Abstract
Due to the rapid growth in the heavy metal-based industries, their effluent and local dumping have created significant environmental issues. In the past, typically, removal of heavy metals was handled by reverse osmosis and ion exchange techniques, but these methods have many disadvantages. Therefore, extensive work into the development of improved techniques has increased, especially for heavy metal removal. Many countries are currently researching new materials and techniques based on nanotechnology for various applications that involve extracting heavy metals from different water sources such as wastewater, groundwater, drinking water and surface water. Nanotechnology provides the possibility of enhancing existing techniques to tackle problems more efficiently. The development in nanotechnology has led to the discovery of many new materials such as magnetic nanoparticles. These nanoparticles demonstrate excellent properties such as surface-volume ratio, higher surface area, low toxicity and easy separation. Besides, magnetic nanoparticles can be easily and efficiently recovered after adsorption compared with other typical adsorbents. This review mainly emphasises on the efficiency of heavy metal removal using magnetic nanoadsorbent from aqueous solution. In addition, an in-depth analysis of the synthesis, characterisation and modification approaches of magnetic nanoparticles is systematically presented. Furthermore, future opportunities and challenges of using magnetic particles as an adsorbent for the removal of heavy metals are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lately, the removal of dye and harmful heavy metals from wastewater released from manufacturing industries is getting much attention. Adsorption is one of the most prominent approaches for water and wastewater de-contamination from heavy metals and organic compounds as well as other inorganic pollutants. Due to this fact, wide ranges of adsorbents with different compositions have been synthesised (Gupta et al., 2010). For instance, low-cost adsorbents like agricultural waste (Bhatnagar et al., 2015, Ahmad and Danish 2018) and biomass-based activated carbon (Yagub et al., 2014) have been considered for effective removal of heavy metals from wastewater. The low price and high porosity of natural clay make them suitable for water cleaning process (Singh et al. 2018a, b). Furthermore, chitosan has found many applications related to adsorption (Rasoulzadeh et al. 2019). The presence of amino and hydroxyl groups subsidizes to vary promising adsorption interaction among pollutants and chitosan (Kyzas and Bikiaris 2015).
Recently, extensive studies have been carried out for considering nanoadsorbents for the pollutant removal from wastewater/water (Lingamdinne et al. 2019; Ruthiraan et al. 2019). In fact, new era nanoadsorbents are mostly in the form of nanoparticles. These nanoparticles are generally created through spark discharge, low-temperature reaction, pulsed laser ablation, photothermal, spray pyrolysis, inert gas condensation, ion sputtering, thermal plasma, pulsed laser ablation and flame spray pyrolysis (Palkar 1999). Techniques like scanning electron microscopy, X-ray photoelectron spectroscopy, transmission electron microscopy, X-ray diffraction and energy-dispersive X-ray spectroscopy are used for the characterization of nanoparticles (Basheer 2018). Primarily, sol-gel procedure assists in the production of improvised adsorbent of titanium/nickel oxide, cadmium sulphide, alumina-silica, sulphide, silica and maghemite. Adsorption rate, as well as efficiencies of nanostructured adsorbents, is higher due to significant surface area, compared with conventional materials. Researchers have proposed different types of efficient, economical and environment friendly nanomaterials for applications related to depollution of industrial discharge, surface, ground and drinking water. An ideal absorbent, however, needs to meet certain requirements which include inexpensive, eco-friendly, high adsorption ability and selectivity. As far as water pollutants are concerned, an ideal absorbent is required to adsorb all contaminants conveniently and efficiently as well as reusable (Sadegh et al. 2017).
In this review paper, a summary of techniques and approaches for the removal of heavy metals using magnetic nanoadsorbent was thoroughly discussed. Also, synthesis and characterisation of the magnetic nanoparticle are elaborated. Furthermore, future opportunities and challenges of magnetic particles for the removal of heavy metals are also collated.
Heavy metals
The sources through which metals are released into the atmosphere include volcanic eruptions, soil and rock weathering and human activities such as the use of polluting chemicals from metals, mining, manufacturing, etc. Metals with density more than 5 g/cm3 are referred to as heavy metals, for instance, lead, mercury, arsenic, zinc and chromium are few of the heavy metals (Barakat 2011). Figure 1 shows the percent of heavy metals present in different fields. Summary of the anthropogenic origin for known heavy metals and maximum contamination levels is standardized by the United Environmental Protection Agency (USEPA) as shown in Table 1. This section comprises of different modified magnetic nanoparticles and their part in heavy metal ions removal. Heavy metals are more often found in rock-forming minerals include those which can conveniently leach because of mineral weathering such as vanadium (V), zinc (Zn), nickel (Ni), copper (Cu) and manganese (Mn), moreover, metals that have intermediate steadiness such as yttrium (Y), scandium (Sc) and many other rare elements, all the way to hafnium (Hf) or uranium (U), which can be found in zircon and are unaffected to weathering. All these heavy metals, later concentrated due to high hydrothermal fluids, permeate the rocks and chemical reactions which lead to precipitation of minerals and ores. These deposits are usually seen inside sedimentary rocks and appropriate for solids storage due to their permeable lattice and extensive permeability (Kobielska et al. 2018).
Soil is also an important sink for heavy metal storage. They are found in relocated rock debris, organic matter (solid phases), water and air trapped inside the soil (fluid phases) as well as insoluble minerals. Both phases, i.e. solid and fluid, interact with one another and different ions through the system (Alloway 2013). However, heavy metal concentration in soil is proportional to the rock type from where the soil originated. The B-horizon is termed as soil third layers from where the heavy metals usually found. Due to the significant concentration of iron oxy-hydroxides and clay enabled B-horizon to attract heavy metals (Kobielska et al. 2018).
Furthermore, heavy metal storage is found on significant depth in surface water that followed from streams/springs to lakes/rivers. However, factors that are contributing to heavy metal concentration and identity in surface waters involve chemical, physical and biological aspects. In addition, surface water that contains oxy-hydroxides/aquatic vegetation trap heavy metals results in bio-accumulation inside the living organisms (Siegel 2002). It is important to keep in mind that a biological system requires the intake of heavy metals, but significant tolerable intake on a daily basis may cause toxicity. The sub-section summarized a few of the heavy metals, i.e. arsenic, cadmium and chromium, available in high content in water and soils. Table 2 displays the adsorption performance along with the host substance of various nanomaterials used for heavy metal removal.
