Abstract
Arsenic in fine air particulate matter (PM2.5) has been identified as an important factor responsible for the morbidity of lung cancer, which has increased sharply in many regions of China. Some reports in China have shown that arsenic in the air exceeds the ambient air quality standard value, while long-term airborne arsenic concentrations in central China and human exposure via inhalation of PM–bound arsenic (inhalable airborne PM) have not been well characterized. In this study, 579 outdoor air PM2.5 samples from Wuhan, a typical city in central China, were collected from 2015 to 2017, and arsenic was measured by inductively coupled plasma-mass spectrometry. Personal exposure to PM-bound arsenic via inhalation and urinary arsenic concentration were also measured. The concentrations of arsenic in PM2.5 were in the range of 0.42–61.6 ng/m3 (mean 8.48 ng/m3). The average concentration of arsenic in 2015 (10.7 ng/m3) was higher than that in 2016 (6.81 ng/m3) and 2017 (8.18 ng/m3), exceeded the standard value. The arsenic concentrations in spring and winter were higher than those in summer and autumn. No significant differences (p > 0.05) were found among different sites. The daily intake of arsenic inhalation based on PM10 samples collected by personal samplers (median, 10.8 ng/m3) was estimated. Urban residents inhaled higher levels of PM-bound arsenic than rural residents. Daily intake of arsenic via inhalation accounted for a negligible part (< 1%) of the total daily intake of arsenic (calculated based on excreted urinary arsenic); however, potential associations between the adverse effects (e.g., lung adenocarcinoma) and inhaled PM-bound arsenic require more attention, particularly for those who experience in long-term exposure. This study is the first report of a 3-year temporal trend of airborne PM2.5-bound arsenic in central China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessing human exposure to arsenic is of considerable interest for public health, particularly in relation to the long-term exposure. To date, arsenic has been widely studied in ambient air (Duan and Tan 2013; Huang et al. 2014a; Li et al. 2015; Sánchez-Rodas et al. 2015; Santos and Fernández-Olmo 2016), water (IARC 2004; Liao et al. 2018; Rahman et al. 2017), soil and dust (Cao et al. 2016), and foodstuffs (Davis et al. 2017; Jiang et al. 2015; Mantha et al. 2016) because of its adverse effects, including cancers (Abernathy et al. 1999).
Based on data from laboratory experiments on animals and from occupational experiments from smelters, inorganic arsenic compounds were classified as group 1 human carcinogens (IARC 1987; IARC 2004). The lung is the principal site for arsenic carcinogenicity (European Commission 2001). When arsenic is inhaled, the accumulation level can be as high as 8 times; thus, a higher risk of lung cancer is expected to be related to a higher amount of inhaled particulate matter (PM)–bound arsenic (European Commission 2001). Inhaled PM-bound arsenic exposure was high during the past decades in mainland China (Duan and Tan 2013), and it could be associated with the increasing morbidity of lung cancer (She et al. 2013; Yoshikawa et al. 2008), particularly lung adenocarcinoma (Guo et al. 2004; Kuo et al. 2017).
Additionally, arsenic in air PM may pose risks to vulnerable populations (Liao et al. 2018; Liu et al. 2018; Rahman et al. 2017; Yang et al. 2015); it has also been associated with heart diseases (Keil and Richardson 2017), and acute lower respiratory infections in children (Wang et al. 2018).
Arsenic contained in airborne PM has been attributed to coal combustion (Liu et al. 2002), resuspended dust, and industrial and vehicle exhaust (Tao et al. 2014). Urbanization and industrialization increase the concentration of arsenic contained in airborne PM, resulting in increased human exposure to airborne PM-bound arsenic through inhalation (Fishbein 1984). More importantly, inorganic arsenic was identified as the predominant species in airborne PM, with a proportion as high as 98% in different cities among various countries (Gonzalez-Castanedo et al. 2015; Huang et al. 2014a; Oliveira et al. 2005; Tsopelas et al. 2008; Yang et al. 2012). Based on the genotoxic properties of arsenic, the WHO has recommended 6.6 ng/m3 as the target value of arsenic in ambient air; China (GB 3095-2012) (Duan and Tan 2013) and several countries in the European Union (EU) have suggested a target value of 6 ng/m3 (European Commission 2004); and the United States (US) recommends a target value of 4.29 ng/m3 (Lewis et al. 2015). However, a study in Japan suggested the association between lung cancer and air PM-bound arsenic concentration of 1.77 ng/m3 or higher (Yoshikawa et al. 2008). Recently, some studies in China (Huang et al. 2014a; Li et al. 2015; Tao et al. 2014) reported that the arsenic concentration in PM has exceeded the recommended standard value (Duan and Tan 2013), while other toxic metals did not. Very high concentrations of arsenic in air (66–70 ng/m3) have been reported in Wuhan, mainly due to the industries, including 11 coal-fired power plants, one of the largest steel companies in China and 11 cement companies (Lv et al. 2006; Querol et al. 2006). High levels of arsenic in atmospheric air (34.7–49.4 ng/m3) were still observed before 2014 (Zhang et al. 2015); however, the current level and trend of airborne PM-bound arsenic in Wuhan has not been reported after the strategy of cutting excess production capacity in the steel and coal industries was implemented in China.
The temporal (several years) profile of PM2.5-bound arsenic in central China has not yet been well documented (Duan and Tan 2013). Since human health can be impacted by chronic exposure to arsenic, long-term and systematic observation is needed to assess dynamic human exposure. In this study, 579 outdoor air samples from Wuhan, central China, collected over 3 years were analyzed to identify whether the general population is still being exposed to high concentrations of PM2.5-bound arsenic and whether there are differences in the exposure levels among various districts. Additionally, human exposure to PM-bound arsenic was calculated via PM10 samples collected by personal samplers (n = 54), and the amount was compared with the total daily intake of arsenic calculated based on their total urinary arsenic concentrations.
