Abstract
This study addresses the different biogeochemical parameters that control the dynamics of Hg, which is a less-studied metal in the Ebrié Lagoon. During two hydrological seasons, the dry season and the rainy season, we regularly sampled and analysed various compartments (e.g. sediments and fishes (Tilapia sp.)) of the lagoon. Thus, the physicochemical parameters were measured in situ (e.g. temperature, pH, salinity, redox potential and dissolved oxygen, total dissolved organic carbon, nitrates and sulphates), and the microbiological parameters (e.g. cultivable cells, total enzymatic activity and catabolic activity) were measured to establish the seasonal variations in the links between Hg and biogeochemical parameters through multivariate statistical analyses. The bioavailability of Hg from an unpolluted site was studied by comparing the ratios of fish and sediment. The results indicated that the seasons influenced the different biogeochemical factors, although for some factors, the variations were not significant. This influence was more pronounced in the dry season than in the rainy season. The impact of microbial activities and organic matter on Hg dynamics was observed in all seasons. However, other factors, such as pH, temperature, salinity, Eh and sulphates, influenced the dynamics of Hg only in the dry season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of aquatic environments by Hg has been a global concern for several decades because Hg is a non-essential metal for the development of living organisms and is recognised as a pollutant with a high degree of toxicity. This is due to its non-biodegradable character, its persistence in ecosystems and its toxicity to living organisms (Cardoso et al. 2014). Hg tends to accumulate in aquatic environments by reversibly associating with sediments and often concentrating in the aquatic food chain. The most toxic form of mercurial derivatives is its organic form, especially MeHg, because of its lipophilic character and its ability to cross biological membranes (King et al. 2000). Hg accumulation in aquatic environments gradually degrades the quality of these environments. The degradation of these aquatic environments particularly affects regions of Africa. While some parts of the world, such as Europe and North America, have declined their contributions to aquatic Hg levels, the levels in Africa have increased in recent decades (Pacyna et al. 2001, 2006). This increase is due to the use of Hg in all anthropic activities, including gold mining, which has led to an increase in Hg fluxes in the terrestrial and aquatic ecosystems in Africa (Fiston 2017). This massive contribution of Hg to terrestrial and aquatic environments is not without risk to human health because these environments are used for either agriculture or fishing.
Lagoons are buffer or exchange zones between inland waters and marine waters. The different movements of these two types of water make this environment an ecosystem with very different seasonal characteristics. This is the case of the Ebrié Lagoon, the largest and most important lagoon of the Ivorian lagoon system, and on its banks has developed Abidjan, a large agglomeration of West Africa. In addition, this lagoon is located in a tropical zone where climatic and environmental conditions (high temperatures, strong fluctuations in salinity and turbidity between the rainy and dry seasons, high biological turnover rates), lead to high variability in many aspects of the Hg cycle (Costa et al. 2012). Coastal sediments are an important compartment for transport, long-term Hg storage and are optimal for Hg methylation (Seelen et al. 2018). Contaminants in sediments can be transferred to the water column via a variety of processes, including diffusion and advection from sediments (often biotically mediated), sediment resuspension and release, and biotransfer through organisms that feed at the sediment–water interface (Mason et al. 1999). The dynamics of Hg at the estuarine level has been addressed by several authors. Seelen et al. (2018) have highlighted the important role of particle inputs in estuaries, particle exchanges between sediments and the water column and organic matter in the distribution of Hg and MeHg. Noh et al. (2013) showed that in saline environments, biogeochemical factors such as suspended matter, primary productivity and sulphate and chloride concentrations influence Hg speciation. To these biogeochemical parameters, the important role of redox chemistry controls for a large part the distribution and fate of MeHg in surface sediments (Mason et al. 1999). Moreover, the effects of redox chemistry can increase with the presence of high levels suspended matter and the intensity of light in the water (Ci et al. 2016). Choi et al. (2019) emphasised the importance of organic matter and total nitrogen in the production and bioavailability of MeHg for organisms in sediments..
However, in the case of the Ebrié Lagoon, although the global levels of Hg and other MTEs are currently known (Soro et al. 2009; Wognin et al. 2017), the mechanisms and control factors regulating the transfer of Hg from one compartment to another compartment remain unknown. Thus, the main objective of this study is to assess the level of Hg contamination in the different compartments of the Ebrié Lagoon (e.g. sediments and fish (Tilapia sp.)) and to constrain the influence of some biogeochemical processes on the dynamics of Hg in these compartments following various climatic seasons. For this, a detailed study was conducted in the Ebrié Lagoon during two hydrological seasons (the dry season and the rainy season). This study concerns the Hg content variation and its link with some physicochemical and microbiological parameters in each compartment.
Material et methods
Study site
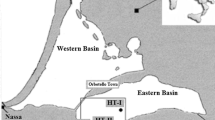
The study was carried out in the Ebrié Lagoon, located in the south of Côte d’Ivoire in the city of Abidjan (Fig. 1). This lagoon is parallel with the equator, and its coordinates are 5° 20′ and 5° 10’ N and 3° 40′ and 4° 50’ W. The study area covers an area of 560 km2 and is 140 km long. Its width does not exceed 7 km, and its mean water volume is estimated at approximately 2.7 × 109 m3, with an average depth of 4.8 m (Varlet 1978). The main climate is tropical and is marked by a period of abundant rain and a long dry period. The Ebrié Lagoon is fed with freshwater by the Agnéby, Mé and Comoé rivers. The lagoon has been in permanent contact with the Atlantic Ocean since 1950 by the artificial channel of Vridi. As part of this study, eight sites were selected for sample collection, including seven in the Abidjan region and one in Cosrou Bay. Cosrou Bay is located about 90 km west of the Abidjan area and is considered a control site. These sampling sites were chosen on the basis of the morphological and hydrobiological criteria proposed by Durand and Guiral (1994), which stipulate that the Abidjan zone is under the influence of the sea and that of Cosrou is stable, with little influence from marine waters and freshwater inputs. This choice is also motivated by the fact that these two zones are very different from the point of view of urbanisation: Abidjan is very urbanised and Cosrou is purely rural.
Sampling and preparation of samples
Samples of sediments, water and fishes (Tilapia sp.) were collected during two sampling campaigns in the dry season (February 2017) and then in the wet season (July 2017). The sediments were collected using a Van Veen grab sampler at the surface. Each sediment sample was split into two parts. The first portion of sediment was used for microbiological analyses and samples were collected in sterile Falcon® vials and then stored at − 18 °C before analysis. The second part of the sample was used for chemical analysis and was stored in freezer bags before analysis. For chemical analyses, the sediment was dried at 105 °C for 24 h. The dried sediment samples were finely ground and stored until analysis.
Water samples were collected at three depths (at the surface, in the middle and at 0.5 m from the bottom) from each site using a 2-l Niskin® bottle. Then, the samples were transferred to Nalgene® polyethylene bottles, which were previously rinsed with a 5% nitric acid solution, rinsed thoroughly with distilled water in the laboratory and rinsed again with an aliquot of the sample from the site before sampling. In the laboratory, the solutions were vacuum filtered using the Whatman® filtration system and a nylon filter (0.45 μm diameter). The filtered samples were stored in Falcon® tubes at − 18 °C until analysis.
The fish samples were obtained at each site by collecting fish from traditional fishermen living around the lagoon. Fish were transported in refrigerated enclosures (4 °C). In the laboratory, the flesh of fishes was delicately separated from the skeleton. These fish flesh samples were then freeze-dried using a lyophiliser Alpha 1-2®, and then, the samples were finely ground and stored before analysis.
Chemical analyses of samples
In situ parameter measurements in water column
During both field seasons, the pH, redox potential, dissolved oxygen and temperature were measured in situ using the multiparameter BANTE 900P®. The salinity of the samples was determined using a multiparameter HANNA HI 9828®. The determination of these parameters was based on an aliquot of the samples taken at each site and at each depth of the water column.
Nitrates, sulphates and dissolved organic carbon analyses in water samples
For the solutions (water column), the anions (sulphates and nitrates) were analysed with kits (test kits, Spectroquant®) using a GENESYS 5® spectrophotometer at wavelengths of 490 and 410 nm, respectively, for sulphates and nitrates. The content of dissolved organic carbon (DOC) was determined using a TOCmeter Shimazu® composed of ASI-V and TOC-Vcsh.
