Abstract
This is the first study on the dinoflagellate cysts in Algerian waters and in Mellah Lagoon (South Western Mediterranean), located within a protected reserve. In total, 42 species of dinocysts belonging to 7 orders, 12 families and 23 genera, were identified in the 26 superficial sediment samples from Mellah Lagoon. The distribution of dinocysts in the sediment of this lagoon is heterogeneous. Indeed, their abundance oscillates between 1 and 315 cysts g−1 dry sediment (DS). Cyst morphotype assemblages were dominated by a few numbers of species: Alexandrium minutum (15.87%), Gonyaulax verior (9.81%), Protoperidinium spp. (7.74%), Alexandrium affine (7.05%), Scrippsiella trochoidea (6.67%), and Alexandrium pseudogonyaulax (6.19%). There is a positive correlation between the density of cysts and the depth (r = 0.61; p < 0.05), organic matter (r = 0.70; p < 0.05), water content (r = 0.71; p < 0.05), and the fine fraction of sediment (r = 0.74; p < 0.05). Surprisingly, although the Mellah Lagoon is almost semi-closed, it holds an important specific richness in dinocysts (42 species) higher than others observed in Mediterranean lagoons. However, cyst abundances are low compared to other lagoons in the Mediterranean Sea. Finally, the presence of dinocysts of Alexandrium catenella/tamarense, A. minutum, and Gymnodinium catenatum associated to paralytic shellfish toxins, A. pseudogonyaulax which produces goniodomin A, also Protoceratium reticulatum and Gonyaulax spinifera complex which produce yessotoxins, needs to implement a monitoring program to prevent a potential human intoxication due to the consumption of contaminated sea products by these potent neurotoxins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton blooms involving toxic dinoflagellates, emerging biological contaminants, have become a common problem in the Mediterranean Sea. Among the 4000 phytoplankton species recorded in the marine environment (Sournia et al. 1991), about 300 species are harmful to aquatic organisms and among them 80 species are known to produce phycotoxins (Granéli and Turner 2006). The Southern Mediterranean countries are experiencing important developments in toxic phytoplankton with periods of seafood intoxication resulting of economic repercussions. Among the studies carried out on these microalgae, we quote those conducted in Algeria (Ounissi and Frehi 1999; Frehi et al. 2007; Illoul et al. 2008, 2012), in Tunisia (Turki et al. 2014; Zmerli Triki et al. 2014, 2015a; Fertouna-Bellakhal et al. 2014, 2015; Ben Gharbia et al. 2017), on the Mediterranean coasts of Morocco (Taleb et al. 2001; EL Madani et al. 2011; Daoudi et al. 2012; Daghor et al. 2016). Coastal lagoons are concerned by expansion of toxic phytoplankton. These ecosystems are often important shellfish production areas, where the emergence of some potentially toxic dinoflagellate species could threat aquaculture activities and human health. The search for areas at risk of developing toxic dinoflagellates, the determination of the resting cysts belonging to toxic species in these ecosystems, can preserve aquaculture activities better.
Despite the ecological importance (contamination of the food web components), economic relevance (interdiction of exploitation of commercial species), and threat to human health, the monitoring of toxic species in the Algerian east coast is not implemented yet (Frehi et al. 2007; Hadjadji et al. 2014; Cheniti et al. 2018). In Mellah Lagoon, with the exception of an inventory of phytoplankton species made during the 1990s (Draredja 2007), there is no study dedicated to toxic dinoflagellates, particularly as regards to their dormancy forms or dinocysts. Mellah lagoon is relatively far (about 50 km) at east of the big industrial port of the city of Annaba which is classified as the industrial capital of Algeria and subject to spills of large quantities of ballast water from large vessels (Hadjadji et al. 2014; Cheniti et al. 2018). However, the currents from the prevailing north-westerly winds, as well as the Atlantic current along the southern coast of the western Mediterranean basin (Millot 1999, 2009), can carry long-distance water masses along the eastern shores, until the Tunisian coasts. The Mellah lagoon supports an important activity of fishing, breeding (mussels) and gathering (clams and hulls), and the proliferation of toxic microalgae could impact the human health via the consumption of the contaminated mollusks. The accumulation of dinoflagellate cysts in the sediment forms seed banks that can trigger phytoplankton blooms and species dispersal (Anderson et al. 1995). In fact, their germination ensures the proliferation of microalgae in the waters and the blooms initiation (McGillicuddy et al. 2003). Consequently, the accumulation of dormant cysts in the sediment is a source of the inoculation of the water column leading to harmful algal blooms (HABs) and their recurrence. Consequently, knowing the distribution of cysts in the surface sediment of lagoons is of great importance.
On the Algerian coasts including the Mellah lagoon, harmful algae events date from the early 2000s. Draredja (2007) was the first author to show the proliferation of Alexandrium catenella (6.4 × 103 cells L−1) and Prorocentrum minimum (P. cordatum) (3.2 × 103 cells L−1) in the Mellah Lagoon waters in April and July 2001, respectively. Frehi et al. (2007) reported the blooms in Annaba Bay of A. catenella (117 × 103 cells L−1) and Gymnodinium catenatum (3.5 × 106 cells L−1) in March and June 2002, respectively. Hadjadji et al. (2014), observed a proliferation of A. catenella (861 × 103 cells L−1) in the same bay in May 2010. In the waters of the Algiers coast (two bays, harbor, and beach), Illoul et al. (2008) reported the occurrence of a bloom of Dinophysis cf. acuminata (1.2 × 103 cells L−1) and Dinophysis sacculus (3.3 × 103 cells L−1) in July–August 2002–2003. In the same region (in 5 beaches), Illoul et al. (2012) mentioned in July 2009 the appearance of a bloom of Ostreopsis spp. (7.9 × 104 cells L−1) resulting of the intoxication and hospitalization of 300 persons.
This study aims to (1) provide detailed description of dinoflagellate cysts, (2) provide information on the distribution and abundance of dinocysts, (3) assess seedbeds in potential risk areas, and (4) identify the potential environmental driving factors of cyst distribution. Results could help aquaculture farm managers and to serve as a basis for future studies of dinoflagellate dynamics in the lagoon.