Materials and methods
Reagents
Standards for arsenic (single-species pentavalent arsenic standard, NIST SRM# 3103a) (Yu et al. 2006) measurement (IV-ICPMS-71A) and internal standard (yttrium in IV-ICPMS-71D) were purchased from Inorganic Ventures (Christiansburg, VA, USA). Nitric acid (68%) was purchased from Fisher Scientific (Gent, Belgium). Ultrapure water (Millipore, Burlington, MA, USA) was used for analysis.
Sample collection
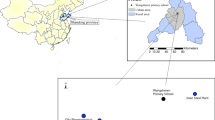
From January 2015 to December 2017, 579 outdoor air PM2.5 samples were collected from 5 sites in Wuhan, the capital city of Hubei Province and a representative city in central China, including Qingshan District (n = 237) and Wuchang District (n = 243) from 2015 to 2017 (5 to 7 samples evenly every month) and Dongxihu, Hanyang, and Jiang’an (n = 33 each, during 2017 only, typically 3 samples each month) (Table S1). The sampling was conducted simultaneously at different sampling sites on days without rain (Table S1 and Figure S1).
Medium-volume samplers (Wuhan Tianhong Environmental Protection Industry Co., Ltd., TH-150C; flow rate 100 L/min) were placed 10–15 m above the ground (HJ/T 194-2005, China) in urban areas characterized by a lack of major industrial activities within a 5-km range from the sampling point. Quartz fiber filters were applied to collect PM2.5 samples for 22 h (90 mm, Whatman International Ltd., Maidstone, England). The sampling date, the standardized air volume, and other related information were recorded. The samples were maintained and transported individually in a polystyrene box at 4°C until analysis as described elsewhere (Li et al. 2018).
Previous studies applied outdoor and indoor airborne PM-bound arsenic from specific sampling sites for human exposure assessment (Cao et al. 2016). To better understand personal dynamic exposure to PM-bound arsenic via inhalation of air, including outdoor air and indoor air, PM10 air samples were collected by personal air samplers carried by the participants in this study, and personal exposure to PM2.5-bound arsenic could be estimated by its partitioning ratio (64% in Taiwan and 63% in Greece) in PM2.5/PM10 according to the previous study (Tsopelas et al. 2008; Ying et al. 2003). Thus, personal PM10 samples were collected by personal air samplers (LP-5, A. P. Buck Inc. Orlando, FL, USA) (Li et al. 2016) from 26 male and 28 female healthy residents aged between 18 and 65 in Wuhan; samples were collected in 3 consecutive days (2 L/min, 37 mm Whatman quartz fiber filter) from October to November 2016, and their spot urine samples (n = 54) were collected on the third day; urine samples were also collected from the participant’s neighbors (n = 66, one urine sample from each participant).
Sample preparation and instrumental analysis
According to our previous reports, total arsenic in the outdoor air PM2.5 samples, personal air PM10 samples (Li et al. 2018), and urine samples (Liu et al. 2018) were analyzed without modification.
Procedural blanks, duplicates, and matrix spikes were performed every 20 samples. The accuracy was assessed by reference materials (NIST SRM 1648A and 2668). The limit of quantification (LOQ) of arsenic was calculated with a signal/noise (S/N) ratio of at least 10, namely 0.10 μg/L. The LOD was set as S/N ≥ 3. As arsenic was not found in any kind of blank samples, the method detection limit (MDL) was calculated from an S/N ratio of 10 (Van den Eede et al. 2015; Wang et al. 2019). The MDLs of arsenic in the PM2.5 and PM10 samples were 0.17 and 0.34 ng/m3, respectively. The MDL of arsenic in urine samples was 0.20 μg/L. The recoveries of arsenic in matrix spiked samples were 92.3–104%.
Estimation of daily exposure
The estimated daily intake (EDI; ng/kg body weight (bw)/day) of arsenic through inhalation of PM10 was calculated with the following Eq. (1) (USEPA 2011), assuming a 100% absorption rate from inhalation to blood circulation.
where C is the arsenic concentration (median and 95th percentile) in personal air samples (ng/m3), AIR is the air inhalation rate (m3/day), and BW is the body weight (kg), as previously described (Li et al. 2018). Details about the parameters are shown in Table S2.
The urinary concentration of arsenic was used to estimate the total daily human exposure to bioavailable arsenic (Li et al. 2017) with the Eq. (2) below, as described elsewhere (Liao et al. 2012).
where C (μg/L) and V (L) represent the total urinary arsenic concentration (Caldwell et al. 2009; Liu et al. 2018) and urine volume, respectively.
Statistical analysis
Statistical analysis was conducted with SPSS 23.0. The residuals were not normally distributed (with Shapiro-Wilk normality test). After natural log-transformation, the residuals were normally distributed. The homoscedasticity of the variance was verified by Levene’s test and the variance was equal. Then, T tests or one-way ANOVA was used to identify the differences between/among groups.
Results and discussion
Concentrations of arsenic in outdoor PM2.5
Arsenic was detected in all collected PM2.5 samples, with values ranging from 0.42 to 61.6 ng/m3 (mean, 8.48 ng/m3; median, 5.89 ng/m3). The mean arsenic concentrations from Wuhan (Wuchang and Qingshan) in 2015 (10.7 ng/m3; median, 7.92 ng/m3) were significantly higher than those in 2016 (6.81 ng/m3; median, 4.85 ng/m3) and 2017 (7.81 ng/m3; median, 5.25 ng/m3), while there was no significant difference between 2016 and 2017 (Table 1). The decrease in arsenic in air from 2015 to 2016 and 2017 could be explained by the reduced steel production of the Wuhan Steel Company, which is one of the largest steel companies in China (Lv et al. 2006).
The mean concentration of PM2.5-bound arsenic in 2017 at each sampling site was, in decreasing order, as follows: Jiang’an (9.56 ng/m3) > Dongxihu (8.57) > Qingshan (8.30) = Hanyang (8.30) > Wuchang (7.31) (Table S3). The median concentration in 2017 was as follows: Jiang’an (6.33 ng/m3) > Qingshan (5.82) = Hanyang (5.42) > Dongxihu (4.87) > Wuchang (4.47) (Table S3), and there were no significant differences among the studied sites. No significant difference was observed between Wuchang and Qingshan (these were the only 2 sites observed for 3 years) during each year (2015–2017).
