Abstract
The microalga Scenedesmus obtusiusculus AT-UAM efficiently captured CO2 from two flue gas streams in a hybrid photobioreactor located in a greenhouse. Uptake rates of CO2, NO, and SO2 from a formulated gas stream were 160.7 mg L−1 day−1, 0.73 mg L−1 day−1, and 1.56 mg L−1 day−1, respectively, with removal efficiencies of 100% for all gases. Exhaust gases of a motor generator were also removed with uptake rates of 111.4 mg L−1 day−1, 0.42 mg L−1 day−1, and 0.98 mg L−1 day−1, obtaining removal efficiencies of 77%, 71%, and 53% for CO2, NOx, and SO2, respectively. On average, 61% of the CO2 from both flue gas streams was assimilated as microalgal biomass. The maximum CO2 uptake rate of 182 mg L−1 day−1 was achieved for formulated flue gas flow rate above 100 mL min−1. The biomass recovery of 88% was achieved using a 20-L electro-coagulation-flotation chamber coupled to a settler with a low specific power consumption of 0.27 kWh kg−1. The photobioreactor was operated for almost 7 months without contamination of invasive species or a decrease in the activity. It is a very encouraging result for long-term operation in flue gas treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, the concentration of carbon dioxide (CO2) in the atmosphere is above 400 ppm, being this the highest value in modern atmospheric records. Likewise, between 2007 and 2017, the average annual increase in atmospheric CO2 was 2.3 ppm (Hartfield et al. 2018), and an alarming temperature rise rate of 1.1 °C was recorded in 2016 (World Meteorological Organization 2018). CO2 is the most important greenhouse gas accounting for approximately 77% of the total emissions. The main industrial sources of CO2 emissions included flue gases from coal (40%), oil (35%), gas (20%), cement (4%), and gas flaring (0.7%) (Bekun et al. 2019). Therefore, environmentally friendly CO2 capture systems have gained importance over the last few decades. Microalgae are an interesting alternative due to their high capacity to assimilate CO2 and produce biomass (Cheah et al. 2015; Aslam et al. 2017) with higher CO2 fixation rates than terrestrial plants (Li et al. 2008; Kao et al. 2014). However, the cultivation of microalgae using flue gas emissions faces some challenges that include tolerance to CO2 (> 10–15%) (Huang et al. 2016; Yun et al. 2016), toxic effects of NOx and SOx concentrations (100–300 ppm) (Lee et al. 2002; Duarte et al. 2016), and resistance to fluctuations in pH, temperature, and light intensity (Singh and Ahluwalia 2013) typically present in systems for outdoor cultivation.
Algal cultivation systems are classified in open and closed systems. Open circulating ponds or raceway ponds (RWPs) are traditionally used for large-scale operation due to the low capital and operating costs (Singh and Ahluwalia 2013; Nagappan et al. 2019). However, they usually present low biomass productivities related to poor mixing, low CO2 mass transfer, and problems to control the operating conditions (Pandey et al. 2014). On the other hand, closed cultivation systems, such as photobioreactors (PBRs), allow easier control of the operating conditions and exhibit higher biomass productivities (Adesanya et al. 2014). Nevertheless, the operating and capital costs are higher, and in some PBR configurations, accumulation of dissolved oxygen may induce cell damage (Fernández et al. 2012; Costache et al. 2013). The latter reduces the carbon fixation efficiency of algal cultures due to the production of reactive oxygen species and competitive inhibition of carboxylation processes in the RUBISCO enzyme (Morales et al. 2018). A promising strategy for overcoming the shortcomings and enhancing the advantages of traditional cultivation systems is hybrid photobioreactors (HPRs). They combine two or more system configurations allowing higher productivities and surface/volume relationship besides lower energy demand and costs. HPRs include the combination of a bubble column with different configurations such as thin layer (Singh and Sharma 2012; Maroneze et al. 2016; Morales-Amaral et al. 2015), illumination platforms (Deprá et al. 2019), or RWPs (Bahr et al. 2014; Liu et al. 2019). In the first two configurations, high biomass productivities are reported, but they are prone to photoinhibition and have low working volumes. In a hybrid configuration of a bubble column and RWP, the CO2 mass transfer is improved by overcoming the major drawback of direct CO2 injection into the RWP, where 80–90% of CO2 is lost to the atmosphere (Putt et al. 2011).
On the other hand, cost-effective microalgal biomass production requires efficient and inexpensive harvesting methods. This operation represents more than 20% of the total production cost (Singh and Ahluwalia 2013). Low biomass concentrations, which range between 0.5 g L−1 in open ponds and 4 g L−1 in PBR (Vandamme et al. 2013), impose difficulties on microalgal biomass harvesting. There are several technologies already developed, such as sedimentation, centrifugation, flocculation, and flotation. However, some of these methods pose economic and operational difficulties or are energy-intensive processes (Rashid et al. 2019). Hence, energy-efficient technologies for biomass harvesting must be developed to reach the economic feasibility of microalgae-based products. Electro-coagulation-flotation (ECF) systems have been widely used for wastewater treatment by removing suspended pollutants (Gao et al. 2010). ECF consists of a floc-generating system through the release of aluminum or iron ions from a metal sacrificial anode and microbubbles produced in situ by a gas-generating cathode (Vandamme et al. 2011; Marrone et al. 2018). The flocs formed from interaction with metal ions float because hydrogen bubbles adhere to them, and biomass can be easily recovered at the top. However, the flocs will eventually fall to the bottom because of their weight. In comparison with coagulation or flocculation, which use compounds like alum, ECF has the advantage of not requiring the addition of other chemicals, such as chloride or sulfate to water (Singh and Patidar 2018). This technology has been used for the removal and recovery of algal biomass and has been reported to be energy and time-efficient (Marrone et al. 2018; Landels et al. 2019). However, most studies on the recovery of microalgal biomass by ECF have been performed on laboratory scale compared to centrifugation or other conventional harvesting techniques.
