Abstract
Heavy metals (HMs) being the notorious and toxic are being introduced into the environment credited to natural and anthropogenic activities. The use of ornamental plants is being ignored as potential candidates for HMs phytoremediation. In this study, pot experiments were conducted on Catharanthus roseus (L.) G.Don to evaluate selected heavy metals tolerance and accumulation potential with reference to the bacterial endophyte (Bacillus cereus) and organic amendments (moss and compost at 5% v/v). Results indicated improvement in uptake of Cd (230 mg kg−1), Cu (229 mg kg−1), and Cr (458 mg kg−1) by C. roseus with B. cereus. The concentration of Ni and Pb was found highest in controls (without strain) that were 420 and 904 mg kg−1, respectively. Conversely, the addition of organic amendments enhanced biomass production, as compared to controls, 441, 471, and 763% by peat moss (T3), compost (T4), and peat moss + compost + inoculum treatments (T6), respectively, while reduction of plant HMs content was observed. Microbial-aided phytoremediation/phytoextraction could be a potential method for removal of Cd, Cr, and Cu, while organic amendments can significantly improve plant growth in the presence of heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among contaminants, heavy metals, being non-biodegradable and indestructible with changing chemical forms, are among priority pollutants (Arshad et al. 2017; Khan et al. 2020). They result in toxicity in living organisms, even at the very low concentrations, which leads to strict regulatory compliance (Iqbal et al. 2019; Khan et al. 2019a). For the removal of metals, i.e., from water and soil, different physical/chemical methods are in use, but often face high cost, energy inefficiency, soil properties disturbance, and secondary pollution limitations. This demands new and novel method of remediation to be identified and investigated such as phytoremediation (Hussain et al. 2018). Phytoremediation is a sustainable technology that relies on the biological species (plants and associated microorganisms) for environmental restoration and cleanup purposes, i.e., soil, water, and sediments (Raza et al. 2019). This technique comprises following advantages: cost effectiveness, high removal competency, environment friendliness, and high public acceptance (Iqbal et al. 2019; Khan et al. 2019b). Among different bioremediation techniques, phytoremediation involves plants and associated microorganisms for the removal/intoxication of contaminants. It has been proved to be effective against many organic and inorganic pollutants, especially against heavy metals like Cd, Cu, Cr, and Zn (Rascio and Navari-Izzo 2011).
Success of this technique is dependent on the plant’s capacity to tolerate and accumulate metal(s) with sufficient biomass production, as well as the availability of metals for plant uptake (Boechat et al. 2017). Regarding its applications, a fundamental option is the involvement of associated microbes that live in close association with plants (Rascio and Navari-Izzo 2011). Bacterial populations are found in various environments including the endosphere and the rhizosphere. Bacteria may enhance metal uptake to plants by enhancing metal mobility in the soil, and through promoting plant growth, which results in larger biomass. The enhanced availability of metals might be due to the production of siderophores (molecule that binds and transports iron to microorganism), like bacillibactin and petrobactin, organic acids (influencing the metal mobility due to their physical and chemical composition), like citric acid, succinic acid, lactobionic acid, and gluconic acid, and biosurfactants (surface active molecules/chemical compounds produced by microorganism to facilitate, solubilize, mobilize and/or accession of hydrophobic substrates), like rhamnolipids, lipoprotein, and surfactin (Arab and Mulligan 2018; Khan et al. 2017a). Apart of microbial influence, the addition of organic amendments also impacts the properties of soil as its addition augment different nutrients and immobilizes heavy metals to decrease their availability for plant uptake (Arshad et al. 2017). Generally, peat moss is composed of cellulose and lignin having functional groups like ketones, alcohols, aldehydes, acids, and ethers. These functional groups take part in binding heavy metals and immobilize them so considered as an efficient material to stabilize heavy metals (Khan et al. 2019b). Compost is derived from dead and decomposed organic matter of plants and animals in aerobic conditions. Due to high organic content, its application improves soil organic carbon content, soil structure, and enzymatic and microbial activities (Iqbal et al. 2019).
Traditional phytoremediation involving hyperaccumulators (HAs) or their transporter (Iqbal et al. 2013) is being replaced by plants with short life cycles (Khan et al. 2019b). One of the potential candidates to replace HAs are ornamental plants (OPs) due to lower chances of biomagnification, and capacity for simultaneous beautification of the environment. An added advantage is that some special OPs especially as hyperaccumulators can remediate metal polluted soils (Forte and Mutiti 2017; Liu et al. 2018; Khan et al. 2019a). There will be more practical benefits of their application if they can be used in combination with microbes (Iqbal et al. 2019; Raza et al. 2019). The purpose of this study was to assess the tolerance and accumulation potential of Catharanthus roseus (L.) G.Don grown in multi-metal system and to identify a suitable combination of selected organic and bacterial amendments for metal accumulation and tolerance. C. roseus commonly known as “Sadabahar” belongs to family Apocynaceae. This plant is grown extensively and valued because of its durability and capability to grow in dry, nutrient scarce, and harsh conditions and tolerance against cold weather (Semenya and Potgieter 2013). Few studies have been reported regarding its potential for accumulating metals, i.e., Cd, Cr, Pb, and Ni (Liu et al. 2008; Khan et al. 2019a). The study that focused on combined use of soil conditioners and bacterial amendments to improve the tolerance of C. roseus in a multi-metal contaminated synthetic wastewater is lacking.
