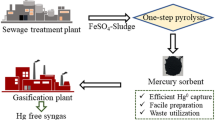
Abstract
The sewage sludge flocculated with ferrous sulfate (SFS) was prepared by one-step pyrolysis to obtain magnetic Fe-containing carbon. Results showed that only a small amount of FexOy as well as extremely weak magnetism were observed at pyrolysis temperatures of less than 500 °C. SFS tended to exhibit intensive agglomeration, leading to the drastic increase of the crystalline-phase particle size at high pyrolysis temperature. The optimal pyrolysis temperature is 700 °C, corresponding to the production of some sulfides, an optimal content of FexOy, and a suitable BET surface. Hg0 removal efficiency of SFS700 (SFS pyrolyzed at 700 °C) reached 80.7% at the reaction temperature of 125 °C. The presence of O2 and low concentration of SO2 enhanced the Hg0 removal, while the H2O vapor and high SO2 concentration inhibited it. Meanwhile, good resistance for the adsorbent to moderate concentrations of SO2 and H2O was observed. Moreover, the good magnetism performance is conducive to the recovery and utilization of the SFS700 in flue gas. Therefore, SFS can be used for Hg0 removal without any chemical modification after undergoing one-step pyrolysis and this study has guiding significance for the resource utilization and engineering practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is one of the most hazardous pollutants due to its toxicity, mobility, and bioaccumulation in the ecosystem and food chain (Yang et al. 2011a). Despite its trace concentration in coal, a large amount of mercury from coal-fired power plants around the world is released into the atmosphere every year (Li et al. 2017a). Different coal ranks and operation conditions lead to various forms of mercury in the flue gas. Three forms of mercury exist in coal-derived flue gas: elemental mercury (Hg0), oxidized mercury (Hg2+), and particulate-bound mercury (Hgp) (Wilcox et al., 2012). Among these forms, Hg2+ and Hgp can be effectively captured by wet flue gas desulfurization or a dust removal system (such as a fabric filter and an electrostatic precipitator) (Scala and Clack, 2008). However, it is difficult to remove Hg0, accounting for greater than 75% of the total mercury (Yang et al. 2011b), by the currently available pollution control devices.
Several technologies have been widely investigated to capture elemental mercury from coal-fired flue gas in accordance with the increasingly stringent environmental emission standards. Thus far, the catalysts or sorbents examined for the capture of elemental mercury can be categorized into three groups: selective catalytic reduction catalysts (Li et al. 2017a; Zhang et al. 2014; Liu et al. 2017), metals and metal oxides, and adsorbents (including carbon-based and non-carbon-based adsorbents) (Liao et al. 2016; Li et al. 2016; Liu et al. 2008; Lee et al. 2009). As the maximum achievable control technology for the capture of mercury from coal-fired power plants, the injection of sorbents is limited by at least three reasons: the poor capture capacity of raw adsorbents, the highest operation cost due to the non-recycling of the adsorbents, and the adverse effects of the sorbents on the utilization of fly ash (Liao et al. 2016; Li et al. 2016).
To obtain a high mercury removal efficiency and decrease the cost for Hg0 capture, several current studies have aimed to develop recyclable adsorbents. As an effective method, the magnetization of the adsorbents permits the separation of spent adsorbents from a mixture with fly ash. Among these, MagZ-Ag0 (Dong et al. 2009a,b), Fe-based spinels (Yang et al. 2011a,c; Xu et al. 2019a; Yang et al. 2010), and Co-MF (Yang et al. 2014) exhibit excellent performance for the capture of gaseous Hg0 from flue gas. Meanwhile, some chemicals such as sulfur, halogen, and noble metals have been used for the modification of sorbents to remedy the poor mercury adsorption capacity (Hamzehlouyan et al. 2014; Zhou et al. 2015; Zou et al. 2017; Yang et al. 2018). Chemical functionalization by sulfur has been considered to be a crucial method for enhancing the mercury adsorption capacity, resulting from the fact that surface sulfur compounds provide sufficient binding sites for mercury, affording stable mercuric sulfides (HgS) (Xu et al. 2018; Liu et al. 2000). Li et al. (2016) have reported that nano-ZnS exhibits the superior mercury adsorption capacity compared with that of commercial activated carbons. Liao et al. (2016; 2019) have prepared magnetic pyrrhotite as a recyclable sorbent to remove Hg0 from the flue gas, which exhibits excellent Hg0 removal capacity at low temperatures. However, the cost of metal sulfides is high and the loading process is complex. Moreover, two or more steps of preparation process (including the preparation of activated carbon, the loading of metal sulfides, and magnetic preparation) evidently increase the manufacturing cost. Therefore, these metal sulfide adsorbents are still extremely low for use in actual industrial applications.
As a typical flocculant, ferrous sulfate (FeSO4) has been widely used in the flocculation and dewatering of excess sludge in sewage treatment plants (Kushwaha et al. 2010; Carmen et al. 2013; Rui et al. 2010). The dosage of chemicals accounts for 3–5% of the dry sludge weight, thereby increasing the sludge volume and subsequently increasing the amount of sludge as well as the difficulty associated with its final disposal (Kushwaha et al. 2010). Accordingly, the use of sludge from municipal wastewater treatment plants as a cost-effective feedstock has always been a key issue (Park and Lee, 2018). In many studies (Pan et al. 2011; Yang et al. 2016), sludge with a chemical flocculant prepared from the pyrolysis of chemical sludge exhibits a higher surface area and pollutant removal efficiency than those prepared from general biological sludge. Moreover, Yang et al. (2016) have examined a ferric-activated-sludge-based adsorbent from biological sludge for the removal of tetracycline and found that magnetite is observed at a pyrolysis temperature range of 550–750 °C. In another study, Yani and Zhang (2010) have discovered that FeSO4 can be converted into FeS when FeSO4 is added to the pyrolysis of Australian lignite. However, it is not clear that whether the SFS can be directly pyrolyzed at appropriate temperature to produce magnetic sulfide-containing biomass carbon for Hg0 removal.