Total mercury content in all samples (sediment and fish)
Total Hg levels (sediment and fish) were analysed by AMA 254®) (Automatic Mercury Analyzer). Based on the method of thermal decomposition atomic absorption spectrometry (AAS) with gold amalgamation, it is a method for the rapid determination of total Hg (Costley et al. 2000). This device is specifically designed for the direct quantification of low Hg concentrations in solid or liquid samples. A sample (without pretreatment) of known weight or volume is placed in the nacelle, which is itself introduced into the catalytic tube. The sample is then dried and decomposed thermally or burned. The decomposition products of the sample are pushed by a flow of oxygen in the second part of the catalytic tube. The decomposition products are then pushed to the amalgam for selective trapping of the Hg, the rest goes into the measuring tanks until the oxygen evacuation of the device. The amalgam and tanks are thermostated at 130 °C to prevent condensation of water. After the decomposition of a sample and the stabilisation of the temperature in the amalgam, the amount of Hg trapped in the amalgam is measured. The same procedure was used for all samples. This technique was used by several authors (Reis et al. 2010; Sahuquillo et al. 2003) to quantify the total Hg in soils as well as in soil solutions. Moreover, our apparatus (AMA 254) is equipped with high-capacity platform and high-sensitivity cell. This cell allows having (1) a theoretical limit of detection at 0.003 ng absolute (i.e. 0.003 ppb for 1 g or ml weighed or injected) and (2) a theoretical limit of quantification of 0.01 ng absolute (i.e. 0.01 ppb for 1 g or ml weighed or injected). Finally, triplicated samples passed the method criteria of < 20% RSD (relative standard deviation) for liquids samples and < 5% RSD for solids samples. All samples passed routine quality assurance metrics included in the standardised analytical method. For example, both standard reference materials such as river clay sediment-Metals (LGC6139), Estuarine sediment (BCR 277R) were recovered within certified limits 1.2 ± 0.05 mg kg−1 and 0.128 ± 0.017 mg kg−1 of Hg, respectively.
Methylmercury extraction from sediment samples
The method used for the extraction of MeHg from sediments was adapted from (Maggi et al. 2009). Briefly, approximately 0.5 g of dry sediment was finely ground and then placed in a 50-ml vial tube and hydrolysed with 2.5 ml of HCl (6 M suprapure®). The sample was homogenised for 5 min and then centrifuged at 2400 rpm for 10 min. The supernatant was removed, and 5 ml of toluene was added to the sediment residues. The entire sample was vigorously stirred for 20 min. After centrifugation (2400 rpm, 20 min), the supernatant containing organo-Hg species was collected in Falcon® tubes. The combined organic extracts were extracted twice with 0.3 ml of a 1% aqueous solution of cysteine to separate the toluene from the MeHg, which ended up in the pellet with L-cysteine. Then, an aliquot of the L-cysteine extract was immediately analysed with the Hg analyser (AMA 254).
Microbiological analyses
Determination of the cultivable microflora was performed according to the NPP technique (Jarvis et al. 2010). One gram of fresh sediment and 9 ml of sterile physiological water were stirred for 1 h with a Stuart® stirrer. The mixture was then centrifuged for 1 min at 500 rpm. One hundred microliters of the obtained supernatant was sampled, and then a series of daughter solutions was obtained by several dilutions (from one tenth to one millionth) using sterile physiological water. Each dilution (20 μl) was used to fill the plates, with four replicates of each. Then, 180 μl of a non-selective culture medium (Nutrient Broth) was added to each dilution. The microplates were then incubated at 28 °C for 48 h. The reading was performed at 620 nm with Multiskan FC® to quantify the total cultivable microflora.
For the determination of the catabolic activity of microorganisms, the protocol was adapted from Garland (1996, 1997). One gram of fresh sediment and 9 ml of sterile physiological water were stirred for 1 h with the Stuart® stirrer. The mixture was then centrifuged for 1 min at 500 rpm. The obtained supernatant was removed and diluted in physiological water according to the enumeration of the total cultivable microflora to obtain the same number of microorganisms. The wells of the Eco-Plate type microplate (Biolog®) were then inoculated with 150 μl of this diluted daughter solution. These microplates have 96 wells ready for use, with 31 carbon substrates of 6 different classes (amines, carbohydrates, complex carbon sources, carboxylic acids, amino acids and carbon phosphates). After incubation for 72 h at 28 °C in the dark, the catabolic activity of the microorganisms was determined by measuring the optical density (OD) using a Multiskan FC® plate reader at 620 nm. The obtained measurements made it possible to calculate the average metabolic activity of the cultivable sediment bacteria known as the average well colour development (AWCD), which is given by the following relation:
Finally, the total enzymatic activity of microorganisms (fluorescein diacetate (FDA)) in sediments was determined in this study following the method developed by Green et al. (2006). This method is a spectrophotometric method in which cleavage by hydrolysis of FDA in fluorescein by several sediment enzymes allows the solutions to appear as fluorescent yellow. For analysis, 10 ml of a phosphate buffer solution and 100 μl of FDA were mixed with 1 g of fresh sediment in sterile 50-ml tubes. The mixture was stirred for 1 h with a Stuart® stirrer and then centrifuged for 1 min at 300 rpm. The optical density of the supernatant was measured at 490 nm using a GENESYS 5 spectrophotometer to determine the total enzymatic activity of the microorganisms using a standard range.
Parameter calculation
The bioaccumulation factors of Hg (BAF) in fish were calculated for each of the sites studied by the following formula:
Data treatment
The results were statistically processed with XLSTAT software version 2018. For chemical and biological characteristics, each analysis was performed in triplicate. Significant differences between each sample were determined by analysing the variance (one-factor ANOVA) and by the Tukey HSD test (significance threshold of P < 0.05, with n = 3). Principal component analysis (PCA) was performed on all the factors studied. Thus, the Pearson correlation coefficient was calculated between the biogeochemical characteristics of the medium and the Hg content of the different compartments of the studied lagoon for each sampling point.
Results
Physicochemical parameters of water in Ebrié Lagoon
The lagoon water temperature values ranged from 25.50 ± 1.80 to 31.17 ± 0.29 °C, with an average of 28.80 ± 1.38 °C in the dry season and an average of 26.54 ± 0.63 °C in the rainy season. The lowest temperature was measured in the waters of the Yopougon site during the rainy season and the highest temperature at the Cosrou site during the dry season. However, for all sites, the dry season temperature values were slightly higher, with an average difference of 2.27 ± 1.08 °C compared to those of the rainy season. This difference was not significant, regardless of the site sampling according to ANOVA and the Tukey test (p = 0.05) (Fig. 2a).
Seasonal variation of the physicochemical parameters (a temperature, b pH, c salinity, d redox potential, e dissolved oxygen content, f dissolved organic carbon content, g nitrate content, h sulphate content) of the Ebrié Lagoon. Each bar corresponds to the average, and the standard deviation is determined from 3 replicates. The letters a, b, c and d correspond to the different groups of significance obtained by analysis of variance and the Tukey test (p = 0.05) on 3 replicates
The pH values ranged from 6.75 ± 0.30 to 7.89 ± 0.09, with an average of 7.62 ± 0.25 in the dry season and 7.44 ± 0.34 in the rainy season. The lowest pH was measured in the waters of the Cosrou site during the rainy season, and the highest pH at the Yopougon site during the dry season. However, for all sites except Marcory and Cocody, the pH values in the dry season were slightly higher, with an average difference of 0.28 ± 0.15, than those in the rainy season. This difference was not significant, regardless of the site sampling according to ANOVA and the Tukey test (p = 0.05) (Fig. 2b).
The salinity values ranged from 0.05 ± 0.00 to 35.23 ± 2.15 ppm, with an average of 27.16 ± 11.12 ppm in the dry season and an average of 10.86 ± 7.31 ppm in the rainy season. The lowest salinity was measured in the waters of the Cosrou site during the rainy season, while the highest salinity was observed at the Port site during the dry season. However, the salinity values of all sites in the dry season were slightly higher, with an average difference of 16.30 ± 7.21 ppm, than those of the rainy season. This difference was not significant regardless of the site according to ANOVA and the Tukey test (p = 0.05) (Fig. 2c).