Materials and methods
Plants selection and growth conditions
The seeds of C. roseus were obtained from Ewan Garden, Islamabad, Pakistan, and grown in pots having sterilized sand. The pots were watered with half-strength Hoagland’s nutrient solution. The Hoagland’s nutrient solution contains 5 mM KNO3, 5 mM Ca(NO3)2.4H2O, 0.06 μM Fe(Na)EDTA, 2 mM MgSO4.7H2O, 1 mM NH4NO3, 46 μM H3BO3, 9 μM MnCl2.4H2O, 0.76 mM ZnSO4.7H2O, 0.204 mM CuSO4.5H2O, 0.450 mM Na2MoO4.2H2O, 0.5 mM KH2PO4, and pH of 5.5 was adjusted by using KOH and H2SO4 solutions. The plants were incubated in a growth chamber at 22 °C, using photo-period with 16:8 day:nighttime. At four leaves stage (4 weeks old), uniform heighted seedlings were transferred to pots.
Experimental procedure
The plastic pots were filled by 350 ml of sieved and sterilized soil. The soil used in pots was a mixture of agriculture soil and sand in 2:1 soil to sand. There was an addition of compost and peat moss with 5% of the respective amendment(s) to the total soil volume (v/v). Compost was prepared from green garden waste at the Horticulture Department, National Agriculture Research Center, Islamabad, Pakistan, and peat moss was bought from Ewan Garden, Islamabad, Pakistan. Culture suspensions of previously isolated plant growth promoting (PGP) Bacillus cereus were prepared in nutrient broth using incubation period of 24 h with 30 °C set temperature with continuous shaking at 200 rpm. After growth cells were harvested by centrifugation at 6000 rpm for 5 min, and cell suspensions were prepared (maintaining the cell density of 108 ml−1) (Khan et al. 2017a). Plants in pots were acclimatized for 3 weeks, and 10 ml bacterial suspensions were added on the first day of the 1st week of acclimatization. After 3 weeks of acclimation, pots for each of the treatment were supplied with synthetic wastewater for 4 weeks (Tables 1 and 2). For each treatment, three pots were used.
Harvesting of plants
Plants were supplied with 13 ml of synthetic wastewater on daily basis (twice daily), and after 4 weeks of exposure plants were harvested. Roots, shoots, leaves, and flowers were separated and stored in zip seal plastic bags, after measurement of the respective fresh weights. Numbers of leaves and average leaf area (measured with ImageJ software) and, root and shoot lengths (in cm) were measured (Khan et al. 2020). Fresh leaf tissues were stored at − 80 °C in freezer for enzymatic and biochemical analysis and rest of plant samples were air-dried at 60 ± 5 °C in oven till constant weight is attained. Air-dried samples were digested for the quantification of HMs uptake and compartmentalization in plant. All the analyses were performed in biological triplicate.
Stress injury assay
Lipid peroxidation was measured in terms of malondialdehyde (MDA) by using thiobarbituric acid (TBA) as done by Khan et al. (2019b). Fresh leaf sample of 100 mg was taken and homogenized using 1 ml of 5% (w/v) TCA in an ice bath. The resulting mixture was centrifuged at 10,000 g for 10 min. Then, 1 ml of 0.67% (w/v) TBA was added in each supernatant, following 30 min heating at 95 °C in the water bath, immediately chilled and centrifuged. The absorbance was recorded at 450, 532, and 600 nm. Final concentration was calculated by using the following equation:
where Vt = 0.001 L; W = 0.1 g.
Chlorophyll a, chlorophyll b, total chlorophyll, and total carotenoids were determined using spectrophotometric method (Arnon 1949). Forty milligrams of fresh biomass of leaves with 2 ml of 80% (v/v) aqueous acetone was used for extraction of these pigments. These extracts were then centrifuged at 5000 rpm for 5 min and supernatant was collected and pellets were re-extracted with 1 ml of 80% (v/v) acetone. They were evaluated at 663, 645, and 470 nm against blank of 80% acetone and respective pigment content was calculated using equation proposed by Lichtenthaler (1987);
Electrolyte leakage (EL) was determined by using the method of Dionisio-sese and Tobita (1998), by using an electrical conductivity meter. Briefly, 5-mm-sized leaf cuts were prepared from stored fresh samples and were dispersed in test tubes having 10 ml of distilled deionized water, with incubation at 32 °C for 2 h and then initial EC (EC1) was measured. Afterwards, all samples were autoclaved for 20 min at 121 °C then were cooled to 25 °C and EC (EC2) was measured again. The EL was determined by using the following formula:
EC1 = Primary electrical conductivity
EC2 = Secondary electrical conductivity
Preparation of plant samples for metals analysis
Acid digestion method was used to prepare samples to evaluate plant body’s metal content. Dried samples of 50 mg were grinded then digested using 3 ml concentrated HNO3 (added and swirled smoothly), 3 ml of H2O2 and 0.5 ml of concentrated HCl per sample (Estefan et al. 2013). Samples were placed on hotplate with set temperature of 150 °C for 10 min with 10-min delay for cooling. Samples were filtered using Whatman filter paper No. 42 and final volume was adjusted up to 25 ml. Digested samples were assessed through atomic absorption spectrometry (AAS), with the help of Perkin Elmer, AAS-700. Certified reference material was used when the metal analysis was performed. For every batch of sample analysis, the blank controls and CRMs were used. The analysis was started after the stabilization of atomic absorption spectrophotometry, reading for CRMs were taken. After confirming the stabilization and analytic accuracy/precision of AAS, the analysis was performed.