The PM2.5-bound arsenic concentrations could be attributed to coal combustion (such as thermal power plants in Wuhan), traffic exhaust, and other industries in Wuhan. No significant differences in air PM2.5-bound arsenic concentrations were observed among the sampling sites, which was consistent with a previous conducted from 2003 to 2004 (Lv et al. 2006), and this result could be explained by the widespread arsenic pollution present in the urban areas of the entire city, regardless of whether the area is located in an industrial district, commercial area, traffic area, or relatively clean air area.
China witnessed a decline in its PM concentration during 2001–2010 (Jacquemin et al. 2015). A similar decline was observed for arsenic in air PM in Wuhan, China (Table 2), from 66–70 ng/m3 in PM10 during 2003–2004 (Lv et al. 2006; Querol et al. 2006) and 34.7–49.4 ng/m3 in PM2.5 during 2012–2013 (Zhang et al. 2015) to 8.48 ng/m3 in PM2.5 during 2015–2017.
Data on air arsenic concentrations from cities in China and other countries are summarized in Table 2 and Table S4. The highest air arsenic concentration from China was reported in Guangxi (a mining town, mean 21.8 μg/m3) (Zhang et al. 2009), followed by Hunan (a school in an industrial city, median 380 ng/m3 (Cao et al. 2016), and another place close to a battery plant, median 210 ng/m3 (Cao et al. 2015)) (Table S4). The arsenic in the air PM of these extremely polluted areas was 10–1000 times higher than that recorded in the Chinese megacities of Beijing (mean 7.6–32.6 ng/m3) (Tao et al. 2014; Wu et al. 2013a; Wu et al. 2013b; Wu et al. 2015), Shanghai (7.9–30.8) (Chen et al. 2008; Ming et al. 2015), Guangzhou (17.6) (Huang et al. 2014a; Huang et al. 2014b), and Shenzhen (28.6) (Duan and Tan 2013) (Table 2) and in some other capital or industrialized cities (Table S4).
Generally, the airborne arsenic concentrations in most areas of China were much higher than those in most cities of Europe and North America (Table 2). The lowest mean concentration of arsenic was reported in New Zealand, at only 5.6 × 10−3 ng/m3 (Marx et al. 2014). The arsenic concentration (mean 8.49 ng/m3) in this study was 10–20 times higher than that of most values reported in European countries, such as in Spain (Barcelona) (0.5 ng/m3) (Rivas et al. 2014) in southern Europe, where the densities of population and motor vehicles were also high. The concentration of arsenic in this study was also higher than those reported in the US, including Washington, DC (mean ~ 1.0 ng/m3) (Greene and Morris 2006) and Greater Houston, TX (Liu et al. 2016); and those in Europe, including Turkey (mean 2.23–5.55 ng/m3) (Onat and Şahin 2014) and Greece (mean 1.42–2.39 ng/m3) (Thomaidis et al. 2003); and those in Asia, including Visakhapatnam, India (2.5–5.3 ng/m3) (Police et al. 2016).
Currently, the airborne arsenic concentrations reported in most cities of China (Duan and Tan 2013) is higher than the ambient air target value of 6.6 ng/m3 recommended by the WHO, In addition to China, only a few other countries (regions) have reported high air arsenic concentrations so far, including Bor, Serbia (Serbula et al. 2010; Serbula et al. 2017), some parts of Poland (Widziewicz et al. 2016), and Pakistan (von Schneidemesser et al. 2010) (Table 2). Meanwhile, in China, the average concentrations of PM-bound arsenic in only a few cities/regions, such as Chengdu (5.9 ng/m3) (Table S4), Lhasa (1.8 ng/m3), Hong Kong (3.9–6.0 ng/m3) (Duan and Tan 2013; Pun et al. 2014), and Taiwan (3.15–4.99 ng/m3) (Fang et al. 2012; Ying et al. 2003) (Table 2), were lower than the target value. Further research on the distribution of arsenic in air PM from China and its health effects is urgently required.
Seasonal variation
The seasonal variation in the arsenic concentrations in air PM2.5 is shown in Table 3 and Table S5. Summer had the lowest concentration of arsenic in air PM2.5 in 2015, while there were no significant differences among spring, autumn, and winter. During 2016, significant differences were observed between spring and summer/autumn. Autumn had the lowest concentration in 2017. Data on arsenic in outdoor air PM2.5 in Wuhan for each month during 2015–2017 are shown in Table S6.
Overall, no significant difference in air PM2.5-bound arsenic was observed between winter and spring in the present study. This result could be explained by the unfavorable atmosphere for diffusion, sandstorm migration from North China (Zhang et al. 2015), or biomass burning during spring (Querol et al. 2006). The seasonal pattern showed that the arsenic in PM2.5 in summer and autumn was lower than that in spring and winter, which was consistent with our previous study about nickel in air PM for most inland regions in mainland China (Li et al. 2018). The seasonal pattern could be explained by meteorological data such as higher air pressure, lower temperature, and lower humidity observed in the winter and spring, as shown in Table 3.
The chronic adverse health effects and PM have differed among studies; a lack of spatially resolved elemental composition data may be partly responsible for these differences (Beelen et al. 2015). Therefore, studies about the temporal trends and seasonal profiles of arsenic concentrations in outdoor air PM in most regions of China are needed to investigate the association between human exposure to air PM-bound arsenic and its adverse health effects and to establish efficient preventive measures and strategies.
Arsenic exposure through personal air PM10 inhalation
The contribution of personal inhalation of airborne PM to arsenic exposure was not yet well characterized. Outdoor air PM inhalation could be used for the calculation of human exposure. However, inhalation exposure will be underestimated based on the outdoor air concentration of arsenic and the outdoor exposure factor (the percentage of outdoor activity, 0.05–0.20) (Li et al. 2018; Rodes et al. 2010; USEPA 2011). To estimate the daily intake of arsenic through the inhalation pathway more accurately, air PM10 samples collected by personal air samplers were used in this study.
