Abstract
The current investigation evaluates metal (loid)s biomonitoring using algae as well as the metal(loid) pollution of seawaters and sediments in the northern part along the Persian Gulf. Algae, seawater, and sediment samples were collected from four coastal areas with different land applications. The concentration of Ni, V, As, and Cd in abiotic samples (seawater and sediment) and four species of algae (Enteromorpha intestinalis, Rhizoclonium riparium, Cystoseira myrica, and Sargassum boveanum) was measured using an ICP-AES device. Concentrations of potentially toxic elements in seawater, sediments, and algae species followed the trend of “Ni˃V˃As˃Cd.” The area of Asaloyeh (with the highest industrial activity) and the Dayyer area (with the lowest industrial activity) provided the highest and lowest amounts of metal(loid)s pollution, respectively. The average concentrations of V and As in four algae species significantly differed for all sampled areas. Obtaining the bio-concentration factor (BCF) > 1 for seawater and < 1 for sediment indicated that the studied algae have the ability to efficiently concentrate metal(loid)s from seawater and the limited accumulation of metals in sediments. According to the Nemerow pollution index, the order of metal(loid)s pollution for the studied areas estimated as Asaloyeh>Ganaveh>Bushehr>Dayyer. Algae species of C. myrica and E. intestinalis can often serve as suitable biological tools for monitoring seawater and sediment quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The metal(loid)s can have harmful effects on growth, cell division, photosynthesis, and cause the destruction of primary metabolites in some marine algae and reduce the chloroplast content, leading to the death of cells and plankton (Ramachandra et al. 2018). In addition, metal(loid)s pollutants have serious consequences for human health due to high toxicity, lack of degradability, and accumulation along with the food web (Teimouri et al. 2018; Jacob et al. 2018). Thus, continuous measurement of metal(loid)s is necessary to inform and prevent their levels in the environment.
Recently, efforts have been focussed on the use of biomonitoring tools to assess the environment pollution by toxic metal(loid)s. In this regard, measurement of the metal(loid)s concentration in marine organisms is preferable to direct measurement of the concentration in the seawater and sediment samples (Singh et al. 2016; Al-Yemni et al. 2011). Due to the wide fluctuations in seawater, the metal(loid)s concentration in seawater is very low. Also, the concentration of metals in sediments is affected by factors such as the amount of organic material, the composition and size of particles, pH, and oxidation-reduction potential (Noronha-D’Mello and Nayak 2016). On the other hand, the biomonitor indicator is effective for comparing the concentration of metal(loid)s in an organism and its surrounding environment and can provide information such as pollutant concentrations in the seawater (Topcuoğlu et al. 2003). So far, some species of sponge (Illuminati et al. 2016), moss (Izquieta-Rojano et al. 2016; Favas et al. 2018), diatom (Gautam et al. 2017; Illuminati et al. 2017), crab (Perry et al. 2015), barnacles (Dionísio et al. 2013), snail (Bian et al. 2016), microalgae (Qiu 2015), and macroalgae (Chakraborty et al. 2014) have been explored to monitor metal(loid)s in aqueous systems. Among these biomonitoring agents, macroalga has many advantages over other agents like low mobility (which make it a useful agent for assessing the pollutants in seawater), considerable biomass, easy identification, long life, availability, and the ability to accumulate metals (Çelekli et al. 2016; Aydın-Önen and Öztürk 2017). Therefore, algae have a unique ability to be used to evaluate metal(loid)s pollution in marine environments. Around the world, the algal species have been used as the metal biomonitor agent. Akcali and Kucuksezgin (2011) have introduced the brown algae of Cystoseira species and green algae of Ulva and Enteromorpha species as metal biomonitoring agent in the Aegean Sea. Brown and green algae have been reported for monitoring heavy metals in rivers, gulfs, and oceans (Chakraborty et al. 2014; Kobzar and Khristoforova 2015; García-Seoane et al. 2018). The metal measurements in water, sediment, and green algae of Ulva species in Lake Pulicat were investigated by Kamala-Kannan et al. (2008) and a higher concentration of metals in algae than in water and sediment samples was found.
Over the past two decades, many potentially polluting industries such as oil, gas, and petrochemicals, as well as downstream industries have flourished in the northern coastlines of the Persian Gulf, Iran, which has led to the release of pollutants like heavy metals into the seawater (Freije 2015; Kafaei et al. 2017a, b). Furthermore, seas can be contaminated with metal laden-wastewaters from small industries, industrial towns, cities, and commercial activities (Jones et al. 2017; Dobaradaran et al. 2018). Various studies (Yümün 2017; Zhang et al. 2017; Nasrolahi et al. 2014) have shown a correlation between the coastal land application and the diversity and quantity of pollutants in the seawater. A review of the published literature showed that although macroalgae have been used for biomonitoring of the marine pollution (Dadolahi-Sohrab et al. 2011; Gomes and Asaeda 2013; Kamala-Kannan et al. 2008), the association of metal(loid)s contents in algae with the type of coastal land application, especially in the Persian Gulf, is still unclear.