Statistical analysis
All values given in this study are mean of three samples, presented with standard deviation. The normality of data was checked using Shapiro-Wilk normality test. Analysis of variance (ANOVA) was performed using SPSS (version 21), ANOVA was followed by Duncan’s multiple range test with significant differences noted at p < 0.05 and significantly different values were shown with different alphabets, with “a” for significantly highest value, followed by the later alphabets for the lower means. Pearson’s correlation method was applied on data to check correlation between studied variables, and values of Pearson’s coefficient correlation (r) were noted at p value < 0.05 was considered as significant. With the help of network analysis, the interaction model of all the studied plant parameters. The descriptive network analysis was performed on JASP (version 0.10.2). The nodes (studied variables) and edges (the estimated relation) were constructed by the Fruchterman-Reingold algorithm.
Results
Plant growth parameters
In terms of leaves production, T6 exhibited 147% increase compared to the plants cultivated without any amendment. Plants in T3, T4, and T5 treatments had shown 126% and 115% and 53% increase in number of leaves, respectively, in comparison to T1. The lowest leaf count was found in T1 plants (Table 3). Highest leaf area value was found in T3 that was 2.64 cm2, 226% higher than T1. Plants in CW treatment had not shown significant increase, while plants in T5 had the least leaf area (64% lesser than T1) (Table 3).
The plants of T6 showed higher root and shoot length, 9.4 and 8.7 cm, while plants in T3 and T4 combined had 9.08 and 8.66 cm and 9.02 and 8.67 cm, respectively. Lowest root and shoot lengths were found in T1, 5.33 and 3.83 cm, respectively (Table 3). While for biomass, T6 showed highest production, compared to all other treatments that was 2937.8 mg fresh (root: 572.4, shoot: 482.8, leaf: 1882.5) and 1524.6 mg DW (root: 307.1, shoot: 248.8, leaf: 968.6). The T4 plants showed 1899.5 mg fresh (root: 231.7, shoot: 366, leaf: 1301.6) and 835.6 mg dry weight (root: 135.6, shoot: 187.74, leaf: 513), while T3 amended plants were found to have 1799 mg fresh (root: 254, shoot: 367.6, leaf: 1222.7) and 798 mg dry weight (root: 139.9, shoot: 142, leaf: 516.4). Inoculum amended plants (T5) have not shown significant variations in the fresh and dry biomass production, as compared to T2 (305 and 156 mg for T5, while 332 and 140 mg for T2, fresh and dry biomass, respectively). The T1 plants had lowest production among all other treatments (Table 3).
Plant physiological parameters
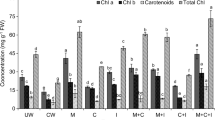
Chlorophyll content was found highest in T6, with chlorophyll a of 281%, chlorophyll b of 105%, and carotenoids 286%, higher as compared to T1 (Fig. 1). With T4 242, 45, and 197%, while with T3 115, 73, and 260% higher chlorophyll a, chlorophyll b, and carotenoid contents were noted compared to the T1. Plants in T5 showed 195, 85, and 259%, while in T2 182, 68, and 173%; higher chlorophyll a, chlorophyll b, and carotenoids, content, respectively, in comparison to the T1 (Fig. 1). EL was assessed in percentage form. The highest EL value was found in plants in T2 that was 73%. Plants in T3, T6, and T1 showed significant reduction in EL content that is 52, 51, and 51%, respectively, as compared to T2. While for plants in T5 and T4 insignificant changes in EL were noted, with 67 and 71% in respective treatments (Fig. 2).
Effects of heavy metals on photosynthetic pigments of C. roseus after 4 weeks of exposure to synthetic wastewater, T1 (plants irrigated with tap water), T2 (plants irrigated with synthetic wastewater), T3 (5% peat moss-amended soil), T4 (5% compost-amended soil), T5 (B. cereus-amended soil), and T6 (5% peat moss, 5% compost- and bacterial strain-amended soil). Bars denoted with different alphabet were significantly different at significance level p < 0.05, and n = 3 ± SD
Effects of heavy metals on electrolyte content of C. roseus after 4 weeks of exposure to synthetic wastewater, T1 (plants irrigated with tap water), T2 (plants irrigated with synthetic wastewater), T3 (5% peat moss amended soil), T4 (5% compost-amended soil), T5 (B. cereus-amended soil), and T6 (5% peat moss, 5% compost- and bacterial strain-amended soil). Bars denoted with different alphabet were significantly different at significance level p < 0.05, and n = 3 ± SD
Lipid peroxidation is presented in terms of malondialdehyde (MDA) content in C. roseus plants (fresh biomass). It was found highest with T2 and T5 that was 0.00173 and 0.00177 μmol g−1 FW, respectively. All other treatments showed significantly lower MDA contents as compared to mentioned above: T6–0.00049, T3–0.00032, and T4–0.00049 μmol g−1 of FW, respectively, while the lowest MDA content was found in T1, which was 0.00024 μmol g−1 FW (Fig. 3).