In previous studies, Scenedesmus obtusiusculus AT-UAM showed the potential for CO2 fixation from flue gas and biodiesel production because of its tolerance to high CO2 concentrations and lipid accumulation (Toledo-Cervantes et al. 2013). It also proved to be resistant to a wide pH range, photosynthetic photon flux density (PPFD), and temperature (Cabello et al. 2015) and was successfully grown outdoors (Morales et al. 2018). However, this microalga has not yet been cultivated in a hybrid photobioreactor nor fed with real flue gas streams.
Therefore, this work aimed to develop a CO2 capture system using the microalga Scenedesmus obtusiusculus AT-UAM for the treatment of flue gases from a formulated gas stream or an exhaust gas of a portable liquefied petroleum gas (LPG)–powered generator. It also comprised the use of a 100-L hybrid photobioreactor with the incorporation of a biomass harvesting stage by an electro-coagulation-flotation system.
Material and methods
Microorganism and culture medium
Scenedesmus obtusiusculus AT-UAM was grown on BG-11 medium, containing the following (in g L−1): NaNO3, 1.5; K2HPO4·2H2O, 0.04; MgSO4·7H2O, 0.075; citric acid, 0.006; ferric ammonium citrate, 0.006; Na2EDTA·2H2O, 0.001; and Na2CO3, 0.02, and 1 mL L−1 of A5 trace mineral solution. The composition of A5 was as follows (in g L−1): H3BO3, 2.86; MnCl2·4H2O, 1.81; ZnSO4·7H2O, 0.22; Na2MoO4·2H2O, 0.39; CuSO4·5H2O, 0.079; and Co(NO3)2·6H2O, 49.4 (Toledo-Cervantes et al. 2013).
Hybrid photobioreactor
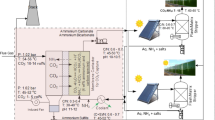
The HPR consisted of a 100-L RWP coupled to a 2.5-L bubble column (BC) (Fig. 1a). Two peristaltic pumps were used to recirculate the culture between the RWP and the bubble column; the liquid flow was 300 mL min−1. The formulated or real flue gas stream was measured using rotameters and distributed from the bottom of the BC through a porous glass disk. A data acquisition system (AlgaeConnect®) was implemented for data logging of pH, dissolved oxygen (DO), and temperature. All sensors were located in the RWP, and the PPFD impinging on the surface of HPR was measured by a light meter (DL-2005; Sper Scientific, USA). The inoculum for both experiments was generated separately in a 20-L bubble column with a working volume of 18 L, supplied with 1% CO2 at a rate of 2.8 L min−1, and operated at 28 °C. The initial biomass concentration in the HPR was 0.11 g L−1, but when it reached the level above 1 g L−1, 50 L of culture was removed and replaced with fresh BG-11 medium to ensure that the HPR maintained growth conditions over the entire operation period. The experimental system was located in a rooftop greenhouse at UAM Cuajimalpa in Mexico City (19° 21′ 5.8″ N, 99° 17′ 0.2″ W) and exposed to the circadian cycle of temperature and PPFD.
Flue gases
Two different sources of flue gases were used: (i) a formulated flue gas stream from a 4.29-m3 certified Praxair cylinder, having the following composition: CO2 (15%), NO (100 ppm), and SO2 (100 ppm) balanced with N2, and (ii) a real flue gas obtained from the exhaust gas of a 3-kVA portable power generator (Generac model LP3250). This generator used LPG as fuel, and the flue gas was collected using a 50-L gas sampling Tedlar® bag, after which the following composition was quantified by a portable flue gas analyzer (see the section “Gas phase analysis”): CO2 (4–5%), CO (0.9–1.23%), NO (12–25 ppm), NO2 (0–25 ppm), and SO2 (12–60 ppm). The formulated flue gas was supplied for 10 h of daylight using a flow rate of 100 mL min−1. The real flue gas was delivered for 18 h at 150 mL min−1 from samples previously collected in three 50-L Tedlar bags, to avoid alkalinization of the culture medium. In both cases, for the rest of the time, only air was supplied.
Finally, different gas flow rates were tested to maximize the simultaneous removal of CO2, NO, and SO2 and to determine the carbon fate in the HPR as well as the pH response. The formulated flue gas was sparged into the BC at 100 mL min−1, 130 mL min−1, 150 mL min−1, and 180 mL min−1, and these flow rates were evaluated in ascending order until steady removals. In all cases, the liquid flow rate was 300 mL min−1.
Biomass harvesting studies
Recovery assays were performed with a centrifuge (Hettich Zentrifugen, model Rotina 380, type 1701-30), using two plastic containers with a total working volume of 500 mL. The initial biomass concentration of Scenedesmus obtusiusculus AT-UAM was adjusted to 1 ± 0.05 g L−1 for every condition tested. Experiments were performed at different rotational speeds (250 rpm, 500 rpm, 750 rpm, 1000 rpm, 1250 rpm, 1500 rpm, 2000 rpm, 3000 rpm, and 4000 rpm) and centrifugation times of 5 min, 10 min, 15 min, and 20 min.
Biomass recovery was also made by ECF in a 0.6-L Pyrex glass beaker with the same working volume and sampling times used for centrifugation assays. The ECF system consisted of 4 aluminum electrodes with a total effective area of 0.024 m2. The following voltages and electric current were applied: 12 V and 0.1 A, 24 V and 0.2 A, and 30 V and 0.3 A.
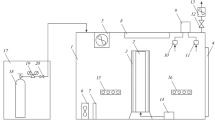
Figure 1b shows the ECF chamber for biomass harvesting after the cultivation stage. The system comprised three stages. The first step was carried out in a 20-L ECF chamber, consisting of 4 aluminum electrodes with a total effective area of 0.16 m2, and operated at 16 V and 0.6 A for 40 min; microalgal culture from the HPR was fed to this chamber at a flow rate of 0.5 L min−1. In the second stage, the biomass flocs resulting from the ECF were passed through a mesh filter screen (0.1 mm), and finally, the non-recovered biomass was sent to a 25-L settler, where it remained for 10 h, and the supernatant was purged.
