Abstract
In the developing world, rapid urbanization and industrialization produces an enormous volume of wastes daily. This study was aimed to explore the potential and risks associated with sewage sludge through the characterization and fractionation technique. Sewage sludge samples were collected from various wastewater treatment in five different cities of Pakistan. Considerable amounts of macro-elements were detected in all types of sewage sludge samples. The pHw of all sewage sludge were neutral to slightly alkaline in reaction. Total organic carbon (TOC) was maximum (18.73%) with Coca-Cola sewage sludge (CSS) while the minimum (14.69%) was with Water and Sanitation Agency (WASA) sewage sludge (WSS). Percent relative distribution of cadmium (Cd) was higher in residual fraction (F4) up to 52% in the Nestle wastewater treatment plant, Sheikhupura (NSS). The chromium (Cr) concentration in Kasur sewage sludge (KSS) was extremely in mobile fraction (exchangeable) as compared with all other sludge samples, therefore showing a higher level of risk assessment code. While in the case of Iron (Fe), mobility was less and its maximum portion was noted in residual fraction (F4) of all sewage sludge samples. Percent distribution of manganese (Mn) showed variable trends for different sewage sludge samples. Zinc (Zn) concentration showed high mobility (exchangeable fraction) in case of NUST wastewater treatment plant, Islamabad (NTS) (31.16%) and WSS (37.83%) as compared with other sewage sludges. The risk assessment code indicated that Zn and Ni had a medium level of risk with I-9 Sector wastewater treatment plant, Islamabad (ISS), CSS, KSS, and NSS whereas these pose a high risk with NTS and WSS. Based on physicochemical properties, nutrients, trace elements, mobility, and risk assessment code, it was concluded that KSS should not be recommended at any application rate while NTS and WSS may be used at low application rates whereas ISS, CSS, and NSS may be used for agricultural crop production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development, industrialization, and urbanization, the massive volume of sewage sludge (SS) is being produced in the urban areas of the major cities of Pakistan (Riaz et al. 2018a). The safe disposal of sewage sludge with minimal impact on the environment has surfaced as a serious concern in recent times (Singh and Agrawal 2008). Among many, land disposal as organic fertilizer is considered as the most popular and economical method of SS management. It is applied to agricultural lands because it supplies many essential nutrients to crop plants and modifies soil physical, chemical, and biological properties (Latare et al. 2014; Riaz et al. 2018a; Cai et al. 2019). Several researches have shown positive, beneficial impacts of SS application like increment in biomass production and crop productivity and improvement in physicochemical properties of soils (Murtaza et al. 2012; Riaz et al. 2018b). However, there is a severe apprehension that SS also contains excessively high concentrations of potentially toxic elements (PTE) that restricts its use as a substitute for fertilizer. Various SSs exhibit wide variations in their physical, chemical, and biological properties depending on the origin of raw wastewater such as municipal or industrial effluents.
Further to this, characteristics of SS typically depend on the type and the degree of treatment processes followed and finishing sludge treatment (Haynes et al. 2009; Smith 2009). Studies showed that the SS processing method significantly affected not only the beneficial elements but also potentially toxic trace element concentration, fractionation, availability, and mobility. These trace elements like cadmium (Cd), chromium (Cr), iron (Fe), lead (Pb), and zinc (Zn) are naturally non-biodegradable with complex chemistry and can accumulate in the soil and water for a long time, enter the food chain, and thereby bioconcentrate in the environment (Zhang et al. 2017; Liu et al. 2018; Riaz et al. 2018b). The total concentrations of trace elements in SS indicate the entire level, while the mobilization capacity and the potential risk to the environment mainly depends on specific chemical speciation/fraction of metals in sewage sludge (Zhang et al. 2017). With the installation of a large number of wastewater treatment plants in Pakistan, a large volume of SS is being produced daily (Riaz et al. 2018b). Farmers use SS on croplands for nutrient enhancement. Information resolved from the total metal analysis in SS was not sufficient to make a decision either it is accessible to plant or not and ultimately affect the food chain. Sequential extraction is the only way to estimate metal concentration associated with mobile or immobile forms. Carbonate-bound concentration of metals is also easily accessible to plants as they can be released at lowered soil reaction. An immobile form of metal can be bound with manganese oxides, amorphic iron oxides, and silicates, and also present in the crystalline structures of minerals. The PTEs are bounded with organic and inorganic contents in the SS (Liu et al. 2016; Braga et al. 2017). Thus, the evaluation of SS toxicity by chemical speciation is essential before making a decision on its suitability for agricultural application. A risk assessment code (RAC) is employed to evaluate the potential environmental risk of trace elements in sewage sludges and has been widely used in the environmental sciences to ascertain trace elements toxicity (Huang et al. 2011). The risk assessment code assesses the available trace elements in SS by applying the percentage of the readily available and toxic fraction (denoted as F1) of the total trace elements. The trace elements that are weakly bound to sewage sludge could pose a greater risk to the environment due to their higher bioavailability potential.
To the best of our knowledge, classification of various SSs from all over Pakistan has not been done so far. This study was conducted to explore the potential risk and fertility status of SSs generated in Pakistan for use in agriculture and classify them based on RAC.