Effects of heavy metals on lipid peroxidation (in terms of MDA content) of C. roseus after 4 weeks of exposure to synthetic wastewater, T1 (plants irrigated with tap water), T2 (plants irrigated with synthetic wastewater), T3 (5% peat moss-amended soil), T4 (5% compost-amended soil), T5 (B. cereus-amended soil), T6 (5% peat moss, 5% compost- and bacterial strain-amended soil). Bars denoted with different alphabet were significantly different at significance level p < 0.05, and n = 3 ± SD
Plant metals uptake
Uptake and distribution of metal content was recorded in selected plant parts (including root, shoot, and leaves, respectively). Uptake, distribution, and accumulation of cadmium (Cd) varied significantly between different treatments. Highest concentration of Cd was found in T5 that is 230 mg kg−1 DW; with roots 147, shoots 71, and leaves having 12 mg kg−1 DW (Fig. 4a). Second highest concentration of Cd was found in T2 that was 214 mg kg−1 DW, with roots 124, shoots 70, and leaves 18 mg kg−1 DW, respectively (Fig. 4a). Lowest concentration of Cd was found in T6 (Fig. 4a). For Cr, the highest concentrations were found in T5 plants with a cumulative level of 229 mg kg−1 DW, with leaves containing 175, shoots 39, and roots 14 mg kg−1 DW, respectively (Fig. 4b). Second highest Cr concentration was found in T2 with total of 195 mg Cr kg−1 DW, with leaves 146, roots 28, and shoots 21 mg kg−1 DW. Lowest Cr content was found in T3 and T4 treatments viz. 42 and 43 mg kg−1 DW (Fig. 4b). Lead was found highest in T2 with total concentration of 904 mg kg−1 DW, having concentrations (mg kg−1 of DW) of 686 in shoots, 159 in roots, and 58 in leaves (Fig. 4c). Second highest Pb concentration was observed in T5 plants having 880 mg Pb kg−1 DW, with shoots 691, roots 118, and leaves having 70 mg Pb kg−1 DW, respectively. Lowest Pb concentration was found in T6 that was 85 mg kg−1 DW (Fig. 4c). With respect to Ni, T2 plants showed highest concentration, that was 420 mg kg−1 of DW, being highest in shoots 234, followed by roots 124, and lowest in leaves that was 61 (Fig. 5a). Second highest Ni concentration was observed in plants in T5 treatment, with cumulative Ni concentration of 353 mg kg−1 DW, where roots contained 186, shoots 131, and leaves was 34 mg kg−1 DW, in the cumulative Ni concentration. Lowest total Ni concentration was found in T4 that was 43 mg kg−1 of DW (Fig. 5a). Copper concentrations also varied in different treatments, being highest again in T5, the total concentration of 457.57 mg kg−1 of DW, the concentrations were 250, 130, and 67 mg kg−1 of DW in shoot, root, and leaves, respectively (Fig. 5b). Cu concentration was found second highest in T2 (396 mg kg−1 in total, with 173, 138, and 83 mg kg−1 of DW in root, shoot, and leaves, respectively). Plants in T4 had shown significantly lowest concentration that was 56 mg kg−1 of DW (Fig. 5b). Same trend existed in treatments where B. cereus was used in combination with amendments. Plants that were in T1 did not show metal uptake as they were irrigated with freshwater (Figs. 4 and 5).
Cadmium, chromium, and copper uptake and compartmentalization by C. roseus after 4 weeks of exposure to synthetic wastewater: a cadmium b chromium c copper content in root, shoot, and leaves of C. roseus, T1 (plants irrigated with tap water), T2 (plants irrigated with synthetic wastewater), T3 (5% peat moss amended soil), T4 (5% compost-amended soil), T5 (B. cereus-amended soil), and T6 (5% peat moss, 5% compost, and bacterial strain-amended soil). Bars denoted with different alphabet were significantly different at significance level p < 0.05, and n = 3 ± SD
Nickel and lead uptake by C. roseus after 4 weeks of exposure to synthetic waste water: a nickel content, b lead content in root, shoot, and leaf of C. roseus, T1 (plants irrigated with tap water), T2 (plants irrigated with synthetic wastewater), T3 (5% peat moss-amended soil), T4 (5% compost-amended soil), T5 (B. cereus-amended soil), and T6 (5% peat moss, 5% compost- and bacterial strain-amended soil). Bars denoted with different alphabet were significantly different at significance level p < 0.05, and n = 3 ± SD
Correlation and network analysis of C. roseus parameters upon synthetic wastewater exposure
The Pearson’s correlation analysis is presented in Fig. 6. Significant strong to moderate positive correlation between the physiological parameters was noted. These physiological parameters included total number of leaves per plant, leaf area, fresh and dried weight, root and shoot length, and fresh and dried weight. The coefficient correlations were found in the range of 0.959 to 0.573, at significance level of 0.050 and 0.000. No significant correlation was noted between root fresh and dry weight and leaf area and leaf dry weight and leaf area, at both levels of significance. Chlorophyll a and total chlorophyll were moderately and positively correlated with all the physiological parameters of C. roseus, except with leaf area, root, and shoot length. The chlorophyll b showed no correlation with any parameters except with chlorophyll a, total chlorophyll, and carotenoid, where the relation was strongly positive. Similarly, the significant carotenoid was found correlated positively chlorophyll a and total chlorophyll. In case of lipid peroxidation, negative correlation was found with leaf area (− 0.532) and shoot fresh weight (− 0.510). Also, EL showed positive correlation only with chlorophyll a and total chlorophyll levels.