Arsenic
Arsenic is known as the twentieth furthermost abundant element and traced in most of the environment matrices, for instance, soil, water and air as well as living matters. In water and soil, it generally exists in both organic and inorganic forms. Arsenite, As (III), and Arsenate, As(V), fall under the category of inorganic form, whereas dimethylarsinic acid and monomethylarsonic acid are organic forms (Rahman and Hasegawa 2011). According to the International Agency for Research on Cancer (IARC), compounds containing arsenic contents referred to as group 1-carcinogen. Every year, thousands of people are affected by poisonous arsenic, as the groundwater that has arsenic contaminants is used for daily use as drinking, irrigation, cooking and cleaning. Rice is a well-known staple food, especially in countries like China, Japan, Pakistan, India and Malaysia, where this is grown using ground or river water. Previous studies reported the primary source of arsenic exposure in non-seafood supplements in Europe (Nisticò et al. 2018). In contrast to organic forms, inorganic forms possess higher toxicity. However, organic form in solid-liquid medium is freely mobilized, due to the weak bond with the solid medium. Nevertheless, there is a lack of interest in researching organic arsenic form environmental behaviour, and only limited studies are available on the removal route from wastewater and water (Cheng et al. 2005).
Based on the literature, the predictable adsorbent used for arsenic removal includes activated alumina, oxides, resins and activated carbon, but they have several limitations such as insignificant adsorption strength, high-cost materials, the formation of sludge and difficult to separate (Hokkanen et al. 2015). There is strong Fe and As interaction that made magnetic-based iron oxide a suitable candidate as an adsorbent for arsenic species removal, particularly from polluted soil and water. Moreover, magnetic nanoparticles can be stabilized by economic bio-related sources such as biomasses and polysaccharides (Magnacca et al. 2014). Table 3 shows the adsorption capacity of various adsorbents used for the removal of inorganic arsenic form. Therefore, studies on magnetic nanoparticle such as Ɣ- Fe2O3 show excellent results via FTIR and XPS analysis in terms of adsorption strength, particularly for arsenite and arsenate. Moreover, it can be reused for more than five times with the addition of 1 M of sodium hydroxide (Lin et al. 2012). Magnetic nanoparticles have shown positive results for the pollutant removal as well as economic and environmental perspectives (Nisticò et al. 2018).
Cadmium
Cadmium (non-degradable) ions mainly hold significant toxicities as well as accessibly developed to organism via food that makes it difficult to rip off and causes biological damage. Cadmium introduces into the environment through gradual erosion and rocks and soil abrasions, such as volcanic eruptions. Furthermore, cadmium is a pollutant that directly affects human health in various ways such as limiting cell growth, bone infections and lung damage. Due to all these health issues, WHO (World Health Organization) has stated that the limit of cadmium in blood should not be more than 0.005 mg/L (Kumar and Chawla 2014).
In this regard, finding efficient material or technique that helps to eliminate/reduce the heavy metal contaminants is getting more attention. Researchers synthesized several materials; however, at present, the most prominent and promising material for the removal of heavy metals, particularly for cadmium removal, is superparamagnetic nanoparticles. Metal contaminants are attracted to the magnetite materials via their exterior active sites. Table 4 shows the different nano-metal oxides along with their adsorption capability towards cadmium removal from water. Recent studies have proved that several surface coating of magnetite exhibit higher adsorption capability towards cadmium (Huang and Keller 2015). Also, ethylene diamine tetra acetic is toxic that may result in skin rashes up to some extent. The coating of inorganic nanoparticles helps to avoid aggregation and remains its magnetic properties. Moreover, metal oxide-based nanocomposites have been significantly analysed, and shown positive results with higher removal capacity for metal removal. For instance, Al2O3-Fe3O4 nanocomposite was remarked as the best nano-sized metal oxide for the removal of cadmium based on adsorption capacity (El-Latif et al. 2013).
Chromium
Among all heavy metals, chromium is one of the toxic pollutants that are carcinogenic, non-biodegradable and mutagenic to a living organism. The biological and toxicological chromium characteristics vary with their chemical forms. Chromium is introduced to the environment via process industries like metal finishing, electroplating, pigments, dyeing, steel manufacturing and leather tanning. However, in aqueous and soil systems, chromium exists in oxidation states as Cr (III) and Cr (VI) (Shariati et al. 2017). Cr (VI) is more toxic compared with Cr (III) and directly affects human health. Due to high toxicity, chromium presence in drinking water and surface water is limited to 0.05 and 0.1 mg/L, respectively (Gupta et al. 2001). In order to remove chromium pollutants, several techniques like chemical precipitation (Özer et al. 1997), electrocoagulation (Shariati et al. 2017) and membrane separation (Chakravarti et al. 1995) are used in the past. The drawbacks of these techniques include significant energy/chemical quantity, high cost and complicated operations (Ghiaci et al. 2004). However, adsorption does not have these limitations and hence used more commonly.
Several researchers used different adsorbents to remove chromium, but most of them have either limited adsorption ability or chromium selectivity, especially for hexavalent form (Sureshkumar et al. 2016). To overcome these limitations, superparamagnetic nanoparticles revealed acceptable results and have advantages like quick separation with higher efficiency, economical cost, reuse and higher surface area (Shariati et al. 2017). Researchers, through experimental studies, noted that the magnetite and maghemite nanoadsorbent adsorption capacity is remarkable. Furthermore, these nanoadsorbents can be easily separated via an external magnetic field. Furthermore, magnetite and maghemite nanoadsorbents holding superparamagnetic characteristics can help to improve its re-dispersion and continual reuse (Singh et al. 2015). However, to enhance the adsorption and surface area, the surface of the magnetic nanoparticles must be coated with significant pore mesoporous such as SiO2 and amine.
Magnetic nanoparticles
Presently, the unique physical properties of nanomaterials are getting significant attention, especially the salient magnetic properties. There are many factors that determine the magnetic properties of nanomaterials such as chemical composition, type and degree of the crystal lattice, shape and size of particles, and configuration (for non-homogeneous structure). Alteration in the characteristics of nanoparticle such as size, composition, shape and structure can enhance the material’s magnetic properties (Khajeh et al. 2013a, b).
Magnetic nanoparticles are highly recyclable, non-toxic, reusable and possess magnetic characteristics that offer advantages of convenient separation upon using an external magnetic field (Lisjak and Mertelj 2018). Magnetic nanoparticles have been also known to have vast potential as catalyst supports. The magnetic characteristics of the support restrict the obligation to let the catalyst separation through centrifugation, filtration or extraction. In addition, the potential of covalent binding among catalyst and magnetic nanoparticles’ surface strongly decreases its dispersion thus products’ purity (Zhu et al. 2013). However, magnetic nanoparticles as catalyst support have attained significant attention in research society in organic synthesis because of green and sustainable chemistry. Certainly, the fabrication of materials can be conducted in environmentally benign solvents that have a good atom economy and produce less environmental contamination (Dalpozzo 2015). Besides, magnetic nanoparticles with controlled size, shape, surface chemistry and magnetic features have been fabricated through chemical synthesis for applications like magnetic energy storage and bio-imaging (Frey et al. 2009).