Materials and methods
Collection and preparation of sewage sludge samples
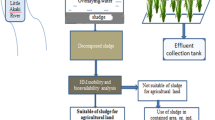
Three sub-samples of SS of six primary wastewater treatment plants (WWTPs) were collected from various cities of Pakistan (Fig. 1). These samples included I-9 Sector wastewater treatment plant, Islamabad (ISS); Coca-Cola Company wastewater plant, Lahore (CSS); Water and Sanitation Agency (WASA) Treatment Plant, Faisalabad (WSS); Kasur wastewater treatment plant (KSS); NUST wastewater treatment plant, Islamabad (NTS); Nestle wastewater treatment plant, Sheikhupura (NSS). The sampling was performed by the grab method; it is the least expensive method of collecting a reprehensive sample from huge WWTPs (Telliard 1989).
Each collected SS sample was adequately dried (air and oven-dry) to achieve constant weight and ground. After grinding, the samples were screened through a 2-mm sieve. The practice of passing only a portion of the ground sample through the sieve and discarding the remainder is erroneous. This introduces a positive bias in the sample as the rejected part may include sludge elements with differential fertility. Therefore, the entire sample was passed through the sieve except for concretions and pebbles of more than 2 mm. Sieving was repeated and samples were grinded until all aggregate particles were fine enough to pass through the sieve and only pebbles, organic residues, and concretions remain.
Measurement of physicochemical properties and trace elements
The pHw and ECw were measured by preparing 1:10 sludge/water suspension (w/v) by adopting the method mention in USEPA ( 2005). The total organic carbon (TOC) contents were determined in the collected samples following Carballa et al. (2005) on TOC Analyzer (Shimadzu: TOC-L CSH, Kyoto, Japan). The TOC concentrations were calculated by the difference between total carbon (TC) and inorganic carbon (IC). Total nitrogen (TN) in sewage sludge was determined by distillation after the digestion of individual SS samples with concentrated sulfuric acid (H2SO4). Total phosphorus (TP) was analyzed after digesting the samples in 3:1 mixture of nitric acid (HNO3) and perchloric acid (HClO4), followed by calorimetric determination using the molybdophosphoric acid method on a spectrophotometer (UV 5100 B, Metash, China) at 470 nm (Olsen and Sommers 1982). The extract of the digestion made for TP analysis was also used for total potassium (TK) analysis. The total concentrations of 19 trace elements like aluminum (Al), antimony (Sb), arsenic (As), barium (Ba), bismuth (Bi), boron (B), calcium (Ca), cadmium (Cd), Chromium (Cr), cobalt (Co), copper (Cu), Iron (Fe), Lead (Pb), Lithium (Li), Magnesium (Mg), Manganese (Mn), Nickel (Ni), selenium (Se), silver (Ag), and zinc (Zn) in SS samples were determined in triplicate following wet digestion with aqua regia (HNO3: HCl, 1:3) (USEPA 2005). The digests were filtered through Whatman No. 42 filter paper and analyzed for elements using an inductively coupled plasma optical emission spectrometer (ICP Optical Emission Spectrometer, Optima 5300DV ICP-OES, Perkin Elmer, USA).
Sequential extraction of trace elements in sewage sludge
The Community Bureau of Reference (BCR) three-step sequential extraction procedure described by Ure et al. (1993) was used to evaluate the distribution of trace elements Cd, Cu, Cr, Fe Mn, Ni, Pb, and Zn in four fractions: acid soluble/exchangeable fraction (F1, exchangeable metal and carbonate-associated fractions); reducible fraction (F2, fraction associated with Fe and Mn oxides); oxidizable fraction (F3, fraction bound to organic matter); and residual fraction (F4). The analysis was performed on four parallel samples of different sewage sludges. Air-dried sludge samples (0.5 g) were taken in 50 mL polypropylene centrifuge tubes, and reagents specified for each fraction were added. After extractions, the liquid phase was separated by centrifugation at 4000 rpm for 20 min. The supernatant was then decanted into a full mouth plastic bottle and kept in the refrigerator at five ±2 °C till estimation. The residual metal fraction was determined by digesting the residues of step 3 under conditions. The extracts were analyzed for different elements on inductively coupled plasma optical emission spectrometer (ICP Optical Emission Spectrometer, Optima 5300DV ICP-OES, Perkin Elmer, USA). The matrix effects were countered by preparing the standards in solutions identical to that of extracting reagent used for each fraction.
Risk assessment
The RAC was also applied to estimate the environmental risk associated with heavy metal pollution in sewage sludge. It classifies the risk levels based on the chemical speciation of heavy metals. RAC assesses the availability of metals by applying a scale to the percentage of metals present in the F1 fraction. Risk classification in terms of RAC (Zhang et al. 2017). RAC was calculated as follows:
where F1 is the concentration of trace elements in exchangeable fraction (mg kg−1), and HM represents the total concentration of trace elements (mg kg−1).
Quality assurance
All chemicals used in this study were chromatographically pure. All laboratory consumables and centrifuge tubes were dipped in 20% nitric acid solution which was prepared with ultrapure water overnight and then flushed thoroughly with ultrapure water. Determination in ICP was carried out in three replicates, and the reported results were the average of three replicated along with the standard deviation. All determinations were performed at room temperature.