Pearson correlation for the studied physiological and stress parameters of C. roseus. The lower diagonal represents the observed p values for the correlation coefficients, gradient filled with yellow color with heavier shades for high p values, while no color for insignificant correlations. The upper diagonal represents the correlation coefficients, with green color indicating positive, red color indicating negative, and no color indicating absence of any significant correlation between two parameters. The higher intensity of green and red color represents strong correlation, and vice versa for the weak correlation, n = 18. LN, total leaves per plant; LA, leaf area; LFW, leaf fresh weight; LDW, leaf dried weight; RL, root length; RFW, root fresh weight; RDW, root dried weight; SL, shoot length; SFW, shoot fresh weight; SDW, shoot dried weight; MDA, malondialdehyde content; EL, electrolyte leakage; Chl a, chlorophyll a; Chl b, chlorophyll b; and Car, carotenoid content
The dispersion of collected data can be visualized with the help of descriptive network analysis using Fruchterman-Reingold algorithm, which is presented in Fig. 7 to visualize networks of parameters with each other. Details related to the analysis are provided in supplementary table 1 and 2. Clearly, the physiological parameters were clustered together, with positive networking, indicating the interdependence on each other. While among the biochemical parameters chlorophyll a was very close to the physiological parameters, suggesting the importance of chlorophyll a in maintaining the response of C. roseus upon wastewater exposure, the impact of carotenoid and chlorophyll b had weakly positive compatibility with the plant physiology. While EL was negatively impacting the leaf area, the relation between malonaldehyde production and EL was positive, indicating the combined negative effect on physiological status, while the malonaldehyde production was found in negative networking status with all the physiological parameters. Among the dispersive, highest dispersion was noted between leaf area (1) and chlorophyll b (− 1) at x-axis, at y-axis between EL (1) and leaf number and shoot length (− 1).
Descriptive network analysis based on Fruchterman-Reingold algorithm for the studied parameters of C. roseus. Left side represent the score of parameters at x- and y-axis, while graph on right side show the sparsity of nodes (parameter including LN, total leaves per plant; LA, leaf area; LFW, leaf fresh weight; LDW, leaf dried weight; RL, root length; RFW, root fresh weight; RDW, root dried weight; SL, shoot length; SFW, shoot fresh weight; SDW, shoot dried weight; MDA, malondialdehyde content; EL, electrolyte leakage; Chl A, chlorophyll a; Chl B, chlorophyll b; and Car, carotenoid content) and connection with lines (green for positive and red for negative relation). Total chlorophyll is not used in the construction of network as it is the sum of Chl A and Chl B
Discussion
Estimation of plant growth parameters with reference to organic amendments
Plants growth depends on soil quality, one of the practices is to use the organic amendments to increase soil nutrient composition and water holding capacity (Tejada and Benítez 2015; Rizwan et al. 2016). The addition of organic amendments has been reported to increase biomass production, to improve plant growth and yield (Beesley and Marmiroli 2011). Moss and compost are two kinds of amendments that were utilized in this experiment, and both amendments enhanced plant growth as compared with non-amended plants (Table 3). They enhanced soil carbon content, improved texture, and microbial activity. Tordoff et al. (2000) stated that organic matter has a strong ability to improve soil physical structure and nutrient supply, to reduce metal mobility that could cause plant toxicity. Compost-amended soil supported higher biomass production of sunflower and reduced Cu availability in Cu-contaminated soil as compared to un-amended soil (Jones et al. 2016). Stanislawska-Glubiak et al. (2015) reported 2–2.5 folds grain, 2–3 folds of roots and 1.2–1.6 folds greater growth of other above ground parts in maize with moss-containing soils. Moss does not condense over time and thus provides better aeration and moisture content for longer periods (Khan et al. 2019b). The bacterial amendment did not contribute to the improvement of plant growth that could be due to low nutrient uptake and deprived quality of soil (Table 3). Combined application of T6 resulted in the highest biomass production, compared to all other treatments (Table 3). The combined application improved availability of nutrients and soil quality. These bacteria may help in plant growth by the production of phytohormones, organic acids, siderophores, and nutrient cycling (Glick 2014; Kong and Glick 2017; Olanrewaju et al. 2017).