Therefore, the aims of the current study were directed to monitor Cd, Ni, V, and As in two green algae species [Enteromorpha intestinalis (also named as Ulva intestinalis Linnaeus) and Rhizoclonium riparium (Roth) Harvey (also named as R. riparium)] and two brown algae species [Cystoseira myrica and Sargassum boveanum] in four coastal areas with different land applications along the northern coast of Persian Gulf, Iran. These metal(loid)s were selected based on their natural and anthropogenic presence in the environment and according to previous studies (Delshab et al. 2017; Safari et al. 2018).
Material and methods
The study area
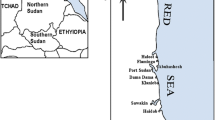
Four areas with an area of 27,653 km2 in Bushehr province, southern Iran were studied. All areas were located on the northern coast of the Persian Gulf. As depicted in Fig. 1, four sampling areas were selected based on the coastal land applications. The sampling areas in the current investigation are:
- (a)
The Asaloyeh area (representative of a highly industrialized area due to containing one of the largest gas reservoirs in the world);
- (b)
The Ganaveh area (representative of a semi-industrial area, due to the presence of industrial towns, oil reservoirs, and combined power plants);
- (c)
The Bushehr area (representative of a residential-commercial area); and
- (d)
The Dayyer area (representative of a fishery environment).
Algae sampling
Four algae species including Enteromorpha intestinalis, Rhizoclonium riparium, Cystoseira myrica, and Sargassum boveanum were manually sampled from each of the studied area (Asaloyeh, Dayyer, Bushehr, and Ganaveh). Sampling was done four times in 2017. Sampling was started in January 2017 and ended in May 2017. The interval between the two sampling stages for each season was about 20 days (because of the stormy sea and/or rainy weather). At each station, a space of about 50 m2 (around 10 m × 5 m) was considered as a sampling point (Kamala-Kannan et al. 2008; Shiber 1980). The wet weight of each algae sample was 300–500 g. Samples were collected in the tidal zone of the sea. The depth of water in the tidal area at sampling stations was about 0.5–1.5 m. Therefore, according to the description, a total of 64 algae samples (4 species of algae × 4 areas × 4 replicates) were provided.
Each algae sample was initially washed with seawater to remove silt and epiphyte then, to avoid any contamination during sampling, the samples were put in polyethylene bags and transferred to the laboratory during 0.5–4 h (depending on the distance of places from the laboratory). In the field, the algae were separated by color and visual appearance, and in the laboratory algae samples were also checked for uniformity. All used glassware and bags involved in the sampling and analyzing were washed with a 10% nitric acid solution and then rinsed thoroughly with deionized water (Merck Company).
Specification of algae species and genus
The algae samples in the laboratory were classified based on their color and appearance. Algae were identified based on microscopic and morphological characteristics and comparison with published literature (Niamaimandi et al. 2017; Jha et al. 2009). Finally, all samples were classified into two colors (green and brown) and four species (Enteromorpha intestinalis, Rhizoclonium riparium, Cystoseira myrica, and Sargassum boveanum). According to International Code of Nomenclature for algae (McNeill et al. 2012), “Rhizoclonium riparium” and “Enteromorpha intestinalis” were also named “Rhizoclonium riparium (Roth) Harvey” and “Ulva intestinalis Linnaeu”, respectively.
Seawater and sediments sampling
Seawater samples were taken at the time of algae sampling, but the sediment samples were collected during low tide in the tidal zone. About 300 mL seawater was sampled at 20–30 cm below the water surface in the acid-washed bottles. Seawater samples were filtered using the 0.42 μm-filter (Whatman, cellulose nitrate membrane) to separate the suspended solids and then acidified (pH < 2 using 1.5 mL/L conc. HNO3) (Achary et al. 2017) in the field to minimize the possibility of metal(loid) loss due to laminar conditions in the sample bottles. After that, the seawater samples were transferred to the laboratory.
The intertidal sediments samples (upper 0–5 cm) were taken using a shovel and drilling tools during low tide. The sampling of sediments was also carried out in the approximate area of 50 m2 (around 10 m × 5 m). Surface sediments were collected in an area of 5 cm × 5 cm. It should be noted that at each station about 4 sediment samples were taken in each station and then homogenized to produce a 500 g- composited sample. The sediment samples were stored in plastic bags and transferred to the laboratory.