Results and discussion
Physicochemical properties
Table 1 shows some selected physicochemical properties of the different sewage sludges. The highest ECw (12.46 ± 0.20 dS m−1) was found for WSS, and the lowest ECw was recorded with ISS (4.11 ± 0.07 dS m−1). The pHw of all the collected samples ranged from 6.69 to 7.56. The lowest pH value was found with ISS (6.69 ± 0.12), while the highest was with CSS (7.56 ± 0.15). The highest total organic carbon (TOC) content was found in CSS (18.73%) followed by ISS (18.58%), while the lowest in WSS (14.69%). The color of the collected samples was mostly black (Table 1). The EC provides information about the number of soluble salts present in the material (Lopez-Moreno et al. 2015). The sewage sludge obtained from Water and Sanitation Agency, Faisalabad, wastewater treatment plant had the highest EC due to more soluble salts present in sewage water as the groundwater of Faisalabad city is brackish (Khaliq 2014). Application of SS having such type of physicochemical properties (e.g., WSS) under arid to semi-arid conditions may cause an increase in soil salinity and affect the soil-water balance, plant nutrient’s availability, microbial activity, and final crop yields (Li et al. 2013), while the application of other sewage sludge samples having low EC, neutral pH, and a significant quantity of N, P, K, and TOC may improve the soil fertility status of certain soil types. The pH of all sewage sludge samples was neutral to slightly alkaline (Table 1). Usually, the sewage sludge produced from municipal/city wastes or industrial wastes has neutral to slightly alkaline pH. It is assumed that at neutral pH, there will be a high availability of trace elements (Latare et al. 2014; Singh and Agrawal 2010). Another property is the color of SS, which indicates the maturity and stability of the SS product (Sullivan and Miller 2001). The color of SS samples ranged from gray to dark black (Table 1) that could be due to the presence of colored chemicals released from various textile industries into wastewater streams. Further, the wastewaters generated from dying industries may contain multiple contaminants like acids, toxic compounds, and color compounds (Fan and Zhang 2008). Out of these, color is the first contaminant to be predictable in SS. Alkaline-black is one of the most commonly used dyes in industries, which is the main reason for the black color in wastewater streams (Solpan and Guven 2002).
All the samples had a considerable amount of N, P, and K nutrients. The highest N contents were found in ISS (4597 mg kg−1 DW), followed by CSS (3124 mg kg−1 DW). The trend for N contents was as ISS > CSS > NTS > NSS > KSS > WSS. The highest concentration of P was found in NSS (5009 mg kg−1 DW), and the least was recorded in WSS (1021 mg kg−1 DW). Maximum K was found in NSS (153 mg kg−1 DW) and least in CSS (27 mg kg−1 DW). The trend for K contents in SS samples was as NSS > NTS > KSS > WSS > ISS > CSS. The primary plant nutrient associated with SS is N (Zebarth et al. 2000). Among the collected SS samples, maximum N contents were found in ISS, which may be attributed to some industrial wastes where nitrogen gas is used for the preparation of their products. In literature, N contents in sewage sludge are reported in the range from 0 to 5.0% (Zebarth et al. 2000). The concentration of P in sewage sludge varies even more broadly than N (Warman and Termeer 2005) while in the soil-plant system, P uptakes by plants are regulated by soil Al, Fe, and Ca content. The K contents in sewage sludge are usually low (0.15–0.40%) in concentration (Haynes et al. 2009). In our collected samples, the concentration of K was low as compared to N and P.
Total concentration of trace elements
The highest concentration of total B was noted in CSS and the lowest was recorded in ISS (Table 2). The highest Cu contents were found in WSS (2.05 mg kg−1 DW), while the lowest Cu contents were recorded in KSS (0.46 mg kg−1 DW). The trend for Cu was WSS > ISS > CSS > NTS > NSS > KSS. Boron is used in the fiberglass and glass industries because of its mechanical qualities. Boron is also used in production of detergents and bleaches as well (Parks and Edwards 2005). The potential sources of Cu in sewage sludge and composts include cleaning and plating, baths, pulp, industrial chemicals (Bouzid et al. 2008). The highest concentration of Cu in WSS may be due to the mixing of municipal/city solid wastes adjacent to the Cu industry. A considerable amount of Mn was also present in all collected samples. A large quantity of Fe was also present in the collected samples, which ranged from 0.1 to 2.0%. The highest (value) Zn concentration was detected with NTS and lowest (value) in KSS, showing the trend as NTS > ISS > CSS > NSS > WSS > KSS. The concentration of Fe was too high in all collected samples of sewage sludge. The main sources of Fe in sewage sludge are industrial wastewater, household waste, and city wastes. The presence of a considerable amount of these beneficial nutrients (B, Co, Mn, Mo, and Zn) in all collected samples (Table 2) makes these products valuable for agricultural application. According to Council Directive of the European Communities (1986), the concentration of B, Cu, Co, Mn, Mo, and Zn were well below the permissible levels in the analyzed sewage sludge samples. Moreover, the highest concentration of T-As was found in CSS (26.54 mg kg−1 DW), followed by NSS (7.05 mg kg−1 DW). The analysis showed that the highest T-Cd concentration was found in ISS (3.29 mg kg−1 DW), followed by NSS (2.05 mg kg−1 DW) as compared with all SS samples (Table 3). In the case of Cr, the highest concentration was recorded in KSS (17,115 mg kg−1 DW). A considerable amount of T-Ni, T-Sb, and T-Se were also present in all