Estimation of plant metal uptake and sequestration
The metal content of plants varied between T2 and T5 with Pb and Ni concentration being the highest in the former and Cu, Cr, and Cd concentration being the highest in the later ones. The trend was as followed: Pb 3% and Ni 17% higher in T2 than in T5 plants, while Cu 13%, Cd 7.7% and Cr 17% higher in T5 as compared to T2. With respect to Cr, the highest concentration was found in leaves showing high translocation (Fig. 4b). Budak et al. (2011) reported that in Lobularia maritima (L.) Desv. the concentrations of Cr were 123 and 234 mg kg−1 in roots and shoots, respectively, showing translocation to aerial parts of a plant. One of the most important factors that mediate the metal uptake is the secretion of organic acids possibly due to result of activities of sources, e.g., root exudates, microorganisms, decomposition of organic matter, and may imitate plant response to biotic and abiotic stresses (Adeleke et al. 2017; Man et al. 2016). Cu also followed the same trend, i.e., plant inoculated with B. cereus showed higher levels of metal uptake and translocation to the above ground parts (Fig. 5b). Similar results were reported by a study, where inoculation of two bacterial strains (P. fluorescens RB4 and B. subtilis 189) increased the uptake and accumulation of Cu and Pb in shoots of C. roseus, in Cu and Pb co-contaminated soil, corresponding to the controls (Khan et al. 2017b). With respect to Cd roots contained the highest amount of Cd showing least translocation of Cd to aerial parts. Liu et al. (2008) also reported the same trend with Althaea rosea (L.) Cav., an ornamental plant that accumulated high amount of Cd in root section. Cui et al. (2013) confirmed this trend in the study; they reported translocation factors in Celosia argentea L. < 1, and the Pb concentration in C. argentea pyramidalis shoots was greater than 1000 mg kg−1, the threshold amount for a Pb hyperaccumulator. Forte and Mutiti (2017) conducted the study to assess the phytoremediation of Helianthus annuus L. and Hydrangea paniculata Siebold, and they reported that both species accumulated significant amounts of Cu and Pb to be classified as hyperaccumulator species. According to studies reported, the higher concentrations of metals in inoculum amended treatments can be attributed to the production of siderophores, organic acids, and biosurfactants depending on the microbial species (Anjum et al. 2010; Braud et al. 2009; Ma et al. 2015). Microbial siderophores, biosurfactant, and organic acids help in enhancement of the bioavailable fractions of metal in soil solution. Braud et al. (2009) inoculated soil with siderophore-producing bacterium Pseudomonas aeruginosa and found significant increase in phytoavailable fractions of Cr and Pb. Prapagdee and Wankumpha (2017) reported that application of cadmium-resistant bacteria Arthrobacter sp. and Micrococcus sp. significantly improved Cd uptake by Chlorophytum laxum R.Br. Ma et al. (2015) reported that the addition of biosurfactant-producing bacterial strain Bacillus sp. SC2b in Sedum plumbizincicola enhanced Cd and Zn uptake in plant body by 15 and 13%, respectively, compared to non-inoculated controls. Rajkumar et al. (2008) studied the effect of inoculation of bacterium Bacillus weihenstephanensis SM3 on Zn uptake of H. annuus and found that the Zn uptake and accumulation increased in roots and shoots by 22 and 35%, respectively in contrast to controls. Rajkumar et al. (2013) found that addition of Bacillus megaterium on the sterilized seeds of 3 plants (Brassica juncea (L.) Czern., Luffa cylindrica (L.) M.Roem., and Sorghum halepense (L.) Pers.) reduced Ni concentration in roots and shoots as compared to the control plants.
Organic amendments have been reported to reduce metal concentration in plants (Bidar et al. 2019). In current study, the addition of organic amendments (compost and peat moss) resulted in immobilization and reduction of bioavailable fractions in soil (Fig. 4a, b, and c, and 5a and b). Based on laboratory experiments, the maximum sorption abilities were measured: “green waste compost 86.5 mg Pb g−1, 30.2 mg Cu g−1, and 13.9 mg Zn g−1; and peat 47.8 mg Pb g−1, 11.8 mg Cu g−1, and 4.2 mg Zn g−1. The partiality of the metals for the amendment materials was Pb > Cu> > Zn (Nwachukwu and Pulford 2008). Amendments used in this experiment significantly reduced C. roseus metal concentration, with compost showing more dominating effects over moss that indicate the reduction in metal mobility in the soil. Compost is reported to induce the double dilution effect, i.e., upon addition to soil, it initially reduced the metal uptake due to the dilution of metal concentration in soil, followed by improved plant growth yield/biomass (McBride et al. 2015; Paltseva et al. 2020). Han-Song et al. (2010) reported 70% decrease in exchangeable Cd fraction due to application of chicken manure compost. Dodangeh et al. (2018) reported that the uptake of Mn, Pb, Ni, and Cd by shoots and bulbs of selected ornamental plants was reduced due to application of organic amendments. Farrell et al. (2010) concluded that green waste compost and municipal solid waste-derived composts improved plant growth yield and depth of rooting system, decreased metal uptake and accumulation, elevated pH, and improved the nutrient content of soil. B. cereus in combination with compost and moss showed no significant contribution to metal uptake. Similar results for Cd uptake were reported by Touceda-González et al. (2015), where they found that Burkholderia phytofirmans PsJN in combination with gravel sludge resulted in reduction of Pb and Zn uptake in plants, while no significant effects were measured on Cd concentration. Yang et al. (2018) investigated the effects of organic amendments: green tea amendment (GTA) and oil cake amendment (OCA) on Cd bioavailability of Pakchoi cabbage (Brassica rapa L.) and concluded that Cd concentration of aerial parts decreased by 8.21–18.05 and 7.77–35.89% in respective treatments.