Recently, metal oxide nanostructures are also broadly considered as magnetic nanoparticles. Metal oxide nanostructures exhibit properties like semi-conductor, insulator or metallic because of its electronic morphology. Convenient and cost-effective approaches have been designed to produce magnetic metal oxides such as Co3O4, NiO2 and Fe2O3 (Sahu et al. 2010). Magnetic metal oxides and magnetic iron oxide nanoparticles have been recommended for an extensive number of applications such as biomedical, supercapacitor, heavy metals and dyes removal, and anti-bacterial agents (Akrami and Niazi 2016),(Prabhu et al. 2015), (Mamani et al. 2014). The magnetic iron oxide nanoparticles that hold superparamagnetic strength include magnetite and maghemite, chemically represents as Fe3O4 and Fe2O3, respectively. Superparamagnetic acts as a huge paramagnetic atom due to significant magnetic moments and it responses faster to the external magnetic field with limited coercivity and remanence (Sonti and Bose 1995). The nanoparticles are conveniently attracted to the magnetic field but lost their magnetic characteristics after their removal. These features and a limited chance of agglomeration make them a suitable candidate for a wide range of applications (Di Marco et al. 2007).
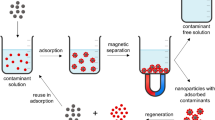
At present, magnetic nanoparticles such as ferrite colloids, maghemite, haematite and magnetite (Roy et al. 2017) hold extensive attention, particularly for water purification. Magnetic nanoparticles like magnetite and maghemite are considered unique nano-sized materials, applicable in biotech and biomedicine fields as well as competent adsorbent. For instance, magnetite with magnetic properties like significant surface area and adsorption capability is eligible for the removal of heavy metals from aqueous solutions and can be extracted and reuse conveniently through the external magnetic field. Nevertheless, bare magnetite nanoparticles oxidize easily and get rusted in the acidic atmosphere, Furthermore, they dispose of aggregation by magnetic force, thus reduce the magnetic strength as well as adsorption capability. Hence, surface protection of magnetic nanoparticles is a must to avoid aggregation. Therefore, oxide/polymeric compounds (biocompatible polymers) and surfactants have been widely used to improvised the stability of magnetic nanoparticles (Stanicki et al. 2015). Recently, modification of magnetic nanoparticles using organic molecules has been used widely in several applications, for example in the biomedical field for cell separation, drug delivery, gene targeting, hyperthermia, etc. (Osaka et al. 2006). Nevertheless, the modification of magnetic nanoparticles with appropriate coating has verified to be one of the utmost effective ways. Wang et al. (2010) fabricated hybrid magnetic nanoparticles based on poly-methyl meth-acrylate and superparamagnetic iron oxide nanoparticles with surface modification for heavy metals (Cu (II), Pb (II), Co (II) and Hg (II)) removal through external magnetic fields. The removal efficiency of heavy metals depends on the capture of metal ions via the amine group (Wanna et al. 2016). In a method that combined magnetic separation and biosorption techniques, metal ions’ adsorption was demonstrated effectively with advantages of eco-friendly, inexpensively low operation cost and flexibility (Li et al. 2008; Mohammed et al. 2017). The advantages of support magnetic nanoparticles, as well as modified magnetic nanoparticles as a suitable candidate for adsorption, are listed as follows: (1) a large number of particles produced using easy and convenient methods. (2) The capacity of adsorption is significant due to the large surface area. (3) Toxicity is lower and offers unique magnetic strength, and (4) metal-laden sorbents conveniently separate from processed wastewater through an external magnetic field (Wu et al. 2016). However, water pollutants are mostly non-magnetic. Therefore, it is preferable that the magnetic nanoparticles mix along with the pollutants competently, and captured carefully with pollutants owing to their maximum ferromagnetism.
Magnetic nanoparticles synthesis
Due to their remarkable strength, several production methods are designed to synthesize these magnetic nanoparticles. Among all synthesis approaches, coprecipitation method is most widely used. The performance of magnetic nanoparticles depends on characteristics like shape, size and composition as well as process factors like ratio of Fe2+/Fe3+, the temperature of the reaction, pH, iron salt and ionic property of the media. An important aspect of the synthesis of magnetic nanoparticles is the production of particles of definite shape and size (Khajeh et al. 2013a, b). The shape and possibilities to produce anisotropic magnetic lattices are exclusively necessary. In order to decrease the interparticle interactions, magnetic nanoparticles mostly require being isolated from others with a coating adjacent to the particle (Bhateria and Singh 2019) (Fig. 2).
Comparison of published work on synthesis of magnetic nanoparticles using three different routes (Mahmoudi et al. 2011)
Table 5 shows the advantages and disadvantages of different methods to synthesize magnetic nanoparticles using a chemical approach. The main aspect in the procedure for the synthesis of magnetic nanoparticles is to keep it comparatively easy, economical and reproducible. The methods to produce magnetic nanoparticles is categorised into physical (electron beam lithography, gas-phase deposition, high energy ball milling, aerosols, laser-induced pyrolysis and pulsed laser ablation), chemical (sol-gel, hydrothermal, electrochemical, aerosol/vapour-phase, chemical coprecipitation, flow injection and sonochemical decomposition reactions) and biological (microbial) methods (Chen et al. 2008), (Ali et al. 2016), (Mahdavi et al. 2013), (Roy et al. 2017), (Tapeinos 2018). All these methods are further classified into two classes, i.e. aqueous and non-aqueous. In aqueous class, producing controlled size water-soluble mono-disperse magnetic iron oxide nanoparticles is a challenging task, whereas magnetic iron oxide nanoparticles that are produced through non-aqueous class can only be dissolved in non-polar solvents. The aqueous route is most preferable than non-aqueous, in terms of sustainability and economy (Abdullah et al. 2019).
Characterization of magnetic nanoparticles
To understand the physicochemical feature and the structural character of magnetic nanoparticles as well as pollutant removal mechanisms using magnetic nanoparticles, it is important to characterize magnetic nanoparticles (Fig. 3). These nanoparticles are characterized through various analytical techniques which include X-ray diffraction (XRD), thermogravimetric analysis (TGA), X-ray spectroscopy (EDS), scanning electron microscopy (SEM), Fourier transforms infrared spectroscopy (FE-SEM), X-ray adsorption near-edge structure (XANES), vibrating sample magnetometer (VSM), Brunauer Emmett Teller (BET), confocal micro X-ray fluorescence (μ-XRF) and extended X-ray absorption fine structure (EXAFS) (Yu et al. 2016).
Modification of magnetic nanoparticles
Magnetic nanoparticles are often stable at solid state, and due to this fact, they are applied in many applications, for instance as catalytic and sensing. The only drawback of using magnetic nanoparticles is its inadequate stability especially in the aqueous phase that leads to aggregation. Figure 4 shows the modification approach of a magnetic nanoparticle. Furthermore, pristine magnetic nanoparticles are usually unstable in a strongly acidic medium. This aspect holds magnetic nanoparticles to be considered as long-lasting or reusable material. Moreover, significant surface area to volume ratio results in decreasing surface energy, owing to the durable magnetic attractions along with particles, which lowers their dispersion in aqueous and matrices. The protein or enzyme exposure to such boundaries may result in activity loss (Roy et al. 2017).