collected SS samples. The highest T-Pb concentration was found in CSS (124.3 mg kg−1 DW) and lowest in KSS (18.8 mg kg−1 DW). The concentration of trace elements in the sewage sludge depends on various aspects such as (a) origin of sewage, (b) processes of sewage treatment, and (c) sludge treatment degrees. In our study, the highest concentration of total As in CSS was due to the use of packing materials of beverages and cold drinks which contain a sufficient amount of As in their composition (Montanari 2015; Mandal and Suzuki 2002). The wide variation of trace elements found among SSs depends on the source of background concentrations. The sewage sludge obtained from municipal wastewater treatment plants (ISS, NTS, WSS, and KSS) showed the higher content of metals (Cd, Cr, and Pb) as compared with food industries (NSS and CSS) sewage sludges. This is because there is no arrangement for the separate disposal of sewage generated from household and small and large industries; even hospital water is being discharged in the common disposable drains. Therefore, the concentration of trace elements was more in sewage sludges produced from municipal wastewater treatment plants than that of industrial wastewater treatment plants. Industrial effluents came from the point source, but in the case of domestic wastewater, the non-point sources are involved. The highest Pb concentration in ISS and CSS samples was due to the use of Pb in various domestic and industrial products in Islamabad and Lahore cities like Pb-batteries in automobiles and Pb-alloys (Iqbal and Shah 2011). The galvanizing and electroplating may be a reason for metal accumulation such as Cu, Cr, Ni, Pb, and Zn, while industrial products may, at the end of their life, be discharged as wastes. Key urban inputs include business effluents (e.g., dental uses, car washes, other originalities), drainage waters, traffic-related emissions (asphalt wear, petrol/oil leakage, linings, brake tires, vehicle exhausts, etc.), and atmospheric deposition, which are transported with domestic and industrial wastewater into the sewage system (Sorme and Lagerkvist 2002).
In Pakistan, during the past decade, the percentage share of leather export has aggravated by an average of over 8%. There are more than 600 tanneries in the formal sector of Pakistan, and 50% of these are located in Kasur, a district of Punjab province, with a long-standing tradition of leather tanning (Afzal et al. 2014). These leather tanneries are significant sources of Cr in groundwater, soils, plants, and wastewater adjacent to those areas (da Costa Cunha et al. 2016). Our results indicated that the highest Cr contents were found in KSS, which was due to the presence of these leather tanneries in Kasur district. Previous studies showed that the concentration of Cr in Kasur tanneries was up to 4000 mg kg−1 soil (Afzal et al. 2014; Ateeq et al. 2016; Tariq et al. 2005). The concentration of other elements like Ag, Al, Ba, Bi, Ca, Li, and Mg, showed a significant amount in all samples (Table 4). The Industrial Estate in I-9 and I-10 sector Islamabad was established in 1963. It comprises of more than 250 industries. The industries include flour mills, re-rolling mills, marble cutting, steel melting furnaces, oil and ghee, pharmaceuticals, soap, auto body shops polishing, and recycling of Pb storage batteries (Nazir et al. 2011). Therefore, the metals detected from ISS include Pb, Zn, Cu, Co, Ni, Cr, and Cd.
Chemical fractionation of trace elements
The highest concentration of Pb was mainly associated with the residual fraction (F4) in all SS samples (Fig. 2b). The reserved concentration of Pb with F4 in ISS, NTS, WSS, CSS, KSS, and NSS was 63, 68.41, 8.19, 71.53, 52.77, and 41.74%, respectively. However, F1 was less than 2.6% in sludge samples and below the detection limit in KSS and NSS. The variable trend of Ni distribution was observed in sludge samples (Fig. 2c). Nickel was broadly distributed in four fractions in all sewage sludge samples due to its mobility. The sum of the two (F1 and F2) fractions ranged from 47% in ISS to 67% in WSS. High mobility of Ni was also reported by Liu and Sun (2013). Nickel associated with F4 ranged from 30.5% in ISS to 13.91% in WSS. This highest concentration was mainly found in F1 and F4 fractions in KSS and CSS, respectively. About 32% and 58% concentration of Cr in KSS and CSS was associated with F4 fraction and about 36–44% distributed in F3 fraction concerning total Cr concentration. Among all sewage sludge samples, the concentration of Zn was detected in all fractions (F1, F2, F3, and F4). The highest level of Zn was detected in the F1 fraction (Fig. 3a). The percentage of concentration distribution of Zn in all samples ranged from 26 to 37% for F1, 24–29% for F2, 19–29% for F3, and 10–23% for F4. A remarkable behavior of Zn was observed in the case of CSS and KSS in which F1 was about 14 and 17% and F3 was 38 and 31%, respectively of total concentration. The total concentration of Cu was very less in all samples (Fig. 3b). Thus, its fractional distribution concentration was below the detection limit in the case of NTS, KSS, and NSS, although it was detected in ISS, WSS, and CSS samples. The maximum concentration of Fe was observed in the F4 fraction in all sewage sludge samples (Fig. 3c). The decreasing order of Fe concentration in four fractions was as F4 (35–57%) > F3 (21–33%) > F2 (11–27%) > F1 (8–16%). Sewage sludge samples showed a variable trend regarding Mn fractions (Fig. 3d). In ISS, about equal concentration was present as F3 and F4. In the case of NTS, CSS, and KSS maximum Mn was associated with F4 (36.65, 38.24, and 37.50%, respectively). Approximately 32% of Mn was associated with F1 fraction in case of WSS. Higher F3 was observed in case of NSS.