The Eh values of the lagoon waters oscillated between − 46.67 ± 6.45 and 19.63 ± 15.72 mV, with an average of − 35.12 ± 9.49 mV in the dry season and an average of − 23.07 ± 19.99 mV in the rainy season. The lowest Eh value was measured in the waters of the Yopougon site during the dry season and that of the highest Eh was measured at the Cosrou site during the rainy season. However, the lagoon water potential of all sites was lower in the dry season, with an average difference of − 12.05 ± 16.88 mV, than that in the rainy season. This difference was not significant regardless of the site, with the exception of the Cosrou site (control site), according to ANOVA and the Tukey test (p = 0.05) (Fig. 2d).
The dissolved oxygen levels (DO) ranged from 3.95 ± 0.18 to 8.78 ± 2.66 mg l−1, with an average of 6.86 ± 1.18 mg l−1 in the dry season and an average of 5.07 ± 0.67 mg l−1 in the rainy season. The lowest OD value was measured in the waters of the Biétri site during the rainy season, and the highest OD at the site of Koumassi in the dry season. At all sites, the DO levels were higher in the dry season, with an average difference of 1.79 ± 1.11 mg l−1, than those in the rainy season, but the difference was not statistically significant according to ANOVA and the Tukey test (p = 0.05) (Fig. 2e).
The dissolved organic carbon (DOC) contents of the lagoon waters ranged from 3.43 ± 0.33 to 12.39 ± 5.30 mg l−1, with an average of 4.64 ± 1.94 mg l−1 in the dry season and 7.26 ± 2.37 mg l−1 in the rainy season. The lowest DOC value was measured in the waters of the Koumassi site during the dry season while the highest value was observed at the Port site during the rainy season. The DOC contents in the water column were slightly lower in the dry season, with an average difference of − 3.27 ± 1.73 mg l−1, than those in the rainy season, except at the Cocody site. However, the differences between the seasons were not significant regardless of the site, except for the Port site, where the difference between seasons was significant according to ANOVA and the Tukey test (p = 0.05) (Fig. 2f).
The nitrate contents were between 0.49 ± 0.43 and 122.24 ± 15.57 mg l−1, with an average of 1.11 ± 0.59 mg l−1 in the dry season; however, the value of nitrate at the Koumassi site was higher than that of the other sites. The average of all sampling sites was 2.16 ± 0.55 mg l−1 during the rainy season. The lowest nitrate content was measured in the waters of the Yopougon site during the dry season and the highest nitrate content at the Koumassi site during the rainy season. At these different sites, the nitrate contents were lower during the dry season than those of the rainy season by an average of − 1.01 ± 0.76 mg l−1, but these differences were not statistically significant, regardless of site, according to ANOVA and the Tukey test (p = 0.05), with the exception of the Koumassi site (Fig. 2g).
The sulphate contents ranged from 6.39 ± 7.16 to 599.23 ± 6.25 mg l−1, excluding Cosrou, which had contents below the limit of detection. All other sites had an average of 549.78 ± 41.42 mg l−1 in the dry season and an average of 360.30 ± 167.39 mg l−1 in the rainy season. The sulphate content was almost zero in all seasons at the Cosrou site; however, at the sites in the Abidjan zone, the lowest sulphate content was measured in the Koumassi site during the rainy season and the highest sulphate level at the Marcory site in the dry season. At these different sampling sites, the sulphate contents were higher during the dry season that those of the rainy season by an average 189 ± 149 mg l−1. However, this difference was not statistically significant, regardless of the site and the season, according to ANOVA and the Tukey test (p = 0.05), with the exception of the Koumassi site (Fig. 2h).
Microbiological activities in Ebrié Lagoon sediments
The total microflora enumeration in sediments ranged from 1.8 × 103 ± 1.1 × 103 to 2.9 × 106 ± 1.4 × 105 cultivable cells per gram of dry sediment. The lowest number of cultivable cells per gram of sediment was obtained in the Cosrou sediments during the rainy season, while the largest number of cultivable cells was obtained in the Koumassi sediments during the dry season. At these different sampling sites, the number of cultivable cells was higher during the dry season than that in the rainy season, except in Cocody and Marcory. However, this difference was not statistically significant regardless of the season according to ANOVA and the Tukey test (p = 0.05), except at the Koumassi site (Fig. 3a).
Seasonal variation of the biological parameters (a number of cultivable cells/g of dry sediment, b total enzymatic activity, c catabolic diversity AWCD) of the Ebrié Lagoon. Each bar corresponds to the average, and the standard deviation is determined from 3 replicates. The letters a, b, c, d, e, f, g, h, i and j correspond to the different groups of significance obtained by analysis of variance and the Tukey test (p = 0.05) on 3 replicates
The total enzymatic activities of microorganisms in sediments ranged from 0.01 ± 0.00 to 0.54 ± 0.00 mg FDA g−1 dry sediment h−1. The enzymatic activity was lowest in the sediments of the Port site during the dry season and highest in the sediments of the Cosrou site in the dry season. At these sites, the enzymatic activity was lower during the dry season, with an average difference of − 0.15 mg FDA g−1 dry sediment h−1 in the rainy season, except for the Cosrou and Banco sites. This difference in the sites between seasons was significant according to ANOVA and the Tukey test (p = 0.05), except for the Koumassi site (Fig. 3b).
The catabolic activity of microorganisms in sediments ranged from 0.03 ± 0.03 to 2.26 ± 0.03. The catabolic activity was lowest in the Banco sediments during the rainy season and highest in the Koumassi sediments during the rainy season. At these different sites, the catabolic activity was higher in the dry season that that in the rainy season, with an average difference of 0.78 ± 0.25 at the Cosrou, Yopougon, Banco and Cocody sites. In contrast, at the Koumassi, Marcory, Port and Biétri sites, the catabolic activity was lower in the dry season that that in the rainy season, with an average difference of 0.51 ± 0.17. The difference at the sites between seasons was significant according to ANOVA and the Tukey test (p = 0.05) (Fig. 3c).
Total Hg content in the different compartments (sediment, fish) of the Ebrié Lagoon
The total sediment Hg content (THgsediment) ranged from 13.79 ± 0.15 to 1165.06 ± 6.44 μg kg−1, with a substantially similar average in the dry and rainy seasons. The Cocody site had the highest content of THgsediment regardless of the season (Fig. 4a). The lowest contents were measured at the Port and Cosrou sites during the dry season and the rainy season, respectively. In general, except for the Cosrou and Marcory sites, the THgsediment contents were higher in the rainy season that those in the dry season, with an average difference of 121.58 ± 155.71 μg kg−1. According to ANOVA and the Tukey test (p = 0.05), these differences between the seasons and the sites were significant, except for the sites near the Vridi Canal (Biétri, Port and Yopougon), where there was no difference between seasons (Fig. 4a).
a Total Hg (HgTS) and b MeHg (MeHgS) contents (μg kg−1) and c methylation rates (%) in sediment for the different sampling sites. Each bar corresponds to the average, and the standard deviation is determined from 3 replicates. The letters a, b, c, d, e, f and g correspond to the different groups of significance obtained by analysis of variance and the Tukey test (p = 0.05) on 3 replicates
The MeHg contents of the lagoon sediments (MeHgsediment) ranged from 2.18 ± 0.13 to 4.41 ± 0.48 μg kg−1, with a similar average of 2.66 μg kg−1 in the dry and rainy seasons. Similar to the total Hg content in the sediments, the Cocody site had the highest levels of MeHgsediment regardless of the season (Fig. 4b). The lowest MeHgsediment contents were measured at the Port and Yopougon sites during the dry and rainy seasons, respectively. At these different sites, the MeHgsediment contents were higher in the rainy season that those in the dry season, with an average difference of 0.39 ± 0.28 μg kg−1 at the sites of Koumassi, Port, Cosrou, Banco and Cocody and Biétri. In contrast, the Cosrou, Yopougon and Marcory sites had MeHgsediment contents that were lower in the rainy season that those in the dry season, with an average difference of − 0.64 ± 0.44 μg kg−1. According to ANOVA and the Tukey test (p = 0.05), the differences between seasons and sites were not significant, except for the Cosrou and Cocody sites (Fig. 4b). The MeHgsediment compared to the THgsediment indicated the methylation rates ranged from 0.5 ± 0.01 (Cocody) to 15.79 ± 0.98% (Port) in the dry season and from 0.38 ± 0.04 (Cocody) to 7.66 ± 0.58% (Cosrou) during the rainy season. The seasonal variation in methylation was marked at Cosrou and Port, with a higher methylation percentage in the dry season than in the rainy season for the Port site and a higher methylation percentage in the rainy season than in the dry season for the Cosrou site (Fig. 4c).