Preparation of algae and sediments samples for measurement
In the laboratory, all sediments and algae samples were oven-dried at 55 °C for 48 h for minimizing the metal(loid)s vaporization and reaching to constant weight (De La Calle et al. 2013). The algae samples were reached to constant weight at this condition. According to the literature (Malea and Kevrekidis 2014), dried samples or the algae and sediments were manually ground and passed through a nylon net (mesh = 63 μm) and subsequently stored in polyethylene containers. For the next milling and sieving, the equipment was completely cleaned up to prohibit cross contaminations between samples. Three sub-samples of each alga and sediment samples were acid-digested. The sample digestion method was done like those reported in our previous study (Safari et al. 2018). The exact amount of 1 g of powdered samples (algae or sediment) was poured into acid-washed tubes and then 8 mL of concentrated nitric acid (Fluka, 35% (w/w), suprapurⓇ) and 2 mL of perchloric acid (Merck Co., Germany, 90% (w/w), suprapurⓇ) were added. The solution was kept at room temperature for 10 h to perform the preliminary digestion. Subsequently, the pre-digested samples were placed at an oven with a temperature of 100 °C for 4 h and then 140 °C for 4 h until a clear solution was obtained. The tubes were again placed at room temperature to be cooled and filtered. Then, the volume of the filtrate was increased to 50 mL with ultrapure demineralized water (Milli-Q System, electrical conductivity: 0.054 μS/cm at 25 °C). The solution was tested for measuring the target contaminants of Cd, As, V, and Ni.
A control sample was used along with each sample to determine the amount of contamination caused by the equipment and digestion. To ensure the accuracy of the method used, the standard solution of metal(loid)s (CASS-5) was provided from the Merck company and the level of recovery was monitored. The recovery rates for all studied elements were within the range of 83–104%. The relative standard deviation (RSD) of the three replicates samples was less than 4.3%. It should be noted that all the materials, solutions, and acids were obtained from internationally accredited companies. Details of the quality assurance requirements were specified elsewhere (Safari et al. 2018).
Measurements
The concentration of Cd, Ni, As, and V was measured in seawater, sediment, and algae samples by Coupled Plasma Atomic Emission Spectroscopy (ICP-AES 4100, Agilent) at wavelengths characteristic of 167–785 nm, which induces excited atoms and ions to emit electromagnetic radiation. The limits of detection were 0.03 μg/L for Cd, 0.11 μg/L for V, 0.24 μg/L for Ni, and 0.03 μg/L for As. Details and specifications of this instrument were presented elsewhere (Safari et al. 2018). Briefly, some operational conditions for using this instrument were as follows: 0 L/min of auxiliary gas flow rate, 18 L/min of plasma gas flow rate, 2.1 kg/cm2 of carrier (nebulizer) gas pressure, 5 s of detector integration time, 1.4 mL/min of peristaltic pump flow rate, and three integrations per solution.
Statistical analysis
The SPSS software version 22 was used for multivariate analysis as well as data correlation analysis. Descriptive statistics (average ± SD) were used to report the amount of metal(loid)s in algae, water, and sediment. The normality of distribution data of metal(loid)s concentration in algae samples for all studied stations was checked based on the Shapiro-Wilk’s test. The differences between the amount of metal(loid)s in studied areas and algae species were determined by analysis of variance (ANOVA) test. The Pearson correlation matrix was used to determine the relationship between the amount of metal(loid)s in algae and those in seawater and sediments. The ability of algae in the accumulation of metal(loid)s were evaluated by linear regression. The p value ≤ 0.05 was identified as the significance level.
Data analysis
Bio-concentration factor
The bio-concentration factor (BCF) was calculated for evaluating the concentration of accumulated metal(loid)s in algae samples based on their measured concentration in seawater and sediment:
where Cn is the mean concentration of metal(loid) in each algae species, Cw and Cs is the average concentration of studied elements in seawater and sediment, respectively.
Metal(loid) pollution index
The metal(loid)s pollution index (MPI) was calculated to measure the overall content of metal(loid)s in the studied algae or sediment samples in each area using the following formula (Khaled et al. 2014):
where Mn is the average concentration of n-th metal (μg/g). For this study n = 4.
Nemerow pollution index (NPI)
The NPI index is used to evaluate the overall contamination of sediments with all the metal(loid)s and is described by the following formula:
where the PMIi is the average of the metal(loid) pollution index of all the pollutants, the PMImax is the maximum MPI among the metal(loid)s, based on the individual pollution index for each sampling area.
Geo-accumulation index (Igeo)
The Geo-accumulation index (Igeo) is calculated based on the individual metal(loid)s approach to evaluate metal(loid)s pollution of the sediment samples. Igeo is computed using the following formula:
where Csed and Bn are the measured and background values of metal(loid)s, respectively.
Health risk assessment
Generally, seawater can cause human health risks through skin absorption. Recreational and fishing activities in coastal areas can expose people to metal pollution. Therefore, it is essential to address the health risks of metals in seawater. The health risk assessment was calculated using the following formula:
where Ddermal-seawater is the dermal exposure dose, Cw is the contaminant concentration (mg/L), P is the permeability coefficient (cm/h), SA is the exposed body surface area (18,000 cm2), ET is the exposure time (1 h/day), CF is the conversion factor (1 L/ 1000 cm3), and BW is the body weight (70 kg) (Nadal et al. 2011).