Material and methods
Study area and sampling strategy
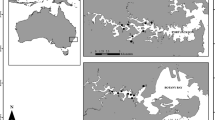
The Mellah Lagoon is located in the extreme eastern side of Algeria (8° 20′ E–36° 54′ N), on the southern shore of the western basin of the Mediterranean Sea (Fig. 1). The Mellah Lagoon is the unique lagoon in Algeria; it covers about 865 ha, with a length of 4.50 km and a width of 2.50 km. It’s formed of a central depression, with a maximum depth not exceeding 5 m. This brackish water ecosystem is an integral part of the wetland complex of El-Kala national park, where there are other freshwater ecosystems: Oubéira and Tonga lakes. Guelorget et al. (1989) described the Mellah Lagoon as an environment that corresponds to a würmian lake endorheic depression invaded by the sea during the Flemish eustatic rise. According to the story this site was a freshwater lake and for aquaculture purposes, a channel was dug by the Maltese Pisani at the end of the eighteenth century for connecting it to the adjacent coast.
Sediment sampling was carried out during April 4 and 5, 2016 on 26 stations covering the entire lagoon (Fig. 2), using cylindrical cores (80 cm long, 8 cm diameter) operated by a professional diver. The majority of stations are located within the expanse (depth > 2 m), where the fine fraction is important; it is the sedimentary typology most favorable to the distribution of dinocysts. Three replicates at each station were adopted. The top 3 cm of undisturbed surface sediment from the three replicates samples for each sampling station were mixed together and then stored in total darkness at 4 °C until the analyses. Indeed, cysts are distributed mostly in the first three centimeters of the sediment surface (Erard-Le Denn et al. 1993). Dinocysts were separated from the sediments according to the modified density gradient method using Ludox CLX described by Yamaguchi et al. (1995); Erard-Le Denn and Boulay (1995) and Genovesi et al. (2007). Once the extraction operation is complete, the dinocysts are stored in tubes covered with aluminum, then placed at 4 °C while waiting for the counting and identification phase.
A fraction of the sampled sediment was used for the analysis of the water content and the proportion of the fine fraction in the sediment. The water content in the sediment is calculated by drying the sediment at 60 °C until the substrate is completely dehydrated. The separation of the fine fraction from the coarse fraction is set at the limit of 63 μm. Sediment samples with a particle size greater than 63 μm were dried at 60 °C, then sieved through a series of the AFNOR type for 15 min, with a mesh size varying from 2000 at 63 μm (2000, 1600, 1400, 1250, 1000, 710, 500, 355, 280, 250, 180, 140, 125, 90, 80 and 63 μm). The separation between the different meshes was conducted using an automatic vibrator “Retch VS 1000.” The contents of the sieves were calculated (in g and %) to determine the median which represents the mean diameters of the grains, it allows us to define the nature of the sediment for each station. The particle size classification of the sediments is referred to ISO 14 688-1. The sediment organic matter is obtained from the difference in weight between the dry sediment and the sediment incinerated at 450 °C for 12 h so to evaporate organics in the form of carbon dioxide. According to Guelorget et al. (1989), this method is justified because of the low sediment content of phyllitic minerals, which alone can lead to errors in this measure.
In parallel with sediment sampling, bathymetric surveys, diving with a depth meter and physicochemical parameters (temperature, salinity, pH and dissolved oxygen), were measured at 26 stations spread over the entire lagoon (Fig. 2), using a multiparameter “HANNA HI9828.” Water transparency was measured using a Secchi disk with a standard diameter of 30 cm.
Resting cyst identification and quantification
The taxonomic identification of cysts was performed according to the Matsuoka and Fukuyo (2000) methodology based on the microscopic observation of their morphological characteristics. Indeed, for each prepared Sedgewick plate, all the morphotypes of the cysts observed are photographed. The identification is carried out according to the morphological characteristics of the cysts under inverted light microscope. This direct method is based on morphological features and characters of cysts using identification keys and from plates illustrated in articles and publications dealing with dinocysts (Head 1996; Zonneveld 1997a, b; Zonneveld and Jurkschat 1999; Rochon et al. 1999; Head et al. 2001; Pospelova and Head 2002; Kim et al. 2007; Matsuoka et al. 2009). Moreover, the observations of the various species of cysts of the lagoon are carried out under the microscope “Olympus IX53,” with photographs taken at × 40 magnification. Part of the final sample was used to quantify dinocysts using a sedimentation chamber and an inverted microscope. Calculation of the abundance of dinocysts in each sample allows to determine the number of resting cysts per gram of wet sediment. For this, solutions (from 5 to 20 ml) of seawater cyst extracts are used. For each, 1 ml is taken and distributed on a Sedgewick count plate allowing the counting of dinocysts under a “Leica DM750” microscope, at × 20 magnification. Expression of cyst densities was first evaluated per gram of wet sediment (Eq. 1). To reduce the variability due to the sediment water content, we expressed cyst densities per gram of dry sediment (Eq. 2).
N: number.
Accordingly, resting cyst quantifications were performed with a single extraction step, and cyst densities in sediments were estimated by applying a twofold correction factor (Genovesi et al. 2013).
Statistical analyses
A principal component analysis (PCA) is performed using R, version 3.4.2 (R Core Team 2017; Ihaka and Gentleman 1996) for Windows, whose objective is to relate the resting cyst distribution pattern to environmental variables. Additionally, a Spearman’s correlation analysis is also used at p < 0.05 to determine the interaction between the resting cyst abundance in the lagoon and the environmental variables.
Results
Physicochemical parameters and sedimentological characteristics
The average water temperature calculated from the 26 stations surveyed during the study period (April 2016) was 19.09 ± 0.48 °C, with a maximum of 19.93 °C, reported at station 13 located in the east shore of the lagoon (depth = 1.90 m), and a minimum of 17.86 °C recorded in station 4 in the throttling zone of the lagoon (depth = 3.30 m) (Table 1). The fluctuations of salinity in the lagoon were directly related to sea-lagoon exchanges and to the inflow of freshwater through the three seasonal rivers. The average salinity of the water was 26.69 ± 0.48. The maximum salinity of 28.29 was recorded at station 1 in the north of the lagoon in front of marine inputs, while the minimum salinity of 26.06 was detected at station 26 at the extreme south of the lagoon far from marine influences (Table 1). The pH of the waters of the lagoon was slightly alkaline and oscillated between 8.15 and 8.31. In addition, the waters of the Mellah lagoon were well oxygenated, particularly with regard to the peripheral stations and the contents vary between 6.25 and 8.88 mg L−1. During the spring season, the water transparency corresponding to the depth of disappearance of the Secchi disk oscillated between 2.10 and 3.10 m for the depth > 3.50 m (Table 1).