With this background, for utilizing the sewage sludge flocculated with ferrous sulfate, the comprehensive effects of different pyrolysis temperatures on the surface features and the forms of Fe, O, and S were investigated. In addition, N2 adsorption/desorption, powder X-ray diffraction (XRD), thermal gravimetry–mass spectrometry (TG–MS), inductively coupled plasma-mass spectrometry (ICP-MS), vibrating sample magnetometer (VSM), and X-ray photoelectron spectroscopy (XPS) measurements as well as Fourier transform infrared (FTIR) spectroscopy were employed for sample characterization. The role of flue gas components (e.g., O2, SO2, and H2O) toward the removal of Hg0 was examined. Furthermore, the mechanisms involved in the removal of Hg0 over SFS were discussed according to the characterization and experimental results. The ultimate goal is to utilize the SFS for Hg0 removal from coal combustion flue gas, which could be significant for both resource utilization and future industry application.
Materials and methods
Preparation of materials
SFS was obtained from a wastewater treatment plant (Wuhan, Hubei Province) in China. First, SFS was dried at 105 °C and then pyrolyzed by using a temperature-programmed tube furnace with a temperature increase rate of 5 °C/min at different temperatures (300 °C, 500 °C, 700 °C, 800 °C, and 900 °C) for 1 h under a nitrogen flow (250 mL/min) (as shown in Figure S1). For each experiment, it took at least 30 min to obtain a pure nitrogen atmosphere. After pyrolysis, all samples were washed with distilled water, dried, and ground using a mortar. The pyrolyzed samples (SFS) were denoted as SFS300, SFS500, SFS700, SFS800, and SFS900.
Adsorbent characterization
The phase distributions of metals in SFS were analyzed by ICP-MS (Thermo Fisher, America) using a sequential extraction procedure. The BET surface area of samples was analyzed on an ASAP 2020 porosimeter using N2 gas as an adsorbate at liquid-nitrogen temperature (77 K). The pore volume and pore size of the samples were calculated by the Barrett–Joyner–Halenda method. The magnetism of samples was determined by using a physical property measurement system using a VSM (PPMS-VSM). The crystalline structure of the samples was characterized by power XRD. XRD patterns were recorded on an X’Pert PRO diffractometer (Cu-Kα radiation, 2θ = 5–90°) with an operating voltage of 40 kV and an emission current of 40 mA. FTIR (VERTEX 70, Bruker, Germany) spectroscopy was employed for the determination of the group species distribution of the samples under different pyrolysis conditions. XPS (ESCALAB 250Xi, Thermo Fisher, USA) was employed to determine the binding energies of Fe 2p, S 2p, O 1s, and Hg 4f with Al-Kα radiation (hv = 1486.6 eV). To examine the evolution of sulfide-related gases during pyrolysis, experiments were also performed using a Perkin Elmer STA 6000 TGA–differential scanning calorimetry (DSC) system coupled to a 403C Aeolos Netzsch mass spectrometer (TGA–MS). The treated SFS was heated from room temperature to 900 °C at a heating rate of 5 °C/min under nitrogen conditions with a flow rate of 40 mL/min.
Measurement of Hg0 adsorption performance
To examine the mercury adsorption performance of samples pyrolyzed at various temperatures, a laboratory-scale fixed-bed adsorption system was established (Fig. 1), including a mercury generation system, a fixed-bed reactor system, a humidification system, a mercury detection system, a gas flow control system, and an exhaust gas treatment system. The Hg0 concentration was maintained at ~ 130 μg/m3 by adjusting N2 to flow through the mercury permeation tube (HE-SR, VICI Metronics, USA) and oil bath temperature. A cold vapor atomic adsorption spectrometer analyzer (SG-921, China) was employed to measure the Hg0 concentration. Hg0 capture experiments were performed in a quartz tube reactor with an internal diameter of 6 mm in a fixed-bed reactor. A total of 50 mg of the prepared samples was used for every experiment with a total gas flow rate of 1000 mL/min, leading to a GHSV of 130,000 h−1. Each of the sample was placed in the middle of the quartz tube reactor which was wrapped in the temperature-programmed tube furnace. The simulated flue gas (SFG) contained balance N2, 5 vol% O2, 0–8 vol% H2O (when used), and 0–2000 ppm SO2 (when used). All the gas pipes after the oil bath were heated by heating tapes to ensure a steady temperature of 95 °C for the purpose of preventing the deposition of Hg. Before commencing each test, it took at least 30 min to obtain steady SFG and Hg concentrations. In mercury detection system, the mercury speciation conversion system was equipped to identify the mercury speciation in the flue gas. The impingers containing 10% of KCl solution (side I) or 0.5 mol L−1 SnCl2/HCl solution (side II) were placed between the reactor and mercury analyzer, respectively. When the flue gas steam was passed through side I, the oxidized mercury (Hg2+) could be captured by KCl solution and the Hg0 concentration (Hg0out) could be measured by the mercury analyzer. When the flue gas steam was turned to other side, the oxidized mercury could be reduced to Hg0 by SnCl2 solution and the total concentration of mercury (HgTout = Hg0out + Hg2+out) could be measured. Mean values were obtained from triplicate sample measurements to reduce uncertainties. As depicted above, the total Hg0 removal efficiency (ηT), the Hg0 oxidation efficiency (ηoxi), and the Hg0 adsorption efficiency (ηads) could be defined as follows:
where Hg0in and Hg0out represent the Hg0 concentration at the inlet and outlet of the reactor, respectively. HgTout represents the total mercury concentration outlet of the reactor. Since Hg0 and Hg2+ in the outlet of flue gas might be both adsorbed on the adsorbents, ηads covers the Hg0 and Hg2+ adsorbed on the adsorbents and ηoxi only represents the part of Hg2+ present in flue gas. t represents the accumulated time of each set of experiments, and t = 180 min in this work.