Future prospects
Phytoremediation using ornamental plants is a good alternative to traditional hyperaccumulators. Still, little knowledge is available regarding ornamental plants and their interaction with microbial species. In this study, we explored their interaction for short period of time, but long-term study is required to confirm the translocation pattern, fate of metals, and long-term response of plants species to metals. Further studies are also needed to assess the time the amendments can continue to immobilize heavy metals in soils. Lastly, it is vital to check the response of strains combination with amendments on plant(s) metal(s) uptake when exposed for longer period of times.
Conclusions
This study highlights the effects of Bacillus cereus and organic amendments (compost and peat moss) on growth and heavy metal tolerance/accumulation in Catharanthus roseus (L.) G.Don against a multi-metal system. C. roseus showed significant improvement in plant physiological and physiochemical parameters, reduced plant stress, lower metal uptake with the addition of organic amendments, and best results in case of compost + moss + inoculum. Bacterial augmentation in rhizosphere (using B. cereus) enhanced accumulation of Cu, Cr, and Cd but did not improve the accumulation of Ni and Pb in plant. With these results, ornamental plants like C. roseus can be presented as an option for the final polishing step of metals containing treated wastewater.
References
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles and fate of organic acids in soils: a review. S Afr J Bot 108:393–406
Anjum F, Bhatti HN, Asgher M, Shahid M (2010) Leaching of metal ions from black shale by organic acids produced by Aspergillus niger. Applied Clay Science 47:356–361
Arab F, Mulligan CN (2018) An eco-friendly method for heavy metal removal from mine tailings. Environ Sci Pollut Res Int 25:16202–16216
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Arshad M, Khan AHA, Hussain H, Anees M, Iqbal M, Soja G, Yousaf S (2017) The reduction of chromium (VI) phytotoxicity and phytoavailability to wheat (Triticum aestivum L.) using biochar and bacteria. Appl Soil Ecol 114:90–98
Beesley L, Marmiroli M (2011) The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ Pollut 159:474–480
Bidar G, Pelfrêne A, Louvel B, Janus A, Douay F (2019) Influence of amendments on metal environmental and toxicological availability in highly contaminated brownfield and agricultural soils. Environ Sci Pollut Res 26:33086–33108
Boechat CL, Giovanella P, Amorim MB, de Sa ELS, de Oliveira Camargo FA (2017) Metal-resistant rhizobacteria isolates improve Mucuna deeringiana phytoextraction capacity in multi-metal contaminated soils from a gold mining area. Environ Sci Pollut Res 24:3063–3073
Braud A, Jézéquel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74:280–286
Budak F, Zaimoglu Z, Bascı N (2011) Uptake and translocation of hexavalent chromium by selected species of ornamental plants. Pol J Environ Stud 20:857–862
Cui S, Zhang T, Zhao S, Li P, Zhou Q, Zhang Q, Han Q (2013) Evaluation of three ornamental plants for phytoremediation of Pb-contaminated soil. Int J Phytoremediat 15:299–306
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Dodangeh H, Rahimi G, Fallah M, Ebrahimi E (2018) Investigation of heavy metal uptake by three types of ornamental plants as affected by application of organic and chemical fertilizers in contaminated soils. Environ Earth Sci 77:473
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region 3
Farrell M, Perkins WT, Hobbs PJ, Griffith GW, Jones DL (2010) Migration of heavy metals in soil as influenced by compost amendments. Environ Pollut 158:55–64
Forte J, Mutiti S (2017) Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil. Water Air Soil Pollut 228:77
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Han-Song C, Qiao-Yun H, Li-Na L, Peng C, Wei L, Ming L (2010) Poultry manure compost alleviates the phytotoxicity of soil cadmium: influence on growth of Pakchoi (Brassica chinensis L.). Pedosphere 20:63–70
Hussain F, Hussain I, Khan AHA, Muhammad YS, Iqbal M, Soja G, Yousaf S (2018) Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ Exp Bot 153:80–88
Iqbal M, Nawaz I, Hassan Z, Hakvoort HW, Bliek M, Aarts MG, Schat H (2013) Expression of HMA4 cDNAs of the zinc hyperaccumulator Noccaea caerulescens from endogenous NcHMA4 promoters does not complement the zinc-deficiency phenotype of the Arabidopsis thaliana hma2hma4 double mutant. Front Plant Sci 4:404
Iqbal A, Mushtaq MU, Khan AHA, Nawaz I, Yousaf S, Iqbal M (2019) Influence of Pseudomonas japonica and organic amendments on the growth and metal tolerance of Celosia argentea L. Environ Sci Pollut Res Int 2019:1–15
Jones S, Bardos RP, Kidd PS, Mench M, de Leij F, Hutchings T, Herzig R (2016) Biochar and compost amendments enhance copper immobilisation and support plant growth in contaminated soils. J Environ Manag 171:101–112
Khan AHA, Tanveer S, Alia S, Anees M, Sultan A, Iqbal M, Yousaf S (2017a) Role of nutrients in bacterial biosurfactant production and effect of biosurfactant production on petroleum hydrocarbon biodegradation. Ecol Eng 104:158–164