The total Hg content in fish (THgfish) varied from 106.33 ± 2.86 to 1110.30 ± 0.76 μg kg−1, with similar averages in the dry and rainy seasons. During the dry season, the lowest THgfish content was measured at the Biétri site, and the highest THgfish content was measured at the Yopougon site. In the rainy season, the lowest THgfish content was observed at the Biétri site, while the highest content was observed at the Cocody site. The THgfish contents were higher in the rainy season than those in the dry season, with an average difference of 176.84 ± 70.48 μg kg−1, except for those at the Yopougon and Marcory sites, according to ANOVA and the Tukey test (p = 0.05) (Fig. 5).
Total Hg content in fish (Tilapia sp.) (μg kg−1) for the different sampling sites. Each bar corresponds to the average, and the standard deviation is determined from 3 replicates. The letters a–k correspond to the different groups of significance obtained by analysis of variance and the Tukey test (p = 0.05) on 3 replicates. n.d. no data
BAFsin fishes
Figure 6 displays the bioaccumulation factors (BAFs) between the fish and sediment of the Ebrié Lagoon. The BAF value obtained in relation to the sediments was between 0.31 ± 0.02 and 25.93 ± 0.46. The factor was higher at the Port site in the dry season, while it was lower at the Biétri site in the dry season. The BAF was less than 1 for Cosrou and Marcory in the dry season, less than 1 for Biétri and Cocody in all seasons, and less than 1 for Yopougon in the rainy season. The BAF was greater than 1 for Cosrou, Marcory and Koumassi in the rainy season, for Port and Banco in all seasons and for Yopougon in the dry season. The BAF was approximately equal to 1 for Marcory and Koumassi in the dry season. Regardless of the sampling site, the BAF was higher in the rainy season than that in the dry season, except at the Port, Yopougon and Cocody sites (Fig. 6).
Discussion
Seasonal effect on physicochemical parameters
The circulation of water in the Ebrié Lagoon is strongly linked to the tide and the fluvial currents (Wango et al. 2008). Thus, during the dry season, continental inputs (e.g. runoff or precipitation) are negligible, evaporation is maximised and marine influence prevails. All of these conditions allow the temperature and salinity to reach their highest levels. In the rainy season, rainfall and river inflow were more important, and the temperature reached its minimum value. Moreover, the rainy season corresponds to the beginning of upwelling in the coastal region. Several studies (Wognin et al. 2017; Tosic et al. 2019) have shown the influence of seasonal variability on water quality parameters. According to our results, there is significant seasonal variation in all of the physicochemical parameters that were studied, except for the nitrate and dissolved oxygen contents.
In our study, a slight decrease in temperature was observed from the dry season to the rainy season. Similar values were observed in various lagoons in West Africa. The obtained temperatures were in the same range as those observed in the same lagoon by previous studies (Kouassi et al. 2005; Inza et al. 2009). The same temperatures were observed in the Kpeshie lagoon in Ghana, where the temperatures ranged from 28.4 to 29.6 °C (Addo et al. 2011), as well as those observed in the Konkouré River Estuary in Guinea—specifically in the bay—in Sangaréah, where the values were from 30.8 to 31 °C (Onivogui et al. 2013). According to Monde et al. (2007), the change in lagoon water temperature is related to rainfall and river fluxes; specifically, in Comoé, the temperature increases during the period of low rainfall and water levels in the dry season and decreases during the period of abundant rainfall and river flow during the rainy season. These observations are consistent with our results.
The same behaviour was observed for pH in the dry season. There is more seawater supply in the zone of Abidjan, which is under marine influence (Durand and Guiral 1994), inducing the slightly basic pH observed at all the sampling sites. In detail, the highest values were observed at the Yopougon and Port sites, which are close to the marine water gate, and the lowest value was observed in Cosrou, which is far from the Vridi Canal and thus far from marine influences. In the rainy season, the pH of the continental waters decreases slightly, becoming more acidic. These results are in agreement with the literature data on the Ebrié Lagoon (Soro et al. 2009; Kouamé et al. 2016; Aka et al. 2017).
The salinities in the Ebrié Lagoon are generally related to its proximity to the Atlantic Ocean. The marine water differs from the continental waters by a very high salinity, i.e. up to 35 ppm (based on the ocean), during the rainy season. The continental waters, in particular those of the Comoé River (salinities close to 0 ppm), will reduce the salinity of the lagoon by the dilution effect or by pushing the marine waters to their front door. In the dry season, the salinities measured at the sampling sites near the Vridi Canal were the highest, while the Cosrou sampling site, which is located far from the ocean, had a very low salinity. In the rainy season, the lagoon was under the influence of continental waters. These observations are in agreement with the results obtained by Aka et al. 2016, 2017.
The redox potential is a good tracer of the pollution gradient (Borch et al. 2010; Tokarz and Urban 2015). As the distance from the pollution source increases, more acceptors of electrons in the medium become oxidising. As the distance from the pollution source decreases, the number of electrons increase and the environment becomes reductive. These observations could explain the redox potential data from our sampling sites. All the sites in the lagoon zone in Abidjan were reducing, and Cosrou, which is in a rural area, was the least reducing in the dry season and became oxidative during the rainy season by the dilution effect. In the Ebrié Lagoon, the redox potential is related to the pollution gradient rather than to the season.
Among the parameters likely to influence the concentration of DO is the light that allows aquatic plants and algae to photosynthesise and thus increase the content of DO in the water column (Hunt and Christiansen 2000). During the rainy season, runoff brings suspended matter to the lagoon, leading to poor light scattering in the water column, decreasing photosynthesis and consequently lowering the production of DO in the water column. This pattern explains the slight difference between the seasons observed in the Ebrié Lagoon. According to the results obtained by Aka et al. (2017), the waters of the lagoon are more loaded with suspended matter during periods of precipitation.
The sulphate contents were significantly similar among sampling sites in the Abidjan zone. These high sulphate levels can be attributed to anthropogenic activities or the intrusion of marine waters. Indeed, sulphate is the major component of dissolved salts in oceans. On the other hand, the Cosrou site, which is located in a rural area of the lagoon, has almost no grades regardless of the season. In the dry season, sulphates are introduced into the lagoon by marine waters. In the rainy season, sulphates are also introduced into the lagoon by the addition of continental water, which slightly dilutes the sulphate concentrations during this season. This slight dilution could explain the zero level observed at Cosrou.
The form of nitrogen in the surface water depends on several factors, including the pH, dissolved oxygen concentration and biological communities present. They may subsequently be driven by surface runoff or leaching through the soil (Hou et al. 2013). These processes could explain the low nitrate concentrations measured at all sampling sites and in all seasons. Nitrates can also come from wastewater, which often releases high concentrations of ammonia and nitrite into the water; these components are oxidised to nitrate through microbial activity. This process could explain the abundant nitrate at the Koumassi site caused by the intensive microbial activities fed by wastewater from the industrial zone and domestic waste from the area around the lagoon site.
In the water column, the DOC contents are slightly higher in the rainy season than in the dry season. Because natural sources of DOC are low, the anthropogenic sources of DOC observed in aquatic systems mainly come from runoff and urban discharge (domestic and industrial), which explains this slight seasonal variation in DOC. This seasonal variation in organic matter has also been observed in other studies (Aka et al. 2016; Bisinoti et al. 2007; Macalady et al. 2000). Runoff from the rainy season is, therefore, an entry point for organic matter into the Ebrié Lagoon.
Very little information is available, or even nonexistent, on all indicators of microbiological activity in sediments. The results show that the sediment microflora is higher in the dry season than in the rainy season. This would be due to a dilution effect in the rainy season caused by resuspension of microorganisms contained in the sediments by the currents induced by the rainy season. This would increase the microflora of the water column in this season (Kouassi et al. 2005). The enzymatic activity is favoured during the season when the microflora is the least (rainy season). This suggests that other parameters have occurred. It should be noted that some environmental factors may influence the microbial community (Lauber et al. 2008, 2009). As for the catabolic activity, it is influenced by the intensity of pollution of the sites. Indeed, effluent discharges contribute to the microbial contamination of the Ebrié lagoon (Kouassi et al. 2005).