The particle size analysis of the prospected stations, in particular those with a depth greater than 2 m near the center of the lagoon, was characterized by an important rate in silt (< 63 μm), with contents that exceeded 50% (Fig. 3; Table 1). Almost all of the sites surveyed are characterized by medium sand. The water content of the sediments shows that the highest levels are found in the muddy bottoms, where the fine fraction (< 63 μm) dominates (Table 1). Extremes in water retention of sediments are recorded in station 13 (19.05%), where the fine fraction represents only 1.68%, and in station 23 (80.58%), with a rate in fine fraction of 95.55% (Table 1). The obtained results show that the content of fine fraction is increasing from the shore to the center of the lagoon. The highest rates (> 90%) are recorded in the deep zones of the lagoon (depth > 3.20 m). Station 17 (depth = 4.90 m), located in the west of Mellah, contains the highest rate (96.84%). The lowest levels of organic matter in sediments are observed in the periphery of the lagoon, with the depth not exceeding 1 m (Table 1). The lowest value (0.83%) is recorded in station 26 (depth = 0.70 m), with a substrate composed with a pure sand. The highest rates are found inside the lagoon, where the fine fraction is dominating (Table 1). Thus, the maximum content of 24.65% is detected in station 20 located in the center of the lagoon (depth = 4.60 m), where the fine fraction is clearly dominant (96.05%). Overall, the distribution of organic matter in the lagoon is very heterogeneous, with an average of (13.96 ± 8.88) %.
Dinocyst distribution and abundance
A total of 42 species of dinocysts belonging to 7 orders, 12 families and 23 genera, were identified in the 26 superficial sediment samples from Mellah Lagoon (Table 2). The distribution of taxa in dinocysts is organized into the following: Peridiniales (3 families, 7 genera, and 14 taxa), Gonyaulacales (3 families, 7 genera, and 15 taxa), Gymnodiniales (2 families, 5 genera, and 9 taxa) (Plate 1), Suessuales, Prorocentrales, Thoracosphaerales, and Tovelliales (1 family, 1 genera, and 1 taxon for each order). The biological, paleontological names, the harmful effects, and relative abundance of dinoflagellate cysts identified in the present study Mellah Lagoon are showed in Table 2. The distribution of dinocysts in the surface sediments of the Mellah Lagoon is very uneven. Indeed, their abundance oscillates between 1.50 cysts g−1 DS in both station 22 (south east of the lagoon) and station 26 (south of the lagoon) and 303 and 315 cysts g−1 DS in station 17 (center of the lagoon) and station 15 (West center of the lagoon), respectively (Fig. 4). The average of cyst abundance in the whole lagoon is 114 cysts g−1 DS. The highest density of dinocysts is found in station 15 characterized by a bottom of sandy silt, where the hydrodynamic intensity is relatively low and in station 17 in the center of the lagoon the deepest zone (4.90 m) of the lagoon, than characterized by bathymetric confinement (Fig. 4), while the lowest concentration of these cysts was detected at both stations 22 and 26 (depth < 1.80 m) near the coast so with a high agitation and characterized by a substrate dominated by pure sands. The specific richness extremes vary between 2 in stations 18 (depth = 1.90 m) and 22 (depth = 1.80 m), near the coast south east of the lagoon and 17 in station 19 (depth = 4.80 m) in the center of the lagoon. Generally, the silt fraction and the deepest sites are the richest in cysts than the sandy fraction near the coast (Fig. 5). The Mellah Lagoon dinocysts are represented mainly by three orders: Gonyaulacales (54.56%), Peridiniales (35.01%), and Gymnodiniales (8.85%) (Fig. 6). The other groups are very poorly represented (1.85%). In Mellah Lagoon, only one species is classified as common or constant (F > 75%): Alexandrium minutum, while the regular species 50% < F < 75%) are as follows: Alexandrium verior, A affine, Scrippsiella trochoidea and Protoperidinium spp. (Table 2). The dinoflagellate cysts were dominated by a few species (Fig. 7): Alexandrium minutum (15.87%), Gonyaulax verior (9.81%), Protoperidinium spp. (7.74%), Alexandrium affine (7.05%), Scrippsiella trochoidea (6.67%), and Alexandrium pseudogonyaulax (6.19%). Among the 42 dinocysts detected in surface sediment of lagoon, 8 are considered to be potentially noxious/toxic as Alexandrium catenella/tamarense, Alexandrium margalefi, Alexandrium minutum, Alexandrium pseudogonyaulax, Gonyaulax spinifera complex, Protoceratium reticulatum, Gymnodinium catenatum, and Prorocentrum minimum (Table 2).