The specific power input (SPI) was calculated according to Fayad et al. (2017)
where U is the voltage (in volts, V) and I is the electric current used (in amperes, A). V is the volume of treated culture (in L), t is the time of exposure to the harvesting technique (in hours, h), BRE is the biomass recovery efficiency, and X0 is the initial biomass concentration at the beginning of the harvesting process (g L−1).
Control experiments were performed in a 1-L plastic settling cone with a working volume of 0.5 L, at the same biomass concentration used in the centrifugation and ECF assays. Sampling was done every hour at 2 cm from the surface of liquid until no change occurred in biomass concentration.
Analytical methods
Biomass content
Fifty milliliters of culture medium was collected daily from the HPR and replaced with non-sterile water. Biomass was quantified by the dry weight method, filtering a known volume of culture medium through a 0.8-μm membrane, and dried at 60 °C in a thermobalance (Sartorius model MA160) until a constant weight was attained.
Liquid phase analysis
The supernatant retrieved from a prefiltered sample was passed through a 0.2-μm membrane to quantify the total dissolved carbon and nitrate. The determination of total inorganic carbon (TIC) was done by a TOC-VCSH analyzer (TOC-L series; Shimadzu, Japan).
Biomass analysis
Protein was quantified by a modified Lowry method for microalgal biomass (Lowry et al. 1951). Total carbohydrate content was determined by a modified phenol–sulfuric acid method (Dubois et al. 1956). Chlorophyll was extracted with methanol 90% v/v. Five milliliters of methanol (90% v/v) was added to 1 mL of the culture sample; this mixture was sonicated at 60 °C for 60 min. Afterward, the absorbance of the supernatant retrieved from each sample was measured at 650 nm and 665 nm; absorbances were corrected for turbidity by subtraction of absorbance values obtained at a wavelength of 750 nm. Chlorophyll content was determined with the following equation: Chlorophyll (μg mL−1) = 16.5 (A665) − 8.3 (A650). All methods were described previously in detail (Toledo-Cervantes et al. 2013).
The total lipid content was quantified with the sulfo-phospho-vanillin assay. For that, 2 mL of concentrated H2SO4 was added to 100 μL of the culture sample and boiled for 15 min. Then, every sample was placed in an ice bath for 5 min; subsequently, 5 mL of the sulfo-phospho-vanillin solution was added and incubated at 37 °C and 200 rpm, for 15 min, and finally read at a wavelength of 530 nm (Mishra et al. 2014). The calibration curve was prepared using commercial olive oil.
Biomass was calcined at 400 °C for 4 h to gravimetrically calculate the inorganic matter present in the biomass. All analyses were performed in triplicate.
Gas phase analysis
Gaseous effluent of the BC was collected in a 10-L Tedlar® bag, and the sample was pumped, precooled, and dried by a portable flue gas analyzer (NOVA model 5006) to simultaneously measure gas composition, including oxygen produced by photosynthesis. O2, CO, SO2, NO, and NO2 were determined by electro-chemical sensors and CO2 by a solid-state infrared sensor.
Calculations
The specific growth rate (μ, day−1) and biomass productivity (Pb, mg L−1 day−1) were calculated for both cultures by adjusting the biomass concentration kinetic data to the Gompertz model (Auria et al. 2000). The flue gas removal in the HPR was expressed in terms of volumetric uptake rate (UR = (Cin − Cout) / HRT) and removal efficiency (RE = (1 − Cout / Cin) × 100). Here, Cin and Cout are the influent and effluent gas concentrations in the BC, respectively; HRT is the hydraulic residence time calculated as HRT = Fg / VL, where Fg is the gas flow and VL is the total liquid culture volume (RWP + BC).
Results and discussion
HPR batch operation with formulated and real flue gases
Figure 2 shows the evolution of biomass, flue gas compound removals, operating conditions (temperature and PPFD), and response variables (pH and DO), related to the photosynthetic activity of S. obtusiusculus AT-UAM in the HPR fed with formulated (Fig. 2a) and real flue gases (Fig. 2b). The experiments lasted 20 days and 30 days, respectively. As can be seen in both experiments, the temperature was in the range of 11–32 °C, although the average temperature for the formulated gas experiment (19.9 °C) was slightly higher than that for real flue gases (18.5 °C) and the maximum PPFD was 640 μmol m−2 s−1. Variation in operating conditions of both experiments was associated with the circadian cycles and climate, with minimum values during the night and maximum values around noon. pH and DO followed the same trend as a result of CO2 and nitrate consumption due to photosynthesis. The initial pH of 8 gradually increased to around 10, when formulated flue gas was fed, and up to 12 in the case of real flue gas. A daily increase in DO occurred from the saturation value (6 mg L−1) recorded at the end of the night period when only air was supplied, up to 20 mg L−1 during the early afternoon; after this time, the DO gradually decreased. According to all the abovementioned conditions, Scenedesmus obtusiusculus AT-UAM was not in the optimum values of 620 μmol m−2 s−1, 35 °C, and pH of 7.5, previously reported by Cabello et al. (2015), and in some cases, operation was close to the critical values (T < 10 °C and pH > 10), when photosynthesis diminished seriously.
