016.11.006
Shuai Y, Zhou H, Mu Q, Zhang D, Zhang N, Tang J, Zhang C (2018) Characterization of a biosurfactant-producing Leclercia sp. B45 with new transcriptional patterns of alkB gene. Ann Microbiol 69:139–150. https://doi.org/10.1007/s13213-018-1409-0
Singh P, Patil Y, Rale V (2019) Biosurfactant production: emerging trends and promising strategies. J Appl Microbiol 126:2–13. https://doi.org/10.1111/jam.14057
Smulek W, Sydow M, Zabielska-Matejuk J, Kaczorek E (2020) Bacteria involved in biodegradation of creosote PAH—a case study of long-term contaminated industrial area. Ecotox Environ Safe 187:109843. https://doi.org/10.1016/j.ecoenv.2019.109843
Spano A, Lagana P, Visalli G, Maugeri TL, Gugliandolo C (2016) In vitro antibiofilm activity of an exopolysaccharide from the marine thermophilic Bacillus licheniformis T14. Curr Microbiol 72:518–528. https://doi.org/10.1007/s00284-015-0981-9
Sun S, Wang Y, Zang T, Wei J, Wu H, Wei C, Qiu G, Li F (2016) A biosurfactant-producing Pseudomonas aeruginosa S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour Technol 281:421–428. https://doi.org/10.1016/j.biortech.2019.02.087
Su C, Xiang Z, Liu Y, Zhao X, Sun Y, Li Z, Li L, Chang F, Chen T, Wen X, Zhou Y, Zhao F (2019) Analysis of the genomic sequences and metabolites of Serratia surfactantfaciens sp. nov. YD25(T) that simultaneously produces prodigiosin and serrawettin W2. BMC Genomics 17:1–19. https://doi.org/10.1186/s12864-016-3171-7
Tian W, Yao J, Liu R, Zhu M, Wang F, Wu X, Liu H (2016) Effect of natural and synthetic surfactants on crude oil biodegradation by indigenous strains. Ecotox Environ Safe 129:171–179. https://doi.org/10.1016/j.ecoenv.2016.03.027
Ueno A, Ito Y, Yumoto I, Okuyama H (2007) Isolation and characterization of bacteria from soil contaminated with diesel oil and the possible use of these in autochthonous bioaugmentation. World J Microb Biot 23:1739–1745. https://doi.org/10.1007/s11274-007-9423-6
Zhou H, Huang X, Bu K, Wen F, Zhang D, Zhang C (2019) Fungal proliferation and hydrocarbon removal during biostimulation of oily sludge with high total petroleum hydrocarbon. Environ Sci Pollut R 26:33192–33201. https://doi.org/10.1007/s11356-019-06432-z
Funding
This work was supported by the XinJiang Keli New Technology Development Co., Ltd. (K18-529102-014, K17-529102-004), the National Natural Science Foundation of China (No. 51978189), and the Research Funds of the Guangxi Key Laboratory of Theory and Technology for Environmental Pollution Control (No.1801 K011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Huang, Y., Zhou, H., Zheng, G. et al. Isolation and characterization of biosurfactant-producing Serratia marcescens ZCF25 from oil sludge and application to bioremediation. Environ Sci Pollut Res 27, 27762–27772 (2020). https://doi.org/10.1007/s11356-020-09006-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09006-6