Light microscopy photographs of selected morphotype cysts isolated from surface sediments in Mellah Lagoon. I—Peridiniales: 1—Diplopsalis lenticula; 2—Protoperidinium conicoides; 3—Preperidinium sp.; 4—Pentapharsodinium dalei; 5—Protoperidinium avellana; 6—Protoperidinium sp.; 7—Scrippsiella trochoidea; 8—Zygabikodinium lenticulatum. II—Gonyaulacales: 9—Alexandrium pseudogonyaulax; 10—Bitectatodinium spongium; 11—Gonyaulax spinifera complex. III—Gymnodiniales: 12—Gymnodinium catenatum; 13—Polykrikos kofoidii; 14—Polykrikos schwartzii. Scale bar 10 μm
Relationship between environmental factors and resting cyst abundance
Spearman’s correlation analyses show that the correlations between the abundance of dinocysts and the environmental factors of the surface of sediment such as silt fraction (r = 0.74; p < 0.05), water content (r = 0.71; p < 0.05), organic matter (r = 0.70; p < 0.05), and the depth (r = 0.61; p < 0.05) are positive and significant. The multivariate analysis (PCA) indicates that the density of dinocysts is significantly correlated with the environmental factors mentioned above (Fig. 8). This PCA shows that the first two factorial axes yielded nearly 74.35% of the information. Axis F1 explains 54.26% of the total variation; it is built mainly by the positive correlation of the variables silt fraction (r = 0.98), water content (r = 0.96), organic matter (r = 0.96), depth (r = 0.76), and total RC counting (r = 0.74) and which also contribute significantly to its construction (cos2 = 0.96, cos2 = 0.93, cos2 = 0.92, cos2 = 0.58 and cos2 = 0.54, respectively) and negatively with the variable coarse fraction (r = −0.98), which strongly contribute to the construction of this axis (cos2 = 0.96). In addition, axis F2 explains 20.09% of the total variation; it is built mainly by the positive correlations of the variable temperature (r = 0.77) and pH (r = 0.65) which remarkably contribute to the construction of this axis (cos2 = 0.59 and cos2 = 0.43, respectively) and the negative correlation of variables salinity (r = −0.74) and dissolved oxygen (r = −0.15) with a difference in contribution (cos2 = 0.55 and cos2 = 0.02, respectively).
Discussion
This is the first study reporting the distribution, abundance and diversity of cyst assemblages in the superficial sediment of Algerian Mediterranean waters (Mellah Lagoon). Dinoflagellate cysts were found in the 26 sampled stations, but with heterogeneous abundances and patchy distribution.
There are many species of microalgae with a benthonic phase in their life cycle during which they are deposited on the seabed where they usually remain dormant. The dinoflagellate species producing the most important HABs are often characterized by cysts production. These cysts can remain in the sediment for months or even years before they germinate when environmental conditions become favorable. Then, the produced vegetative cells multiply exponentially to form blooms. Despite being a lesser known aspect of life cycle of HAB species, the dormancy phase of dinoflagellates (cyst) is often a key factor to understand HAB development. Many studies have been performed on the dinocyst assemblages in recently deposited sediment of the coastal ecosystems of Western Mediterranean basin (Montresor et al. 1998; Bravo et al. 2006, 2008; Satta et al. 2010, 2013; Rubino et al. 2010; Feki et al. 2013). Mediterranean lagoons were also investigated (Genovesi et al. 2009, 2013; Bouchouicha Smida et al. 2012; Satta et al. 2014; Fertouna-Bellakhal et al. 2014, 2015; Zmerli Triki et al. 2014; Zmerli Triki et al. 2015b; Zmerli Triki et al. 2016; Daghor et al. 2016; Dhib et al. 2016; Zmerli Triki et al. 2017). Unfortunately, no such studies have been conducted to date in Algerian waters.
A detailed spatial distribution of resting cysts present in superficial sediment (< 5 cm) is reported for the first time in Mellah Lagoon. Our results show that cyst densities in this unique preserved ecosystem are characterized by moderate values (up to 315 cysts g−1 DS) compared to some Mediterranean coastal waters. Indeed, the found cysts densities are lower than those in Bizerte lagoon (Tunisia) (20,126 cysts g−1 DS and 2742 cysts g−1 DS reported by Fertouna-Bellakhal et al. 2014 and Zmerli Triki et al. 2017, respectively) and in Izmir Bay (Turkey) (3292 cysts g−1 DW reported by Aydin et al. 2011). However, the cyst abundances of Mellah lagoon are similar to those found in Ghar El Melh lagoon (Tunisia) with up to 229 cysts g−1 DS (Dhib et al. 2016) and Cabras in Sardinia (Italy) with up to 287 cysts g−1 DW (Satta et al. 2014), but higher than that found in Homa Lagoon (Turkey) with up to 71 cysts g−1 DW (Aydin et al., 2014). Regarding to species composition, interestingly, despite the restricted surface (865 ha) and the relatively recent age of the Mellah, this lagoon contains a relatively high species richness (42 species) when compared to all the Mediterranean lagoons mentioned above except the lagoon of Bizerte which contains the same number of species as the Mellah Lagoon. Our results show that six species dominate: Alexandrium minutum, A. affine, A. pseudogonyaulax, Protoperidinium spp., Gonyaulax verior, and Scrippsiella trochoidea. Among these species, two species are associated to HABs, the first A. minutum (15.87%) producing paralytic shellfish toxins (PSTs) (Anderson et al. 2012) is the most dominant and the second one is A. pseudogonyaulax (6.19%) which was shown to produce goniodomin A, a potent toxin (Zmerli Triki et al. 2016). These species could in the near future form HABs and therefore impact negatively the biological components of the Mellah lagoon with potential human intoxication.
The accumulation of dormant cysts in the sediment resulting of “cyst banks” could be a source of seeding allowing the initiation of blooms and the recurrence of these phenomena. The mapping of the different resistance cysts present in the superficial sediment of the Mellah Lagoon is important ecologically. Generally, dinocysts size is ranged between 20 and 100 μm, allowing them to behave like fine silt particles in the natural environment (Dale 1983; Lacasse et al. 2013). Fine grain sized sediments are characterized by higher cyst concentration when compared to sandy sediment (Matsuoka et al. 2003; Horner et al. 2011). In the Mellah lagoon, the highest abundances are found in the fine-rich bottoms by moving towards the center of the lagoon from 2.5 m. This also applies to the sheltered areas located in the North West and in the West center of the lagoon. Fertouna-Bellakhal et al. (2014) reported that the spatial distribution and cyst abundance are controlled by local currents in Bizerte Lagoon (Tunisia).