Therefore, in order to improve the stability of the magnetic nanoparticles, extensive studies have been conducted and designed various modification approaches, for example surfactants (C18H34O2, C12H24O2, alkane sulphonic acids, alkyl phosphonic acids, lauric acid and oleic acid), polymers (polyethylene glycol, poly-vinyl-pyrrolidone, poly-vinyl alcohol and poly-acrylic acid) and natural dispersants (starch, chitosan, albumin, ethyl-cellulose, gelatin and dextran) (Roy et al. 2017); (Shete et al. 2015); (Mamani et al. 2013); (Nemati et al. 2012); (Zhang et al. 2010); (Lee, Lee et al. 2006); (Dung et al. 2009); (Fang et al. 2014); (Lüdtke-Buzug et al. 2009); (Lu et al. 2006). Table 6 shows the various modification approaches of iron oxide nanoparticles along with their merits and demerits (Xu et al. 2014b). Moreover, modification approaches enhance biocompatibility, biodegradability and surface strength. It also replaces the iron oxide nanoparticles’ hydrophobic nature into hydrophilic (Sadeghi et al. 2012). With suitable modification approach, the dispersibility, biocompatibility and stability of iron oxide nanoparticles can be upgraded; moreover, the oxidation process can also be slowed down significantly (Widder et al. 1981); (Lu et al. 2010). Table 6 shows the various modification approaches of iron oxide nanoparticles along with their merits and demerits (Xu et al. 2014a):
Surface modification of magnetic nanoparticles for heavy metal removal
Surface modification is a path of selective incorporation of the functional group onto nanomaterials’ surface without altering or affecting the materials’ core. It offers the material a new surface chemical characteristic such as stability, melting point, electronic morphology and reactivity that vary from that of original material thus building it homogenously in size, providing better shape distribution and maintaining its stability in the aqueous phase (Zhu et al. 2010).
Recently, there is a renewed attention in magnetic nanoparticle modification as a result of the capability of magnetic nanoparticles to make bonds with various terminal groups of different compounds, specifically ligands. Thus, considerable efforts have been progressed with the pollutant removal from wastewater via magnetic nanoparticles through reinforcing it onto different nanomaterials (Rebuttini 2014). Recently, magnetic nanocomposite materials comprising of two/more different nanomaterials have been considered for water and wastewater purification. This is due to the gain of permitting for enhancement in magnetic nanoparticles’ adsorption property, manipulation of purification technologies, prevention of aggregation and cost. Nanocomposite materials support to restrict the challenging task of suitable coating the magnetic core deprived of altering bare magnetic nanoparticle properties as well as avoid leaching of surfactant during separation (Ojemaye et al. 2017a, b). For example, titanium dioxide (TiO2) with extremely strong photo-catalytic characteristic are reinforced into magnetic nanoparticles to enhance the latter photo-catalytic features for their use in pollutant removal from water or wastewater. Typically, nanocomposite materials are renewed to be very active for pollutant removal from wastewater compared with bare magnetic nanoparticles (Koduru et al. 2019; Lingamdinne et al. 2019). Magnetic TiO2, magnetic carbon nanotubes, graphene magnetic nanocomposites, TiO2 carbon nanotubes nanocomposites, etc. have all been stated for organic, inorganic and microbial pollutants removal from wastewater (Ojemaye et al. 2017a, b; Sahu et al. 2019). Nonetheless, challenges like lower magnetic and adsorption properties are few of the challenges that come across with these materials.
When modified through ligand exchange, maleimide coupling or covalent linkage, magnetic nanoparticles can be very effective for pollutant removal from wastewater. Synthesized ligands have great adsorbing chelating properties/functional groups that can make bonds via their terminal groups with bare/coated magnetic nanoparticles to remove pollutants, organic and inorganic, from wastewater and with enhanced adsorption efficiency. The prospective of ligands and modification of magnetic nanoparticles improves their adsorption efficiency; also, it helps convenient separation and avoids the nanoparticles’ toxicity channelled into the environment (Ojemaye et al. 2017a, b). Modified nanoadsorbents allows several techniques to target pollutants even at low concentration and advances pollutants movement on its exterior surface.
The following four techniques for multi-modification of magnetic nanoparticles for wastewater purification application are possible, and these have brought massive progress in environmental nanotechnology sector:
-
Covalent bond formation: This technique requires the attachment of amine, carboxylic and hydroxyl group on nanoparticles’ surface. For a nanoparticle with the attachment of carboxylic group, the ligand should have amine/hydroxyl group on magnetic nanoparticles’ surface. The attachment of carboxylic group on magnetic nanoparticles’ surface is activated by using 1-ethyl-3-(dimethyl-amino-propyl)-carbodiimide and N-hydroxy-succinimide. On the other hand, a nanoparticle with amine group attachment can make a covalent bond with a ligand of the carboxylic group. The ligand is initially activated with 1- ethyl-3- (dimethyl-amino-propyl) and N-hydroxy-succinimide led by its coupling with amine containing magnetic nanoparticles attach with an amine group. Both aforementioned compounds, 1-ethyl-3-(dimethyl-amino-propyl)-carbodiimide and N-hydroxy-succinimide, are used for carboxylic activation only if carboxylic acid comprising compound is soluble in water; however, 4-dimethyl-amino-pyridine and N,N-Dicyclo-hexyl-carbodiimide are used for water-insoluble compound (Sun et al. 2015).
-
Ligand exchange: Ligand molecules on nanoparticles’ surface can be exchanged for another to offer an enhancement in magnetic nanoparticles’ stability and adsorption capability. Here, novel ligand coming in must have an extremely high affinity for magnetic nanoparticles, therefore, capable of exchanging the molecules that were initially used for magnetic nanoparticle coating. Moreover, ligand joins more strongly on to the inorganic nanoparticles’ surface. Based on these techniques, the incoming ligand is a bi-functional, where one of its functional group attaches to the surface of the nanoparticle and other for additional modification. Typically, this technique is usually considered for silver/gold magnetic nanoparticles via tetra-octyl-ammonium bromide (Thanh and Green 2010).
Magnetic nanoparticles coated with oleic acid are replaced with triazolyl fatty acids using the ligand technique. Nonetheless, modified magnetic nanoparticles displayed good superparamagnetic features and provided steady colloidal suspensions. Huang and co-workers developed mercapto-modified magnetic nanoparticles using solvent-assisted ligand exchange techniques (mild conditions) for mercury (Hg2+) removal from water with a removal efficiency of 282 mg/g. In comparison with other available modified techniques, this technique offers high stability and dispersibility in an aqueous medium; moreover, the technique is convenient to operate and efficient (Huang et al. 2016a); (Huang et al. 2016b).