Distribution pattern of a Cd, b Pb, c Ni, and d Cr fractions in different sewage sludge generated in Pakistan. F1: Acid soluble/exchangeable fraction, Cd: Cadmium, Pb: Lead, Ni: Nickel, Cr: Chromium, action F2: Reducible fraction F3: oxidizable fraction, F4: residual fraction, I-9 Sector, wastewater treatment plant sewage sludge, Islamabad, NTS: NUST wastewater treatment plant, Islamabad sewage sludge, WSS: Water and Sanitation Agency (WASA) Treatment Plant sewage sludge, Faisalabad, CSS: Coca-Cola Company wastewater plant sewage sludge, Lahore, KSS: Kasur wastewater treatment plant sewage sludge, NSS: Nestle wastewater treatment plant sewage sludge, Sheikhupura
Distribution pattern of a Zn, b Cu, c Fe, and d Mn fractions in different sewage sludge generated in Pakistan. F1: Acid soluble/exchangeable fraction, Zn: Zinc, Cu: Copper, Fe: Iron, Mn: Manganese, F2: Reducible fraction F3: oxidizable fraction, F4: residual fraction, I-9 Sector, wastewater treatment plant sewage sludge, Islamabad, NTS: NUST wastewater treatment plant, Islamabad sewage sludge, WSS: Water and Sanitation Agency (WASA) Treatment Plant sewage sludge, Faisalabad, CSS: Coca-Cola Company wastewater plant sewage sludge, Lahore, KSS: Kasur wastewater treatment plant sewage sludge, NSS: Nestle wastewater treatment plant sewage sludge, Sheikhupura
Every change in environmental conditions, such as acidity, redox potential, inorganic, and organic ligand concentrations results in mobility changes, and thereby in metal bio-accessibility. Insoluble salts such as phosphates can immobilize the main portion of the total Pb (Walker et al. 2003). The second most abundant fraction in case of Pb is F3, which ranges from 14 to 38%. These results agreed with other studies (Liang et al. 2013; Liu and Sun 2013). Braga et al. (2017) described metal fractionation results of six sewage sludge treatment plants of Brazil and reported that approximately 66% of total Pb contents were associated with F4 followed by F3 (29%). The pH is the major factor governing Pb distribution in different fractions in SS (Sungur et al. 2015). Nickel is often found in greater quantities in the mobile fractions (Kabata-Pendias and Pendias 2002). Within organic matter, they have a preferential bonding with the largely soluble fulvic acids, which partly explains their high degree of mobility (Brummer 1986). Hanay et al. (2008) also indicated the predominant form of Ni in the exchangeable form. Liang et al. (2013) concluded that Ni was abundantly distributed in F1 and F2, but in some samples, F4 fraction was high due to the alkaline stabilization process. Preferential affiliation of Cd with F3 in sludge was also stated by Singh et al. (2015) and Chen et al. (2014). Liu and Sun (2013) reported similar results after extracting various forms of Cd in different sewage sludge samples from China. Hanay et al. (2008) reported the dominant form of Cr in the oxidizable form. This may correlate with the oxidizing character of Cr. The trivalent cation is relatively immobile because this form has a strong affinity for negatively-charged ions and colloids (Fendorf 1995). Egiarte et al. (2009) also reported that Cr was mainly found with the metal oxides (50%), while 26% was organically bound and 24% with the residual fraction. Braga et al. (2017) reported that, on average, about 78% of total Cr was bound with organic matter and 19% associated with the residual fraction. The organically bound portion is hardly considered very mobile or available because the metals remain associated with the stable, high-molecular-weight humic substances, which decompose slowly (Singh et al. 1998). However, under oxidative conditions, the metals bound to OM and sulfurs have been reported to be re-released. In this fraction, the metal ion acts as the central ion, and the active OM group serves as the ligand or perhaps through the reaction of the sulfide ion and trace elements (Wang et al. 2010). F1 was only present in WSS, CSS, and KSS. The sum of the first two fractions (F1 and F2) hardly exceeds 10% of the total Cr concentration. Liu and Sun (2013) found that F1 and F2 were less than 5 and 15% respectively. Co-precipitation and occlusion of Zn with Fe and Al oxyhydroxides may become an important mechanism for retention of Zn in these wastes. (Rosazlin et al. 2007) reported Zn concentration mainly in the residual form (43%) followed by Fe-Mn oxides (27%). The high concentration of Zn associated with organically bound fraction was due to the formation of stable Zn sulfide precipitates (Braga et al. 2017). McLean and Bledsoe (1992) stated that Zn could exist in a considerable fraction in F1, mainly as carbonates, and its stability is pH-dependent. Zn is also seen to absorb largely on oxide, organics, and residual fractions and the stability of Zn increases with increasing pH (Brummer 1986). Co-precipitation and occlusion of Zn with Fe and Al oxyhydroxides may become an important mechanism for retention of Zn in these wastes. Copper is immobilized by binding with the mineral fraction of the sludge (iron oxides) and in the bonds of organic and residual fractions. The presence of humic acids that are only slightly soluble is of significance in the context of the stability of Cu by binding with such compounds. Several authors found Cu mainly associated with the organic fraction (Rosazlin et al. 2007). This finding is consistent with the known affinity of Cu for organic matter ligands. Hanay et al. (2008) also found Cu predominantly in the oxidizable fraction. The mobility and bioavailability of Cu if any may be controlled by the binding of Cu to the soluble part of organic matter because of the special affinity of Cu to organic matter (Kabata-Pendias and Pendias 2002). Braga et al. (2017) also reported that up to 15% Fe was presented as exchangeable and carbonate bound, whereas 35% Fe as a residual fraction. Fe can be precipitated as Fe3(PO4)2, after reaction with phosphate, or can form FeS and Fe thiol after interacting with inorganic and organic forms of sulfur, respectively. Diverse distribution of Mn in different fractions was also noticed by Liu and Sun (2013). Manganese is typically found in nature as its oxide and hydroxide forms, e.g., as manganite or braunite. The source of Mn in sewage may be related to discharge from industrial facilities or leachate from landfills and soils. Our results of Mn are in agreement with Braga et al. (2017) about the variable behavior of Mn fractions in different SS samples.