Results and discussion
Characterization of adsorbents
Adsorbent properties of SFS under various pyrolysis temperatures
ICP-MS technology was employed to determine the elemental composition and atomic ratios of the SFS pyrolyzed at various temperatures. Table 1 summarizes the yield and most of the abundant metal elements of SFS. With the increase of the pyrolysis temperature from 300 to 900 °C, the yield decreased from 72.3 to 36.1% owing to the decomposition of organic matter and the release of gases at higher temperatures. Among the detection of raw SFS, seven metal elements, i.e., iron (Fe), sodium (Na), calcium (Ca), potassium (K), aluminum (Al), zinc (Zn), magnesium (Mg), respectively, exhibited the highest content. Owing to the addition of ferrous sulfate as a sludge flocculant in a wastewater treatment plant, the content of S in raw sludge (R-S) reached 3.51%. Specifically, with the increase of the pyrolysis temperature, the content of all elements increased for the SFSs. Compared with the most abundant transition-metal element of Fe, the others can be ignored because their contents are low and they are inactive for mercury removal. Above all, the dominant abundance of Fe metal might play a crucial role for the removal of Hg0, which is far beyond the effect of the other metal elements.
BET analysis
Table 1 summarizes the textural properties of SFSs at various pyrolysis temperatures. Clearly, the BET-specific surface area, total pore volume, and average pore diameter were considerably affected by the pyrolysis temperature. The measured specific surface area was extremely small for SFS pyrolyzed at a temperature of less than 500 °C, but it rapidly increased from 5.5 to 338.4 m2/g with the increase in the temperature from 500 to 900 °C, indicative of the carbonaceous, aromatic bio-char matrix structure (Hung et al. 2017). Moreover, the increase in the pyrolysis temperature led to a high total pore volume and decreased average pore diameter. Kumar et al. (2017) and Lee et al. (2013) have reported that the result is related to the removal of greater amounts of labile material and release of volatile gases at a high pyrolysis temperature. Hence, SFS tends to exhibit a high specific surface area and total pore volume at high pyrolysis temperature, thereby facilitating mass transfer in adsorption and making it favorable for the Hg0 adsorption and oxidation (Li et al. 2017a).
XRD analysis
Figure 2 shows the XRD patterns of samples prepared under different pyrolysis temperatures. Clear peaks, except peaks corresponding to NaSO4 (PDF 24-1132) at 2θ values of 31.831°, 33.981°, 37.817°, and 52.490°, were not observed for the R-S. ICP-MS analysis revealed that Fe is more abundant than Na, but a crystal phase for FeSO4 is not detected in the XRD patterns, indicative of its high dispersion in R-S. At a pyrolysis temperature of 300 °C, peaks were observed at 2θ values of 24.138°, 33.152°, 35.611°, 40.854°, 54.089°, and 62.449°, corresponding to α-Fe2O3 (PDF 33-0664), related to the decomposition of FeSO4 (Yani and Zhang, 2010). With the further increase in the pyrolysis temperature, diffraction peaks at 2θ values of 30.272°, 35.684°, 57.400°, and 63.011° corresponding to γ-Fe2O3 (PDF 25-1402) were clearly detected, indicative of its magnetism. In case of SFS700, clear diffraction peaks were observed at 30.095°, 35.422°, 56.942°, and 62.515°, corresponding to magnetic Fe3O4 (PDF 19-0629) particles. This result may be related to the comprehensive effects of the reduction of Fe2O3 and decomposition of ferric sulfate at elevated temperatures, leading to the transformation of iron oxides to Fe3O4. For SFS800 and SFS900, a series of peaks corresponding to FeC3 revealed that an excessively high pyrolysis temperature enhances the reduction of iron oxide. According to the above analysis, SFS decomposing at an appropriate temperature possibly exhibited certain magnetism, which will be suitable as a prerequisite for the removal of mercury by using magnetic adsorbents. A series of performances between the pyrolysis temperature and magnetic properties will be further discussed in the sections of magnetic properties and XPS analysis.
Magnetic properties
As discussed in above XRD analysis, SFS showed certain magnetism after pyrolysis. A VSM technology was used to determine the magnetism property and the results are shown in Fig. 3. SFS prepared at a temperature of less than 500 °C exhibited an extremely low value for the maximum magnetization (Mm), but it clearly increased for SFS prepared at temperatures ranging from 500 to 800 °C. However, with the increase in the temperature to 900 °C, the measured Mm significantly decreased due to the reduction of iron oxides under high pyrolysis temperature conditions. The generated magnetic properties of these samples may be related to the decomposition of ferric sulfate at high temperatures to produce magnetic iron oxides (Yani and Zhang, 2010), which will be further discussed in the sections on TG-MS and XPS analyses. Results revealed that the sample prepared under the appropriate pyrolysis temperature exhibits superparamagnetism due to its minimized coercivity and a negligible magnetization hysteresis. This magnetization characteristic ensured that SFS700-900 can be easily separated from a fly ash mixture by magnetic separation, but it does not aggregate with the removal of the external magnetic field, demonstrating potential as a magnetic adsorbent.