Cheniti et al. (2018) demonstrated the potential introduction of several HAB species by ballast water in the Annaba harbor, the second most important industrial and commercial port in Algeria. Also, Annaba bay holds an important HAB species diversity (Frehi et al. 2007; Hadjadji et al. 2014). Mellah Lagoon is located only 50 km from Annaba bay and harbor. One can suppose the transfer of HAB species present in Annaba waters thanks to the currents along the coast and to permanent water exchange between the open Mediterranean Sea and Mellah Lagoon (Millot 1999, 2009). However, this hypothesis needs to be verified by further investigations. Results from a survey of an annual spatio-temporal variation of phytoplankton in three stations distributed along a longitudinal axis (North-South) in Mellah Lagoon (Draredja et al. 2019) show a high correlation (r = 0.99) between the abundance of total phytoplankton cells in the column of water and the density of cyst assemblages in the superficial sediment. In addition, a positive correlation (r = 0.66) was observed between the abundance of the Alexandrium minutum cysts and its vegetative form in the water column.
Several studies have reported that high cyst accumulations are recorded in fine grained rather than in sandy sediments in various coastal marine systems (Yamaguchi et al. 1996; Kremp 2000; Matsuoka et al. 2003; Anglés et al. 2010). In addition, the highest level of sedimentary organic matter in which dinocysts is usually observed in the stations with the highest proportions of fine particles (< 63 μm). Sedimentary organic matter and dinocysts have a strong affinity for fine sediment particles because they adsorb on mineral surfaces. This adsorption process contribute to a better conservation of the organic matter in a general way and thus lead to a correlation between the sedimentary organic matter and the proportion of fine particles (< 63 μm) (r = 0.90) (Draredja 2007; Magni et al. 2008; Yu et al. 2009). Our study corroborates the previous work as in the Mellah Lagoon; the highest concentrations of sedimentary organic matter are observed in the stations with the highest proportion of fine particles (Draredja et al. 2013).
The highest abundances (between 121 and 303 cysts g−1 DS) were registered in the sediment characterized by > 85% of the fine fraction, 70–78% of the water content, and 19.89–22.75% of the organic matter content. Interestingly, bathymetry positively affects the abundance of dinocysts in the superficial sediments of Mellah Lagoon. The deepest central stations (between 3.10 and 4.90 m) are the richest in cysts. The low hydrodynamic, especially in the center of the Mellah, the deepest zone of the lagoon (between 4 and 4.90 m), and the sheltered zone in the center and northwest of the lagoon (Guelorget et al. 1989), facilitates sedimentation of the cysts. The stations of these mentioned zones (stations 10, 11, 14, 15, 17, 19, and 21) show important abundances in cysts (between 141 and 315 cysts g−1 DS). It is contrary to the sites located in the banks (depth < 1.50 m) and exposed to the prevailing north–west winds where the densities in cysts are relatively low (between 1 and 70) with the exception of the station 5 located in the north–east of the lagoon (178 cysts g−1 DS) having a depth of 4 m.
The present study shows the following: (1) Although the Mellah Lagoon is almost semi-closed, because of its remoteness from the adjacent coast, where it is connected to it by a long (900 m), narrow (5–10 m), and winding channel, it encloses a relatively high specific richness in cysts (48 species) in comparison to other Mediterranean lagoons; (2) a heterogeneous and patchy cyst distribution and a relatively dinoflagellate cyst abundances when compared with some southern Mediterranean ecosystems as Bizerte lagoon in Tunisia; (3) as in the majority of marine and lagoon environments, soils rich in fine fractions, organic matter and water (soft vases), so deep or sheltered contain a higher number of cysts compared to the banks of the banks with hydrodynamic ford, with a dominant coarse fraction poor in organic matter. To conclude, the presence of some dinocysts belonging to HAB species related to PSTs such as Alexandrium catenella/tamarense, Alexandrium minutum, and Gymnodinium catenatum; PTP (potentially toxin producer) such as Alexandrium margalefi; Goniodomin A such as Alexandrium pseudogonyaulax; and Yessotoxin such as Gonyaulax spinifera complex and Protoceratium reticulatum, needs to implement a monitoring program to detect toxic species in order to prevent potential human intoxication due to the consumption of contaminated shells or/and fishes. A parallel study on the distribution of phytoplankton in the Mellah shows a positive correlation between the density of total microalgae in the water column and that of cyst assemblages in sediments from north to south of the lagoon (Draredja et al. 2019). However, to understand better the potential impact of the highlighted HAB species present in Mellah lagoon by their vegetative and benthic forms, additional studies should be conducted including the isolation of cells and establishment of clonal cultures, genetic and toxin characterization of the HAB species. We also have to investigate mollusks intoxications by LC-MS/MS as Mellah Lagoon holds since several years the exploitation of shells and fishes. In addition to HAB species proliferation monitoring program, we should also include cyst community studies for early warning of HAB development and also as an important precautionary management policy to prevent any transfer of HAB species by sediment dredging.