In autumn 2016, personal air PM10-bound arsenic (median 10.8 ng/m3, 95th percentile 13.8 ng/m3) (Table 4 and Fig. 1a) and the values estimated for PM2.5-bound arsenic (median 6.93 ng/m3, 95th percentile 8.84 ng/m3) (Table S7, based on a partitioning ratio of 64% for PM2.5/PM10) (Ying et al. 2003) were both higher than those detected in outdoor PM2.5 (median 2.69 ng/m3, 95th percentile 7.32 ng/m3) (Table 3); these results suggested atmospheric arsenic easily penetrated indoors spaces, with small-sized PM, or there were other indoor sources of arsenic, such as smoking and fuel combustion.
The exposure doses could be influenced by factors such as gender, residential and working area, age, and inhalation rate. To estimate the EDI of arsenic through inhalation, the population was categorized into five age groups (Table S2) (USEPA 2011). The median and 95th percentile values of arsenic concentrations were used for the calculation of the EDIs.
The EDIs of PM10-bound arsenic inhalation for adults are shown in Table 5. The median arsenic exposure doses from air PM10 samples collected by personal air samplers in Wuhan were calculated. The gender differences in body weight were considered; the results, in decreasing order, are ranked as follows: urban female (GM, 3.56 ng/kg bw/day) > urban male (2.97) > rural female (1.77) and rural male (1.76). Additionally, the highest median EDIs of infants were twice those of teenagers (Table S8).
There were uncertainties in the estimation of daily intake. First, the mean values of AIR were extracted from the US Environmental Protection Agency (USEPA) exposure handbook (USEPA 2011), which could be not sufficiently appropriate for China. Second, the sampling sites in this study included only urban and suburban rural areas and might not be representative of residents who live in remote rural areas. Finally, personal air PM10 samples were collected from adults; thus, the results might not represent the exposure dose of other age groups due to their different behavior patterns.
Arsenic exposure via air particulate matter compared with total bioavailable daily intake based on urinary arsenic concentration
Generally, the elimination half-life of arsenic and its metabolites is approximately 2–4 days (Lauwerys and Hoet 2001), and urinary arsenic can reflect recent personal exposure. The total urinary arsenic concentration in this study (GM, 15.9 μg/L) was higher than that (GM, 1.69–4.45 μg/L) from the US NHANES during 2003–2014 (samples were limited to individuals who had urinary arsenobetaine concentrations below the MDL) (Welch et al. 2018). When urinary arsenic was stratified by considering rural-urban and gender differences, the distribution is (Fig. 1b and Table S9) as follows: rural male (median, 20.3 μg/L) > urban male (19.7) > urban female (17.6) > rural female (12.3). The results of a previous study on urinary arsenic in women who were pregnant and in their first trimester in urban Wuhan, China, (median, 18.1 μg/L) (Liu et al. 2018) were consistent with the values observed (17.6 μg/L) for urban females in the present study. The median urinary total arsenic (17.7 μg/L) in this study was close to the 95th percentile of inorganic-related arsenic in the US population (18.9 μg/L) (Caldwell et al. 2009), and the 95th percentile for the highest subgroup (urban male, 33.9 μg/L) in this study was close to the occupational biological effect index (35 μg/L) provided by the American Conference of Governmental Industrial Hygienists (ACGIH 2001).
The withdrawn provisional tolerable daily intake of inorganic arsenic had been previously recommended by WHO as 2.1 μg/kg bw/day (Halder et al. 2013), and the total daily intake based on urinary arsenic (EDIurine) observed in this study (Table 5) was lower than this value, but higher than that recommended by USEPA (0.3 μg/kg bw/day) (USEPA 2018). The median values of total EDIurine of arsenic, in decreasing order, were as follows: urban female (497 ng/kg bw/day) > rural male (417) > urban male (405) > rural female (348). Compared with the total EDIurine, air exposure accounted for less than 1% (Table 5), which was consistent with a previous document (European Communities 2001).
However, the comparison should be interpreted with caution. When total urinary arsenic is used for the estimation of total daily intake, the daily intake could be underestimated as only approximately 66% (54–74%) of the total dose in human was excreted in urine (Buchet et al. 1981; Hays et al. 2010), although urinary excreted arsenic may represent the bioavailability of arsenic in diet to a large extent—a critical parameter to better estimate arsenic exposure (Li et al. 2017). Additionally, inorganic-related arsenic metabolites and seafood-related harmless arsenic metabolite arsenobetaine should be quantified separately in central China in future studies to better characterize the harmful part of human exposure to arsenic, since data are only available from Nanjing, China, (Wang et al. 2016; Zhang et al. 2014) and Inner Mongolia, China, (Liu et al. 2017) so far.
In most areas around the world, rice and water are reported to be the main sources of ingested arsenic, especially for inhabitants of Asia (Davis et al. 2017; Mantha et al. 2016). According to a previous report about arsenic exposure in children from a hometown with nonferrous metals, arsenic exposure via diet was the predominant exposure pathway of residents, accounted for approximately 82% of the total EDI; the second major exposure pathway was via soil and dust, accounted for approximately 12%. The PM-bound arsenic accounted for approximately 3% (Cao et al. 2016), which was higher than the ratio observed in this study. The differences might be attributed to the fact that Cao et al.’s study was conducted in an area polluted by arsenic severely.
Coal combustion and its related air PM components have been suggested to be related to lung adenocarcinoma, which is the predominant type of lung cancer in some specific areas, such as Xuanwei, Yunnan, China (Wu et al. 2016). Epidemiological evidence suggests that the arsenic concentration in air is a reliable predictor of lung/respiratory cancer risk (Lewis et al. 2015). Thus, although the exposure of arsenic via inhalable PM was minor or even negligible compared with the daily intake via oral ingestion, the problem caused by inhalation of inorganic arsenic in areas where airborne arsenic exceeds the standard, requires more attention.