Risk assessment code
Risk assessment results of various SS samples are in Table 5. RAC has graded the risk into five classes, i.e., RAC < 1% no risk, 1–10% low risk, 11–30% medium risk, 31–50% high risk, and > 50% very high risk (Zhang et al. 2017). Accordingly, RAC results suggested that Cd was at low risk (relatively safe to the environment) in ISS and CSS. The RAC value for Cu was below 1% in all samples; therefore, it poses no risk to the environment. As shown in Fig. 1, KSS falls into the high-risk category due to the high value of Cr having an RAC of 52%. The RAC analysis showed that Zn posed a medium risk with ISS, CSS, KSS, and NSS while it posed a high risk with NTS and WSS. Data also showed that Ni had a medium threat with all SS samples except in the case of WSS, which posed a high risk as the RAC value was high enough (40%). It could be inferred that Fe showed a low risk to the environment in all SS samples except in the case of KSS, which showed a medium risk as its value ranges between 11 and 33%, the only single sample not showing a risk class regarding all trace elements.
Maximum allowable limits of potentially toxic elements in different countries
The maximum permissible limits of potentially toxic elements for the land application of SS in various countries shown in Table 6. The comparison of our results with these limits explored that the concentration of Zn in all analyzed samples was lower than the permissible limits of 86/278/EEC, Sweden, France, and the USA (Part 503), but higher than the Netherlands permissible limits except in the case of KSS. The comparison of current study results depicted that the concentration of Cu in all analyzed samples were lower than permissible limits. The Ni concentration in present study results was lower than permissible limits defined by 86/278/EEC, France and the USA (Part 503). The concentration of Ni in ISS, WSS, CSS, and NSS were higher than Sweden and the Netherlands permissible limits of potentially toxic elements in sewage sludge. The concentration of Cd in the case of ISS, CSS, and NSS were higher than the permissible limits of Sweden and the Netherlands. In the case of Pb, only the concentration of Pb in CSS was higher than the permissible limits of Sweden and the Netherlands. The permissible limits for Cr were 100 mg kg−1, 75 mg kg−1, 1000 mg kg−1, and 3000 mg kg−1 for Sweden, Netherlands, France, and the USA (Part 503), respectively.
Conclusion
The presence of beneficial nutrients (B, Co, Mo, Mn, and Zn) in a significant quantity in all collected samples makes these products valuable for agricultural land application. On the other hand, these products also possess some PTEs in their composition like CSS contains As above the critical limit, and KSS showed a high RAC due to the high concentration of Cr (52%), which may become a threat to the soil-plant system if applied to croplands. The RAC revealed that Zn and Ni stood a medium risk with ISS, KSS, and NSS while stances a high risk with NTS and WSS. Thus, ISS may be applied to croplands but in lower application rates.
References
Afzal M, Shabir G, Iqbal S (2014) Assessment of heavy metal contamination in soil and groundwater at leather industrial area of Kasur, Pakistan. Clean Soil Air Water 42:1133–1139. https://doi.org/10.1002/clen.201100715
Ateeq M, Hameed UR, Rehman SZ, Ullah F (2016) Evaluation of health risks among the workers employed in the tannery industry in Pakistan. J Entomol Zool Stud 4:244–246
Bouzid J, Elouear Z, Ksibi M (2008) A study on removal characteristics of copper from aqueous solution by sewage sludge and pomace ashes. J Hazard Mater 152:838–845. https://doi.org/10.1016/j.jhazmat.2007.07.092
Braga AFM, Zaiat M, Silva GHR (2017) Metal fractionation in sludge from sewage UASB treatment. J Environ Manag 193:98–107. https://doi.org/10.1016/j.jenvman.2017.01.070
Brummer, G.W., 1986. Heavy metals species, mobility and availability in soils. In: Bernard M, Brinckman FE, Sadler PJ (eds) The importance of chemical speciation in environment processes. Dahlem Konferenzen, Berlin, Heidelberg, Springer-Verlag, pp. 169–192. https://doi.org/10.1007/978-3-642-70441-3_11
Cai H, Liu J, Kuo J, Buyukada M, Evrendilek F (2019) Thermal characteristics, kinetics, gas emissions and thermodynamic simulations of (co-) combustions of textile dyeing sludge and waste tea. J Clean Prod 239:118113. https://doi.org/10.1016/j.jclepro.2019.118113
Carballa M, Omil F, Lema JM, Llompart M, Garcia C, Rodriguez I (2005) Behaviour of pharmaceuticals and personal care products in a sewage treatment plant of northwest Spain. Water Sci Technol 52:29–35