References
Anderson DM, Sullivan JJ, Reguera B (1989) Paralytic shellfish poisoning in northwest Spain: the toxicity of the dinoflagellate Gymnodinium catenatum. Toxicon 27:665–674
Anderson DM, Fukuyo Y, Matsuoka K (1995) Cyst methodologies. In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual on harmful marine microalgae, IOC manuals and guides, vol 33. UNESCO, Paris, pp 229–245
Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M (2012) The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14:10–35
Anglés S, Jordi A, Garcés E, Basterretxea G, Palanques A (2010) Alexandrium minutum resting cyst distribution dynamics in a confined site. Deep-Sea Res II 57:210–221
Aydin H, Matsuoka K, Minareci E (2011) Distribution of dinoflagellate cysts in recent sediments from Izmir Bay (Aegean Sea, Eastern Mediterranean). Mar Micropaleontol 80:44–52
Aydin H, Yürür EE, Uza S (2014) Dinoflagellate cyst assemblages in surface sediments from Homa Lagoon (Izmir Bay, Eastern Aegean Sea, the Mediterranean). Fresenius Environ Bull 23(8):1795–1801
Ben Gharbia H, Kéfi-Daly Yahia O, Cecchi P, Masseret E, Amzil Z, Herve F, Rovillon G, Nouri H, M’Rabet C, Couet D, Zmerli Triki H, Laabir M (2017) New insights on the species-specific allelopathic interactions between macrophytes and marine HAB dinoflagellates. PLoS One 12(11):e0187963. https://doi.org/10.1371/journal.pone.0187963
Bouchouicha Smida D, Sahraoui I, Hadj Mabrouk H, Sakka Hlaili A (2012) Seasonal dynamics of genus Alexandrium (potentially toxic dinoflagellate) in the lagoon of Bizerte (North of Tunisia) and controls by the abiotic factors. C R Biol 335(6):406–416
Bravo I, Garcé E, Diogène J, Fraga S, Sampedro N, Figueroa RI (2006) Resting cysts of the toxigenic dinoflagellate genus Alexandrium in recent sediments from the Western Mediterranean coast, including first description of cysts of A. kutnerae and A. peruvianum. Eur J Phycol 41(3):293–302
Bravo I, Vila M, Masó M, Figueroa IR, Ramilo I (2008) Alexandrium catenella and Alexandrium minutum blooms in the Mediterranean Sea: toward the identification of ecological niches. Harmful Algae 7:515–522
Cheniti R, Rochon A, Frihi H (2018) Ship traffic and the introduction of diatoms and dinoflagellates via ballast water in the port of Annaba, Algeria. J Sea Res 133:154–165
Daghor L, Hssaïda T, Chakir S, Limani H, Mouflih M, Hamoumi N, El Madani F, Ennafah B, Fraikech M, El Bouhmadi K (2016) Dinoflagellate cyst study of surface sediments from the Moroccan Atlantic lagoon system Oualidia - Sidi Moussa and the Mediterranean lagoon of Nador. Bull Inst Sci Rabat Earth Sciences Section 38:1–18 (in French)
Dale B (1983) Dinoflagellate resting cysts: “benthic plankton”. In: Fryxell GA (ed) Survival strategies of the algae. Cambridge University Press, New York, pp 69–136
Daoudi M, Serve L, Rharbi N, El Madani F, Vouvé F (2012) Phytoplankton distribution in the Nador lagoon (Morocco) and possible risks for harmful algal blooms. Transit Waters Bull 6:4–19
Dhib A, Fertouna Bellakhal M, Turky S, Aleya L (2016) Driving factors of dinoflagellate cyst distribution in surface sediments of a Mediterranean lagoon with limited access to the sea. Mar Pollut Bull 112(1–2):303–312
Draredja B (2007) Structure and functioning of a Mediterranean lagoon environment: Mellah lagoon (El-Kala, North-East Algeria). University of Annaba, Algeria, Dissertation (in French)
Draredja B, Melouah K, Beldi H (2013) Current granulometric characteristics of the Mellah lagoon (Northeast Algeria): clogging effects of the channel of communication with the sea. Rapp Comm Int Mer Médit 40:82 (in French)
Draredja MA, Frihi H, Boualleg C, Goffart A, Abadie E, Laabir M (2019) Seasonal variations of phytoplankton community in relation to environmental factors in a protected meso-oligotrophic southern Mediterranean marine ecosystem (Mellah lagoon, Algeria) with an emphasis of HAB species. Environ Monit Assess 191:603
EL Madani F, Chiaâr A, Chafi A (2011) Phytoplankton composition and abundance assessment in the Nador lagoon (Mediterranean coast of Morocco). Acta Bot Croat 70(2):269–288
Erard-Le Denn E, Desbruyeres E, Olu K (1993) Alexandrium minutum: resting cyst distribution in the sediments collected along the Brittany coast, France. In: Smayda TJ, Shimizu Y (eds) Toxic phytoplankton blooms in the sea. Elsevier Science Publishers, Amsterdam, pp 109–114
Erard-Le Denn E, Boulay V (1995) Resting cysts of Alexandrium minutum in marine sediments: quantification by three methods. In: Lassus P, Arzul G, Erard-Le Denn E, Gentien P, Marcaillou-Le Baut C (eds) Harmful marine algal blooms. Lavoisier Publishing, Paris, pp 257–730
Feki W, Hamza A, Frossard V, Abdennadher M, Hannachi I, Jacquot M, Belhassen M, Aleya L (2013) What are the potential drivers of blooms of the toxic dinoflagellate Karenia selliformis? A 10-year study in the Gulf of Gabes, Tunisia, southwestern Mediterranean Sea. Harmful Algae 23:8–18
Fertouna-Bellakhal M, Dhib A, Béjaoui B, Turki S, Aleya L (2014) Driving factors behind the distribution of dinocyst composition and abundance in surface sediments in a western Mediterranean coastal lagoon: report from a high resolution mapping study. Mar Pollut Bull 84:347–362
Fertouna-Bellakhal M, Dhib A, Fathalli A, Bellakhal M, Chom N, Masseret E, Laabir M, Turki S, Aleya L (2015) Alexandrium pacificum Litaker sp. nov (group IV): resting cyst distribution and toxin profile of vegetative cells in Bizerte Lagoon (Tunisia, Southern Mediterranean Sea). Harmful Algae 48:69–82
Frehi H, Couté A, Mascarell G, Perrette-Gallet C, Ayada M, Kara MH (2007) Dinoflagellés toxiques et/ou responsables de blooms dans la baie d’Annaba (Algérie). C R Biol 330:615–628
Figueroa RI, Bravo I, Fraga S, Garcès E, Llaveria G (2009) The life history and cell cycle of Kryptoperidinium foliaceum, a dinoflagellate with two eukaryotic nuclei. Protist 160:285–300