-
Click reaction: This technique includes cyclo-addition of Cu (I)-catalysed terminal alkyne-azide under a wide range of conditions with significant stereospecificity and efficiency. There are also other known click reactions whereby functional groups and molecules are grafted on magnetic nanoparticles’ surface (Tucker-Schwartz et al. 2011). Thus, extensive reactions concerning with click reaction for magnetic nanoparticle modification have been testified, but limited or none has stated for water purification application (Erathodiyil and Ying 2011). This modification technique has been reported and is known as an important technique in terms of magnetic nanoparticles’ modification due to the significant number of advantages; however, it is surprisingly underutilized for modification of magnetic nanoparticles for water purification applications.
-
Maleimide coupling: In comparison with covalent coupling, this technique includes conjugation of thiols with primary amines. This technique for magnetic nanoparticles and ligand coupling is barely employed and acknowledged. Sulfo-succinimidyl-4-(maleimide-methyl)-cyclo-hexane-1- carboxylate is used as a coupling agent for maleimide technique. Huh and co-workers developed cross-linking of magnetic nanocrystal with Herceptin using an abovementioned coupling agent. The developed material was 14 nm in size and Herceptin has been conjugated successfully to magnetic nanoparticles. Besides, the maleimide technique can also be used for both carboxyl and amino group modification of magnetic nanoparticles (Huh et al. 2005) (Table 7).
Prospective and future challenges of magnetic nanoparticles
Magnetic nanoparticles’ development as an effective adsorbent for heavy metals removal has been studied significantly, due to its high adsorption efficiency and chemical stability, good recyclability and convenient separation. A significant number of researches associated with the fabrication of magnetic nanoparticles through various methods are discussed herein. The biocompatibility of iron oxide nanoparticles may confirm the safety of fabricated materials in water treatment as well as to magnetic properties, which help the benefits of convenient separation by an external magnetic field. Based on the literature, the summary of how the fabrication methods can affect the production of iron oxide nanoparticles on the overall matrix also have been discussed. Moreover, it is realized that maghemite and magnetite are two of the primary phases that contributed to the magnetism of nanoparticles. In comparison with maghemite, magnetite nanoparticles displayed great magnetization (Lu et al. 2015). Thus, its production with high saturation magnetization is still a challenging task by researchers as it can be oxidized easily and needed an oxygen-free environment during production. Additionally, maghemite nanoparticles have good stability as compared with magnetite. Regarding large-scale production, maghemite nanoparticles’ fabrication is more economical and facile as compared with magnetite nanoparticles that typically carried out in inert surroundings. While, maghemite displays lower magnetism strength; however, with size-controlled production within the solid matrix and suitable weight ratio, they may offer adequate magnetism for the recovery process and ease of separation. Consequently, considerable studies must be carried out to explore promising materials to produce effective adsorbent in water and wastewater treatment (Fulekar et al. 2009). Nevertheless, there have been significant studies on heavy metal ions’ adsorption, but the mechanism of how the reaction happens is not completely understood, therefore, still require to be investigated further (Esakkimuthu et al. 2014). The in-depth understanding of physical and chemical features of adsorbents can offer a good understanding of the nature of chemical interaction/bonding among nanoparticles and composites to develop efficient adsorbent.
Iron oxide nanoparticles incorporation with solid matrices can improve the adsorbent properties with interesting features of ease of separation, high-specific surface area and sage to be used. Parameters like heavy metal ion concentration, pH, temperature, contact time and adsorbent dosage are considered as the utmost significance, as any change in the abovementioned parameters can greatly change the adsorbent’ removal efficiency (Zhang et al. 2016). Hereafter, in-depth knowledge of fabrication methods and controlled parameters in adsorption process will help in producing magnetic iron oxide nanoparticles for water purification. Also, limited literature has been reported related to magnetic iron oxide nanoparticle regeneration. Therefore, it is important to have profound studies on the regeneration process of magnetic iron oxide nanoparticles for environmental sustainability. Lastly, it is still a massive challenge for research society to find an appropriate solution for effective water treatment with a reliable and economical approach. Hence, it is noteworthy that all the abovementioned issues must be addressed properly so that for the upcoming generation, clean water can be preserved.
Conclusions
The use of nanotechnology to treat water and wastewater is receiving a lot of attention. Magnetic nanoparticles and incredible strength, as well as their integration with existing treatment technologies, give tremendous opportunities to effectively treat water and wastewater. Whereas in many areas, different magnetic nanoparticle structures can offer alternative applications. Furthermore, magnetic nanoparticles are effectively considered for contamination removal such as heavy metals, dyes, fluoride and chlorinated organic compound, which are commonly found in ground water, drinking and wastewater. Even though several characteristics of magnetic nanoparticles were discussed in this review paper, however, most of the magnetic nanoparticles are still at the laboratory research stage. In addition, the obstacles faced in the remediation of water and wastewater by nanomaterials are essential, but many of these obstacles are only impermanent, such as expensive cost and technical handling. To overcome these issues, co-operation between research societies, government and industries as well as a stakeholder is important. It is expected that evolving nanotechnology by cautious handling to evade unexpected results can continuously offer robust explanations to water and wastewater remediation challenges.