All of these physicochemical parameters can therefore influence the microbial activity and the distribution of Hg in the different compartments of the Ebrié Lagoon.
Distribution of Hg in fish and sediment of the lagoon
The total Hg contents in all sediment sampling sites were well above the continental crustal mercury (UCC) value of 56 μg kg−1 (Wedepohl 1995), except at the Cosrou site during the rainy season and the Port site during the dry season. In addition, the Yopougon, Banco, Biétri and Cocody sites had total Hg contents in their sediments that were greater than the total Hg contents in unpolluted sediments (i.e. 50 to 300 μg kg−1) (Calamari and Naeve 1994). Indeed, the sampling sites of Banco and Yopougon belong to the municipality of Yopougon, the largest municipality of Côte d’Ivoire, and in addition, Yopougon is home to the largest industrial area of the city of Abidjan. According to a previous study (Koffi 2009), the banks of the lagoon at Yopougon were heavily polluted by agricultural activities, as are the areas upstream from this industrial zone. The Biétri site is also close to the town of Port-Bouet (large residential town) and close to an industrial zone with refinery industries. The Cocody site is located in Cocody Bay, which is the mouth of the wastewater outlet of the northern and eastern parts of the city of Abidjan. Inza and Yao (2015) reported that sewage was a significant source of trace metals in this part of the lagoon (Cocody Bay). On the other hand, the sediments of the Port, despite the establishment of large industry and all the port activities, remained even lower than the Hg content of the continental crust. This result is probably due to its intense hydrodynamism and its great depth (Yao et al. 2017). The Cosrou site, located in a rural area of the Ebrié Lagoon far from the agglomeration of Abidjan, has unpolluted sediments compared to the sediments of other sites that are located in the central basin of the lagoon. Indeed, the central part of the Ebrié Lagoon is an industrial, municipal and agricultural waste disposal site (Brenon et al. 2009; Koffi 2009). However, according to some research (Oliveri et al. 2016), anthropogenic activities are responsible for the Hg enrichment of the sediment. In general, the lagoon sediments do not meet the quality criteria based on the ecotoxicological data used to estimate a risk of toxicity on benthic organisms in relation to the toxic effect concentration (TEC) and the probable effect concentration (PEC). The TEC corresponds to the threshold below which toxicity to benthic invertebrates is unlikely, and the PEC corresponds to the threshold above which toxicity to benthic invertebrates is likely. Their values for Hg are 180 μg kg−1 and 1100 μg kg−1 (MacDonald et al. 2000), respectively. Unlike other sites, the sediments of the Port site during the two seasons, as well as those of Cosrou during the rainy season, do not pose a risk to benthic organisms. In conclusion, the sediments of Cocody during the rainy season have a high probability of toxicity, and the other sampling sites have relatively lower toxic effects on the benthic organisms.
The total Hg contents in the sediments of other hydrological systems of the world, as well as those obtained by some authors in the sediments of the Ebrié Lagoon, are presented in Table 1. The total Hg contents measured in this study are of the same order of magnitude as those obtained by Coulibaly et al. 2009 (12.06 to 1460 μg kg−1) in the estuarine bays of Abidjan. Nevertheless, they are much higher than those measured by Coulibaly et al. (2012) in the same lagoon (Bay of Biétri), with values ranging from 0.68 to 0.88 μg kg−1. The values obtained from the sediments in the Ebrié lagoon in our study have higher Hg concentrations than the sediments of the Konkouré River Estuary in the Republic of Guinea (Onivogui et al. 2013) and the Wadi Hanifah in Saudi Arabia (Abdel-Baki et al. 2013). They are of the same order of magnitude as the Marginal sea of Pacific Ocean in the China (Kim et al. 2019), the Mekong River Delta in Vietnam (Choi et al. 2019), and Narragansett Bay in the northeastern USA (Taylor et al. 2012). They are less polluted than the Benya Lagoon in Ghana (Vowotor et al. 2014), Seine bay and Caux in France (Cossa et al. 2002), the Bandar Imam Khomeini port in Iran (Peery et al. 2018) and Augusta Bay in Sicily (Oliveri et al. 2016).
For MeHg analyses in the Ebrié Lagoon sediments, the data indicate the presence of MeHg at concentrations ranging from 2.18 to 4.41 μg kg−1. This methylation varies from 0.38 to 15.79% and is 2 times higher than the methylation of Hg in the sediments of Brazilian mangrove ecosystems (de Oliveira et al. 2015). This difference in the methylation rate is probably due to the presence of the mangrove, which serves as a filter for these ecosystems, because most of the Ebrié Lagoon study sites are located in the urban zone, i.e. without aquatic vegetation. Indeed, aquatic plants can contain 10 to 30% MeHg (Bridou et al. 2011), which forms in their roots (Gentès 2012) and comes directly from sediments (Mason et al. 1999). Several studies have shown that this reaction is driven by the geochemical processes of the environment in which the reaction takes place (Celo et al. 2006; Paranjape and Hall 2017).
Fish is one of the most traded foods in the world, and its consumption is more than 19 kg per capita (FAO 2004). Thus, standards have been established around the world to prevent Hg contamination resulting from human consumption. The total Hg concentrations obtained from fish flesh (Tilapia sp.) of the Ebrié Lagoon in this study ranged from 106.3 to 1110.3 μg kg−1, depending on the sampling site and season. Currently, the lack of standard in Côte d’Ivoire does not limit fish consumption and therefore Hg poisoning. However, these Hg contents were compared to the reference values established by the European Economic Community (EEC) and the Canadian Food Inspection Agency (CFIA) (500 μg kg−1) for all fish (Serreta 2000), and the results showed that the Hg content in fish exceeded these standards at the Banco and Cocody sites in all seasons, the Yopougon site in the dry season, and the Port site in the rainy season. Our values are in the same range as that obtained by Coulibaly et al. (2012), in Biétri Bay (Table 2). The comparison of the Hg content of fish from the Ebrié Lagoon with other fish in other rivers in the world shows that the fish from the Enrié lagoon are more concentrated in Hg than in Wadi Hanifa in Saudi Arabia (Abdel-Baki et al. 2013) and in Florida Bay in USA (Kannan et al. 1998). The fish in the Ebrié Lagoon are on average identical to those of Mekong River Delta in Vietnam (Choi et al. 2019) while they are less polluted than the fish of Coastal Lagoon in Mexico (Aguilar-Betancourt et al. 2016)(Table 2).
In general, the accumulation of Hg in the trophic chain begins with the assimilation of phytoplankton, and according to Tessier (2004), phytoplankton accumulates Hg from the dissolved phase up to more than 104 times the concentration of the medium. In addition, some studies (Semyalo et al. 2011) have shown that Tilapia filter feeds and that its diet consists mainly of detritus, zooplankton and phytoplankton. Tables 3 and 4 show that Hg content in fish was positively correlated with the sediment Hg (r = 0.415) during the rainy season. In other words, apart from phytoplankton, it seemed that the Hg in fish came mainly from the sediment. A good positive correlation was observed between sediment Hg and sediment MeHg (r = 0.895 in the dry season and r = 0.650 in the rainy season) and between fish Hg and sediment MeHg (r = 0.560) in the Ebrié Lagoon. Indeed, several studies (Baralkiewicz et al. 2006; Bridou et al. 2011; Watras 1992) have shown that the main form of Hg in fish is monomethylmercury. However, MeHg, which is bioaccumulative in benthic and pelagic populations, can be provided by sediments and aquatic plants (Guimarães et al. 2000; Mason et al. 1999; Mauro et al. 1999), which is consistent with our results.
Biogeochemical parameters controlling Hg distribution
In our study, the Pearson correlation tables were made between the Hg contents of sediment and fish and the bio-physicochemical parameters measured according to the sampling season (Tables 3 and 4) to determine which factors control the presence of Hg in the Ebrié Lagoon. Several studies (Costa and Liss 1999; Fitzgerald and Lamborg 2013) have highlighted the important role of organic matter and microorganisms on the behaviour of mercury in an aquatic environment.