The pH decreases when CO2 solubilizes from the gaseous phase to the liquid, according to the reversible reaction CO2 + H2O ⇄ H+ + HCO3−, then H+ is consumed during HCO3− conversion to CO2, which is ultimately fixed during photosynthesis. Thus, steady-state usage of HCO3− as the original carbon source for photosynthesis leaves OH− in the cell, and it is neutralized by H+ uptake from the extracellular environment. Therefore, the reduction of H+ in the culture medium unavoidably leads to an increase in pH. High pH provides a greater drive for CO2 transport due to the shift in carbon dissolution in water. However, a neutral or acidic pH is more favorable for carbon fixing microalgae at the cellular level because bicarbonate and dissolved CO2 are preferred over CO32− (Imamura et al. 1983), which is present at extremely high pH, affecting microalgal growth. Nitrate consumption during microalgal growth is also linked to a rise in pH due to a net uptake of H+ (Scherholz and Curtis 2013). On the other hand, some of the problems associated with outdoor algal cultivation that limit the CO2 capture and biomass productivity are light and temperature variations and DO accumulation (Morales et al. 2018). Low PPFD reduces photosynthesis, and extremely high PPFD on the surface causes photoinhibition (Janssen 2016). Temperature influences both the physicochemical CO2 availability for the cells and the metabolic processes within the cell (Costache et al. 2013; Paliwal et al. 2017); therefore, non-optimal temperatures due to climate or seasonal conditions affect the operation of cultivation systems. DO is an indicator of photosynthetic activity by microalgae, but excessive oxygen buildup is one of the major problems in obtaining CO2 fixation rate and high biomass productivity. DO may exceed 250% of saturation during daytime (Fernández et al. 2012; Costache et al. 2013), which may lead to the photo-oxidative death of the culture under prolonged exposure to full sunlight (Fernández et al. 2012).
Figure 2a and b also shows the removal of the main components in both flue gases. As can be seen in both experiments, the microalgal culture quickly started to mitigate flue gas compounds, particularly when the system was fed with the formulated flue gas stream. RE of all compounds was kept at a value of 100% after the first 10 days of operation until the end of the experiment. Table 1 summarizes the main results for both experiments. As can be seen, URs for CO2, NOx, and SO2 were 160.7 mg L−1 day−1, 0.73 mg L−1 day−1, and 1.56 mg L−1 day−1, respectively, when formulated flue gas was fed. On the other hand, when real flue gas was supplied, the maximum RE and UR for CO2 (76.7%, 111.4 mg L−1 day−1), NOx (72%, 0.42 mg L−1 day−1), and SO2 (53.3%, 0.98 mg L−1 day−1) were lower than those obtained with formulated flue gas, which could be associated with the pH value maintained during the culture (7–11 and 8–12 with formulated and real flue gas, respectively). In the latter case, at a pH value of 12, 100% of the inorganic carbon is found as insoluble CO32−, affecting the carbon availability and cell activity. It could have contributed to the lower performance when the real gas was fed. It is important to point out that pH drop is commonly reported after the injection of flue gas, reducing the CO2 fixation and microalgal growth through the generation of acid compounds derived from the injection of SO2 and NOx into the culture medium (Lee et al. 2002; Yen et al. 2015). However, acidification was not observed in our case.
Flue gas removals obtained in this study were in the high range of most values reported in the literature using RWP systems (see Table 2), and normal values of UR were in the range of 27 mg L−1 day−1 to 69.54 mg L−1 day−1. Similar values to those obtained in our work were reported by Cheng et al. (2018) for the treatment of a coal-fired power plant–derived flue gas (12 ± 2% CO2, 120 ± 10 ppm NOx, 50 ± 10 ppm SO2) by direct injection into an RWP (2500 L min−1, 310 m3). They found maximum CO2, SO2, and NOx RE of 55%, 95%, and 66% with UR of 120.4 mg L−1 day−1, 0.715 mg L−1 day−1, and 0.72 mg L−1 day−1, respectively. However, REs of CO2 and NOx were lower than those obtained here with both formulated and real flue gas. In a similar way to our work, de Godos et al. (2014) studied the influence of a sump located in an RWP to promote the CO2 mass transfer of a flue gas from a diesel-fueled heating boiler with a composition of 10.6% CO2, 18.1 ppm CO, and 38.3 ppm NOx, finding similar UR values (see Table 2). Finally, in the study performed by Suriya Narayanan et al. (2019), flue gas (10.6% CO2, 150 ppm NOx, 6 ppm SO2) generated by an LPG burner was supplied directly to an open rectangular pond (200 L), obtaining CO2 UR values between 116 and 202 mg L−1 day−1. A major difference with our work was the temperature range recorded during the supply of flue gas with values between 30 and 40 °C. In the case of our assays with Scenedesmus obtusiusculus AT-UAM (see Table 1 and Fig. 2), the temperature was below optimum values (Cabello et al. 2015), which may partially account for the difference in CO2 UR.
Growth of Scenedesmus obtusiusculus AT-UAM fed with formulated and real flue gas
Figure 2 also shows that Scenedesmus obtusiusculus AT-UAM grew faster in the HPR fed with the formulated flue gas than with the real flue gas, reaching similar biomass concentrations (≈ 1.2 g L−1) in two thirds of the time. Table 1 shows the specific growth rates of 0.6 day−1 and 0.38 day−1 and biomass productivities of 69 mg L−1 day−1 and 41.8 mg L−1 day−1 for the formulated and real flue gas, respectively. The differences in growth rates and biomass productivities could be explained by CO2 concentrations and the feeding period with each flue gas.
As with CO2 UR, biomass productivity values obtained in this work were in the range of those achieved in the experiments performed in open systems with a supply of flue gas (see Table 2). Zhu et al. (2014) studied the growth and lipid accumulation in different strains of Nannochloropsis oceanica in an RWP with the supply of flue gas (13% CO2, 129 ppm NOx, and 30 ppm SOx), obtaining the values of Pb between 15.09 and 17.14 mg L−1 day−1, considerably lower than those achieved in our study. Dineshbabu et al. (2017) reported a Pb value of 35 mg L−1 day−1 for the operation of a cylindrical tank (550 L) inoculated with Phormidium valderianum and fed with flue gas. Similar Pb values (38–35 mg L−1 day−1) were reported by Pawlowski et al. (2014) for microalgal cultivation in an RWP (20 m3) with the flue gas (100 L min−1, 10.6% CO2, 38.3 ppm NOx). These results and the comparison with the operation of RWP without the bubble column (biomass productivity around 25 mg L−1 day−1) show the advantage of coupling a bubble column to cultures grown in RWP systems, not only in the enhancement of CO2 UR but also in growth parameters such as Pb.