Results and discussion
Metal(loid) pollution of seawater and sediments
Figure 2 shows the average concentration of metal(loid)s in seawater and sediment samples. According to Fig. 2, the lowest value of metal(loid)s (except Cd in seawater) was observed in seawater and sediment samples of the Dayyer coast, which was less affected by urban, commercial, and industrial pollution than other areas. On the other hand, in most cases (with the exception of As in seawater), the highest average concentration of metal(loid)s, which was notably higher than other samples, were detected in sediments and seawater of the Asaloyeh where the very large industrial sites are located. After Asaloyeh, samples of the Ganaveh area had the highest metal(loid) pollution as it receives a metal(loid)s load from commercial and industrial sources. The samples collected from the Bushehr area, rank third, reflecting the impact of urbanization, as well as trade and ship ports on the pollution of the sea (Dobaradaran et al. 2018).
In all samples collected from seawater and sediments, the average concentration of metal(loid)s showed the trend of “Ni > V > As > Cd.” The metal(loid)s concentration in seawater and sediments in ten islands in the Gulf, the average concentration of the four metal(loid)s in seawater in our study was higher than the values measured in the mentioned study in Table 1. Also, the average concentrations in sediments for Ni, As, and V (in some studied areas) were close to those reported by Jafarabadi et al. (2017).
The highest concentration of the measured metal(loid)s was seen for Ni, which was analyzed in the range of 2.20–30.80 μg/L in seawater and 12.50–88.90 μg/g in sediments and then followed by V with a average concentration of 2.30–17.82 μg/L in seawater and 5.50–33.75 μg/g in sediments. Increasing the concentrations of Ni and V is related to human activities and these two metals are the pollution indication of the gas and oil industries (Safari et al. 2018). So, petrochemical activities and gas refineries probably play a major role in the distribution of these metal(loid)s in the Persian Gulf (Ranjbar Jafarabadi et al. 2018). Rahmanpour et al. (2014) have assessed the concentration of metals in the Qeshm Island and referred to the impact of oil activities on the prevalence of more Ni and V in seawater than other studied elements. Although the average concentration of Ni in this region was almost twice as high as our most polluted area (Asaloyeh), the concentration of V was similar to the lowest concentration in the Dayyer area. Neyestani et al. (2016) was found an average concentration of Ni and V in the sediment samples higher than in our most polluted area, and have attributed the concentration of metals to oil and petrochemical activities. In a study on the western side of the Persian Gulf (Abdollahi et al. 2013), which is exposed to severe oil pollution, the concentration of Ni (54–58.33 μg/g) and V (30.66–32.56 μg/g) in sediments samples was close to our measurements for Asaloyeh.
The average concentration of As (0.99–1.67 μg/L for seawater and 1.80–11.30 μg/g for sediments) was much lower than the average of Ni and V concentrations. Cadmium with a concentration of 0.01–1.00 μg/L in seawater and 0.18–1.09 μg/g in sediments showed the lowest concentration among measured metal(lid)s. Other studies in Table 1 reported lower concentrations of As than our investigation. The average concentration of Cd in seawater in the Khark Island (Ranjbar Jafarabadi et al. 2018) was twice as high as in our most polluted area (Asaloyeh) and in Larak Island it is less than the Asaloyeh, but more than the other 3 areas in our study. In a recently published study (Sharifinia et al. 2018), the Cd concentration (3.90–17.65 μg/g) in sediments was also reported much higher than our measurements.
Accumulation of metal(loid)s in the studied algae
The average concentrations of metal(loid)s in the studied algae in investigated areas are compared and results are depicted in Fig. 3. This figure shows the superiority of the algal species studied in the Asaloyeh area for accumulating metal(loid)s in their tissues. The algae collected from Ganaveh, Bushehr, and Dayyer ranked next in terms of metal(loid)s concentration. The results reflect the impact of the metal(loid) contamination load on the marine environment and the bio-accumulation of metal(loid)s in the algae in different areas. In most cases, the trend of metal(loid)s accumulation in algae species was similar to that of seawater and sediments (i.e., Ni > V > As > Cd). In the Asaloyeh area as the highest polluted area, the sequence of “V˃ Ni˃ As˃ Cd” was observed for E. intestinalis, R. riparium, and C. myrica algae. The algae studied were intermediate between water and sediments for condensation of metal(loid)s, because the pollutant concentration in most cases in the studied species was measured to be more than seawater and less than sediment.
Nickel was the most abundant pollutant read in the four studied algae species. The lowest average concentration of Ni was reported in E. intestinalis and at Dayyer area (7.10 μg/g) and the highest value was seen in R. riparium species in Asaloyeh (50.60 μg/g). The average Ni concentration in the algae species decreased as follows: R. riparium > S. boveanum > C. myrica > E. intestinalis. The results showed that the Ni accumulation in E. intestinalis and C. myrica species was dependent on the sampling area. Thus, the average Ni concentration in the four areas was significantly differed (p value < 0.05, N = 16). The Pearson correlation analysis, which shows the relationship between the concentration of metal(loid)s in algae and those in the seawater/sediment of the sampling areas is presented in Table 2. According to Table 2, the Ni metal accumulated in algae species did not show a wide association with the seawater/sediments Ni, and only in the Ganaveh the relationship between Ni in C. myrica and seawater was significant (p value < 0.01, N = 16).