Total Hg content in sediment was not influenced by any parameters studied. However, sediment MeHg was positively correlated with the redox potential (r = 0.507), temperature (r = 0.501), DOC (r = 0.628) and the total enzymatic activity (r = 0.484) and negatively correlated with the pH (r = − 0.627), salinity (r = − 0.542) and sulphate content (r = − 0.476). According to several studies (Graham et al. 2017; Taylor et al. 2012), DOC forms strong organometallic complexes with Hg and promotes Hg methylation by bacteria by acting as a substrate for mineralisation. In addition, the proportion of MeHg increases with the concentration of organic matter (Roulet 2016). DOC is one of the key parameters of Hg distribution in the Ebrié Lagoon. In the water column, the DOC contents are slightly higher in the rainy season than in the dry season. Because natural sources of DOC are low, the anthropogenic sources of DOC observed in aquatic systems mainly come from runoff and urban discharge (domestic and industrial), which explains this slight seasonal variation in DOC. This seasonal variation in organic matter has also been observed in other studies (Aka et al. 2016; Bisinoti et al. 2007; Macalady et al. 2000). Runoff from the rainy season is, therefore, an entry point for organic matter into the Ebrié Lagoon. Moreover, methylation increased with increasing temperature and redox potential, which is consistent with the literature data (Mauro et al. 1999). All the sites of the Ebrié Lagoon had a reducing potential favourable to the reduction of sulphate into sulphide, favouring the methylation of Hg, which explains the positive correlation between MeHg and Eh and negative correlations between MeHg and sulphates and between sulphate content and Eh (r = − 0.640). On the other hand, when the salinity, pH and sulphates increased, the methylation decreased. These results are consistent with the work of Noh et al. (2013), which state that elevation of salinity and sulphates decreases the rate of Hg methylation in sediments. Our results are also consistent with the literature data (Riba et al. 2004), which shows that metal mobility increases when pH and salinity are low. Nevertheless, our results contradict other studies (de Oliveira et al. 2015; Graham et al. 2017), which have highlighted that the formation of MeHg in the superficial sediment fraction tends to increase with salinity and dissolved organic carbon.
In rainy seasons, no correlation is observed probably due to a resuspension of Hg sediments (Brown et al. 2015) by the current induced by the flow of inland water during this period.
The bioaccumulation of Hg in an aquatic environment is a function of the characteristics of this environment (Marusczak 2010). However, our results show that in the Ebrié Lagoon the bioaccumulation of Hg is not directly influenced by any of the parameters studied outside AWCD (r = − 0.55) (Table 4). Microorganisms play an important role in the cycling of elements such as carbon, carbon, nitrogen and mercury. Very little or no information is available on the microbial biomass and diversity of the lagoon sediments, which could possibly contribute to the biogeochemical processes related to the mercury cycle. Our results show that the microbial biomass is more developed in the dry season than in the rainy season due to a dilution effect during the rainy season. Positive correlations were found during the dry season, including those between the number of cultivable microorganisms, AWCD (r = 0.777) corresponding to microorganism catabolic diversity, nitrate content (r = 0.957) and dissolved oxygen (r = 0.439), and negative correlations were found with the concentration of sulphate (r = − 0.433) in the rainy season, confirming the previous seasonal variation. The increase in dissolved oxygen and nitrates in the Ebrié Lagoon may favour the development of microbial biomass in sediments (Bryant et al. 2012; Silvennoinen et al. 2008). Indeed, the Koumassi site had a larger microbial biomass than the other sampling sites, as well as higher contents of dissolved oxygen and nitrate during the dry season.
Microorganisms are also responsible for inducing Hg methylation and MeHg demethylation. The total enzymatic activity measured in our study is a good way of linking the activity of microorganisms to the speciation of Hg in the lagoon (Graham et al. 2017). There was significant spatial and seasonal variation in our results. The enzymatic activity was negatively correlated with sulphates (r = − 0.819), dissolved oxygen (r = − 0.858), and salinity (r = − 0.711). The total enzymatic activity was positively correlated with the redox potential (r = 0.663), the temperature (r = 0.717) and the MeHg content. Our results confirm that bacterial activity plays a role in the formation of MeHg, which is even more marked when the temperature increases (Bridou et al. 2011; Macalady et al. 2000). Indeed, the correlations observed in our study are still much more accentuated in the dry season than in the rainy season (temperature effect and dilution).
Conclusion
The study of the seasonal dynamics of Hg in the Ebrié Lagoon is interesting in that it allows us to draw several conclusions. The seasons influence various biogeochemical factors, although for some factors, this variation is not significant. This influence is more pronounced in the dry season than in the rainy season. The impact of microbial activities and organic matter on Hg dynamics is observed in all seasons. The microorganisms, by their enzymatic activity, induced the speciation of Hg through the formation of MeHg in sediments. This methylation of Hg increases as the temperature and redox potential increase. In addition, when the salinity, pH and sulphates increase, the methylation of Hg in the sediments decreases. For dissolved organic carbon (DOC), it is one of the key parameters of the distribution of Hg in the Ebrié Lagoon because it is assumed that it plays the role of trapping Hg and is a source of nutrients for microorganisms. The results also showed that when dissolved oxygen and nitrates increase in the Ebrié Lagoon, the microbial biomass of the sediments increases. Hg in fish comes either directly from sediment or indirectly through the assimilation of phytoplankton. It should also be noted that pH, temperature, salinity, Eh and sulphates, rather influenced the dynamics of Hg only in the dry season.
References
Abdel-Baki A, Dkhil M, Al-Quraishy S (2013) Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia. Afr J Biotechnol 10:2541–2547. https://doi.org/10.5897/AJB10.1772
Addo MA, Okley GM, Affum HA, Acquah S, Gbadago JK (2011) Water quality and level of some heavy metals in water and sediments of Kpeshie Lagoon, La-Accra, Ghana. Res J Environ Earth Sci 3:487–497
Aguilar-Betancourt CM, González-Sansón G, Kidd KA, Munkittrick KR, Curry RA, Kosonoy-Aceves D, Lucano-Ramírez G, Ruiz-Ramírez S, Flores-Ortega JR (2016) Fishes as indicators of untreated sewage contamination in a Mexican coastal lagoon. Mar Pollut Bull 113:100–109. https://doi.org/10.1016/j.marpolbul.2016.08.073
Aka AM, Wognin AV, Irie BTJ, Coulibaly AS, Monde S, Aka K (2016) Seasonal fluctuations of the content of metals (Ni, Cu, Zn and Cd) from the sediments of the estuarine bays of the Ebrié lagoon in Côte d’Ivoire. J Chem Biol Phys Sci 6:970–981
Aka AM, Wognin AV, Amani EM, Irie BTG, Coulibaly AS, Monde S (2017) Analyse des Parametres Physico-Chimiques et Bacteriologiques des Eaux de L’estuaire de la Lagune Ebrie (Sud-Est de la Cote D’ivoire). Eur J Sci Res 147:301–314
Baralkiewicz D, Gramowska H, Gołdyn R (2006) Distribution of total mercury and methyl mercury in water, sediment and fish from Swarze¸dzkie lake. Chem Ecol 22:59–64
Bisinoti MC, Sargentini E, Jardim WF (2007) Seasonal behavior of mercury species in waters and sediments from the Negro River Basin, Amazon, Brazil. J Braz Chem Soc 18:544–553