Table 1 also shows the microalgal biomass composition. On average, protein, carbohydrate, lipid, and protein contents were 43.75%, 34.25%, 11.75%, and 1.8%, respectively. This is in agreement with the results previously reported by Toledo-Cervantes et al. (2018a) for S. obtusiusculus AT-UAM. However, the main difference was the high carbohydrate accumulation obtained in our work. González-Fernández and Ballesteros (2012) suggested that in some microalgal strains, both the supply of CO2 and the light/dark cycles affect key enzymes related to the biosynthesis of carbohydrates. Both conditions were present in the experiments performed in this study. According to the carbohydrate and protein percentages, the biomass produced could be used either as a dietary supplement or in energy production–related processes (Spolaore et al. 2006).
Carbon fate
On average, the carbon fate for the experiments considering biomass, inorganic carbon, and desorbed carbon was 61%, 23%, and 14%, respectively (see Table 1). These values are similar to those obtained by de Godos et al. (2014), with 66% of carbon recovered as biomass. The outgassing in that work was lower (4%), which could be associated with feeding strategy since the flue gas was supplied on demand or with a higher L/G ratio (≈ 27). It suggests that increased CO2 retention time significantly enhanced fixation efficiency. Although this approach seems promising for CO2 RE enhancement, the volume of flue gas treated is lower. Cheng et al. (2018) found that around 10% of the supplied CO2 was utilized to produce biomass and 0.2% as dissolved inorganic carbon; the rest of the carbon (≈ 90.8%) was desorbed. Lower values for biomass fixation (2.29% and 0.84%) were reported by Suriya Narayanan et al. (2019) and Zhu et al. (2014), respectively; as expected, a high percentage of carbon was desorbed in open systems (Ali and Park 2017). The gas–liquid mass transfer, promoted by the CO2 injection system to the bubble column coupled to RWP, reduced directly the CO2 released to the atmosphere, increasing the CO2 fixation efficiency and the environmental benefits for the flue gas mitigation.
HPR batch operation at different flow rates of formulated flue gas
The performance of the HPR at different flow rates of formulated flue gas is shown in Fig. 3. As can be seen, CO2 RE decreased from 100 to 63% for 100 mL min−1 and 180 mL min−1, respectively; besides, NO and SO2 REs were 100% for all conditions tested (see Table 2), and although the CO2 load increased 1.8 times, the maximum CO2 UR (182 mg L−1 day−1) was just 14% higher than the value for 100 mL m−1; this slight raise indicates that maximum CO2 capture was exceeded under the HPR operation conditions (irradiance and temperature, pressure, flue gas CO2 concentration, and culture medium composition). Furthermore, the pH increased from 7.2 to 7.9, depending on the flow rate tested, to maximum temporary pH values between 10.5 and 9.2 (see Fig. 3) reached during daylight period for 100 mL min−1 and 180 mL min−1, respectively. As it was previously mentioned, CO2 and pH are two interdependent factors that critically affect growth. Consequently, it is important to achieve a suitable pH regulation to allow proper growth, maximizing the CO2 fixation rate and minimizing losses from injected flue gas (Wang et al. 2018). In our case (see Fig. 3), between 62 and 26% of the carbon fed in the flue gas was fixed as biomass for 100 mL min−1 to 180 mL min−1, respectively, and CO2 losses by desorption in the RWP and release in the BC gas phase increased globally from 20.9 to 54.1% for the abovementioned flow rates. All these experiments allowed changing the L/G ratio between 3 and 1.7 and proved that CO2 absorption/capture was enhanced at a higher L/G ratio. This behavior was also observed by Toledo-Cervantes et al. (2016) with a maximum CO2 RE of 98.8 at an L/G ratio of 1 for photosynthetic biogas upgrading with 29.5% of CO2, using a similar HPR configuration.
HPR performance at different flow rates of formulated flue gas. Filled square, CO2 UR; filled circle, CO2 RE; empty square, maximum pH during the daylight period. Vertical bars indicate carbon fate: the black zone is biomass, the gray zone is dissolved inorganic carbon in liquid, the white zone is desorbed carbon, and the diagonal-stripe pattern zone is carbon released in the gas phase
Semicontinuous operation of the HPR with Scenedesmus obtusiusculus AT-UAM
Figure 4 shows HPR performance under a semicontinuous regime. Formulated flue gas was fed during 3 cycles, after which the system was restarted only with air, and finally, formulated flue gas was restored. As can be seen, the CO2, NO, and SO2 REs were 100%, keeping a CO2 UR of 160.7 mg L−1 day−1. Scenedesmus obtusiusculus AT-UAM grew well during these 3 cycles, in contrast to the period when the air was fed, reaching 1 g biomass L−1 in approximately 50 days. The lower activity was reflected in the pH with noticeably lower values compared to those observed with the flue gas. This could be related not only to the low CO2 concentration but also to cultivation conditions (T < 10 °C). Toledo-Cervantes et al. (2018b) operated an HPR system consisting of a tubular photobioreactor (45.5 L) with an absorption column (3.5 L), initially fed with biogas (29.5% CO2, 0.5% H2S, and 70% CH4) and later with flue gas (20% CO2 and 80% N2) during 150 days. The CO2 RE was 100% during the period when HPR was fed with flue gas stream, and CO2 UR was, on average, 115.8 mg CO2 L−1 day−1 during the whole experiment. This value is slightly lower than the one maintained for 205 days of operation in this study (152.7 mg CO2 L−1 day−1, RE of 95%). Marín et al. (2018) reported the effect of seasonal variations on CO2 removal with the HPR system, consisting of an RWP (180 L), connected to an absorption column (2.5 L) supplied with a formulated biogas stream. The experiments were carried out during 330 days obtaining CO2 RE between 66 and 99.7% and a maximum CO2 UR of 108.95 mg CO2 L−1 day−1. In our study, the lowest value for CO2 RE was 95%, suggesting that the culture system was effective at capturing CO2 in the long term.
Biomass recovery
As can be seen in Fig. 5, the highest biomass recovery efficiency (92%) was obtained for centrifugation at 4000 rpm from ECF assays (Fig. 6). The highest value (81%) was attained at 30 V and 0.3 A. Both biomass recovery techniques achieved similar values of recovery efficiency in a considerably shorter time than in the control assay performed in a settling cone (90% after 7 h).