The V accumulation in the algae species was highly dependent on the studied areas. The average concentration of V in all algal species was significantly different among the sampling areas (p value < 0.01 for each of the algae species). Vanadium, which showed the second-highest concentration in the algae species biomass, has the widest average range of concentrations (5.90–55.57 μg/g). The decrease in the accumulation of V in algae species was observed as follows:
R. riparium ˃ C. myrica ˃ E. intestinalis ˃ S. boveanum
The analysis of Pearson correlation showed no significant association between vanadium in algae and seawater (p value ˃ 0.05). In contrast, a significant association was found between the amount of vanadium in sediments and algal tissue in the investigated areas (Asaloyeh: C. myrica, p value ˂ 0.01, Dayyer: E. intestinalis, p value ˂ 0.05, and Ganaveh: R. riparium, p value ˂ 0.05).
The As accumulation in the algae was also dependent on the sampling area. Because the difference in average As concentration between areas for each alga was significant. The lowest average concentration of As was related to C. myrica in Dayyer area (0.85 μg/g) and the highest one has belonged to E. intestinalis in the Asaloyeh area (3.09 μg/g). Among the algae species, the highest difference was observed between As in E. intestinalis (0.99–3.09 μg/g). According to Table 2, a significant association between As in seawater with R. riparium (in Asaloyeh, p value < 0.01), with S. boveanum (in Dayyer, p value < 0.01), and with C. myrica (in Bushehr, p value ˂ 0.05). As well, a significant association was seen for arsenic in the sediments and C. myrica in the Dayyer area (p value < 0.05).
Cadmium with an average concentration of 0.09–0.52 μg/g in four algae species had the lowest concentration among the studied metal(loid)s. As a result, only in E. intestinalis in Asaloyeh, the Cd average concentration exceeded 0.50 μg/g dry weight. Based on the Pearson correlation, the association between the amount of Cd in algae and seawater/sediments was most significant in comparison with other studied metal(loid)s. For example, in the Bushehr area, the correlations between Cd of seawater with S. boveanum and Cd in sediments with E. intestinalis and R. riparium, as well as the correlations between Cd in Asaloyeh sediments and Dayyer seawater with C. myrica were significant (p value < 0.05 or ˂ 0.01).
As a comparison, in a study on brown and green algae in the Strait of Hormuz (Dadolahi-Sohrab et al. 2011), the Cd concentration (2.10–5.50 μg/g) was much higher than our study and the concentration of Ni (21.50–71.60 μg/g) were reported higher than our measurements. For C. myrica, the concentration of Cd (3.33–7.50 μg/g) (Alahverdi and Savabieasfahani 2012), was significantly higher than our study (0.06–0.71 μg/g) and the minimum and maximum Ni concentrations (10.00–43.06 μg/g) were greater than in our study (5.80–33.30 μg/g). In contrast, in the E. intestinalis species on the coasts of the sea of Oman, the Cd concentration (ND-0.003) was significantly lower than our cadmium measurements (Manavi 2013). In studies in other parts of the world, in Sargassum species studied in two regions of Todos os Santos Bay, Brazil, the concentration of Cd (0.38, 0.71 μg/g) and As (16.10, 19.70 μg/g) were higher than our measured levels, V (25.10, 14.10 μg/g) was similar to our concentration range, and Ni (7.47, 9.93 μg/g) was lower than our values (Brito et al. 2012). For E. intestinalis in the Gulf of Kutch in India (Chakraborty et al. 2014), the amount of Cd (0.88–2.20 μg/g) was higher than our study, whereas for nickel the reported values (1.10–2.10 μg/g) were much lower than our measured values. In the Aegean Sea, Akcali and Kucuksezgin (2011) have reported a similar Cd concentration in the Cystoseira species to that of our work, but in the Enteromorpha species, cadmium was approximately 10 times lower than in our measured case.
The high concentrations of Ni and V in the algal species tissue (also seawater and sediments) in the Asaloyeh area might be originated from the petroleum and gas sources. Nickel and vanadium are two major components of gas and petrochemical industries located in the Asaloyeh, and thus, the release of these elements into the environment is unavoidable (Soltanieh et al. 2016; Kafaei et al. 2017a). Environmental pollution such as ambient air pollution (Chakraborty et al. 2014; Safari et al. 2018) can also affect the biological accumulation of metal(loid)s. Various factors such as structural difference of algae, physical and chemical properties of seawater and sediments, environmental concentration of metal(loid), age and growth conditions of algae, contact time, sampling time, metabolic processes, competition of metal(loid)s in absorption to algae, and final concentrations of element in algae modulators affect the accumulation of the metal in algae (Afonso et al. 2018; Brito et al. 2012; Ramachandra et al. 2018; Billah et al. 2017).