Chen H, Zhai Y, Xu B (2014) Fate and risk assessment of heavy metals in residue from co-liquefaction of Camellia oleifera cake and sewage sludge in supercritical ethanol. Bioresour Technol 167:578–581. https://doi.org/10.1016/j.biortech.2014.06.048
Council of the European Communities (1986) Council Directive of 12 June 1986 on the Protection of Environment and in Particular of the Soil, when Sewage Sludge is used in Agriculture (86/278/EEC). Off J Eur Comm., No. L 181/6–12
da Costa Cunha G, Peixoto JA, de Souza DR (2016) Recycling of chromium wastes from the tanning industry to produce ceramic nanopigments. Green Chem 18:5342–5356. https://doi.org/10.1039/C6GC01562J
Egiarte G, Corti G, Pinto M, Arostegui J, Macías F, Ruíz-Romero E, Camps M, Arbestain M (2009) Fractionation of Cu, Pb, Cr, and Zn in a soil column amended with an anaerobic municipal sewage sludge. Water Air Soil Pollut 198:133–148. https://doi.org/10.1007/s11270-008-9832-7
Fan X, Zhang X (2008) Adsorption properties of activated carbon from sewage sludge to alkaline-black. Mater Lett 62:1704–1706. https://doi.org/10.1016/j.matlet.2007.09.085
Fendorf S (1995) Surface reactions of chromium in soils and waters. Geoderma 67:55–71. https://doi.org/10.1016/0016-7061(94)00062-F
Hanay O, Hasar H, Kocer NN, Aslan S (2008) Evaluation for agricultural usage with speciation of heavy metals in a municipal sewage sludge. Bull Environ Contam Toxicol 81:42–46. https://doi.org/10.1007/s00128-008-9451-4
Haynes R, Murtaza G, Naidu R (2009) Inorganic and organic constituents and contaminants of biosolids: implications for land application. Adv Agron 104:165–267. https://doi.org/10.1016/S0065-2113(09)04004-8
Huang H, Yuan X, Zeng G (2011) Quantitative evaluation of heavy metals’ pollution hazards in liquefaction residues of sewage sludge. Bioresour Technol 102:10346–10351. https://doi.org/10.1016/j.biortech.2011.08.117
Iqbal J, Shah MH (2011) Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J Hazard Mater 192:887–898. https://doi.org/10.1016/j.jhazmat.2011.05.105
Kabata-Pendias A, Pendias H (eds) (2002) Trace elements in soils and plant, 3rd edn. CRC Press, Boca Raton
Khaliq A (2014) Modeling the effects of groundwater pumping on watertable of a Faisalabad water supply scheme. PhD diss., University Of Agriculture, Faisalabad
Latare A, Kumar O, Singh S (2014) Direct and residual effect of sewage sludge on yield, heavy metals content and soil fertility under rice-wheat system. Ecol Eng 69:17–24. https://doi.org/10.1016/j.ecoleng.2014.03.066
Li P, Cheng X, Xue B (2013) Evaluation of composted sewage sludge application to soil. IERI Proc 5:202–208. https://doi.org/10.1016/j.ieri.2013.11.093
Liang X, Ning XA, Chen G (2013) Concentrations and speciation of heavy metals in sludge from nine textile dyeing plants. Ecotoxicol Environ Saf 98:128–134. https://doi.org/10.1016/j.ecoenv.2013.09.012
Liu JY, Sun SY (2013) Total concentrations and different fractions of heavy metals in sewage sludge from Guangzhou, China. Trans Nonferrous Met Soc China 23:2397–2407. https://doi.org/10.1016/S1003-6326(13)62747-8
Liu J, Zeng J, Sun S, Huang S, Kuo J, Chen N (2016) Combined effects of FeCl3 and CaO conditioning on SO2, HCl and heavy metals emissions during the DDSS incineration. Chem Eng J 299:449–458. https://doi.org/10.1016/j.cej.2016.04.132
Liu J, Zhuo Z, Xie W, Kuo J, Lu X, Buyukada M, Evrendilek F (2018) Interaction effects of chlorine and phosphorus on thermochemical behaviors of heavy metals during incineration of sulfur-rich textile dyeing sludge. Chem Eng J 351:897–911. https://doi.org/10.1016/j.cej.2018.06.158
Lopez-Moreno M, Aviles LL, Roman F (2015) Sludge and compost amendments in tropical soils: impact on coriander (Coriandrum sativum) nutrient content. World Academy of Science, Engineering and Technology. Int J Biol Biomol Agric Food Biotechnol Eng 9:388–394. https://doi.org/10.1999/1307-6892/10001053
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235. https://doi.org/10.1016/S0039-9140(02)00268-0
McLean JE, Bledsoe BE (1992) Behavior of metals in soils. EPA ground water issue EPA/540/S-92/018
Montanari A (2015) Inorganic contaminants of food as a function of packaging features. In food packaging hygiene, pp. 17–41. Springer International Publishing. https://doi.org/10.1007/978-3-319-14827-4_2
Murtaza G, Haynes RJ, Kim KR (2012) Effect of aging biosolids with soils of contrasting pH on subsequent concentrations of Cu and Zn in pore water and on their plant uptake. Environ Sci Pollut Res 19:636–645. https://doi.org/10.1007/s11356-011-0592-3
Nazir A, Malik RN, Ajaib M (2011) Hyperaccumulators of heavy metals of industrial areas of Islamabad and Rawalpindi. Pak J Bot 43:1925–1933
Olsen S, Sommers L (1982) Chemical and microbiological properties of phosphorous. In: Page AL et al (eds) Methods of soil analysis. Part 2. Soil Sci. Sot. Am., Madison