References
Abdullah NH, Shameli K, Abdullah EC, Abdullah LC (2019) Solid matrices for fabrication of magnetic iron oxide nanocomposites: synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos Part B 162:538–568
Ahmad T, Danish M (2018) Prospects of banana waste utilization in wastewater treatment: a review. J Environ Manag 206:330–348
Akrami A, Niazi A (2016) Synthesis of maghemite nanoparticles and its application for removal of Titan yellow from aqueous solutions using full factorial design. Desalin Water Treat 57(47):22618–22631
Ali, A., M. Z. Hira Zafar, I. ul Haq, A. R. Phull, J. S. Ali and A. Hussain (2016). Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9: 49
Aliramaji S, Zamanian A, Sohrabijam Z (2015) Characterization and synthesis of magnetite nanoparticles by innovative sonochemical method. Procedia Mater Sci 11:265–269
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. Springer, Heavy metals in soils, pp 11–50
Babel S and Kurniawan T (2005). Various treatment technologies to remove arsenic and mercury from contaminated groundwater: an overview. Southeast Asian Water Environment 1
Badruddoza A, Tay A, Tan P, Hidajat K, Uddin M (2011) Carboxymethyl-β-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: synthesis and adsorption studies. J Hazard Mater 185(2–3):1177–1186
Barakat M (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4(4):361–377
Basheer AA (2018) New generation nano-adsorbents for the removal of emerging contaminants in water. J Mol Liq 261:583–593
Bhateria R, Singh R (2019) A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J Water Process Eng 31:100845
Bhatnagar A, Sillanpää M, Witek-Krowiak A (2015) Agricultural waste peels as versatile biomass for water purification–a review. Chem Eng J 270:244–271
Chakravarti A, Chowdhury S, Chakrabarty S, Chakrabarty T, Mukherjee D (1995) Liquid membrane multiple emulsion process of chromium (VI) separation from waste waters. Colloids Surf A Physicochem Eng Asp 103(1–2):59–71
Chen F, Gao Q, Hong G, Ni J (2008) Synthesis and characterization of magnetite dodecahedron nanostructure by hydrothermal method. J Magn Magn Mater 320(11):1775–1780
Chen R, Zhi C, Yang H, Bando Y, Zhang Z, Sugiur N, Golberg D (2011) Arsenic (V) adsorption on Fe3O4 nanoparticle-coated boron nitride nanotubes. J Colloid Interface Sci 359(1):261–268
Cheng Z, Van Geen A, Louis R, Nikolaidis N, Bailey R (2005) Removal of methylated arsenic in groundwater with iron filings. Environ Sci Technol 39(19):7662–7666
Chou C-M, Lien H-L (2011) Dendrimer-conjugated magnetic nanoparticles for removal of zinc (II) from aqueous solutions. J Nanopart Res 13(5):2099–2107
Cruz IF, Freire C, Araújo JP, Pereira C, Pereira AM (2018) Multifunctional ferrite nanoparticles: from current trends toward the future. Elsevier, Magnetic Nanostructured Materials, pp 59–116
Dalpozzo R (2015) Magnetic nanoparticle supports for asymmetric catalysts. Green Chem 17(7):3671–3686
De Carvalho J, De Medeiros S, Morales M, Dantas A, Carriço A (2013) Synthesis of magnetite nanoparticles by high energy ball milling. Appl Surf Sci 275:84–87
Di Marco M, Sadun C, Port M, Guilbert I, Couvreur P, Dubernet C (2007) Physicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. Int J Nanomedicine 2(4):609–622
Dung T, Danh T, Hoa L, Chien D, Duc N (2009) Structural and magnetic properties of starch-coated magnetite nanoparticles. J Exp Nanosci 4(3):259–267
El-Latif MMA, Ibrahim AM, Showman MS, Hamide RRA (2013) Alumina/Iron Oxide nano composite for cadmium ions removal from aqueous solutions. International Journal of Nonferrous Metallurgy 2(02):47
Erathodiyil N, Ying JY (2011) Functionalization of inorganic nanoparticles for bioimaging applications. Acc Chem Res 44(10):925–935
Esakkimuthu T, Sivakumar D, Akila S (2014) Application of nanoparticles in wastewater treatment. Pollut Res 33(03):567–571
Fang C, Xiong Z, Qin H, Huang G, Liu J, Ye M, Feng S, Zou H (2014) One-pot synthesis of magnetic colloidal nanocrystal clusters coated with chitosan for selective enrichment of glycopeptides. Anal Chim Acta 841:99–105
Feng L, Cao M, Ma X, Zhu Y, Hu C (2012) Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J Hazard Mater 217:439–446
Frey NA, Peng S, Cheng K, Sun S (2009) Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem Soc Rev 38(9):2532–2542
Fulekar M, Singh A, Bhaduri AM (2009) Genetic engineering strategies for enhancing phytoremediation of heavy metals. Afr J Biotechnol 8(4)
Ghafoor S, Ata S (2017) Synthesis of carboxyl-modified Fe3O4@ SiO2 nanoparticles and their utilization for the remediation of cadmium and nickel from aqueous solution. J Chil Chem Soc 62(3):3588–3592
Ghiaci M, Kia R, Abbaspur A, Seyedeyn-Azad F (2004) Adsorption of chromate by surfactant-modified zeolites and MCM-41 molecular sieve. Sep Purif Technol 40(3):285–295
Ghosh Chaudhuri R, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112(4):2373–2433
Giménez J, Martinez M, de Pablo J, Rovira M, Duro L (2007) Arsenic sorption onto natural hematite, magnetite, and goethite. J Hazard Mater 141(3):575–580
Goon IY, Zhang C, Lim M, Gooding JJ, Amal R (2010) Controlled fabrication of polyethylenimine-functionalized magnetic nanoparticles for the sequestration and quantification of free Cu2+. Langmuir 26(14):12247–12252
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Water Res 35(5):1125–1134
Gupta VK, Jain R, Malathi S, Nayak A (2010) Adsorption–desorption studies of indigocarmine from industrial effluents by using deoiled mustard and its comparison with charcoal. J Colloid Interface Sci 348(2):628–633
Hao R, Xing R, Xu Z, Hou Y, Gao S, Sun S (2010) Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv Mater 22(25):2729–2742
Hernández-Hernández AA, Álvarez-Romero GA, Castañeda-Ovando A, Mendoza-Tolentino Y, Contreras-López E, Galán-Vidal CA, Páez-Hernández ME (2018) Optimization of microwave-solvothermal synthesis of Fe3O4 nanoparticles. Coating, modification, and characterization. Mater Chem Phys 205:113–119
Hokkanen S, Repo E, Lou S, Sillanpää M (2015) Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem Eng J 260:886–894
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Huang L, He M, Chen B, Hu B (2016a) A mercapto functionalized magnetic Zr-MOF by solvent-assisted ligand exchange for Hg 2+ removal from water. J Mater Chem A 4(14):5159–5166
Huang S, Ma C, Liao Y, Min C, Du P, Jiang Y (2016b) Removal of mercury (II) from aqueous solutions by adsorption on poly (1-amino-5-chloroanthraquinone) nanofibrils: equilibrium, kinetics, and mechanism studies. J Nanomater 2016
Huang Y, Keller AA (2015) EDTA functionalized magnetic nanoparticle sorbents for cadmium and lead contaminated water treatment. Water Res 80:159–168