Previously, studies on the health effects of PM-related air pollution in China mainly focused on PM10 or PM2.5 (Fang et al. 2016). Studies are transitioning from PM2.5 to select toxic components such as PAHs or arsenic. The associations between arsenic in air PM and lung cancer are expected to be analyzed in developed countries (Raaschou-Nielsen et al. 2016), while more attention is required on these issues in developing countries, such as China, Serbia, Pakistan, and Vietnam. In particular, the regional and gender differences in PM-bound arsenic exposure via inhalation observed in this study may provide some clues to the associations between arsenic inhalation exposure and adverse respiratory effects.
It is difficult to determine whether or not a threshold exists for the carcinogenicity of arsenic because the current available data were insufficient to justify the assumption of a threshold (European Commission 2001). Developing countries with arsenic air pollution, such as China, are appropriate sites to investigate the potential causality.
The results of this study will be helpful for relevant policy-making, and enhance public awareness of the health risks, as lung cancer appears to be a critical problem following chronic inhalation exposure to PM-bound arsenic (European Communities 2001). Although the atmospheric arsenic concentration within a year in some cities had been previously reported in China (Duan and Tan 2013), studies on the dynamic long-term (several years or longer) investigation of arsenic in PM2.5 or PM10 and its health effects are needed in the future. This study implicated the importance of PM abatement technology (Campa et al. 2018; Minguillón et al. 2009) and the emergence of clean energy such as wind, water, and sunlight instead of coal combustion.
Conclusion
Tested PM-bound arsenic levels in this study were higher than those found in cities from most other countries. Clean energy instead of coal combustion is needed to lower the arsenic emissions in China. Infants are vulnerable to high levels of PM-bound arsenic. High concentrations of arsenic were observed in outdoor air PM2.5 during 2015–2017, with a slight decreasing trend. The concentrations of arsenic in air PM2.5 samples in winter and spring were higher than those in summer and autumn. Inhaled PM-bound arsenic in Wuhan exceeded the recommended target values for a long time; its lung accumulation and associations with diseases such as lung adenocarcinoma require more attention, although the contribution of inhaled air PM-bound arsenic to the total arsenic intake is minor.
This study described personal exposure via the inhalation of PM-bound arsenic in air and its contribution to the total daily intake based on excreted urinary arsenic. The sources of high levels of arsenic in air and its distribution throughout China, including remote rural areas and other developing countries, need further investigation.
References
Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B et al (1999) Arsenic: health effects, mechanisms of actions, and research issues. Environ Health Perspect 107:593–597
ACGIH (2001) Documentation of biological exposure indices. 7th edition. . Cincinnati (OH): ACGIH Worldwide: American Conference of Government Industrial Hygienists (ACGIH)
Beelen R, Hoek G, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer PH, Nieuwenhuijsen MJ, Xun WW, Katsouyanni K, Dimakopoulou K, Marcon A, Vartiainen E, Lanki T, Yli-Tuomi T, Oftedal B, Schwarze PE, Nafstad P, de Faire U, Pedersen NL, Östenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Sørensen M, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita HB, Sugiri D, Krämer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Jaensch A, Ineichen A, Tsai MY, Schaffner E, Probst-Hensch NM, Schindler C, Ragettli MS, Vilier A, Clavel-Chapelon F, Declercq C, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Katsoulis M, Trichopoulou A, Keuken M, Jedynska A, Kooter IM, Kukkonen J, Sokhi RS, Vineis P, Brunekreef B (2015) Natural-cause mortality and long-term exposure to particle components: an analysis of 19 European cohorts within the multi-center ESCAPE project. Environ Health Perspect 123:525–533
Buchet J-P, Lauwerys R, Roels H (1981) Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int Arch Occup Environ Health 48:111–118
Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD (2009) Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003–2004. J Expo Sci Environ Epidemiol 19:59–68
Campa AMSDL, Sánchez-Rodas D, Alsioufi L, Alastuey A, Querol X, Rosa JDDL (2018) Air quality trends in an industrialised area of SW Spain. J Clean Prod 186:465–474
Cao S, Duan X, Zhao X, Wang B, Ma J, Fan D, Sun C, He B, Wei F, Jiang G (2015) Health risk assessment of various metal(loid)s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ Pollut 200:16–23
Cao S, Duan X, Zhao X, Chen Y, Wang B, Sun C et al (2016) Health risks of children’s cumulative and aggregative exposure to metals and metalloids in a typical urban environment in China. Chemosphere 147:404
Chen J, Tan M, Li Y, Zheng J, Zhang Y, Shan Z, Zhang G, Li Y (2008) Characteristics of trace elements and lead isotope ratios in PM(2.5) from four sites in Shanghai. J Hazard Mater 156:36–43
Cong Z, Kang S, Luo C, Li Q, Jie H, Gao S et al (2011) Trace elements and lead isotopic composition of PM in Lhasa, Tibet. Atmos Environ 45:6210–6215
Davis MA, Signespastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T et al (2017) Assessment of human dietary exposure to arsenic through rice. Sci Total Environ 586:1237