Genovesi B, Mouillot D, Vaquer A, Laabir M, Pastoureaud A (2007) Towards an optimal sampling strategy for Alexandrium catenella (Dinophyceae) benthic resting cysts. Harmful Algae 6:837–848
Genovesi B, Laabir M, Masseret E, Collos Y, Vaquer A, Grzebyk D (2009) Dormancy and germination features in resting cysts of species complex (Dinophyceae) can facilitate bloom formation in a shallow lagoon (Thau, southern France). J Plankton Res 31(10):1209–1224
Genovesi B, Mouillot D, Laugier T, Fiandrino A, Laabir M, Vaquer A, Grzebyk D (2013) Influences of sedimentation and hydrodynamics on the spatial distribution of Alexandrium catenella/tamarense resting cysts in a shellfish farming lagoon impacted by toxic blooms. Harmful Algae 25:15–25
Granéli E, Turner JT (2006) An introduction to harmful algae. In: Granéli E, Turner JT (eds) Ecology of harmful algae. Ecological Studies 189:3–7
Guelorget O, Frisoni GF, Ximenes MC, Perthuisot JP (1989) Biogeological expressions of confinement in a Mediterranean lagoon: Lake Melah (Algeria). Rev Hydrobiol Trop 22:87–99 (in French)
Hadjadji I, Fréhi H, Ayada L, Abadie E, Collos Y (2014) A comparative analysis of Alexandrium catenella/tamarense blooms in Annaba Bay (Algeria) and Thau lagoon (France); phosphorus limitation as a trigger. C R Biol 337(2):117–122
Hallegraeff GM, Bolch CJ, Blackburn SI, Oshima Y (1991) Species of the toxigenic dinoflagellate genus Alexandrium in Southeastern Australian waters. Bot Mar 34:575–587
Head M (1996) Modern dinoflagellate cysts and their biological affinities. In: Jansonius J, McGregor DC (eds) Palynology: principles and applications. American Association of Stratigraphic Palynologists Foundation, Dallas, pp 1197–1248
Head MJ, Harland R, Matthiessen J (2001) Cold marine indicators of the late quaternary: the new dinoflagellate cyst genus Islandinium and related morphotypes. J Quat Sci 16:621–636
Horner RA, Greengrove CL, Davies-Vollum KS, Gawel JE, Postel JR, Cox AM (2011) Spatial distribution of benthic cysts of Alexandrium catenella in surface sediments of Puget Sound, Washington, USA. Harmful Algae 11:96–105. https://doi.org/10.1016/j.hal.2011.08.004
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Illoul H, Maso M, Fórtuño JM, Cros L, Morales-Blake A, Séridi R (2008) Potentially harmful microalgae in coastal waters of the Algiers area (Southern Mediterranean Sea). Cryptogam Algol 29(3):261–278
Illoul H, Hernández FR, Vila M, Djas N, Aït Younes A, Bournissa M, Koroghli A, Marouf N, Rabia S, Ameur FLK (2012) The genus Ostreopsis along the Algerian coastal waters (SW Mediterranean Sea) associated with a human respiratory intoxication episode. Cryptogam Algol 33(2):209–216
Kim CJ, Kim HG, Kim CH, Oh HM (2007) Life cycle of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides in Korean coastal waters. Harmful Algae 6:104–111
Klein G, Martin JL, Kaczmarska I (2010) Biological synopsis of phytoplankton new to the Bay of Fundy. Can Manuscr Rep Fish Aquat Sci 2919: v + 28
Kremp A (2000) Distribution dynamics and in situ seeding of potential of Scrippsiella hangoei (Dinophyceae) cyst population of Baltic Sea. J Plankton Res 22(11):2155–2169
Laabir M, Collos Y, Masseret E, Grzebyk D, Abadie E, Savar V, Sibat M, Amzil Z (2013) Influence of environmental factors on the paralytic shellfish toxin content and profile of Alexandrium catenella (Dinophyceae) isolated from the Mediterranean Sea. Mar Drugs 1:1583–1601
Lacasse O, Rochon A, Roy S (2013) High cyst concentrations of the potentially toxic dinoflagellate Alexandrium tamarense species complex in Bedford Basin, Halifax, Nova Scotia, Canada. Mar Pollut Bull 66(1–2):230–233
Magni P, Falco G, Como S, Casu D, Floris A, Petrov AN, Castelli A, Perilli A (2008) Distribution and ecological relevance of fine sediments in organic-enriched lagoons: the case study of the Cabras lagoon (Sardinia, Italy). Mar Pollut Bull 56(3):549–564
Matsuoka K, Fukuyo Y (2000) Technical guide for modern dinoflagellate cyst study. WESTPAC-HAB/WESTPAC/IOC, Asian Natural Environmental Science Center, Tokyo
Matsuoka K, Joyce BL, Kotani Y, Matsuyama Y (2003) Modern dinoflagellate cysts in hypertrophic coastal waters of Tokyo Bay, Japan. J Plankton Res 25:1461–1470
Matsuoka K, Kawami H, Nagai S, Iwataki M, Takayama H (2009) Reexamination of cyst-motile relationships of Polykrikos kofoidii Chatton and Polykrikos schwartzii Butschli (Gymnodiniales, Dinophyceae). Rev Palaeobot Palynol 154:79–90
McGillicuddy DJ, Signell RP, Stock CA (2003) A mechanism for offshore initiation of harmful algal blooms in the coastal Gulf of Maine. J Plankton Res 25:1131–1138
Millot C (1999) Circulation in the Western Mediterranean Sea. J Mar Syst 20(1–4):423–442
Millot C (2009) Another description of the Mediterranean Sea outflow. Prog Oceanogr 82(2):101–124
Montresor M, Zingone A, Sarno D (1998) Dinoflagellate cyst production at a coastal Mediterranean site. J Plankton Res 20(12):2291–2312
Ounissi M, Fréhi H (1999) Variability of microphytoplankton and Tintinnida (Ciliated Protozoa) in an eutrophic sector of the Annaba Gulf (S.W. Mediterranean). Cah Biol Mar 40(2):141–153
Paz B, Riobó P, Fernandez ML, Fraga S, Franco JM (2004) Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 44:251–258
Pospelova V, Head MJ (2002) Islandinium brevispinosum sp. nov. (Dinoflagellata), a new species of organic-walled dinoflagellate cyst from modern stuarine sediments of New England (USA). J Phycol 38:593–601
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.r-project.org/
Reñé A, Satta CT, Garcés E, Massana R, Zapata M, Anglès S, Camp J (2011) Gymnodinium litoralis sp. nov. (Dinophyceae), a newly identified bloom-forming dinoflagellate from the NW Mediterranean Sea. Harmful Algae 12:11–25