Khan WU, Ahmad SR, Yasin NA, Ali A, Ahmad A (2017b) Effect of Pseudomonas fluorescens RB4 and Bacillus subtilis 189 on the phytoremediation potential of Catharanthus roseus (L.) in Cu and Pb-contaminated soils. Int J Phytoremediat 19:514–521
Khan AHA, Butt TA, Mirza CR, Yousaf S, Nawaz I, Iqbal M (2019a) Combined application of selected heavy metals and EDTA reduced the growth of Petunia hybrida L. Sci Rep 9:4138
Khan AHA, Nawaz I, Yousaf S, Cheema AS, Iqbal M (2019b) Soil amendments enhanced the growth of Nicotiana alata L. and Petunia hydrida L. by stabilizing heavy metals from wastewater. J Environ Manag 242:46–55
Khan AHA, Nawaz I, Qu Z, Butt TA, Yousaf S, Iqbal M (2020) Reduced growth response of ornamental plant Nicotiana alata L. upon selected heavy metals uptake, with co-application of ethylenediaminetetraacetic acid. Chemosphere 241:125006
Kong Z, Glick BR (2017) The role of plant growth-promoting bacteria in metal phytoremediation. Adv Microb Physiol 71:97–132
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu J-n, Q-x Z, Sun T, Ma LQ, Wang S (2008) Growth responses of three ornamental plants to Cd and Cd–Pb stress and their metal accumulation characteristics. J Hazard Mater 151:261–267
Liu J, Xin X, Zhou Q (2018) Phytoremediation of contaminated soils using ornamental plants. Environ Rev 26:43–54
Ma Y, Oliveira RS, Wu L, Luo Y, Rajkumar M, Rocha I, Freitas H (2015) Inoculation with metal-mobilizing plant-growth-promoting rhizobacterium Bacillus sp. SC2b and its role in rhizoremediation. J Toxic Environ Health A 78:931–944
Man YB, Chung AKC, Wong MH (2016) Changes in low molecular weight organic acids and antioxidative enzyme activities of wetland plants under metal stresses. Environ Eng Manag J 15:1657–1663
McBride MB, Shayler HA, Russell-anelli JM, Spliethoff HM, Marquez-bravo LG (2015) Arsenic and lead uptake by vegetable crops grown on an old orchard site amended with compost. Water Air Soil Pollut 226:1–10
Nwachukwu OI, Pulford ID (2008) Comparative effectiveness of selected adsorbant materials as potential amendments for the remediation of lead, copper and zinc-contaminated soil. Soil Use Manag 2:199–207
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 33:197
Paltseva AA, Cheng Z, Perl S, Groffman PM (2020) Remediation of an urban garden with elevated levels of soil contamination. Sci Total Environ 722:137965
Prapagdee B, Wankumpha J (2017) Phytoremediation of cadmium-polluted soil by Chlorophytum laxum combined with chitosan-immobilized cadmium-resistant bacteria. Environ Sci Pollut Res Int 23:19249–19258
Rajkumar M, Ma Y, Freitas H (2008) Characterization of metal-resistant plant-growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J Basic Microbiol 48:500–508
Rajkumar M, Ma Y, Freitas H (2013) Improvement of Ni phytostabilization by inoculation of Ni resistant Bacillus megaterium SR28C. J Environ Manag 128:973–980
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Raza A, Khan AHA, Nawaz I, Qu Z, Yousaf S, Ali MA, Iqbal M (2019) Evaluation of arsenic-induced stress in Dahlia pinnata Cav.: morphological and physiological response. Soil Sediment Contam 28:716–728
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23:17859–17879
Semenya SS, Potgieter MJ (2013) Catharanthus roseus (L.) G.Don.: extraordinary Bapedi medicinal herb for gonorrhoea. J Med Plant Res 7:1434–1438
Stanislawska-Glubiak E, Korzeniowska J, Kocon A (2015) Effect of peat on the accumulation and translocation of heavy metals by maize grown in contaminated soils. Environ Sci Pollut Res Int 22:4706–4714
Tejada M, Benítez C (2015) Application of vermicomposts and compost on tomato growth in greenhouses. Compost Sci Util 23:94–103
Tordoff G, Baker A, Willis A (2000) Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Touceda-González M, Brader G, Antonielli L, Ravindran VB, Waldner G, Friesl-Hanl W, Sessitsch A (2015) Combined amendment of immobilizers and the plant growth-promoting strain Burkholderia phytofirmans PsJN favours plant growth and reduces heavy metal uptake. Soil Biol Biochem 91:140–150
Yang Z, Liu L, Lv Y, Cheng Z, Xu X, Xian J, Zhu X, Yong Y (2018) Metal availability, soil nutrients, and enzyme activity in response to application of organic amendments in Cd-contaminated soil. Environ Sci Pollut Res Int 22:2425–2435
Funding
The present study was financially supported by the specialized University Research Funds (URF) of Quaid-i-Azam University (QAU), Islamabad.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing or conflict of interest.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb).
Rights and permissions
About this article
Cite this article
Mushtaq, M.U., Iqbal, A., Nawaz, I. et al. Enhanced uptake of Cd, Cr, and Cu in Catharanthus roseus (L.) G.Don by Bacillus cereus: application of moss and compost to reduce metal availability. Environ Sci Pollut Res 27, 39807–39818 (2020). https://doi.org/10.1007/s11356-020-08839-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08839-5