The ability of algae as biomonitoring agent of metal(loid)s in seawater and sediment
The results of the regression analysis showed a positive relationship between the concentration of some metal(loid)s in seawater- algae and sediment- algae (Table 3). The correlations between the Cd concentration in algae species and seawater showed very strong correlations for algae of C. myrica (maximum value R2 = 0.998), R. riparium, and S. boveanum. In contrast, for Ni, a weak to moderate correlation between seawater and algae species was seen (R2 = 0.129–0.525). In the case of vanadium, the moderate to strong correlations were evaluated (R2 = 0.526 for S. boveanum—R2 = 0.975 for E. intestinalis). For arsenic metalloid, a solid-to-strength (R2 = 0.133 for E. intestinalis and R2 = 0.816 for C. myrica) correlation was observed. In sediments, in contrast to seawater, the correlation was weak to moderate, although, like water, the highest correlation was found for the brown algae of C. myrica (R2 = 0.521). Similar to seawater, the correlation for Ni in sediments was in the range of R2 = 0.153–0.616. For V metal, the correlation for R. riparium, S. boveanum, and E. intestinalis was observed to be very strong (> 0.8). The highest correlation was observed for As in sediments and E. intestinalis species with R2 = 0.687.
The C. myrica species, in both seawater and sediment samples, was recognized as the most suitable biomonitoring tool for Cd. This species was also recognized as the best seawater As biomonitor and an appropriate biomonitor agent for Ni in sediments and V in seawater. The E. intestinalis species was the best agent for assessing V and Ni in seawater and a suitable agent for monitoring V, Cd, and As in sediments. The R. riparium species was the best biomonitor of V in the sediments and an appropriate monitoring agent of V, Cd, and Ni in the seawater among studied algae. The S. boveanum species was also known as a suitable biomonitor for Cd in seawater, Ni in the sediments, and V in seawater and sediments. Therefore, the studied algae species seem to be good biomonitoring agents for reflecting the Ni, V, Cd, and As pollution of the marine environment.
Assessment of metal(loid) pollution through indexes
Bio-concentration factor (BCF)
The BCF values of metal(loid)s for all algae in each area are given in Table 4. For most of the studied areas for seawater and sediments samples, the lowest and highest BCF values were calculated in R. riparium and E. intestinalis (for Cd), in E. intestinalis and R. riparium (for Ni), and in S. boveanum and R. riparium (for V), respectively. For arsenic in both seawater and sediment, in most areas, the highest BCF was found for E. intestinalis and the minimum values were varied for different algae species in different areas. The calculated values clearly showed that the ability of each alga to absorb various metal(loid)s from seawater and sediments is very variable. For instance, both of the green algae have shown a very different behavior in the absorption of Cd and Ni. The values of BCFw for all metal(loid)s were higher than BCFs (the sediments BCF). Based on Table 4, the largest amount of bio-concentration was related to BCFw of Cd in E. intestinalis and C. myrica species, while BCFw calculated for V had also high value for R. riparium and C. myrica species. In most cases, the BCFw factor was calculated to be greater than one, indicating the high ability of selected algae in the bio-accumulation of metal(loid)s from seawater. On the other hand, BCFs, except for a few cases related to V and Ni, for the R. riparium species in the Ganaveh area was calculated to be less than 1, showing a limited accumulation of metal(loid)s from sediments to algae. For nearly all samples, the BCFs value of V (V-BCFs) was higher than other metal(loid)s except for E. intestinalis in Bushehr, in which V-BCFs was less than As- and Cd-BCFs. Other researchers have also reported a BCFw more than one and BCFs less than one for green algae (Jitar et al. 2015; Manavi 2013; Diop et al.), which emphasized the bio-concentration of metal(loid)s from seawater to algae and the limited accumulation from sediments to algae.
Metal(loid)s pollution index (MPIseawater)
The MPIseawater, which represents the total accumulation of metal(loid)s in algae, showed the trend of “Asaloyeh ˂ Ganaveh ˂ Bushehr ˂ Dayyer” (Table 4) for all algae species. The highest MPIseawater value (5.80) for the green algae of R. riparium in Asaloyeh and the lowest level (1.66) for brown algae of S. boveanum in the Dayyer showed a 5-fold variation in the accumulation of metal(loid)s from a highly polluted area to the safe one. The MPIseawater trend for four areas was as follows:
Asaloyeh: R. riparium ˃ C. myrica ˃ E. intestinalis ˃ S. boveanum.
Ganaveh: C. myrica ˃ E. intestinalis ˃ R. riparium ˃ S. boveanum.
Bushehr: C. myrica ˃ E. intestinalis ˃ R. riparium ˃ S. boveanum.
Dayyer: E. intestinalis ˃ R. riparium ˃ C. myrica ˃ S. boveanum.
Based on Table 4, for all areas, the green algae of R. riparium had a high MPIseawater rate among other species and lowest MPIseawater was allocated to S. boveanum.