As shown in Table 3, ECF is a more efficient technique than centrifugation in terms of energy consumed for biomass recovery. The specific power inputs were between 20 and 99 times lower than those required by centrifugation. Richardson et al. (2014) analyzed the effect of centrifugation and ECF on the economic viability of algae-based fuels and also found that ECF was more efficient in terms of energy consumption, resulting in a higher revenue return by 8.88%. Similarly, Shi et al. (2019) showed that centrifugation required almost 4 times more electricity than ECF technologies.
Vandamme et al. (2011) studied the harvesting of Chlorella vulgaris in an ECF system with two aluminum electrodes. Ninety-five percent recovery efficiency was obtained after 20 min at a current density of 1.5 mA cm−2 and a power input of 8.1 kWh kg−1. Although the biomass recovery efficiency was higher, the time and the current density were higher than any in our study (see Table 3). Fayad et al. (2017) achieved 100% recovery efficiency for Chlorella sorokiniana after 50 min of treatment with a current density of 2.9 mA cm−2 and a specific power consumption of 1 kWh kg−1. This low value was attributed to the addition of 1.5 g L−1 NaCl to the culture medium at the beginning of the ECF process, which would enhance conductivity in the liquid medium and facilitate the harvesting process. It is contrary to the results presented in this study, where ECF was tested directly in the culture medium without the addition of any salts or other compounds.
ECF system
An electro-coagulation-flotation system was implemented and tested with 40 L of the biomass culture fed with real flue gases (see Fig. 1b). The recovery efficiency of the ECF chamber was 73%, and the global efficiency of 88% was obtained when it was coupled to a settler. In comparison with the first ECF assays, conducted on a smaller scale, the specific power consumption was also improved by operating an ECF chamber in continuous mode, with 0.27 kWh kg−1. This value only takes into account the energy supplied to the ECF chamber, while biomass recovery efficiency was lower (73–83%). It is mainly due to a reduction in the surface area (from 0.096 to 0.008 m2 L−1) regarding the assays performed with a working volume of 0.5 L, which resulted in a less efficient biomass harvesting. Additionally, the continuous injection of the culture broth reduced the residence time of biomass in the ECF chamber, resulting in a similar recovery efficiency in less time. On the other hand, the reduction in specific power consumption could be associated with the working volume (0.5–20 L), which allowed retrieving a higher amount of biomass during this assay, thus reducing the SPI. Marrone et al. (2018) reported that a bench-scale ECF equipment (nominal flow of 568 L h−1) required 5 times less power input per liter of processed culture than the centrifugation method and the energy consumption was almost 9 times lower when a settling stage was coupled. The results obtained in our study confirmed that ECF harvesting is a promising approach to enhancing the feasibility of algae-derived processes and products by reducing energy input and processing time. Although these results are a forward step to couple cultivation/harvesting stages, ECF application requires more studies to develop electrodes with more efficient, non-fouling, and less-toxic properties.
Conclusions
S. obtusiusculus AT-UAM was able to grow in an HPR fed with both formulated and real flue gas, exhibiting high removal efficiencies for CO2, SO2, and NOx. CO2 biomitigation is a long-term ecological alternative for the treatment of combustion effluents rich in CO2. Moreover, products of commercial interest could be obtained from the harvested microalgal biomass. Electro-coagulation-flotation is an energy-efficient technique for the recovery of microalgal biomass that may enhance the viability of microalgae-based biorefineries by reducing the costs associated with energy consumption in the biomass harvesting stage. This study demonstrated the possibility of coupling a biomass recovery stage to the treatment of flue gas streams as well as the use of an HPR configuration to enhance microalgal growth and pollutant removal in long-term operation.
References
Adesanya VO, Cadena E, Scott SA, Smith AG (2014) Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system. Bioresour Technol 163:343–355. https://doi.org/10.1016/j.biortech.2014.04.051
Ali H, Park CW (2017) Numerical multiphase modeling of CO2 absorption and desorption in microalgal raceway ponds to improve their carbonation efficiency. Energy 127:358–371. https://doi.org/10.1016/j.energy.2017.03.143
Aslam A, Thomas-Hall SR, Mughal TA, Schenk PM (2017) Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour Technol 233:271–283. https://doi.org/10.1016/j.biortech.2017.02.111
Auria R, Frere G, Morales M, Acuña ME, Revah S (2000) Influence of mixing and water addition on the removal rate of toluene vapors in a biofilter. Biotechnol Bioeng 68(4):448–455. https://doi.org/10.1002/(SICI)1097-0290(20000520)68:4<448::AID-BIT10>3.0.CO;2-2
Bahr M, Díaz I, Dominguez A, González Sánchez A, Muñoz R (2014) Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ Sci Technol 48(1):573–581. https://doi.org/10.1021/es403596m
Bekun FV, Alola AA, Sarkodie SA (2019) Toward a sustainable environment: nexus between CO2 emissions, resource rent, renewable and nonrenewable energy in 16-EU countries. Sci Total Environ 657:1023–1029. https://doi.org/10.1016/j.scitotenv.2018.12.104