TG–MS analysis
TG–MS was employed to investigate the thermal decomposition of SFS to evaluate the evolution of sulfide-related gases during pyrolysis. As can be observed from the shape of the TG and DTG curves, five distinct stages of thermal degradation were identified (Fig. 4a). The initial weight loss of 1.73% corresponded to the loss of physically adsorbed water molecules at temperatures less than 150 °C. The second weight loss stage occurred between 150 and 500 °C, and it accounted for approximately 31.69% of the total weight of the SFS weight loss, mainly corresponding to the volatilization and decomposition of organic matter in the sludge. In the process, SO2 was evolved on the basis of TG–MS detection (Fig. 4b), probably related to the decomposition of polysulfide compounds. Accompanied with a large amount of CO2 release, carbon-containing organic compounds were further decomposed at 500–700 °C. In the fourth stage, with the increase in the pyrolysis temperature from 700 to 850 °C, the low weight loss of 0.6% mainly corresponded to the reduction of iron oxides under the action of carbon-containing substances. Combined with Fig. 4a, the final stage weight loss of 0.3% revealed that it might correspond to the release of SO2 from sulfate decomposition. Throughout pyrolysis, only SO2 was detected as the evolved sulfide-related gases. The evolution of sulfite will be further examined by XPS analysis.
XPS analysis
To identify the chemical states of the major elements and obtain a better insight on the effect of pyrolysis temperature on SFS, XPS spectra of the samples were recorded. Fe 2p3/2 was deconvoluted into three peaks (Fig. 5a), where the presence of a peak at 711.2 eV and a satellite at ~ 713.0 eV revealed the presence of Fe3+, and the peak at 710.2 corresponded to Fe2+ (Xu et al. 2019b). Based on the quantitative analysis of the Fe 2p3/2 XPS spectra, Table 2 shows the results. In the R-S, Fe mainly existed as Fe2+. However, with the increase in the pyrolysis temperature from 300 to 800 °C, the Fe2+ concentration decreased from 67.1 to 36.0%. This change is attributed to the decomposition of FeSO4 to form iron oxide, releasing sulfur dioxide (Yani et al. 2010). Thus, the decomposition of ferrous sulfate can be represented as follows:
However, the Fe3+ concentration sharply decreased at 900 °C. In combination with XRD analysis, iron oxides were reduced to form iron–carbon compounds at 900 °C, corresponding to the pyrolysis at high temperature.
Figure 5 b and Table 2 summarize the XPS O 1s spectra. The O 1s peaks were deconvoluted into three peaks: absorbed –OH groups or molecular water at 532.8 eV (denoted as Oα′), chemisorbed oxygen at 531.4 eV (denoted as Oα), and the lattice oxygen at ~ 530.4 eV (denoted as Oβ) (Li et al. 2017b). Compared with that observed in other samples under various pyrolysis temperature, Oβ was not detected in R-S, and only 24.1% and 21.6% of Oβ were observed in SFS300 and SFS500, respectively, implying that marginal metal oxides are observed at a low pyrolysis temperature. That is, Fe2+ and Fe3+ mainly existed as metal salts at low-temperature pyrolysis conditions. Owing to the suitable decomposition temperature of FeSO4 from 813 to 953 K (Yani et al. 2010) (as discussed in reaction R1), Oβ increased to the highest percentage of 56.0% at 700 °C. Several studies have proposed that the presence of FexOy contributes to the formation of oxygen vacancies and unsaturated chemical bonds on the catalyst surface, favoring the removal of Hg0 (Li et al. 2017a; Liu et al. 2016). In combination with the XPS analysis of Fe 2p3/2 and O 1s, a high pyrolysis temperature contributed to the sharp decrease in the lattice oxygen content, leading to the decrease of the iron oxide content and significantly reducing the magnetic properties and catalytic activity.
In the S 2p spectra at various pyrolysis temperature, peaks were observed at 161.8, 162.8, 164.0, 166.7, 168.8, and 169.9 eV, corresponding to S2−, \({S}_2^{2-}\), polysulfur, SO32−, SO42−, and HSO4−, respectively (Liao et al. 2016; Li et al. 2018; Kong et al. 2018). Combined with the illustration shown in Table 2, S2− and \({S}_2^{2-}\) were not observed for the R-S or pyrolysis at temperatures of 300 °C and 500 °C, but S2− and \({S}_2^{2-}\) were clearly observed at 700 °C and 800 °C. Yani et al. (2010) reported that the presence of S2− and \({S}_2^{2-}\) corresponding to the reduced sulfite was generated through the reduction reaction. Meanwhile, reducing gases such as H2 and CO could be released from the decomposition of organic compounds at an appropriate pyrolysis temperature, which will facilitate the production of sulfide (including S2− and \({S}_2^{2-}\)) (Yani et al. 2010). In several studies, sulfide has high affinity to Hg0 and is considered as an effective active component for Hg0 removal (Xu et al. 2018; Liu et al. 2000; Kong et al. 2018). Meanwhile, no crystal phase of sulfide is detected in XRD patterns, indicative of its high dispersion on the sorbent surface, which will be obviously beneficial for the Hg0 removal. Owing to the highly oxygenated functional groups present in SFS, FeSx decomposed to evolve SO2 at a pyrolysis temperature of 900 °C, leading to the decrease of the S2− and \({S}_2^{2-}\)content. The results revealed that SFS pyrolyzed at the temperature of 700 °C and 800 °C can produce some sulfides, which will be of significance to the removal of Hg0.
