Abstract
Silver nanoparticles are potent antimicrobials and could be used as a promising alternative of conventional antibiotics. The aim of this study was to isolate bacteria from soil that have ability to produce AgNPs by secondary metabolite activity and their elucidation against human pathogens. These strains Escherichia coli, Exiguobacterium aurantiacumm, and Brevundimonas diminuta with NCBI accession number MF754138, MF754139, and MF754140 respectively were grown for secondary metabolite production. The nanoparticles were confirmed and characterized by UV-Vis spectroscopy and transmission electron microscopy. The optimization study was also carried out to obtain the maximum production of silver nanoparticles. Three parameters, temperature, pH, and AgNO3 concentration, were used to optimize the production of silver nanoparticles. Antimicrobial potential of these nanoparticles was addressed on the Muller-Hinton Agar, and their zones of inhibitions were measured. TEM analysis revealed the size and shape of the silver nanoparticles. All types of AgNPs were spherical in shape; their size range is from 5 to 50 nm. The findings of optimization study showed the maximum production of silver nanoparticles at the pH 9, temperature 37 °C, and 1 mM AgNO3 concentration. All the strains exhibited the great potential as antimicrobial agents against MRSA and several other MDR bacteria with minimum 10 mm to maximum 28 mm zone of inhibition. It was concluded that the present study is an eco-friendly approach for the synthesis of AgNPs that will be beneficial to control the nosocomial infections triggered by MRSA and other human pathogens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is an emergent field which is influencing all the areas of life, and soon it will be the next industrial revolution. This technology introduces the innovation in the field of manufacturing, medicine industry, biotechnology, nano-electronics, and energy. Several physical, chemical, and biological methods are used for the synthesis of nanoparticles, but biological approach is more reliable, because biological synthesis is ecofriendly and cost effective and required less time for the production (Gandhi and Khan 2016). Nanoparticles are the particles of nano sized and occupying the exceptional characteristics just like size, morphology, and distribution (Muthukumaran et al. 2015). Due to high absorption in the visible region (surface plasmon resonance), silver nanoparticles are gaining much attention as compared with other metal nanoparticles. It can be simply detected by UV-visible spectrophotometry (Krishnaraj et al. 2010). The chemical synthesis of silver nanoparticles includes the methods of chemical reduction, electrochemical reduction, ultrasonic-assisted reduction, microwave-assisted synthesis nonaqueous chemical reduction, aqueous solution chemical reduction, and the micro emulsion methods. But use of these chemical methods also accompanies some shortcomings like the use of toxic chemicals, energy depletion, and hazardous for environment and human life (Awwad et al. 2013). The biological approach of silver nanoparticle synthesis could be used as an alternate to avoid such toxic chemical methods. The biological synthesis of silver nanoparticles includes the synthesis by plants, fungi, yeast, algae, and bacteria (Deljou 2016). Biosynthesis of AgNPs by using the extracellular activity is simpler and more economical, while intracellular synthesis needs some ultrasonic treatments and extra detergents (Paul and Sinha 2014). It was reported that silver nanoparticles have great potential as antimicrobial and antiviral as well as mosquitocidals (Suresh et al. 2015). Nowadays, antibiotic resistance is the key challenge, and many human pathogenic bacteria develop resistance to the number of conventional antibiotics. So, there is a need to develop some alternative methodology against these multidrug-resistant (MDR) pathogens. Resistance pattern of MRSA is shocking, and it becomes a problem all over the world. So, there is a need to introduce some new approaches and new agents which are active against the MRSA (methicillin-resistant Staphylococcus aureus) (Singh et al. 2014). The present study has aimed to evaluate the eco-friendly approach for the synthesis of AgNPs to control antibiotic-resistant bacteria pathogens at large scale.
Materials and methods
Isolation of bacteria from different soil samples
Total 39 soil samples were collected from the industrial and agricultural soil of different regions of South Punjab, Pakistan. The samples were collected in the sterilized zip lock bags and stored in the ice box and transported to the laboratory. The soil sample of about 1 g was serially diluted and spread over the LB (Luria-Bertani) agar for the isolation of bacteria (Hayakawa 2008). In experiment 364 bacterial strains were isolated from the 39 different soil samples named as S1 to S39. The isolated strains were used in the screening test for silver nanoparticles synthesis.