Cabello J, Toledo-Cervantes A, Sánchez L, Revah S, Morales M (2015) Effect of the temperature, pH and irradiance on the photosynthetic activity by Scenedesmus obtusiusculus under nitrogen replete and deplete conditions. Bioresour Technol 181:128–135. https://doi.org/10.1016/j.biortech.2015.01.034
Cheah WY, Show PL, Chang JS, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol 184:190–201. https://doi.org/10.1016/j.biortech.2014.11.026
Cheng J, Yang Z, Zhou J, Cen K (2018) Improving the CO2 fixation rate by increasing flow rate of the flue gas from microalgae in a raceway pond. Korean J Chem Eng 35:498–502. https://doi.org/10.1007/s11814-017-0300-1
Costache TA, Acien FG, Morales MM, Fernández JM, Stamatin I, Molina E (2013) Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl Microbiol Biotechnol 97:7627–7637. https://doi.org/10.1007/s00253-013-5035-2
de Godos I, Mendoza JL, Acién FG, Molina E, Banks CJ, Heaven S, Rogalla F (2014) Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour Technol 153:307–314. https://doi.org/10.1016/j.biortech.2013.11.087
Deprá MC, Mérida LGR, de Menezes CR, Zepka LQ, Jacob-Lopes E (2019) A new hybrid photobioreactor design for microalgae culture. Chem Eng Res Des 144:1–10. https://doi.org/10.1016/j.cherd.2019.01.023
Dineshbabu G, Uma VS, Mathimani T, Deviram G, Ananth DA, Prabaharan D, Uma L (2017) On-site concurrent carbon dioxide sequestration from flue gas and calcite formation in ossein effluent by a marine cyanobacterium Phormidium valderianum BDU 20041. Energ Convers Manage 141:315–324. https://doi.org/10.1016/j.enconman.2016.09.040
Duarte JH, Fanka LS, Costa JAV (2016) Utilization of simulated flue gas containing CO2, SO2, NO and ash for Chlorella fusca cultivation. Bioresour Technol 214:159–165. https://doi.org/10.1016/j.biortech.2016.04.078
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Fayad N, Yehya T, Audonnet F, Vial C (2017) Harvesting of microalgae Chlorella vulgaris using electro-coagulation-flocculation in the batch mode. Algal Res 25:1–11. https://doi.org/10.1016/j.algal.2017.03.015
Fernández I, Acién FG, Fernández JM, Guzmán JL, Magán JJ, Berenguel M (2012) Dynamic model of microalgal production in tubular photobioreactors. Bioresour Technol 126:172–181. https://doi.org/10.1016/j.biortech.2012.08.087
Gao S, Yang J, Tian J, Ma F, Tu G, Du M (2010) Electro-coagulation–flotation process for algae removal. J Hazard Mater 177(1–3):336–343. https://doi.org/10.1016/j.jhazmat.2009.12.037
González-Fernández C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30(6):1655–1661. https://doi.org/10.1016/j.biotechadv.2012.07.003
Hartfield G, Blunden J, Arndt DS (2018) State of the climate in 2017. Bull Am Meteorol Soc 99(8):Si–S332. https://doi.org/10.1175/2018BAMSStateoftheClimate.1
Huang G, Chen F, Kuang Y, He H, Qin A (2016) Current techniques of growing algae using flue gas from exhaust gas industry: a review. Appl Biochem Biotechnol 178(6):1220–1238. https://doi.org/10.1007/s12010-015-1940-4
Imamura M, Tsuzuki M, Shiraiwa Y, Miyachi S (1983) Form of inorganic carbon utilized for photosynthesis in Chlamydomonas reinhardtii. Plant Cell Physiol 24(3):533–540. https://doi.org/10.1093/oxfordjournals.pcp.a076545
Janssen M (2016) Microalgal photosynthesis and growth in mass culture. In: Legrand J (ed) Photobioreaction engineering, 1st edn. Elsevier, Amsterdam, pp 185–256
Kao CY, Chen TY, Chang YB, Chiu TW, Lin HY, Chen CD, Chang JS, Lin CS (2014) Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour Technol 166:485–493. https://doi.org/10.1016/j.biortech.2014.05.094
Landels A, Beacham TA, Evans CT, Carnovale G, Raikova S, Cole IS, Goddard P, Chuck C, Allen MJ (2019) Improving electrocoagulation floatation for harvesting microalgae. Algal Res 39:101446. https://doi.org/10.1016/j.algal.2019.101446
Lee JS, Kim DK, Lee JP, Park SC, Koh JH, Cho HS, Kim SW (2002) Effects of SO2 and NO on growth of Chlorella sp. KR-1. Bioresour Technol 82(1):1–4. https://doi.org/10.1016/S0960-8524(01)00158-4
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Prog 24(4):815–820. https://doi.org/10.1021/bp070371k
Liu W, Chen Y, Wang J, Liu T (2019) Biomass productivity of Scenedesmus dimorphus (Chlorophyceae) was improved by using an open pond–photobioreactor hybrid system. Eur J Phycol 54:127–134. https://doi.org/10.1080/09670262.2018.1519601
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Marín D, Posadas E, Cano P, Pérez V, Lebrero R, Muñoz R (2018) Influence of the seasonal variation of environmental conditions on biogas upgrading in an outdoors pilot scale high rate algal pond. Bioresour Technol 255:354–358. https://doi.org/10.1016/j.biortech.2018.01.136
Maroneze MM, Siqueira SF, Vendruscolo RG, Wagner R, de Menezes CR, Zepka LQ, Jacob-Lopes E (2016) The role of photoperiods on photobioreactors—a potential strategy to reduce costs. Bioresour Technol 219:493–499. https://doi.org/10.1016/j.biortech.2016.08.003
Marrone BL, Lacey RE, Anderson DB, Bonner J et al (2018) Review of the harvesting and extraction program within the National Alliance for advanced biofuels and bioproducts. Algal Res 33:470–485. https://doi.org/10.1016/j.algal.2017.07.015
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang JW (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333. https://doi.org/10.1016/j.biortech.2013.12.077
Morales M, Sánchez L, Revah S (2018) The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol Lett 365(3):fnx262. https://doi.org/10.1093/femsle/fnx262
Morales-Amaral MM, Gómez-Serrano C, Acién FG, Fernández-Sevilla JM, Molina-Grima E (2015) Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res 12:99–108. https://doi.org/10.1016/j.algal.2015.08.020