Borch T, Kretzschmar R, Skappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23. https://doi.org/10.1021/es9026248
Brenon I, Audouin O, Pouvreau N, Maurin J-C (2009) Impact of vertical structure on water mass circulation in a tropical lagoon (Ebrié, Ivory Coast). J Afr Earth Sci 55:47–51. https://doi.org/10.1016/j.jafrearsci.2008.12.005
Bridou R, Monperrus M, Gonzalez PR, Guyoneaud R, Amouroux D (2011) Simultaneous determination of mercury methylation and demethylation capacities of various sulfate-reducing bacteria using species-specific isotopic tracers. Environ Toxicol Chem 30:337–344. https://doi.org/10.1002/etc.395
Brown LE, Chen CY, Voytek MA, Amirbahman A (2015) The effect of sediment mixing on mercury dynamics in two intertidal mudflats at Great Bay Estuary, New Hampshire, USA. Mar Chem 177:731–741. https://doi.org/10.1016/j.marchem.2015.10.011
Bryant LD, Little JC, Bürgmann H (2012) Response of sediment microbial community structure in a freshwater reservoir to manipulations in oxygen availability. FEMS Microbiol Ecol 80:248–263. https://doi.org/10.1111/j.1574-6941.2011.01290.x
Calamari D, Naeve H (1994) Revue de la pollution dans l’environnement aquatique africain. Document Technique du CPCA. Rome
Cardoso PG, Pereira E, Duarte AC, Azeiteiro UM (2014) Temporal characterization of mercury accumulation at different trophic levels and implications for metal biomagnification along a coastal food web. Mar Pollut Bull 87:39–47. https://doi.org/10.1016/j.marpolbul.2014.08.013
Celo V, Lean DRS, Scott SL (2006) Abiotic methylation of mercury in the aquatic environment. Sci Total Environ 368:126–137. https://doi.org/10.1016/j.scitotenv.2005.09.043
Choi H, Jeong E, Nguyen VH, Hanh DVB, Dan NP, Shin KH, Han S (2019) Characteristics of sediment affecting monomethylmercury accumulation in benthic fish of the Mekong Delta. Environ Toxicol Chem 38:503–510. https://doi.org/10.1002/etc.4327
Ci Z, Zhang X, Yin Y, Chen J, Wang S (2016) Mercury redox chemistry in waters of the eastern Asian seas: from polluted coast to clean open ocean. Environ Sci Technol 50:2371–2380. https://doi.org/10.1021/acs.est.5b05372
Cossa D, Laurier F, Ficht A (2002) Mercury contamination in the Seine estuary. In: Biogeochemistry of Environmentally Important Trace Elements. ACS Symposium Series, Washington, D.C, pp 298–320
Costa M, Liss PS (1999) Photoreduction of mercury in sea water and its possible implications for Hg0 air-sea fluxes. Mar Chem 68:87–95. https://doi.org/10.1016/S0304-4203(99)00067-5
Costa MF, Landing WM, Kehrig HA, Barletta M, Holmes CD, Barrocas PRG, Evers DC, Buck DG, Claudia Vasconcellos A, Hacon SS, Moreira JC, Malm O (2012) Mercury in tropical and subtropical coastal environments. Environ Res 119:88–100. https://doi.org/10.1016/j.envres.2012.07.008
Costley CT, Mossop KF, Dean JR, Garden LM, Marshall J, Carroll J (2000) Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal Chim Acta 405:179–183. https://doi.org/10.1016/S0003-2670(99)00742-4
Coulibaly A, Monde S, Wognin V, Aka K (2009) Analyse des éléments traces métalliques (ETM) dans les baies estuariennes d’Abidjan en Côte d’Ivoire. Afrique Sci 5:77–96
Coulibaly S, Atse BC, Koffi KM, Sylla S, Konan KJ, Kouassi NJ (2012) Seasonal accumulations of some heavy metal in water, sediment and tissues of black-chinned tilapia Sarotherodon melanotheron from biétri bay in ebrié lagoon, ivory coast. Bull Environ Contam Toxicol 88:571–576. https://doi.org/10.1007/s00128-012-0522-1
de Oliveira DCM, Correia RRS, Marinho CC, Guimarães JRD (2015) Mercury methylation in sediments of a Brazilian mangrove under different vegetation covers and salinities. Chemosphere 127:214–221. https://doi.org/10.1016/j.chemosphere.2015.02.009
Durand J, Guiral D (1994) Hydroclimat et Hydrochimie In Environnement et Ressources aquatiques de Cote d’Ivoire, Tome II, in: Milieux Lagunaires. ORSTOM, pp 59–90
FAO (2004) SITUATION MONDIALE DES PÊCHES ET DE L’AQUACULTURE 2004, FAO. https://doi.org/10.1017/CBO9781107415324.004
Fiston BK (2017) Etude sur l’utilisation du mercure et du cyanure dans l’exploitation artisanale de l’or au Nord et Sud-Kivu, IPIS. ed
Fitzgerald WF, Lamborg CH (2013) Geochemistry of mercury in the environment. In: Treatise on geochemistry, Second edn, p 129. https://doi.org/10.1016/B978-0-08-095975-7.00904-9
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221. https://doi.org/10.1016/0038-0717(95)00112-3
Garland JL (1997) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol Ecol 24:289–300. https://doi.org/10.1016/S0168-6496(97)00061-5
Gentès S (2012) Les micro-organismes colonisant les racines de plantes aquatiques dans les écosystèmes landais: diversité et risques liés à la méthylation du mercure. Université de Pau et des Pays de l’ADOUR.
Graham EB, Knelman JE, Gabor RS, Schooler S, McKnight DM, Nemergut DR (2017) Trends in dissolved organic matter cycling, sediment microbiomes, and methylmercury production across vegetation heterogeneity in a Great Lakes wetland. bioRxiv in press. https://doi.org/10.1101/072017
Green VS, Stott DE, Diack M (2006) Assay for fluorescein diacetate hydrolytic activity: optimization for soil samples. Soil Biol Biochem 38:693–701. https://doi.org/10.1016/j.soilbio.2005.06.020
Guimarães JRD, Meili M, Hylander LD, Silva EDCE, Roulet M, Mauro JBN, De Lemos RA (2000) Mercury net methylation in five tropical flood plain regions of Brazil: high in the root zone of floating macrophyte mats but low in surface sediments and flooded soils. Sci Total Environ 261:99–107. https://doi.org/10.1016/S0048-9697(00)00628-8
Hou D, He J, Lü C, Sun Y, Zhang F, Otgonbayar K (2013) Effects of environmental factors on nutrients release at sediment-water interface and assessment of trophic status for a typical shallow lake, northwest China. Sci World J:1–16. https://doi.org/10.1155/2013/716342
Hunt R, Christiansen I (2000) Understanding dissolved oxygen in streams: information kit. CRC for Sustainable Sugar Production, Australia
Inza B, Yao K (2015) Paramètres physiques et chimiques et métaux lourds des eaux de la Lagune Ebrié (Côte d’Ivoire): influence de la marée et des effluents liquides urbaines. J Mater Environ Sci 6:1321–1329
Inza B, Soro M, Etchian A, Trokourey A, Bokra Y (2009) Caractérisation des sédiments de surface de la baie des milliardaires, lagune ébrie, Côte d’Ivoire. Rev Int des Sci Technol 13:139–154
Jarvis B, Wilrich C, Wilrich PT (2010) Reconsideration of the derivation of most probable numbers, their standard deviations, confidence bounds and rarity values. J Appl Microbiol 109:1660–1667. https://doi.org/10.1111/j.1365-2672.2010.04792.x
Kannan K, Smith RG, Lee RF, Windom HL, Heitmuller PT, Macauley JM, Summers JK (1998) Distribution of total mercury and methyl mercury in water, sediment, and fish from South Florida estuaries. Arch Environ Contam Toxicol 34:109–118. https://doi.org/10.1007/s002449900294
Kim H, Lee K, Lim DI, Nam SI, heeHan S, Kim J, Lee E, Han IS, Jin YK, Zhang Y (2019) Increase in anthropogenic mercury in marginal sea sediments of the Northwest Pacific Ocean. Sci Total Environ 654:801–810. https://doi.org/10.1016/j.scitotenv.2018.11.076
King JK, Kostka JE, Frischer ME, Saunders FM (2000) Sulfate-reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl Environ Microbiol 66:2430–2437. https://doi.org/10.1128/AEM.66.6.2430-2437.2000
Koffi B (2009) L’environnement et la santé des populations riveraines de la lagune Ebrié. Le J des Sci Soc 6:103–116
Kouamé K, Yapo O, Méité L (2016) Contamination des sédiments d’une Lagune tropicale urbaine par les éléments traces métalliques (As, Cd, Cr, Pb, Zn): Cas des baies lagunaires de La ville d’Abidjan (Côte D’ivoire). Int J Pure Appl Biosci 4:204–217
Kouassi A, Tidou A, Kamenan A (2005) Caractéristiques hydrochimiques et microbiologiques des eaux de la lagune Ebrié (Côte d\‘Ivoire). Partie I: Variabilité saisonnière des paramètres hydrochimiques. Agron Afr 17:117–136. https://doi.org/10.4314/aga.v17i2.1663