Huh Y-M, Jun Y-w, Song H-T, Kim S, Choi J-s, Lee J-H, Yoon S, Kim K-S, Shin J-S, Suh J-S (2005) In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J Am Chem Soc 127(35):12387–12391
Karami H (2013) Heavy metal removal from water by magnetite nanorods. Chem Eng J 219:209–216
Khajeh M, Laurent S, Dastafkan K (2013a) Nanoadsorbents: classification, preparation, and applications (with emphasis on aqueous media). Chem Rev 113(10):7741
Khajeh M, Laurent S, Dastafkan K (2013b) Nanoadsorbents: classification, preparation, and applications (with emphasis on aqueous media). Chem Rev 113(10):7735
Khan K, Rehman S, Rahman HU, Khan Q (2014) Synthesis and application of magnetic nanoparticles. Gonzalez Estevez JM. Nanomagnetism. One Central Press (OCP): UK
Kobielska PA, Howarth AJ, Farha OK, Nayak S (2018) Metal–organic frameworks for heavy metal removal from water. Coord Chem Rev 358:92–107
Koduru JR, Karri RR, Mubarak NM (2019) Smart materials, magnetic graphene oxide-based nanocomposites for sustainable water purification. Springer International Publishing, Sustainable Polymer Composites and Nanocomposites, pp 759–781
Kumar R, Chawla J (2014) Removal of cadmium ion from water/wastewater by nano-metal oxides: a review. Water Qual Expo Health 5(4):215–226
Kyzas GZ, Bikiaris DN (2015) Recent modifications of chitosan for adsorption applications: a critical and systematic review. Marine drugs 13(1):312–337
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064–2110
Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S (2006) Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc 128(22):7383–7389
Li H, Chi Z, Li J (2014) Covalent bonding synthesis of magnetic graphene oxide nanocomposites for Cr (III) removal. Desalin Water Treat 52(10–12):1937–1946
Li H, Li Z, Liu T, Xiao X, Peng Z, Deng L (2008) A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads. Bioresour Technol 99(14):6271–6279
Lin S, Lu D, Liu Z (2012) Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles. Chem Eng J 211:46–52
Lingamdinne LP, Koduru JR, Karri RR (2019) A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J Environ Manag 231:622–634
Lisjak D, Mertelj A (2018) Anisotropic magnetic nanoparticles: a review of their properties, syntheses and potential applications. Prog Mater Sci
Lu H, Qiao X, Wang W, Tan F, Sun F, Xiao Z, Chen J (2015) Effective removal of cadmium ions from aqueous solution using chitosan-stabilized nano zero-valent iron. Desalin Water Treat 56(1):256–265
Lu R, Tao K, Sun K, Dou H, Xu B (2010) Facile synthesis of magnetic microcapsules by synchronous formation of magnetite nanoparticles. Colloid Polym Sci 288(3):353–357
Lu S, Cheng G, Zhang H, Pang X (2006) Preparation and characteristics of tryptophan-imprinted Fe3O4/P (TRIM) composite microspheres with magnetic susceptibility by inverse emulsion–suspension polymerization. J Appl Polym Sci 99(6):3241–3250
Lüdtke-Buzug K, Biederer S, Sattel T, Knopp T and Buzug TM (2009) Preparation and characterization of dextran-covered Fe 3 O 4 nanoparticles for magnetic particle imaging. 4th European Conference of the International Federation for Medical and Biological Engineering, Springer
Lunge S, Singh S, Sinha A (2014) Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal. J Magn Magn Mater 356:21–31
Magnacca G, Allera A, Montoneri E, Celi L, Benito DE, Gagliardi LG, Gonzalez MC, Mártire DO, Carlos L (2014) Novel magnetite nanoparticles coated with waste-sourced biobased substances as sustainable and renewable adsorbing materials. ACS Sustain Chem Eng 2(6):1518–1524
Mahdavi M, Ahmad MB, Haron MJ, Namvar F, Nadi B, Rahman MZA, Amin J (2013) Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 18(7):7533–7548
Mahdavian AR, Mirrahimi MA-S (2010) Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem Eng J 159(1–3):264–271
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T (2011) Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev 63(1–2):24–46
Mamani J, Costa-Filho AJD, Cornejo DR, Vieira E, Gamarra L (2013) Synthesis and characterization of magnetite nanoparticles coated with lauric acid. Mater Charact 81:28–36
Mamani JB, Gamarra LF, Brito GEDS (2014) Synthesis and characterization of Fe3O4 nanoparticles with perspectives in biomedical applications. Mater Res 17(3):542–549
Mazarío E, Mayoral A, Salas E, Menéndez N, Herrasti P, Sánchez-Marcos J (2016) Synthesis and characterization of manganese ferrite nanoparticles obtained by electrochemical/chemical method. Mater Des 111:646–650
Mohammed L, Gomaa HG, Ragab D, Zhu J (2017) Magnetic nanoparticles for environmental and biomedical applications: a review. Particuology 30:1–14
Mohapatra S, Nguyen TA, Nguyen-Tri P (2018) Noble metal-metal oxide hybrid nanoparticles: fundamentals and applications. Elsevier
Nemati F, Heravi MM, Rad RS (2012) Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for highly efficient Knoevenagel condensation and Michael addition reactions of aromatic aldehydes with 1, 3-cyclic diketones. Chin J Catal 33(11–12):1825–1831
Nisticò R, Celi LR, Prevot AB, Carlos L, Magnacca G, Zanzo E, Martin M (2018) Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J Hazard Mater 342:260–269
Ojemaye MO, Okoh OO, Okoh AI (2017a) Performance of NiFe2O4-SiO2-TiO2 magnetic photocatalyst for the effective photocatalytic reduction of Cr (VI) in aqueous solutions. J Nanomater 2017
Ojemaye MO, Okoh OO, Okoh AI (2017b) Surface modified magnetic nanoparticles as efficient adsorbents for heavy metal removal from wastewater: Progress and prospects. Mater Express 7(6):439–456
Osaka T, Matsunaga T, Nakanishi T, Arakaki A, Niwa D, Iida H (2006) Synthesis of magnetic nanoparticles and their application to bioassays. Anal Bioanal Chem 384(3):593–600
Özer A, Altundoğan H, Erdem M, Tümen F (1997) A study on the Cr (VI) removal from aqueous solutions by steel wool. Environ Pollut 97(1–2):107–112
Palkar V (1999) Sol-gel derived nanostructured γ-alumina porous spheres as an adsorbent in liquid chromatography. Nanostruct Mater 11(3):369–374
Prabhu Y, Rao KV, Kumari BS, Kumar VSS, Pavani T (2015) Synthesis of Fe 3 O 4 nanoparticles and its antibacterial application. Int Nano Lett 5(2):85–92
Rahman MA, Hasegawa H (2011) High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci Total Environ 409(22):4645–4655
Ranjan D, Talat M, Hasan S (2009) Biosorption of arsenic from aqueous solution using agricultural residue ‘rice polish’. J Hazard Mater 166(2–3):1050–1059
Rasoulzadeh H, Dehghani MH, Mohammadi AS, Karri RR, Nabizadeh R, Nazmara S, Kim K.-H and Sahu J.N (2019) Parametric modelling of Pb (II) adsorption onto chitosan-coated Fe3O4 particles through RSM and DE hybrid evolutionary optimization framework. J Mol Liq: 111893
Rebuttini V (2014). Functional iron oxide-based hybrid nanostructures.