Extracellular biosynthesis of silver nanoparticles
In the screening test all the fresh bacterial strains were inoculated into the Erlenmeyer flask containing the LB broth and incubated at 37 °C for 24 h. After incubation the suspension was centrifuged at 10,000 rpm for 10 min and cell free supernatant was obtained. The bacterial secondary metabolites were taken in the flask and treated with the 1 mM AgNO3 (final volume concentration) and incubated in the shaker incubator at 37 °C for 72 h. The cell free supernatant without addition of AgNO3 was maintained as control (Priyadarshini et al. 2013). During the experiment conditions were maintained as dark. After incubation, the color change from pale yellow to dark brown indicated the formation of silver nanoparticles in the flask containing the silver nitrate. There was no color change observed in the flask without silver nitrate (control). The formation of silver nanoparticles was observed and monitored by the UV-visible spectroscopy.
Molecular identification by 16S rRNA sequencing
Molecular identification was carried out for the selected isolates by the method of 16S rRNA. The total genomic DNA was isolated from the selected strains of bacteria for the PCR analysis. The sequence data was then used for the BLAST analysis and further phylogenetic analysis using neighbor-joining method in MEGA 7.
Characterization of silver nanoparticle
UV-visible spectroscopy
The bacterial culture filtrate which showed dark brown color when treated with the AgNO3 was analyzed by the UV-Vis spectroscopy (Wiley et al. 2006). About 1 ml solution in sample aliquots was taken and monitored on the UV-visible spectrophotometer.
Transmission electron microscopy analysis
Morphological characteristics of silver nanoparticles like size and shape were analyzed by TEM analysis (Gowramma et al. 2015). In the TEM analysis one drop of culture supernatant with AgNO3 was taken and loaded on the copper grid and dried before measurement. The TEM micrographs were taken which confirmed the size and shape of silver nanoparticles.
Optimization study for rapid production of AgNPs
The optimization study was carried to check the effect of different physical parameters on the production of silver nanoparticles. The AgNO3 concentration (0.5 to 2 mM), temperature (28 to 45 °C), and pH (5 pH to 9 pH) are the different reaction parameters which were maintained to check their effect on the rapid and maximum production of silver nanoparticles.
Antimicrobial analysis
Antimicrobial activity of biogenic AgNPs was studied against the number of pathogenic bacteria by using the method of Agar well diffusion assay on Muller-Hinton agar (MHA). The pathogenic microorganisms which were used in antimicrobial assay include a group of gram-positive bacteria Bacillus subtilis, Staphylococcus aureus, Bacillus cereus, and methicillin-resistant Staphylococcus aureus (MRSA), and gram-negative bacteria Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Proteus, salmonella typhi, and Enterobacter vermicularis. The overnight culture of pathogenic bacteria was swabbed by a sterilized cotton swab on Muller-Hinton agar plate, and wells were made by the help of a gel puncture. About 80 to 100 μl suspension of biologically synthesized silver nanoparticles was loaded in each well and incubated for 24 h at 37 °C. The zones of inhibitions were measured after the incubation by measuring the diameter around each well. The study was done in triplicate (Singh et al. 2013).
Results and discussion
Isolation and selection of AgNP-producing bacteria
The physical characteristics of soil samples showed clay, loamy, and sandy texture. The pH range of the soil samples was 7 to 8.1, and the temperature range was 15 to 35 °C. After the screening test, out of 364 bacterial strains only 3 strains were found to have good ability to produce AgNPs by showing dark brown color in the screening study. These three strains were selected and initially named as sa2, sa3, and sa4 (Table 1).
Extracellular biosynthesis of silver nanoparticles
The formation of silver nanoparticles was observed by using the secondary metabolites of selected strains and treated with the 1 mM silver nitrate solution at 37 °C temperature. The reaction mixture turns the pale yellow to dark brown solution which indicated the synthesis of silver nanoparticles (Fig. 1). The phenomenon of surface plasmon resonance is involved in the formation of silver nanoparticles which gives the dark brown color to the solution. The UV-visible spectrum with the scanning speed 300 to 900 was used to monitor and confirm the silver nanoparticles.
Molecular identification
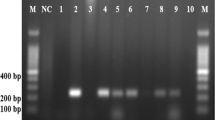
After the confirmation of AgNP production by selected strain, these strains were identified by the 16S rRNA sequencing study. The selected strains showed 99% similarity with the Escherichia coli strain AR_0055, Exiguobacterium aurantiacum strain Q20, and Brevundimonas diminuta strain BZC3. Thus, these strains identified as Escherichia coli sa2, Exiguobacterium aurantiacumm sa3, and Brevundimonas diminuta sa4 after 16S rRNA analysis (Fig. 2). These sequences are submitted to the Gene Bank data base, and accession number for these strains is MF754137 MF754138, MF754139, and MF754140 respectively.