Rochon A, de Vernal A, Turon JL, Matthiessen J, Head MJ (1999) Distribution of recent dinoflagellate cysts in surface sediments from the North Atlantic Ocean and adjacent seas in relation to sea-surface parameters. AASP Contribution 35:1–152. https://doi.org/10.1594/PANGAEA.55715
Rhodes L, McNabb P, de Salas M, Briggs L, Beuzenberg V, Gladstone M (2006) Yessotoxin production by Gonyaulax spinifera. Harmful Algae 5:148–155
Rubino F, Belmonte M, Caroppo C, Giacobbe M (2010) Dinoflagellate cysts from surface sediments of Syracuse Bay (Western Ionian Sea, Mediterranean). Deep-Sea Res II 57:243–247
Satta CT, Anglès S, Garcés E, Lugliè A, Padedda BM, Sechi N (2010) Dinoflagellate cyst in recent sediments from two semi-enclosed areas of the Western Mediterranean Sea subject to high human impact. Deep-Sea Res II 57:256–267
Satta CT, Anglès S, Lugliè A, Guillén J, Sechi N, Camp J, Garcés E (2013) Studies on dinoflagellate cyst assemblages in two estuarine Mediterranean bays: a useful tool for the discovery and mapping of harmful algal species. Harmful Algae 24:65–79
Satta CT, Garcés E, Anglès S, Sechi N, Pulina S, Padedda BM, Stacca D, Lugliè A (2014) Dinoflagellate cyst assemblages in surface sediments from three shallow Mediterranean lagoons (Sardinia, North Western Mediterranean Sea). Estuaries Coast 37:646–663
Sournia A, Chrétiennot-Dinet MJ, Ricard M (1991) Marine phytoplankton: how many species in the world ocean? J Plankton Res 13:1093–1099
Taleb H, Vale P, Jaime E, Blaghen M (2001) Study of paralytic shellfish poisoning toxin profile in shellfish from the Mediterranean shore of Morocco. Toxicon 39:1855–1861
Tang YZ, Gobler CJ (2012) The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 20:71–80
Turki S, Dhib A, Fertouna-Bellakhala M, Frossard V, Baltia N, Kharrat R, Aleya L (2014) Harmful algal blooms (HABs) associated with phycotoxins in shellfish: what can be learned from five years of monitoring in Bizerte Lagoon (Southern Mediterranean Sea)? Ecol Eng 67:39–47
Vlamis A, Katikou P, Rodriguez I, Rey V, Alfonso A, Papazachariou A, Zacharaki T, Botana AM, Botana LM (2015) First detection of tetrodotoxin in Greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 7:1779–1807. https://doi.org/10.3390/toxins7051779
Yamaguchi M, Itakura S, Imai I, Ishida Y (1995) A rapid and precise technique for enumeration of resting cysts of Alexandrium spp. (Dinophyceae) in natural sediments. Phycologia 34:207–214
Yamaguchi M, Itakura S, Nagasaki K, Imai I (1996) Distribution and abundance of resting cysts of the toxic dinoflagellates Alexandrium tamarense and Alexandrium catenella in sediments of the eastern Seto Inland Sea, Japan. In: Yasumoto T, Oshima Y, Fukuyo Y (Eds.) Harmful and toxic algal blooms, Intergovernmental Oceanographic Commission of UNESCO. Laboratory of Bioorganic Chemistry, Tohoku University, Japan, pp. 177–180
Yu B, Dong H, Jiang H, LVG, Eberl D, Li S, Kim J (2009) The role of clay minerals in the preservation of organic matter in sediments of Qinghai Lake, NW China. Clay Clay Miner 57(2): 213–226
Zmerli Triki H, Kefi Daly-Yahia O, Malouche D, Komiha Y, Deidun A, Brahim M, Laabir M (2014) Resting cysts distribution of the potentially toxic dinoflagellate Alexandrium pseudogonyaulax in recent sediment of Bizerte Lagoon (Mediterranean coast, Tunisia). Mar Pollut Bull 84:172–181
Zmerli Triki H, Laabir M, Kefi Daly-Yahia O (2015a) Life history, excystment features and growth characteristics of the Mediterranean harmful dinoflagellate Alexandrium pseudogonyaulax. J Phycol 51(5):980–989
Zmerli Triki H, Ben amor O, Deidun A, Kefi Daly-Yahia O (2015b) Investigating the possible influence of different environmental factors on the Alexandrium catenella/tamarense resting cyst distribution and HAB occurrence within Bizerte lagoon (Mediterranean coast of Tunisia). Mar Life 18:43–53
Zmerli Triki H, Laabir M, Moeller P, Chomérat N, Kéfi Daly-Yahia O (2016) First report of goniodomin A production by the dinoflagellate Alexandrium pseudogonyaulax developing in southern Mediterranean (Bizerte Lagoon, Tunisia). Toxicon 111:91–99
Zmerli-Triki H, Laabir M, Lafabrie C, Malouche D, Bancon-Montigny C, Gonzalez C, Deidun A, Princault O, Kéfi Daly-Yahi O (2017) Do the levels of industrial pollutants influence the distribution and abundance of dinoflagellate cysts in the recently-deposited sediment of a Mediterranean coastal ecosystem? Sci Total Environ 595:380–392
Zonneveld KAF (1997a) Dinoflagellate cyst distribution in surface sediments of the Arabian Sea (Northwestern Indian Ocean) in relation to temperature and salinity gradients in the upper water column. Deep-Sea Res II 44:1411–1443
Zonneveld KAF (1997b) New species of organic walled dinoflagellate cysts from modern sediments of the Arabian Sea (Indian Ocean). Rev Palaeobot Palynol 97:319–337
Zonneveld KAF, Jurkschat T (1999) Bitectatodinium spongium from modern sediments and sediment trap samples of the Arabian Sea (northwestern Indian Ocean): taxonomy and ecological affinity. Rev Palaeobot Palynol 106:153–169
Funding
This work was supported by the program funded by IRD (French Institute for Research and Development) and from LAGUNOTOX research project funded by TOTAL Foundation. The Algerian Ministry of Higher Education and Scientific Research provided funding for Mohamed Anis Draredja PhD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Vitor Manuel Oliveira Vasconcelos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Draredja, M.A., Frihi, H., Boualleg, C. et al. Distribution of dinoflagellate cyst assemblages in recent sediments from a southern Mediterranean lagoon (Mellah, Algeria) with emphasis on toxic species. Environ Sci Pollut Res 27, 25173–25185 (2020). https://doi.org/10.1007/s11356-020-08830-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08830-0