Rishton S, Lu Y, Altman R, Marley A, Bian X, Jahnes C, Viswanathan R, Xiao G, Gallagher W, Parkin S (1997) Magnetic tunnel junctions fabricated at tenth-micron dimensions by electron beam lithography. Microelectron Eng 35(1–4):249–252
Roy A, Bhattacharya J (2013) A binary and ternary adsorption study of wastewater Cd (II), Ni (II) and Co (II) by γ-Fe2O3 nanotubes. Sep Purif Technol 115:172–179
Roy E, Patra S, Karfa P, Madhuri R, Sharma PK (2017) Role of magnetic nanoparticles in providing safe and clean water to each individual. Springer, Complex Magnetic Nanostructures, pp 281–316
Ruthiraan M, Mubarak NM, Abdullah EC, Khalid M, Nizamuddin S, Walvekar R, Karri RR (2019) An overview of magnetic material: preparation and adsorption removal of heavy metals from wastewater. Magnetic Nanostructures, Springer, Cham, pp 131–159
Sadegh H, Ali GA, Gupta VK, Makhlouf ASH, Shahryari-ghoshekandi R, Nadagouda MN, Sillanpää M, Megiel E (2017) The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J Nanostructure Chem 7(1):1–14
Sadeghi S, Azhdari H, Arabi H, Moghaddam AZ (2012) Surface modified magnetic Fe3O4 nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. J Hazard Mater 215:208–216
Sahu JN, Acharya J, Meikap BC (2010) Optimization of production conditions for activated carbons from tamarind wood by zinc chloride using response surface methodology. Bioresour Technol 101(6):1974–1982
Sahu JN, Karri RR, Zabed HM, Shams S, Qi X (2019) Current perspectives and future prospects of nano-biotechnology in wastewater treatment. Sep Purif Rev:1–20
Saravanan P, Vinod V, Sreedhar B, Sashidhar R (2012) Gum kondagogu modified magnetic nano-adsorbent: an efficient protocol for removal of various toxic metal ions. Mater Sci Eng C 32(3):581–586
Shariati S, Khabazipour M, Safa F (2017) Synthesis and application of amine functionalized silica mesoporous magnetite nanoparticles for removal of chromium (VI) from aqueous solutions. J Porous Mater 24(1):129–139
Shete P, Patil R, Tiwale B, Pawar S (2015) Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J Magn Magn Mater 377:406–410
Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer
Singh N, Nagpal G and Agrawal S (2018a) Water purification by using adsorbents: a review. Environmental Technology & Innovation
Singh NB, Nagpal G, Agrawal S, Rachna (2018b) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240
Singh P, Tiwary D, Sinha I (2015) Chromium removal from aqueous media by superparamagnetic starch functionalized maghemite nanoparticles. J Chem Sci 127(11):1967–1976
Singh S, Barick K, Bahadur D (2011) Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J Hazard Mater 192(3):1539–1547
Sonti SV, Bose A (1995) Cell separation using protein-A-coated magnetic nanoclusters. J Colloid Interface Sci 170(2):575–585
Stanicki D, Vander Elst L, Muller RN, Laurent S (2015) Synthesis and processing of magnetic nanoparticles. Curr Opin Chem Eng 8:7–14
Sun Z, Guo D, Li H, Zhang L, Yang B, Yan S (2015) Multifunctional Fe 3 O 4@ SiO 2 nanoparticles for selective detection and removal of Hg 2+ ion in aqueous solution. RSC Adv 5(15):11000–11008
Sureshkumar V, Daniel SK, Ruckmani K, Sivakumar M (2016) Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl Nanosci 6(2):277–285
Tapeinos C (2018) Magnetic nanoparticles and their bioapplications. Elsevier, Smart nanoparticles for biomedicine, pp 131–142
Thanh NT, Green LA (2010) Functionalisation of nanoparticles for biomedical applications. Nano Today 5(3):213–230
Tombácz E, Turcu R, Socoliuc V, Vékás L (2015) Magnetic iron oxide nanoparticles: recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem Biophys Res Commun 468(3):442–453
Tucker-Schwartz AK, Farrell RA, Garrell RL (2011) Thiol–ene click reaction as a general route to functional trialkoxysilanes for surface coating applications. J Am Chem Soc 133(29):11026–11029
Tuutijärvi T, Lu J, Sillanpää M, Chen G (2009) As (V) adsorption on maghemite nanoparticles. J Hazard Mater 166(2–3):1415–1420
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D (2010) Amino-functionalized Fe3O4@ SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349(1):293–299
Wanna Y, Chindaduang A, Tumcharern G, Phromyothin D, Porntheerapat S, Nukeaw J, Hofmann H, Pratontep S (2016) Efficiency of SPIONs functionalized with polyethylene glycol bis (amine) for heavy metal removal. J Magn Magn Mater 414:32–37
Widder KJ, Morris RM, Poore G, Howard DP, Senyei AE (1981) Tumor remission in Yoshida sarcoma-bearing rts by selective targeting of magnetic albumin microspheres containing doxorubicin. Proc Natl Acad Sci 78(1):579–581
Wu T, Pan H, Chen R, Luo D, Li Y, Wang L (2016) Preparation and properties of magnetic Fe3O4 hollow spheres based magnetic-fluorescent nanoparticles. J Alloys Compd 689:107–113
Wu W, Wu Z, Yu T, Jiang C, Kim W-S (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16(2):023501
Xu J-K, Zhang F-F, Sun J-J, Sheng J, Wang F, Sun M (2014a) Bio and nanomaterials based on Fe3O4. Molecules 19(12):21506–21528
Xu J, Sun J, Wang Y, Sheng J, Wang F, Sun M (2014b) Application of iron magnetic nanoparticles in protein immobilization. Molecules 19(8):11465–11486
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interf Sci 209:172–184
Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG (2007) Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ Sci Technol 41(14):5114–5119
Yu F, Ma J, Wang J, Zhang M, Zheng J (2016) Magnetic iron oxide nanoparticles functionalized multi-walled carbon nanotubes for toluene, ethylbenzene and xylene removal from aqueous solution. Chemosphere 146:162–172
Zhang Y, Liu J-Y, Ma S, Zhang Y-J, Zhao X, Zhang X-D, Zhang Z-D (2010) Synthesis of PVP-coated ultra-small Fe 3 O 4 nanoparticles as a MRI contrast agent. J Mater Sci Mater Med 21(4):1205–1210
Zhang Y, Nan Z (2015) Modified magnetic properties of MnFe2O4 by CTAB with coprecipitation method. Mater Lett 149:22–24
Zhang Y, Wu B, Xu H, Liu H, Wang M, He Y, Pan B (2016) Nanomaterials-enabled water and wastewater treatment. NanoImpact 3-4:22–39
Zhu J, Wei S, Chen M, Gu H, Rapole SB, Pallavkar S, Ho TC, Hopper J, Guo Z (2013) Magnetic nanocomposites for environmental remediation. Adv Powder Technol 24(2):459–467
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22(35):3906–3924
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, F.S.A., Mubarak, N.M., Khalid, M. et al. Magnetic nanoadsorbents’ potential route for heavy metals removal—a review. Environ Sci Pollut Res 27, 24342–24356 (2020). https://doi.org/10.1007/s11356-020-08711-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08711-6