Duan J, Tan J (2013) Atmospheric heavy metals and arsenic in China: situation, sources and control policies. Atmos Environ 74:93–101
European Commission (2001) E. Opinion on: Position paper on: Ambient air pollution by arsenic compounds - final version, October 2000. Opinion expressed at the 24th CSTEE PLENARY MEETING, BRUSSELS, 12 JUNE 2001. 2018
European Commission (2004) E. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. 2018
European Communities (2001) E. Ambient air pollution by AS, CD and NI compounds. Position Paper. Office for Official Publications of the European Communities, Luxembourg
Fang GC, Huang YL, Huang JH (2012) Atmospheric arsenic (As) study at five characteristic sampling sites in Taiwan. Environ Monit Assess 184:729–740
Fang D, Wang Q, Li H, Yu Y, Lu Y, Qian X (2016) Mortality effects assessment of ambient PM2.5 pollution in the 74 leading cities of China. Sci Total Environ 569-570:1545–1552
Figueroa DA, Rodriguez-Sierra CJ, Jimenez-Velez BD (2006) Concentrations of Ni and V, other heavy metals, arsenic, elemental and organic carbon in atmospheric fine particles (PM2.5) from Puerto Rico. Toxicol Ind Health 22:87–99
Fishbein L (1984) Overview of analysis of carcinogenic and/or mutagenic metals in biological and environmental samples. I. Arsenic, beryllium, cadmium, chromium and selenium. Int J Environ Anal Chem 17:113–170
Gonzalez-Castanedo Y, Sanchez-Rodas D, Sanchez de la Campa AM, Pandolfi M, Alastuey A, Cachorro VE et al (2015) Arsenic species in atmospheric particulate matter as tracer of the air quality of Donana Natural Park (SW Spain). Chemosphere 119:1296–1303
Greene NA, Morris VR (2006) Assessment of public health risks associated with atmospheric exposure to PM2.5 in Washington, DC, USA. Int J Environ Res Public Health 3:86–97
Guo HR, Wang NS, Hu H, Monson RR (2004) Cell type specificity of lung cancer associated with arsenic ingestion. Cancer Epidemiol Biomark Prev 13:638–643
Halder D, Bhowmick S, Biswas A, Chatterjee D, Nriagu J, Guha Mazumder DN, Šlejkovec Z, Jacks G, Bhattacharya P (2013) Risk of arsenic exposure from drinking water and dietary components: implications for risk management in rural Bengal. Environ Sci Technol 47:1120–1127
Hays SM, Aylward LL, Gagné M, Nong A, Krishnan K (2010) Biomonitoring equivalents for inorganic arsenic. Regul Toxicol Pharmacol 58:1–9
Ho KF, Lee SC, Chan CK, Yu JC, Chow JC, Yao XH (2003) Characterization of chemical species in PM2.5 and PM10 aerosols in Hong Kong. Atmos Environ 37:31–39
Huang M, Chen X, Zhao Y, Yu Chan C, Wang W, Wang X et al (2014a) Arsenic speciation in total contents and bioaccessible fractions in atmospheric particles related to human intakes. Environ Pollut 188:37–44
Huang M, Wang W, Chan CY, Cheung KC, Man YB, Wang X et al (2014b) Contamination and risk assessment (based on bioaccessibility via ingestion and inhalation) of metal(loid)s in outdoor and indoor particles from urban centers of Guangzhou, China. Sci Total Environ 479–480:117–124
IARC (1987) IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Overall evaluations of carcinogenicity : an updating of IARC monographs, volumes 1 to 42, Supplement 7
IARC (2004) Some drinking-water disinfectants and contaminants, including arsenic. Iarc monographs on the evaluation of carcinogenic risks to humans. 84. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, pp. 1
Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, Bellisario V, Buschka A, Bono R, Brunekreef B, Cai Y, Cirach M, Clavel-Chapelon F, Declercq C, de Marco R, de Nazelle A, Ducret-Stich RE, Ferretti VV, Gerbase MW, Hardy R, Heinrich J, Janson C, Jarvis D, al Kanaani Z, Keidel D, Kuh D, le Moual N, Nieuwenhuijsen MJ, Marcon A, Modig L, Pin I, Rochat T, Schindler C, Sugiri D, Stempfelet M, Temam S, Tsai MY, Varraso R, Vienneau D, Vierkötter A, Hansell AL, Krämer U, Probst-Hensch NM, Sunyer J, Künzli N, Kauffmann F (2015) Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE). Environ Health Perspect 123:613–621
Jiang Y, Zeng X, Fan X, Chao S, Zhu M, Cao H (2015) Levels of arsenic pollution in daily foodstuffs and soils and its associated human health risk in a town in Jiangsu Province, China. Ecotoxicol Environ Saf 122:198–204
Keil AP, Richardson DB (2017) Reassessing the link between airborne arsenic exposure among anaconda copper smelter workers and multiple causes of death using the parametric g-formula. Environ Health Perspect 125:608–614
Kuo YC, Lo YS, Guo HR (2017) Lung cancer associated with arsenic ingestion: cell-type specificity and dose response. Epidemiology 28(Suppl 1):S106–S112
Lauwerys R, Hoet P. (2001) Arsenic. In: Industrial chemical exposure. Guidelines for biological monitoring. 3rd Ed. Boca Raton (FL). : Lewis Publishers
Lewis AS, Beyer LA, Zu K (2015) Considerations in deriving quantitative cancer criteria for inorganic arsenic exposure via inhalation. Environ Int 74:258–273
Li H, Wang J, Wang QG, Qian X, Qian Y, Yang M et al (2015) Chemical fractionation of arsenic and heavy metals in fine particle matter and its implications for risk assessment: a case study in Nanjing, China. Atmos Environ 103:339–346
Li T, Cao S, Fan D, Zhang Y, Wang B, Zhao X, Leaderer BP, Shen G, Zhang Y, Duan X (2016) Household concentrations and personal exposure of PM 2.5 among urban residents using different cooking fuels. Sci Total Environ 548-549:6–12
Li H-B, Li J, Zhao D, Li C, Wang X-J, Sun H-J, Juhasz AL, Ma LQ (2017) Arsenic relative bioavailability in rice using a mouse arsenic urinary excretion bioassay and its application to assess human health risk. Environ Sci Technol 51:4689–4696