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120. https://doi.org/10.1128/AEM.00335-09
Macalady JL, Mack EE, Nelson DC, Scow KM (2000) Sediment microbial community structure and mercury methylation in mercury-polluted Clear Lake, California. Appl Environ Microbiol 66:1479–1488. https://doi.org/10.1128/aem.66.4.1479-1488.2000
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31. https://doi.org/10.1007/s002440010075
Maggi C, Berducci MT, Bianchi J, Giani M, Campanella L (2009) Methylmercury determination in marine sediment and organisms by Direct Mercury Analyser. Anal Chim Acta 641:32–36. https://doi.org/10.1016/j.aca.2009.03.033
Marusczak N (2010) Etude du transfert du mercure et du méthylmercure dans les écosystèmes lacustres alpins. Université de Grenoble, France
Mason RP, Laporte JM, Andres S (1999) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 28:302–306. https://doi.org/10.1007/s002449910038
Mauro JBN, Guimarães JRD, Melamed R (1999) Mercury methylation in a tropical macrophyte: Influence of abiotic parameters. Appl Organomet Chem 13:631–636. https://doi.org/10.1002/(SICI)1099-0739(199909)13:9<631::AID-AOC905>3.0.CO;2-E
Monde S, Affian K, Amani E, et al (2007) Analyse temporelle de l’hydrodynamisme du secteur estuarien de la lagune Ebrié à Abidjan (Côte d’Ivoire). Impact de la variabilité climatique. Rev CAMES- Série A 5:32–38
Noh S, Choi M, Kim E, Dan NP, Thanh BX, Van Ha NT, Sthiannopkao S, Han S (2013) Influence of salinity intrusion on the speciation and partitioning of mercury in the Mekong River Delta. Geochim Cosmochim Acta 106:379–390. https://doi.org/10.1016/j.gca.2012.12.018
Oliveri E, Salvagio Manta D, Bonsignore M, Cappello S, Tranchida G, Bagnato E, Sabatino N, Santisi S, Sprovieri M (2016) Mobility of mercury in contaminated marine sediments: biogeochemical pathways. Mar Chem 186:1–10. https://doi.org/10.1016/j.marchem.2016.07.002
Onivogui G, Balde S, Bangoura K, Barry M (2013) Évaluation des risques de pollution en métaux lourds (Hg, Cd, Pb, Co, Ni, Zn) des eaux et des sédiments de l’estuaire du fleuve Konkouré (Rep. de Guinée). Afrique Sci 9:36–44
Pacyna EG, Pacyna JM, Pirrone N (2001) European emissions of atmospheric mercury from anthropogenic sources in 1995. Atmos Environ 35:2987–2996. https://doi.org/10.1016/S1352-2310(01)00102-9
Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S (2006) Global anthropogenic mercury emission inventory for 2000. Atmos Environ 40:4048–4063. https://doi.org/10.1016/j.atmosenv.2006.03.041
Paranjape AR, Hall BD (2017) Recent advances in the study of mercury methylation in aquatic systems. FACETS 2:85–119. https://doi.org/10.1139/facets-2016-0027
Peery S, Doraghi A, Ronagh M, Safahieh A (2018) Mercury bioaccumulation in sediment and root of mangrove forest, Avicennia marina from Emam Khomein Port, north part of the Persian Gulf. Glob Adv Res J Agric Sci 7:183–190
Reis AT, Rodrigues SM, Davidson CM, Pereira E, Duarte AC (2010) Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere 81:1369–1377. https://doi.org/10.1016/j.chemosphere.2010.09.030
Riba I, Del Valls TÁ, Forja JM, Gómez-Parra A (2004) The influence of pH and salinity on the toxicity of heavy metals in sediment to the estuarine clam Ruditapes philippinarum. Environ Toxicol Chem 23:1100–1107. https://doi.org/10.1897/023-601
Roulet M (2016) Annexe 1. Le mercure: son cycle biogéochimique et sa répartition aux échelles planétaire et amazonienne, in: Le Mercure En Amazonie. p 120. https://doi.org/10.4000/books.irdeditions.2533
Sahuquillo A, Rauret G, Bianchi M, Rehnert A, Muntau H (2003) Mercury determination in solid phases from application of the modified BCR-sequential extraction procedure: A valuable tool for assessing its mobility in sediments. Anal Bioanal Chem 375:578–583. https://doi.org/10.1007/s00216-002-1732-x
Seelen EA, Massey GM, Mason RP (2018) Role of sediment resuspension on estuarine suspended particulate mercury dynamics. Environ Sci Technol 52:7736–7744. https://doi.org/10.1021/acs.est.8b01920
Semyalo R, Rohrlack T, Kayiira D, Kizito YS, Byarujali S, Nyakairu G, Larsson P (2011) On the diet of Nile tilapia in two eutrophic tropical lakes containing toxin producing cyanobacteria. Limnologica 41:30–36. https://doi.org/10.1016/j.limno.2010.04.002
Serreta S (2000) Normes internationales applicable à la consommation chimique des poissons marins. Annexe 21:8
Silvennoinen H, Liikanen A, Torssonen J, Florian Stange C, Martikainen PJ (2008) Denitrification and nitrous oxide effluxes in boreal, eutrophic river sediments under increasing nitrate load: a laboratory microcosm study. Biogeochemistry 91:105–116. https://doi.org/10.1007/s10533-008-9262-z
Soro G, Metongo B, Soro N, Ahoussi E, Kouamé F, Zade S, Soro T (2009) Métaux lourds (Cu, Cr, Mn et Zn) dans les sédiments de surface d’une lagune tropicale africaine : cas de la lagune Ebrie (Côte d’Ivoire). Int J Biol Chem Sci 3:1408–1427. https://doi.org/10.4314/ijbcs.v3i6.53161
Taylor DL, Linehan JC, Murray DW, Prell WL (2012) Indicators of sediment and biotic mercury contamination in a southern New England estuary. Mar Pollut Bull 64:807–819. https://doi.org/10.1016/j.marpolbul.2012.01.013
Tessier E (2004) Etude de la réactivité et du transfert du tributyletain et du mercure dans les environnements aquatiques. Université de Pau et des pays de l’ADOUR
Tokarz E, Urban D (2015) Soil redox potential and its impact on microorganisms and plants of wetlands. J Ecol Eng 16:20–30. https://doi.org/10.12911/22998993/2801
Tosic M, Restrepo JD, Lonin S, Izquierdo A, Martins F (2019) Water and sediment quality in Cartagena Bay, Colombia: Seasonal variability and potential impacts of pollution. Estuar Coast Shelf Sci 216:187–203. https://doi.org/10.1016/j.ecss.2017.08.013
Varlet F (1978) Le régime de la lagune Ebrié, Côte d’Ivoire. Paris
Vowotor MK, Odumah Hood C, Sackey SS, et al (2014) An Assessment of Heavy Metal Pollution in Sediments of a Tropical Lagoon: A Case Study of the Benya Lagoon, Komenda Edina Eguafo Abrem Municipality (KEEA) — Ghana. J Heal Pollut 6:26–39. https://doi.org/10.5696/2156-9614-4-6.26
Wango T, Moussa T, Monde S (2008) Modèle Bi-Dimensionnel de la Lagune Ebrié (Côte d’Ivoire). Eur J Sci Res 24:229–243
Watras C (1992) Mercury and mthylmercury in individual zooplankton: implications for bioaccumulation. Limnol Oceanogr 37:1313–1318
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
Wognin AV, N’guessan YM, Assale FJP, Aka AM, Coulibaly AS, Monde S, Aka K (2017) Les éléments traces métalliques dans la lagune Ebrié: distribution saisonnière, niveau de contamination et qualité environnementale des sédiments. Int. J. Biol. Chem. Sci. 11:911–923. https://doi.org/10.4314/ijbcs.v11i2.30
Yao MK, Brou YS, Trokourey A, Soro MB (2017) Metal pollution and ecological risk assessment in sediment of artificial estuary: case of Vridi Channel, Côte d’Ivoire. J Appl Sci Environ Manag 21:785–792. https://doi.org/10.4314/jasem.v21i4.20
Acknowledgements
This work would not have been possible without the logistical support provided by two laboratories in France and Côte d’Ivoire. We would like to thank Regis Moilleron for providing access to his hydrobiology lab for experimentation. We gratefully acknowledge Alexandre Livet for his analytical assistance.
Funding
This study is financially supported by the PASRES program of the Swiss Center for Scientific Research since 2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Severine Le Faucheur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kouame, L.B.C., Bolou Bi, E.B., Aka, N. et al. Seasonality of Hg dynamics in the Ebrié Lagoon (Côte d’Ivoire) ecosystem: influence of biogeochemical factors. Environ Sci Pollut Res 27, 19810–19825 (2020). https://doi.org/10.1007/s11356-020-08471-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08471-3