FTIR analysis
Figure 6 shows the FTIR spectra of the samples prepared at various pyrolysis temperatures. The broad band observed at ~ 3427 cm−1 corresponded to the –OH stretching vibrations of hydrogen-bound hydroxyl groups (Yu et al. 2018). In addition, the peak observed at 2072 cm−1 corresponded to carbonyl sulfide from the polysulfide in R-S and SFS300 (Ohbi et al. 2008), but this peak disappeared at high pyrolysis temperatures due to decomposition. Additionally, the band visible at 1610 cm−1 was attributed to C–O stretching and bending vibrations (Yu et al. 2018) and the band observed at 865 cm−1 corresponded to the C=O or C=C aromatic-ring stretching vibrations (Yu et al. 2018; Wang et al. 2015). The band observed at 1425 cm−1 corresponded to the –CH2 stretching vibrations (Zhao et al. 2016; Milicevic et al. 2012), which became stronger at 700 °C, but this band disappeared with increasing temperature. The band observed at 1101 cm−1 corresponded to the C–O–H bonds in cellulose as well as to C–O stretching vibration of alcohols (R–OH) and aliphatic ethers (Milicevic et al. 2012; Wang et al. 2015). According to above analysis, SFS300 and SFS500 obviously have higher amount of oxygen functional groups and the content of these oxygen functional groups was decreased with the increase of pyrolysis temperature. The above results indicated that different pyrolysis temperatures can affect the quantity even the presence of some functional groups in the SFS, possibly affecting the removal of Hg0.
Hg0 removal performance
Hg0 removal tests
The Hg0 removal efficiency at various pyrolysis temperatures of SFSs was investigated at various reaction temperatures. As shown in Fig. 7, SFS300 and SFS500 exhibited a poor Hg0 removal efficiency. Previous studies (Tan et al. 2012) have revealed that oxygen functional groups such as carbonyl groups (C=O) and ester/lactone group (C(O)–O–C) are the active sites for Hg0 capture, which were conducive to the improvement of mercury removal. Compared with more oxygen functional groups present at a low pyrolysis temperature as shown in the FTIR analysis, the low Hg0 removal efficiency of SFS300 and SFS500 apparently proved that oxygen functional groups do not dominate the Hg0 removal efficiency relative to the comprehensive effects of more FexOy on the SFS surface, the production of some sulfides, and a larger BET surface (Tables 1 and 2; Fig. 5) generated at high-temperature pyrolysis. With the reaction temperature rising, the Hg0 removal efficiencies of SFS700 and SFS800 first increased at low-temperature range and then decreased at the temperature above 125 °C. This might be attributed to the combination effects of the inapparent catalytic activity at low temperature and inhibition of physisorption for Hg0 at high reaction temperature (Zhang et al. 2014; Liu et al. 2018). SFSs exhibited the highest mercury removal efficiency at 125 °C and increased in the following order: SFS900 < SFS800 < SFS700. It can be seen from the Figure S2 that, although the BET surface of SFS700 was less than those of SFS800 and SFS900 (Table 1), it exhibited the highest Hg0 adsorption capacity and oxidation ability and the total removal efficiency was reached to 80.7%. Further, the adsorption capacity and oxidation ability were all decreased with the pyrolysis temperature. From the results of previous XRD (Fig. 2) and XPS analyses (Fig. 5; Table 2), high-temperature pyrolysis condition leads to the decrease in the sulfides (including S2− and \({S}_2^{2-}\)) and iron oxide content, as well as the formation of a large amount of iron carbide, possibly related to the reduction of the mercury adsorption and oxidation capacity. As for SFS900, owing to the high specific surface area and marginal FexOy on the sample surface, the mercury removal was likely to occur dominantly in the form of physisorption, leading to the decrease in the mercury removal efficiency with the increase in the reaction temperature. The results indicated that the optimal reaction temperature is ~ 125 °C and that the optimal pyrolysis temperature is 700 °C. Most of the mercury adsorption materials can be regenerated by heating treatment at 500 °C under the condition of nitrogen atmosphere (Li et al. 2018; Liao et al. 2016). Figure S3 shows the mercury removal capacity and magnetism of SFS700 over 4 Hg0 capture and regeneration cycles. During the 4 cycles, the Hg0 removal efficiency and magnetization of SFS700 exhibited no significant decrease, which indicated that SFS700 could be used as recyclable adsorbent for mercury removal.
Effect of flue gas components
According to the composition of real coal-fired flue gas, the effects of gases including O2, SO2, and H2O were investigated on the Hg0 removal at 125 °C. As shown in Fig. 8, the mercury removal over SFS700 in the absence of O2 was 75.5% during 180 min of reaction. With the introduction of 5% O2 into the flue gas atmosphere, the mercury removal efficiency was increased to 80.7%. This value is in agreement with the reports by Duan et al. (2019). Li et al. (2017c) agreed that Hg0 adsorbed on the adsorbent surface can react with lattice oxygen and/or chemisorbed oxygen to form chemical adsorption sites and then form HgO, which can improve Hg0 removal. Subsequently, the consumed lattice oxygen and/or chemisorbed oxygen can be replenished by gas-phase oxygen (Liu et al. 2018; Qiao et al. 2009). However, with the increase in the O2 content to 10%, the mercury removal efficiency did not clearly increase, indicating that 5% of O2 concentrations can achieve the supplementation of the lost lattice O and promote the removal.
As an inevitable influencing factor, high SO2 concentrations can lead to the deactivation of conventional adsorbents such as active carbon or transition-metal oxides. In this work, the effect of SO2 on Hg0 removal was investigated under 5% O2. With the introduction of 500 ppm SO2 into the flue gas, the mercury removal efficiency was slightly increased from 80.7 to 81.3%, indicative of the positive impact on mercury removal (Fig. 8). As the SO2 content increased to 1000 ppm and 2000 ppm, the mercury removal efficiency was 79.1% and 78.3%, respectively. The results indicated that high concentration of SO2 exhibited a slight inhibition effect on the mercury removal, possibly owing to the strong affinity of S2− to Hg0. The mercury removal over sulfide adsorbent was not easily interfered by SO2 concentration (Li et al. 2016; Liu et al. 2019; Xu et al. 2017). Hence, SFS700 exhibited sulfur resistance for the capture of gaseous Hg0 under a low or high SO2 concentration.