Parks JL, Edwards M (2005) Boron in the environment. Crit Rev Environ Sci Technol 35(2):81–114
Riaz U, Murtaza G, Saifullah, Farooq M (2018a) Comparable effect of commercial composts on chemical properties of sandy clay loam soil and accumulation of trace elements in soil-plant system. Int J Agric Biol 20:85–92. https://doi.org/10.17957/IJAB/15.0433
Riaz U, Murtaza G, Saifullah, Farooq M (2018b) Influence of different sewage sludges and composts on growth, yield, and trace elements accumulation in rice and wheat. Land Degrad Dev:1–10. https://doi.org/10.1002/ldr.2925
Rosazlin A, Che Fauziah I, Rosenani AB, Zauyah S (2007) Domestic sewage sludge application to an acid tropical soil: part III. Fractionation study of heavy metals in sewage sludge and soils applied with sewage sludge. Malays J Soil Sci 11:81–95
Singh R, Agrawal M (2008) Potential benefits and risks of land application of sewage sludge. Waste Manag 28:347–358. https://doi.org/10.1016/j.wasman.2006.12.010
Singh R, Agrawal M (2010) Variations in heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicol Environ Saf 73:632–641. https://doi.org/10.1016/j.ecoenv.2010.01.020
Singh SP, Tack FM, Verloo MG (1998) Heavy metal fractionation and extractability in dredged sediment derived surface soils. Water Air Soil Pollut 102:313–328. https://doi.org/10.1023/A:1004916632457
Singh H, Singh P, Singh D (2015) Chemical fractionation of heavy metals and nutrients in sludge and waste water generated by Coca-Cola soft drink industry. Arch Agron Soil Sci 61:119–138. https://doi.org/10.1080/03650340.2014.924106
Smith SR (2009) A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ Int 35:142–156. https://doi.org/10.1016/j.envint.2008.06.009
Solpan D, Guven O (2002) Decoloration and degradation of some textile dyes by gamma irradiation. Radiat Phys Chem 65:549–558. https://doi.org/10.1016/S0969-806X(02)00366-3
Sorme L, Lagerkvist R (2002) Sources of heavy metals in urban wastewater in Stockholm. Sci Total Environ 298:131–145. https://doi.org/10.1016/S0048-9697(02)00197-3
Sullivan DM, Miller RO (2001) Compost quality attributes, measurements, and variability, pp 95–120. CRC Press, Boca Raton
Sungur A, Soylak M, Yilmaz E (2015) Characterization of heavy metal fractions in agricultural soils by sequential extraction procedure: the relationship between soil properties and heavy metal fractions. Soil Sediment Contam Int J 24:1–15. https://doi.org/10.1080/15320383.2014.907238
Tariq SR, Shah MH, Shaheen N (2005) Multivariate analysis of selected metals in tannery effluents and related soil. J Hazard Mater 122:17–22. https://doi.org/10.1016/j.jhazmat.2005.03.017
Telliard WA (1989) Sampling procedures and protocols for the national sewage sludge survey (no. PB-93-156974/XAB). Environmental Protection Agency, Washington, DC Office of the Assistant Administrator for Water
Ure AM, Quevauviller P, Muntau H (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51:135–151. https://doi.org/10.1080/03067319308027619
USEPA (US Environmental Protection Agency) (2005) Process design manual for land treatment of municipal and industrial wastewater, Center for Environmental Research Information (CERI). U.S. Environmental Protection Agency, Cincinnati
Walker DJ, Clemente R, Roig A (2003) The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ Pollut 122:303–312. https://doi.org/10.1016/S0269-7491(02)00287-7
Wang L, Yu R, Hu G, Tu X (2010) Speciation and assessment of heavy metals in surface sediments of Jinjiang river tidal reach, southeast of China. Environ Monit Assess 165:491–499. https://doi.org/10.1007/s10661-009-0961-2
Warman PR, Termeer WC (2005) Evaluation of sewage sludge, septic waste, and sludge compost applications to corn and forage: yields and N, P, and K content of crops and soils. Bioresour Technol 96:955–961. https://doi.org/10.1016/j.biortech.2004.08.003
Zebarth BJ, McDougall R, Neilsen G, Neilsen D (2000) Availability of nitrogen from municipal biosolids for dryland forage grass. Can J Plant Sci 80:575–582. https://doi.org/10.4141/P99-160
Zhang J, Tian Y, Zhang J, Li N, Kong L, Yu M, Zuo W (2017) Distribution and risk assessment of heavy metals in sewage sludge after ozonation. Environ Sci Pollut Res 24:5118–5125. https://doi.org/10.1007/s11356-016-6313-1
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riaz, U., Murtaza, G., Saifullah et al. Chemical fractionation and risk assessment of trace elements in sewage sludge generated from various states of Pakistan. Environ Sci Pollut Res 27, 39742–39752 (2020). https://doi.org/10.1007/s11356-020-07795-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07795-4