Li H, Wan Y, Chen X, Cheng L, Yang X, Xia W, Xu S, Zhang H (2018) A multiregional survey of nickel in outdoor air particulate matter in China: implication for human exposure. Chemosphere 199:702–708
Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon H-B, Nakata H, Kannan K (2012) Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 46:6860–6866
Liao KW, Chang CH, Tsai MS, Chien LC, Chung MY, Mao IF, Tsai YA, Chen ML (2018) Associations between urinary total arsenic levels, fetal development, and neonatal birth outcomes: a cohort study in Taiwan. Sci Total Environ 612:1373–1379
Liu J, Zheng B, Aposhian HV, Zhou Y, Chen ML, Zhang A, Waalkes MP (2002) Chronic arsenic poisoning from burning high-arsenic-containing coal in Guizhou, China. Environ Health Perspect 110:119–122
Liu S, Ganduglia CM, Li X, Delclos GL, Franzini L, Zhang K (2016) Short-term associations of fine particulate matter components and emergency hospital admissions among a privately insured population in Greater Houston. Atmos Environ 147:369–375
Liu T, Guo H, Xiu W, Wei C, Li X, Di Z et al (2017) Biomarkers of arsenic exposure in arsenic-affected areas of the Hetao Basin, Inner Mongolia. Sci Total Environ 609:524–534
Liu H, Lu S, Zhang B, Xia W, Liu W, Peng Y, Zhang H, Wu K, Xu S, Li Y (2018) Maternal arsenic exposure and birth outcomes: a birth cohort study in Wuhan, China. Environ Pollut 236:817–823
Lv W, Wang Y, Querol X, Zhuang X, Alastuey A, López A et al (2006) Geochemical and statistical analysis of trace metals in atmospheric particulates in Wuhan, Central China. Environ Geol 51:121–132
Mantha M, Yeary E, Trent J, Creed PA, Kubachka K, Hanley T et al (2016) Estimating inorganic arsenic exposure from U.S. rice and total water intakes. Environ Health Perspect:125
Marx SK, Lavin KS, Hageman KJ, Kamber BS, O'Loingsigh T, Mctainsh GH (2014) Trace elements and metal pollution in aerosols at an alpine site, New Zealand: sources, concentrations and implications. Atmos Environ 82:206–217
Ming HU, Zhang Y, Zhao Q (2015) Characteristics and sources of inorganic elements in PM_(2.5) during wintertime in Shanghai. Acta Sci Circumst 35:1993–1999
Minguillón MC, Monfort E, Querol X, Alastuey A, Celades I, Miró JV (2009) Effect of ceramic industrial particulate emission control on key components of ambient PM10. J Environ Manag 90:2558–2567
Oliveira V, Gómez-Ariza JL, Sánchez-Rodas D (2005) Extraction procedures for chemical speciation of arsenic in atmospheric total suspended particles. Anal Bioanal Chem 382:335–340
Onat B, Şahin ÜA (2014) An assessment of particulate mercury and arsenic concentrations in size-fractioned total suspended particulate matter in urban areas. Air Quality Atmosphere & Health 7:131–141
Police S, Sahu SK, Pandit GG (2016) Chemical characterization of atmospheric particulate matter and their source apportionment at an emerging industrial coastal city, Visakhapatnam, India. Atmos Pollut Res 7:725–733
Pun VC, Yu IT, Qiu H, Ho KF, Sun Z, Louie PK, Wong TW, Tian L (2014) Short-term associations of cause-specific emergency hospitalizations and particulate matter chemical components in Hong Kong. Am J Epidemiol 179:1086–1095
Querol X, Zhuang X, Alastuey A, Viana M, Lv W, Wang Y, López A, Zhu Z, Wei H, Xu S (2006) Speciation and sources of atmospheric aerosols in a highly industrialised emerging mega-city in Central China. J Environ Monit 8:1049–1059
Raaschou-Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B, Stafoggia M, Samoli E, Weinmayr G, Dimakopoulou K, Nieuwenhuijsen M, Xun WW, Fischer P, Eriksen KT, Sørensen M, Tjønneland A, Ricceri F, de Hoogh K, Key T, Eeftens M, Peeters PH, Bueno-de-Mesquita HB, Meliefste K, Oftedal B, Schwarze PE, Nafstad P, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Penell J, de Faire U, Korek M, Pedersen N, Östenson CG, Pershagen G, Fratiglioni L, Concin H, Nagel G, Jaensch A, Ineichen A, Naccarati A, Katsoulis M, Trichpoulou A, Keuken M, Jedynska A, Kooter IM, Kukkonen J, Brunekreef B, Sokhi RS, Katsouyanni K, Vineis P (2016) Particulate matter air pollution components and risk for lung cancer. Environ Int 87:66–73
Rahman ML, Kile ML, Rodrigues EG, Valeri L, Raj A, Mazumdar M et al (2017) Prenatal arsenic exposure, child marriage, and pregnancy weight gain: associations with preterm birth in Bangladesh. Environ Int 112:23–32
Rivas I, Viana M, Moreno T, Pandolfi M, Amato F, Reche C, Bouso L, Àlvarez-Pedrerol M, Alastuey A, Sunyer J, Querol X (2014) Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environ Int 69:200–212
Rodes CE, Lawless PA, Thornburg JW, Williams RW, Croghan CW (2010) DEARS particulate matter relationships for personal, indoor, outdoor, and central site settings for a general population. Atmos Environ 44:1386–1399
Sánchez-Rodas D, Campa AMSDL, Alsioufi L (2015) Analytical approaches for arsenic determination in air: a critical review. Anal Chim Acta 898:1–18
Santos G, Fernández-Olmo I (2016) A proposed methodology for the assessment of arsenic, nickel, cadmium and lead levels in ambient air. Sci Total Environ 554:155–166
Serbula SM, Antonijević MM, Milosević NM, Milić SM, Ilić AA (2010) Concentrations of particulate matter and arsenic in Bor (Serbia). J Hazard Mater 181:43–51
Serbula SM, Milosavljevic JS, Radojevic AA, Kalinovic JV, Kalinovic TS (2017) Extreme air pollution with contaminants originating from the mining-metallurgical processes. Sci Total Environ 586:1066–1075
She J, Yang P, Hong Q, Bai C (2013) Lung cancer in China: challenges and interventions. Chest 143:1117–1126