To examine the effect of H2O vapor on the mercury removal over SFS700, flue gases under water vapor conditions (5% H2O, 8% H2O, and SFG conditions) were prepared. The presence of H2O exhibited an obvious inhibition effect on the Hg0 removal (Fig. 8). With the introduction of 5% H2O and 8% H2O into flue gases, the mercury removal efficiency was maintained at 69.1% and 64.3%, respectively. However, the mercury removal capacity under the SFG condition (5% O2, 5% H2O, 500 ppm SO2) did not exhibit a significant inhibition effect compared with that under 5% O2. Morris et al. (2012) have reasoned that H2SO4 can be formed on the adsorbent surface under SFG conditions, which does not lead to the pore blockage, but it improves the Hg0 adsorption via surface oxidation. Acidic materials can increase the amount of micropores as well as the surface area and improve the removal efficiency. In conclusion, SFS700 exhibited a certain H2O vapor resistance under the simulated flue gas condition.
Identification of Hg0 removal mechanism
XPS was employed to analyze the valence chemical states of the elements on spent SFS700 under SFG conditions. Figure 9 shows the Fe 2p, O 1s, S 2p, and Hg 4f XPS spectra of spent SFS700. Compared with the XPS analysis of fresh SFS700 (Fig. 5; Table 2), the spent ISBC700 sample exhibited that the content of Fe3+ (~ 711.2 eV and ~ 713.0 eV) (Xu et al. 2019b) and lattice oxygen (Li et al. 2017b) decreased by 5.4% and 22.5%, respectively, while the ratio of chemisorbed oxygen (Oα, ~ 531.4 eV) and the content of molecular water or absorbed –OH groups (Oα′, ~ 532.8 eV) clearly increased. The results indicated that a part of lattice oxygen (Oβ) is consumed during the removal of mercury where FexOy is involved in the reaction. The S 2p spectra of spent SFS700 showed peaks at 168.8 and 163.2, corresponding to SO42− and S2−, respectively (Fig. 9c) (Xu et al. 2017). Compared with that observed in fresh SFS700 (Fig. 5c), in spent ISBC700, the position of sulfide peaks slightly changed, indicating that sulfides are involved in mercury removal. To verify the hypothesis for the role of Fe, O, and S during the removal of elemental mercury, Hg 4f was further monitored. As shown in Fig. 9d, two binding energies were observed at 101.8 eV and 100.5 eV, which were attributed to HgO and HgS, respectively (Xu et al. 2017; Ma et al. 2019). The presence of HgO indicated that the adsorbed mercury is oxidized by the surface-active sites of SFS700. According to the Mars-Maessen mechanism (Ambrosy et al. 2019; Granite et al. 2000), elemental mercury can be first adsorbed on the surface-active sites, followed by the facile oxidation of adsorbed Hg0 (Hg(ad)) to HgO by surface oxygen species (Oα, Oβ). Subsequently, the consumed surface oxygen species can be replenished by gas-phase oxygen (Zhang et al. 2014; Li et al. 2017b). In addition, sulfides can react with Hg(ad) to form HgS on its surface with the participation of metal ions. Results revealed that both active oxygen and sulfides over sample surface contributed to the Hg0 oxidation removal. Above analyses indicate that the SFS has optimal content of FexOy, suitable BET surface and the production of some sulfides and it can be utilized for Hg0 removal after one-step pyrolysis at optimal temperature of 700 °C instead of any chemical modification and complex preparation process. Moreover, the good magnetism performance was obtained and it will be conducive to the recovery and utilization of these adsorbents in flue gas. In conclusion, this study has guiding significance for this sludge resource utilization and engineering practices.
Conclusions
In this study, SFS was pyrolyzed at various temperatures for investigating its efficiency for Hg0 removal. At the optimal pyrolysis temperature of 700 °C, SFS700 exhibited the highest Hg0 removal efficiency of 80.7% at 125 °C (5% O2 condition) due to the production of some sulfides, optimal content of FexOy, and a suitable BET surface. In addition, the good magnetism performance will be conducive to the recovery and utilization of adsorbents in flue gas. Only a small amount of FexOy and extremely weak magnetism were generated at a pyrolysis temperature of less than 500 °C. Pyrolysis at high temperatures led to the significant increase of the BET surface. However, with the increase in the pyrolysis temperature to greater than 800 °C, SFS exhibited intensive agglomeration and led to the drastic increase in the crystalline-phase particle size, inhibiting the removal of Hg0. Performance activity test also indicated that O2 and low concentration of SO2 improved the Hg0 removal, while the H2O vapor and SO2 with high concentration showed an inhibition effect. Good resistance for the adsorbent to moderate concentrations of SO2 and H2O was observed. This study indicated that the SFS can be used as recyclable adsorbent for Hg0 removal without any chemical modification after undergoing one-step pyrolysis, which is of great significance for the resource utilization and engineering practices.