Nagappan S, Tsai PC, Devendran S, Alagarsamy V, Ponnusamy VK (2019) Enhancement of biofuel production by microalgae using cement flue gas as substrate. Environ Sci Pollut Res 1–16. https://doi.org/10.1007/s11356-019-06425-y
Paliwal C, Mitra M, Bhayani K, Bharadwaj SVV, Ghosh T, Dubey S, Mishra S (2017) Abiotic stresses as tools for metabolites in microalgae. Bioresour Technol 244:1216–1226. https://doi.org/10.1016/j.biortech.2017.05.058
Pandey A, Lee DJ, Chisti Y, Soccol C (2014) Biofuels from algae. Elsevier, Amsterdam
Pawlowski A, Mendoza JL, Guzmán JL, Berenguel M, Acién FG, Dormido S (2014) Effective utilization of flue gases in raceway reactor with event-based pH control for microalgae culture. Bioresour Technol 170:1–9. https://doi.org/10.1016/j.biortech.2014.07.088
Putt R, Singh M, Chinnasamy S, Das KC (2011) An efficient system for carbonation of high-rate algae pond water to enhance CO2 mass transfer. Bioresour Technol 102:3240–3245. https://doi.org/10.1016/j.biortech.2010.11.029
Rashid N, Nayak M, Suh WI, Lee B, Chang YK (2019) Efficient microalgae removal from aqueous medium through auto-flocculation: investigating growth-dependent role of organic matter. Environ Sci Pollut Res 26:27396–27406. https://doi.org/10.1007/s11356-019-05904-6
Richardson JW, Johnson MD, Lacey R, Oyler J, Capareda S (2014) Harvesting and extraction technology contributions to algae biofuels economic viability. Algal Res 5:70–78. https://doi.org/10.1016/j.algal.2014.05.007
Scherholz ML, Curtis WR (2013) Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnol 13:39. https://doi.org/10.1186/1472-6750-13-39
Shi R, Handler RM, Shonnard DR (2019) Life cycle assessment of novel technologies for algae harvesting and oil extraction in the renewable diesel pathway. Algal Res 37:248–259. https://doi.org/10.1016/j.algal.2018.12.005
Singh UB, Ahluwalia AS (2013) Microalgae: a promising tool for carbon sequestration. Mitig Adapt Strateg Glob 18:73–95. https://doi.org/10.1007/s11027-012-9393-3
Singh G, Patidar SK (2018) Microalgae harvesting techniques: a review. J Environ Manag 217:499–508. https://doi.org/10.1016/j.jenvman.2018.04.010
Singh RN, Sharma S (2012) Development of suitable photobioreactor for algae production—a review. Renew Sust Energ Rev 16(4):2347–2353. https://doi.org/10.1016/j.rser.2012.01.026
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. https://doi.org/10.1263/jbb.101.87
Suriya Narayanan G, Kumar G, Seepana S, Elankovan R, Premalatha M (2019) Utilization of unfiltered LPG-burner exhaust-gas emission using microalga Coelastrella sp. J CO2 Util 29:283–295. https://doi.org/10.1016/j.jcou.2018.12.020
Toledo-Cervantes A, Morales M, Novelo E, Revah S (2013) Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresour Technol 130:652–658. https://doi.org/10.1016/j.biortech.2012.12.081
Toledo-Cervantes A, Serejo ML, Blanco S, Pérez R, Lebrero R, Muñoz R (2016) Photosynthetic biogas upgrading to biomass productivity control. Algal Res 17:46–52. https://doi.org/10.1016/j.algal.2016.04.017
Toledo-Cervantes A, Garduño Solórzano G, Campos J, Martinez-García M, Morales M (2018a) Characterization of Scenedesmus obtusiusculus AT-UAM for high-energy molecules accumulation: deeper insight into biotechnological potential of strains of the same species. Biotechnol Rep 17:16–23. https://doi.org/10.1016/j.btre.2017.11.009
Toledo-Cervantes A, Morales T, González A, Muñoz R, Lebrero R (2018b) Long-term photosynthetic CO2 removal from biogas and flue-gas: exploring the potential of closed photobioreactors for high-value biomass production. Sci Total Environ 640-641:1272–1278. https://doi.org/10.1016/j.scitotenv.2018.05.270
Vandamme D, Pontes SCV, Goiris K, Foubert I, Pinoy LJJ, Muylaert K (2011) Evaluation of electro-coagulation–flocculation for harvesting marine and freshwater microalgae. Biotechnol Bioeng 108(10):2320–2329. https://doi.org/10.1002/bit.23199
Vandamme D, Foubert I, Muylaert K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol 31(4):233–239. https://doi.org/10.1016/j.tibtech.2012.12.005
Wang Z, Wen X, Xu Y, Ding Y, Geng Y, Li Y (2018) Maximizing CO2 fixation and lipid productivity of oleaginous microalga Graesiella sp. WBG-1 via CO2-regulated pH in indoor and outdoors open reactors. Sci Total Environ 619–620:827–833. https://doi.org/10.1016/j.scitotenv.2017.10.127
World Meteorological Organization (2018) WMO statement on the state of the global climate in 2017. WMO 212 https://library.wmo.int/doc_num.php?explnum_id=4453. Accessed 10 Dec 2018
Yen HW, Ho SH, Chen CY, Chang JS (2015) CO2, NOx and SOx removal from flue gas via microalgae cultivation: a critical review. Biotechnol J 10(6):829–839. https://doi.org/10.1002/biot.201400707
Yun HS, Ji MK, Park YT, Salama ES, Choi J (2016) Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production. Environ Sci Pollut Res 23:17831–17839. https://doi.org/10.1007/s11356-016-6971-z
Zhu B, Sun F, Yang M, Lu L, Yang G, Pan K (2014) Large-scale biodiesel production using flue gas from coal-fired power plants with Nannochloropsis microalgal biomass in open raceway ponds. Bioresour Technol 174:53–59. https://doi.org/10.1016/j.biortech.2014.09.116
Acknowledgments
The authors thank the scholarship provided by the National Council of Science and Technology (CONACYT).
Funding
This study was funded by the project FSE SENER-CONACYT 247006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Estrada-Graf, A., Hernández, S. & Morales, M. Biomitigation of CO2 from flue gas by Scenedesmus obtusiusculus AT-UAM using a hybrid photobioreactor coupled to a biomass recovery stage by electro-coagulation-flotation. Environ Sci Pollut Res 27, 28561–28574 (2020). https://doi.org/10.1007/s11356-020-08240-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08240-2