Geo-accumulation index (Igeo)
The Igeo values for the metal(loid)s accumulation in the coastal sediments of the Persian Gulf are listed in Table 5. The behavior of cadmium was very different from other metal(loid)s, and based on this metal, all areas are considered to be non-polluted. Based on other metal(loid)s, the pollution of all areas in the class is ‘strong to extreme’. The Asaloyeh area had the highest Igeo values among studied areas, which could be due to industrial activities and the discharge of sewage to the sea.
Nemerow pollution index (NPI)
The value of the Nemerow pollution index was calculated for four areas (Table 5). For all areas, the value of this indicator is higher than one, which indicates the pollution of these areas. The order of this index for the metal(loid)s studied in the areas was as follows: Asaloyeh> Ganaveh> Bushehr> Dayyer. The most severe contamination for Asaloyeh is due to the undeveloped growth of industries in this area.
Metal(loid)s pollution index (MPIsediment)
Like the NPI index, a value greater than one for the MPIsediment index is a sign of metal(loid)s pollution. Therefore, according to Table 5, all sampled areas are polluted and the most severe metal(loid)s pollution has occurred in Asaloyeh.
Health risk assessment
Dermal exposure to cadmium and nickel through seawater was only calculated to assess the potential risk. Hazard index (HI) for Cd and Ni exposure for four areas was estimated smaller than unity (threshold number). In the coastal areas the HI value was decreased as “Asaluyeh > Ganaveh > Dayyer > Bushehr” and “Asaluyeh> Ganaveh> Bushehr> Dayyer” for Cd and Ni, respectively. The carcinogenic risk was not possible for none of the metal(loid)s. Accordingly, exposure to the studied metal(loid)s by dermal contact not assumed to be a source of dermal cancer. However, the non-cancer effect may be minor and the seawater might not be completely safe for residents of the coastal areas.
As we have seen, anthropogenic factors (gas and petrochemical industries, commercialization, and urbanization) had a direct impact on increasing the burden of metal pollution of seawater and sediments compared to the control area. Therefore, environmental protection and especially the marine environment in areas with anthropogenic activity are vital. Ecological indicators such as algae species can be used to identify the influence of metal(loid)s contaminants.
Conclusions
In this investigation, the concentrations of Cd, V, Ni, and As in seawater, sediment, and green/brown algae were investigated in four areas (with different coastal land applications) in the north of the Persian Gulf. The accumulation of metal(loid)s in seawater, sediment, and algae of the studied areas had the pattern of “Ni> V> As> Cd.” The concentration of metals in the seawater, sediments, and algae studied in the Asaloyeh was more intense than the other areas and the lowest amount was found in Dayyer. The quality of seawater and sediment was assessed by using the geo-accumulation index (Igeo), Nemerow pollution index (NPI) metal(loid)s pollution index (MPIsediment). Human activities appear to be the most important factor in the distribution of metals in the studied ecosystem. The algae of C. myrica, S. boveanum, and R. riparium showed a high ability to absorb metal(loid)s from seawater. The C. myrica species for As and Cd in seawater and Ni and Cd in sediments, the E. intestinalis species for Ni and V in seawater and As in sediments and the R. riparium species for V in sediments were identified as the best indicator species. Thus, the algae species can be a Real-Time tool for monitoring the toxic elements in the seawater and sediments of the coastline environment.
References
Abdollahi S, Raoufi Z, Faghiri I, Savari A, Nikpour Y, Mansouri A (2013) Contamination levels and spatial distributions of heavy metals and PAHs in surface sediment of Imam Khomeini Port, Persian Gulf, Iran. Mar Poll Bull 71(1):336–345. https://doi.org/10.1016/j.marpolbul.2013.01.025
Achary MS, Satpathy K, Panigrahi S, Mohanty A, Padhi R, Biswas S, Prabhu R, Vijayalakshmi S, Panigrahy R (2017) Concentration of heavy metals in the food chain components of the nearshore coastal waters of Kalpakkam, southeast coast of India. Food Control 72:232–243
Afonso C, Cardoso C, Ripol A, Varela J, Quental-Ferreira H, Pousão-Ferreira P, Ventura M, Delgado I, Coelho I, Castanheira I (2018) Composition and bioaccessibility of elements in green seaweeds from fish pond aquaculture. Food Res Int 105:271–277
Akcali I and Kucuksezgin F (2011) A biomonitoring study: heavy metals in macroalgae from eastern Aegean coastal areas. Mar Poll Bull 62(3):637–645