References
Ambrosy JM, Pasel C, Luckas M, Bittig M, Bathen D (2019) A detailed investigation of adsorption isotherms, enthalpies, and kinetics of mercury adsorption on nonimpregnated activated carbon. Ind Eng Chem Res 58:4208–4221
Carmen SDR, Luís MM, Rui ARB (2013) Treatment of textile dye wastewaters using ferrous sulphate in a chemical coagulation/flocculation process. Environ Technol 34:719–729
Dong J, Xu ZH, Kuznicki SM (2009a) Magnetic multi-functional nano composites for environmental applications. Adv Funct Mater 19:1268–1275
Dong J, Xu ZH, Kuznicki SM (2009b) Mercury removal from flue gases by novel regenerable magnetic nanocomposite sorbents. Environ Sci Technol 43:3266–3271
Duan XL, Yuan CG, Jing TT, Yuan XD (2019) Removal of elemental mercury using large surface area micro-porous concob activated carbon by zinc chloride activation. Fuel 239:830–840
Granite EJ, Pennline HW, Hargis RA (2000) Novel sorbents for mercury removal from flue gas. Ind Eng Chem Res 39:1020–1029
Hamzehlouyan T, Sampara C, Li J, Kumar A, Epling W (2014) Experimental and kinetic study of SO2 oxidation on a Pt/γ-Al2O3 catalyst. Appl Catal B 152:108–116
Hung CY, Tsai WT, Chen JW, Lin YQ, Chang YM (2017) Characterization of biochar prepared from biogas digestate. Waste Manage Assoc 66:53–60
Kong LN, Zou SJ, Mei J, Geng Y, Zhao H, Yang SJ (2018) Outstanding resistance of H2S-modified Cu/TiO2 to SO2 for capturing gaseous Hg0 from non-ferrous metal smelting flue gas:performance and reaction mechanism. Environ Sci Technol 52(1000):3–10010
Kushwaha JP, Srivastava VC, Mall ID (2010) Treatment of dairy wastewater by inorganic coagulants: parametric and disposal studies. Water Res 44:5867–5874
Kumar U, Maroufi S, Rajarao R, Mayyas M, Mansuri I, Joshi RK, Sahajwalla V (2017) Cleaner production of iron by using waste macadamia biomass as a carbon resource. J Clean Prod 158:218–224
Lee SS, Lee JY, Keener TC (2009) Bench-scale studies of in-duct mercury capture using cupric chloride-impregnated carbons. Environ Sci Technol 43:2957–2962
Lee Y, Eum PRB, Ryu C, Park YK, Jung JH, Hyun S (2013) Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour Technol 130:345–350
Liu W, Vidic RD, Brown TD (2000) Impact of flue gas conditions on mercury uptake by sulfur-impregnated activated carbon. Environ Sci Technol 34:154–159
Liu Y, Kelly DJA, Yang H, Lin CCH, Kuznicki SM, Xu Z (2008) Novel regenerable sorbent for mercury capture from flue gases of coal-fired power plant. Environ Sci Technol 42:6205–6210
Li HL, Zhu L, Wang J, Li LQ, Shi KM (2016) Development of nano-sulfide sorbent for efficient removal of elemental mercury from coal combustion fuel gas. Environ Sci Technol 509:551–9557
Liu T, Man CY, Guo X, Zheng CG (2016) Experimental study on the mechanism of mercury removal with Fe2O3 in the presence of halogens:role of HCl and HBr. Fuel 173:209–216
Liao Y, Chen D, Zou SJ, Xiong SC, Xiao X, Dang H, Chen TH, Yang SJ (2016) A recyclable naturally derived magnetic pyrrhotite for elemental mercury recovery from the flue gas. Environ Sci Technol 50:10562–10569
Liu DJ, Zhou WG, Wu J (2017) Effect of Ce and La on the activity of CuO/ZSM-5 and MnOx/ZSM-5 composites for elemental mercury removal at low temperature. Fu el 194:115–122
Li HH, Wang Y, Wang SK, Wang X, Hu JJ (2017a) Removal of elemental mercury in flue gas at lower temperatures over Mn-Ce based materials prepared by co-precipitation. Fuel 208:576–586
Li HH, Wang SK, Wang X, Tang N, Pan SW, Hu JJ (2017b) Catalytic oxidation of Hg0 in flue gas over Ce modified TiO2 supported Co-Mn catalysts: characterization, the effect of gas composition and co-benefit of NO conversion. Fuel 202:470–482
Li HH, Wang SK, Wang X, Hu JJ (2017c) Activity of CuCl2-modified cobalt catalyst supported on Ti-Ce composite for simultaneous catalytic oxidation of Hg0 and NO in a simulated pre-sco process. Chem Eng J 316:1103–1113
Li N, Wei HQ, Duan YF, Tang HJ, Zhao SL, Hu P, Ren SJ (2018) Experimental study on mercury adsorption and adsorbent regeneration of sulfur-loaded activated carbon. Energy Fuel 32:11023–11029
Liu ZY, Yang W, Xu W, Liu YX (2018) Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine. Chem Eng J 339:468–478
Liu W, Xu HM, Liao Y, Quan ZW, Li SC, Zhao SJ, Qu Z, Yan NQ (2019) Recyclable CuS sorbent with large mercury adsorption capacity in the presence of SO2 from non-ferrous metal smelting flue gas. Fuel 23:5847–5854
Ma YP, Mu BL, Zhang XJ, Yuan DL, Ma C, Xu HM, Fang SM (2019) Graphene enhanced Mn-Ce binary metal oxides for catalytic oxidation and adsorption of elemental mercury from coal-fired flue gas. Chem Eng J 358:1499–1506
Milicevic S, Boljanac T, Martinovic S, Vlahovic M, Milosevic V, Babic B (2012) Removal of copper from aqueous solutions by low cost adsorbent-Kolubara lignite. Fuel Process Technol 95:1–7
Morris EA, Kirk DW, Jia CQ, Morita K (2012) Roles of sulfuric acid in elemental mercury removal by activated carbon and sulfur-impregnated activated carbon. Environ Sci Technol 46:7905–7912
Ohbi DS, Purewal TS, Shah T, Siores E (2008) Crosslinking reaction mechanism of diisopropyl xanthogen polysulfide accelerator in bromobutyl elastomer for medical device applications. J Appl Polym Sci 107:4013–4020