Primary characterization of AgNPs
UV-visible spectroscopy
Due to the surface plasmon resonance UV-visible absorption spectrum of silver nanoparticles showed the strong peaks at 420 to 450 nm. The strains Exiguobacterium aurantiacumm sa3 showed highest peak at 420 nm while other strains Escherichia coli sa2 and Brevundimonas diminuta sa4 showed highest peak at 440 nm. The findings of UV-visible analysis confirmed the formation of silver nanoparticles in the reaction mixture (Fig. 3).
Transmission electron microscopy
For the size and morphological characterization TEM (transmission electron microscopy) was utilized. The TEM analysis revealed the size and shape of the silver nanoparticles. All types of AgNPs were spherical in shape, their size range is from 5 to 50 nm; however, few particles of a larger and shorter diameter were also found (Fig. 4).
Effect of different physical factors on AgNP production
Different concentrations (0.5–2 mM) of silver nitrate were used for the production of silver nanoparticles to check their effect on AgNPs. Silver nanoparticle biosynthesis from bacterial metabolites was found best at 1 mM AgNO3 concentration (Fig. 5). To obtain the maximum production of silver nanoparticles, culture supernatant with different pH ranging from 5 to 9 was used and treated with the silver nitrate. It was observed that maximum UV-visible absorption spectrum was shown by the culture supernatant at the pH 9 and AgNPs were remaining stable (Fig. 6). Effect of temperature on the production of silver nanoparticles was checked at 3 different temperatures ranging from 28–45 °C. It was observed that maximum UV-visible absorption was shown by the culture supernatant at 37 °C and remains stable. Maybe at 37 °C temperature there was maximum kinetic energy of AgNPs; therefore, more stable and faster production of silver nanoparticles was observed (Fig. 7). The production of silver nanoparticles is significantly influenced by the pH of the reaction as the formation of silver nanoparticles started at pH 5 to 6 but eventually increasing with the increase in pH. Temperature also influences the formation and stability of the silver nanoparticles. This study shows the higher temperature from 30 to 37 °C supported the production and stability of the silver nanoparticles. The metallic nanoparticles like silver nanoparticles retain the abundance of free electrons that are accountable for the surface plasmon resonance absorption band. The vibration of these electrons by resonance of the light waves produced surface plasmon resonance (SPR). The optimum conditions help to reveal the surface plasmon resonance (SPR) spectrum at 410 to 460 nm with dark brown color. Okafor et al. (2013) also observed similar results in the previous study.
Antimicrobial analysis
Silver nanoparticles have high antimicrobial activity due to the high surface area to volume ratio. Agar well diffusion assay was used to analyze the antimicrobial potential of bacterial-mediated silver nanoparticles. For the study of antibacterial assay, 13 different pathogenic bacteria were used. The clear zones around well were measured separately. Antimicrobial study revealed that silver nanoparticles were effective against number of multidrug-resistant bacteria and other pathogenic bacteria. The results of antimicrobial activity clearly indicated that the Bacillus cereus was sensitive to all 3 types of AgNPs, while Escherichia coli sa2, Exiguobacterium aurantiacumm sa3, and Brevundimonas diminuta sa4 showed zones of inhibition of 28 mm, 29 mm, and 28 mm respectively (Fig. 8). The Escherichia coli silver nanoparticle showed the clear zones against MRSA7, MRSA8, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter vermicularis, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus and S.typhi. The zones of inhibition measured were 14 mm, 15 mm, 14 mm, 11 mm, 10 mm, 11 mm, 17 mm, 14 mm, 28 mm, and 12 mm respectively. The Exiguobacterium aurantiacumm silver nanoparticle showed the clear zones against the Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter vermicularis, Proteus vulgaris, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, and salmonella typhi. The measured zones of inhibition were 15 mm, 12 mm, 12 mm, 15 mm, 11 mm, 15 mm, 11 mm, 29 mm, and 12 mm respectively. The Brevundimonas diminuta silver nanoparticles showed the clear zones against MRSA6, MRSA8, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Staphylococcus aureus, and Bacillus cereus. The measured zones of inhibition were 12 mm, 16 mm, 15 mm, 12 mm, 12 mm, 21 mm, and 28 mm respectively (Table 2).