Tao J, Zhang RJ, Duan JC, Jing JS, Zhu LH, Chen ZM et al (2014) Seasonal variation of carcinogenic heavy metals in PM2.5 and source analysis in Beijing. Huan Jing Ke Xue 35:411–417
Thomaidis NS, Bakeas EB, Siskos PA (2003) Characterization of lead, cadmium, arsenic and nickel in PM2.5 particles in the Athens atmosphere, Greece. Chemosphere 52:959–966
Tsopelas F, Tsakanika L-A, Ochsenkühn-Petropoulou M (2008) Extraction of arsenic species from airborne particulate filters—application to an industrial area of Greece. Microchem J 89:165–170
USEPA (2011) United States Environmental Protection Agency. Exposure factors handbook. http://www.epa.gov/ncea/efh/report.html
USEPA. (2018) U.S. Environmental Protection Agency. 2018 edition of the drinking water standards and health advisories tables. EPA 822-F-18-001. https://www.epa.gov/sdwa/2018-drinking-water-standards-and-advisory-tables
Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF et al (2015) Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int 74:1–8
von Schneidemesser E, Stone EA, Quraishi TA, Shafer MM, Schauer JJ (2010) Toxic metals in the atmosphere in Lahore, Pakistan. Sci Total Environ 408:1640–1648
Wang X, Zhang J, Xu W, Huang Q, Liu L, Tian M, Xia Y, Zhang W, Shen H (2016) Low-level environmental arsenic exposure correlates with unexplained male infertility risk. Sci Total Environ 571:307–313
Wang Q, Wang J, He MZ, Kinney PL, Li T (2018) A county-level estimate of PM2.5 related chronic mortality risk in China based on multi-model exposure data. Environ Int 110:105–112
Wang Y, Li W, Martínez-Moral MP, Sun H, Kannan K (2019) Metabolites of organophosphate esters in urine from the United States: concentrations, temporal variability, and exposure assessment. Environ Int 122:213–221
Welch B, Smit E, Cardenas A, Hystad P, Kile ML (2018) Trends in urinary arsenic among the U.S. population by drinking water source: results from the National Health and nutritional examinations survey 2003–2014. Environ Res 162:8–17
Widziewicz K, Rogula-Kozłowska W, Loska K (2016) Cancer risk from arsenic and chromium species bound to PM 2.5 and PM 1 – Polish case study. Atmos Pollut Res 7:884–894
Wu S, Deng F, Hao Y, Shima M, Wang X, Zheng C, Wei H, Lv H, Lu X, Huang J, Qin Y, Guo X (2013a) Chemical constituents of fine particulate air pollution and pulmonary function in healthy adults: the Healthy Volunteer Natural Relocation study. J Hazard Mater 260:183–191
Wu S, Deng F, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Wei H, Hao Y, Lv H, Lu X, Guo X (2013b) Blood pressure changes and chemical constituents of particulate air pollution: results from the healthy volunteer natural relocation (HVNR) study. Environ Health Perspect 121:66–72
Wu S, Yang D, Wei H, Wang B, Huang J, Li H, Shima M, Deng F, Guo X (2015) Association of chemical constituents and pollution sources of ambient fine particulate air pollution and biomarkers of oxidative stress associated with atherosclerosis: a panel study among young adults in Beijing, China. Chemosphere 135:347–353
Wu H, Meng S, Xu Q, Wang X, Wang J, Gong R, Song Y, Duan Y, Zhang Y (2016) Gene expression profiling of lung adenocarcinoma in Xuanwei, China. Eur J Cancer Prev 25:508–517
Yang G, Ma L, Xu D, Li J, He T, Liu L, Jia H, Zhang Y, Chen Y, Chai Z (2012) Levels and speciation of arsenic in the atmosphere in Beijing, China. Chemosphere 87:845–850
Yang YY, Liu LY, Guo LL, Lv YL, Zhang GM, Lei J et al (2015) Seasonal concentrations, contamination levels, and health risk assessment of arsenic and heavy metals in the suspended particulate matter from an urban household environment in a metropolitan city, Beijing. China Environ Monit Assess 187:409
Ying IT, Kuo SC, Lin YH (2003) Temporal characteristics of inhalable mercury and arsenic aerosols in the urban atmosphere in southern Taiwan. Atmos Environ 37:3401–3411
Yoshikawa M, Aoki K, Ebine N, Kusunoki M, Okamoto A (2008) Correlation between the arsenic concentrations in the air and the SMR of lung cancer. Environ Health Prev Med 13:207
Yu LL, Butler TA, Turk GC (2006) Effect of valence state on ICP-OES value assignment of SRM 3103a arsenic spectrometric solution. Anal Chem 78:1651–1656
Zhang XY, Tang LS, Gan Z, Wu HD (2009) Heavy metal contamination in a typical mining town of a minority and mountain area, South China. Bull Environ Contam Toxicol 82:31
Zhang J, Shen H, Xu W, Xia Y, Barr DB, Mu X, Wang X, Liu L, Huang Q, Tian M (2014) Urinary metabolomics revealed arsenic internal dose-related metabolic alterations: a proof-of-concept study in a Chinese male cohort. Environ Sci Technol 48:12265–12274
Zhang F, Wang ZW, Cheng HR, Lv XP, Gong W, Wang XM et al (2015) Seasonal variations and chemical characteristics of PM 2.5 in Wuhan, central China. Sci Total Environ 518-519:97–105
Funding
This work was supported by the National Key Research and Development Program of China (2016YFC0206200), the Natural Science Foundation of Hubei (2016CFB274), the Special Project of Chinese Ministry of Environmental Protection (2111101-03 and 2111101-04), and the Health and Family Planning Commission of Wuhan Municipality (WG18A05).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 5413 kb)
Rights and permissions
About this article
Cite this article
Mao, X., Hu, X., Wang, Y. et al. Temporal trend of arsenic in outdoor air PM2.5 in Wuhan, China, in 2015–2017 and the personal inhalation of PM-bound arsenic: implications for human exposure. Environ Sci Pollut Res 27, 21654–21665 (2020). https://doi.org/10.1007/s11356-020-08626-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08626-2