Pan Z, Tian J, Xu G, Li J, Li G (2011) Characteristics of adsorbents made from biological, chemical and hybrid sludges and their effect on organics removal in wastewater treatment. Water Res 45:819–827
Park J, Lee SS (2018) Adsorption of mercury by activated carbon prepared from dried sewage sludge in simulated flue gas. J Air Waste Manage Assoc 68:1077–1084
Qiao SH, Chen J, Li JF, Qu Z, Liu P, Yan NQ (2009) Adsorption and catalytic oxidation of gaseous elemental mercury in flue gas over MnOx/alumina. Ind Eng Chem Res 48:3317–3122
Rui B, António P, Marco SL, José AP (2010) Combination of long term aerated storage and chemical coagulation/flocculation to winery wastewater treatment. Desalination 263:226–232
Scala F, Clack H (2008) Mercury emissions from coal combustion: modeling and comparison of Hg capture in a fabric filter versus an electrostatic precipitator. J Hazard Mater 152:616–623
Tan ZQ, Sun LS, Xiang J, Zeng HC, Liu ZH, Hu S (2012) Gas-phase elemental mercury removal by novel carbon-based sorbents. Carbon 50:362–371
Wilcox J, Rupp E, Ying SC, Lee DH, Negreira AS, Kerchofer A (2012) Mercury adsorption and oxidation in coal combustion and gasification processes. Int J Coal Geol 90:4–20
Wang XQ, Wang P, Ning P, Ma YX, Wang F, Guo XL, Lan Y (2015) Adsorption of gaseous elemental mercury with activated carbon impregnated with ferric chloride. RSC Adv 5:24899–24907
Xu HM, Yuan Y, Liao Y, Xie JK, Qu Z, Yan NQ (2017) [MoS4]2− cluster bridges in Co-Fe layered double hydroxides for mercury uptake from S-Hg mixed flue gas. Environ Sci Technol 51:10109–10116
Xu W, Hussain A, Liu YX (2018) A review on modification methods of adsorbents for elemental mercury from flue gas. Chem Eng J 346:692–711
Xu Y, Luo GQ, He SW, Deng FF, Pang QC, Xu YQ, Yao H (2019a) Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe(NO3)3−laden wood and polyvinyl chloride waste. Fuel 239:982–990
Xu Y, Luo GQ, Pang QC, He SW, Deng FF, Xu YQ, Yao H (2019b) Adsorption and catalytic oxidation elemental mercury over regenerable magnetic Fe-Ce mixed oxides modified by non-thermal plasma treatment. Chem Eng J 358:1454–1463
Yang S, Guo Y, Yan N, Wu D, He H, Xie J, Qu Z, Yang C, Jia J (2010) A novel muti-functional magnetic Fe-Ti-V spinel catalyst for elemental mercury capture and callback from flue gas. Chem Commun 46:8377–8379
Yani S, Zhang DK (2010) An experimental study of sulphate transformation during pyrolysis of an Australian lignite. Fuel Process Technol 91:313–321
Yang SJ, Yan NQ, Guo YF, Wu DQ, He HP, Qu Z, Li JF, Jia PZ (2011a) Gaseous elemental mercury capture from flue gas using magnetic nanosized (Fe3-xMnx)1-δO4. Environ Sci Technol 45:1540–1546
Yang SJ, Guo YF, Yan NQ, Wu DQ, He HP, Xie JK, Qu Z, Jia JP (2011b) Remarkable effect of the incorporation of titanium on the catalytic activity and SO2 poisoning resistance of magnetic Mn-Fe spinel for elemental mercury capture. Appl Catal B Environ 101:698–708
Yang SJ, Guo YF, Yan NQ, Wu DQ, He HP, Qu Z, Jia JP (2011c) Elemental mercury capture from flue gas by magnetic Mn-Fe spinel: effect of chemical heterogeneity. Ind Eng Chem Res 50:9650–9656
Yang JP, Zhao YC, Zhang JY, Zheng CG (2014) Regenerable cobalt oxide loaded magnetosphere catalyst from fly ash for mercury removal in coal combustion flue gas. Environ Sci Technol 48:14837–14843
Yang X, Xu GR, Yu HR, Zhang Z (2016) Preparation of ferric-activated sludge-based adsorbent from biological sludge for tetracycline removal. Bioresour Technol 211:566–573
Yu J, Sun LS, Berrueco C, Fidalgo B, Paterson N (2018) Influence of temperature and particle size on structural characteristics of chars from Beechwood pyrolysis. J Anal Appl Pyrolysis 130:127–134
Yang S, Liu C, Liu Z, Yang B, Xiang K, Zhang C (2018) High catalytic activity and SO2-poisoning resistance of Pd/CuCl2/γ-Al2O3 catalyst for elemental mercury oxidation. Catal Commun 105:1–5
Zhang AC, Zheng WW, Song J, Hu S, Liu ZC, Xiang J (2014) Cobalt manganese oxides modified titania catalysts for oxidation of elemental mercury at low flue gas temperature. Chem Eng J 236:29–38
Zhou Q, Duan YF, Hong YG, Zhu C, She M, Zhang J (2015) Experimental and kinetic studies of gas-phase mercury adsorption by raw and bromine modified activated carbon. Fuel Process Technol 134:325–332
Zhao B, Yi HH, Tang XL, Li Q, Liu DD, Gao FY (2016) Copper modified activated coke for mercury removal from coal-fired flue gas. Chem Eng J 286:585–593
Zou S, Liao Y, Huang SN, Geng Y, Yang S (2017) H2S-modified Fe-Ti spinel:a recyclable magnetic sorbent for recovering gaseous elemental mercury from flue gas as a co-benefit of wet electrostatic precipitators. Environ Sci Technol 51:3426–3434
Acknowledgments
This work was supported by the Test Center of Wuhan University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 582 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Li, H., He, Z. et al. Removal of elemental mercury from flue gas using the magnetic Fe-containing carbon prepared from the sludge flocculated with ferrous sulfate. Environ Sci Pollut Res 27, 30254–30264 (2020). https://doi.org/10.1007/s11356-020-08133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08133-4