Nowadays, production of metal nanoparticles by the use of microbes is more feasible as compared with other approaches. It is reported that among all metal nanoparticles silver has shown very good antimicrobial activity. Bacteria as a biological agent used for the synthesis of AgNPs, because handling and manipulation of bacteria is very simple (Rai et al. 2012). This field of study is an emerging trend, but only a few bacterial strains are able to synthesize AgNPs. It was reported by (Priyadarshini et al. 2013) that 127 bacterial strains were collected from the silver mine but only one strain synthesized the silver nanoparticles and showed positive results. The bacteria which have ability to survive on high concentration of metal, specifically on high silver concentration, can synthesize silver nanoparticles (Iravani 2014). This is the reason that above 300 strains were tested and only five strains showed positive results in our study. Five isolates were selected as positive because in the primary screening they showed the positive results. Silver nanoparticle syntheses by the Bacillus species and E. coli were reported in many studies (Sunkar and Nachiyar 2012). It was observed that most of the nanoparticles were spherical in shape and well settled. The size range of these silver nanoparticles was 15 to 50 nm, but average sizes of 8 nm AgNPs were also observed; these findings correlate with Rajora et al. (2016). The silver nanoparticles breach into the bacterial membrane and disrupt their functions. Therefore, metal nanoparticles show antimicrobial activity against multidrug-resistant bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, and salmonella typhi (Saravanan et al. 2018).
Conclusion
The extracellular synthesis of silver nanoparticles from bacterial metabolites is one of the environment-friendly approaches. In this study, the isolated bacteria have shown the potential to synthesize the silver nanoparticles. The occurrence of MRSA is an alarming signal and needs to be controlled on emergency basis. This unique study focused on utilization of natural metabolites towards eradication of MRSA instead of using other synthetic antibiotics and proved to be best alternative giving significant results that will provide valuable information to control the multidrug-resistant pathogens.
References
Awwad AM, Sale NM, Abdeen AO (2013) Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Infect Control 1:29
Deljou A (2016) Green extracellular synthesis of the silver nanoparticles using thermophilic Bacillus sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iran J Biotechnol 14(2):25–32
Gandhi H, Khan S (2016) Biological synthesis of silver nanoparticles and its antibacterial activity. J Nanomed Nanotechnol 7:366
Gowramma B, Keerthi U, Rafi M, Rao DM (2015) Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech 5(2):195–201
Hayakawa M (2008) Studies on the isolation and distribution of rare actinomycetes in soil. Actinomycetologica 22(1):12–19
Iravani S (2014) Bacteria in nanoparticle synthesis: current status and future prospects. International scholarly research notices
Krishnaraj C, Jagan EG, Rajsekar S, Selvakumar P, Kalaichelvan PT, Mohan N (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B: Biointerfaces 76(1):50–56
Muthukumaran U, Govindarajan M, Rajeswary M, Hoti SL (2015) Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol Res 114(5):1817–1827
Okafor F, Janen A, Kukhtareva T, Edwards V, Curley M (2013) Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int J Environ Res Public Health 10(10):5221–5238
Paul D, Sinha SN (2014) Extracellular synthesis of silver nanoparticles using Pseudomonas aeruginosa KUPSB12 and its antibacterial activity. Jordan J Biol Sci 7(4):245–250
Priyadarshini S, Gopinath V, Priyadarshini NM, MubarakAli D, Velusamy P (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf B: Biointerfaces 102:232–237
Rai MK, Deshmukh SD, Ingle AP, Gade AK (2012) Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol 112(5):841–852
Rajora N, Kaushik JA, Kothari SL (2016) Rapid synthesis of silver nanoparticles by Pseudomonas stutzeri isolated from textile soil under optimised conditions and evaluation of their antimicrobial and cytotoxicity properties. IET Nanobiotechnology 10(6):367–373
Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A (2018) Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog 116:221–226
Singh R, Wagh P, Wadhwani S, Gaidhani S, Kumbhar A, Bellare J, Chopade B (2013) Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int J Nanomedicine 8:4277
Singh D, Rathod V, Ninganagouda S, Hiremath J, Singh AK, Mathew J (2014) Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg Chem Appl
Sunkar S, Nachiyar CV (2012) Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed 2(12):953–959
Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Chandramohan B (2015) Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res 114(4):1551–1562
Wiley BJ, Im SH, Li ZY, McLellan J, Siekkinen A, Xia Y (2006) Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem 110:15666–11567
Acknowledgments
The authors would like to thank Department of Microbiology and Molecular Genetics, The Women University Multan and Higher Education Commission Pakistan for providing support and all the facilities.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saeed, S., Iqbal, A. & Ashraf, M.A. Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environ Sci Pollut Res 27, 37347–37356 (2020). https://doi.org/10.1007/s11356-020-07610-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07610-0