Alahverdi M, Savabieasfahani M (2012) Metal pollution in seaweed and related sediment of the Persian Gulf, Iran. Bull Environ Contam Toxicol 88(6):939–945
Al-Yemni MN, Sher H, El-Sheikh MA, Eid EM (2011) Bioaccumulation of nutrient and heavy metals by Calotropis procera and Citrullus colocynthis and their potential use as contamination indicators. Sci Res Essays 6(4):966–976
Aydın-Önen S, Öztürk M (2017) Investigation of heavy metal pollution in eastern Aegean Sea coastal waters by using Cystoseira barbata, Patella caerulea, and Liza aurata as biological indicators. Environ Sci Poll Res 24(8):7310–7334
Bian B, Zhou Y, Fang BB (2016) Distribution of heavy metals and benthic macroinvertebrates: impacts from typical inflow river sediments in the Taihu Basin, China. Ecol Indic 69:348–359
Billah MM, Mustafa Kamal AH, Idris MH, Ismail J (2017) Mangrove macroalgae as biomonitors of heavy metal contamination in a tropical estuary, Malaysia. Water Air Soil Pollut 228(9):347–314. https://doi.org/10.1007/s11270-017-3500-8
Brito GB, de Souza TL, Bressy FC, Moura CW, Korn MGA (2012) Levels and spatial distribution of trace elements in macroalgae species from the Todos os Santos Bay, Bahia, Brazil. Mar Poll Bull 64(10):2238–2244
Çelekli A, Arslanargun H, Soysal Ç, Gültekin E, Bozkurt H (2016) Biochemical responses of filamentous algae in different aquatic ecosystems in south East Turkey and associated water quality parameters. Ecotoxicol Environ Safe 133:403–412
Chakraborty S, Bhattacharya T, Singh G, Maity JP (2014) Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: a biomonitoring approach for pollution assessment. Ecotoxicol Environ Safe 100:61–68
Dadolahi-Sohrab A, Nikvarz A, Nabavi S, Safahyeh A, Ketal-Mohseni M (2011) Environmental monitoring of heavy metals in seaweed and associated sediment from the Strait of Hormuz, IR Iran. WJFMS 3(6):576–589
De La Calle I, Cabaleiro N, Romero V, Lavilla I, Bendicho C (2013) Sample pretreatment strategies for total reflection X-ray fluorescence analysis: a tutorial review. Spectrochim Acta B Atom Spectrosc 90:23–54. https://doi.org/10.1016/j.sab.2013.10.001
Delshab H, Farshchi P, Keshavarzi B (2017) Geochemical distribution, fractionation and contamination assessment of heavy metals in marine sediments of the Asaluyeh port, Persian Gulf. Mar Poll Bull 115(1–2):401–411
Dionísio M, Costa A, Rodrigues A (2013) Heavy metal concentrations in edible barnacles exposed to natural contamination. Chemosphere 91(4):563–570
Dobaradaran S, Soleimani F, Nabipour I, Saeedi R, Mohammadi MJ (2018) Heavy metal levels of ballast waters in commercial ships entering Bushehr port along the Persian Gulf. Mar Poll Bull 126:74–76. https://doi.org/10.1016/j.marpolbul.2017.10.094
Favas PJC, Pratas J, Rodrigues N, D'Souza R, Varun M, Paul MS (2018) Metal(loid) accumulation in aquatic plants of a mining area: potential for water quality biomonitoring and biogeochemical prospecting. Chemosphere 194:158–170. https://doi.org/10.1016/j.chemosphere.2017.11.139
Freije AM (2015) Heavy metal, trace element and petroleum hydrocarbon pollution in the Arabian Gulf. Arab Univ Basic Appl Sci 17:90–100
García-Seoane R, Fernández JA, Villares R, Aboal JR (2018) Use of macroalgae to biomonitor pollutants in coastal waters: optimization of the methodology. Ecol Indic 84:710–726. https://doi.org/10.1016/j.ecolind.2017.09.015
Gautam S, Pandey LK, Vinayak V, Arya A (2017) Morphological and physiological alterations in the diatom Gomphonema pseudoaugur due to heavy metal stress. Ecol Indic 72:67–76. https://doi.org/10.1016/j.ecolind.2016.08.002
Gomes PI, Asaeda T (2013) Phytoremediation of heavy metals by calcifying macro-algae (Nitella pseudoflabellata): implications of redox insensitive end products. Chemosphere 92(10):1328–1334
Illuminati S, Annibaldi A, Truzzi C, Scarponi G (2016) Heavy metal distribution in organic and siliceous marine sponge tissues measured by square wave anodic stripping voltammetry. Mar Poll Bull 111(1–2):476–482
Illuminati S, Annibaldi A, Romagnoli T, Libani G, Antonucci M, Scarponi G, Totti C, Truzzi C (2017) Distribution of Cd, Pb and Cu between dissolved fraction, inorganic particulate and phytoplankton in seawater of Terra Nova Bay (Ross Sea, Antarctica) during austral summer 2011–12. Chemosphere 185:1122–1135. https://doi.org/10.1016/j.chemosphere.2017.07.087
Izquieta-Rojano S, Elustondo D, Ederra A, Lasheras E, Santamaría C, Santamaría JM (2016) Pleurochaete squarrosa (Brid.) Lindb. As an alternative moss species for biomonitoring surveys of heavy metal, nitrogen deposition and δ15N signatures in a Mediterranean area. Ecol Indic 60:1221–1228. https://doi.org/10.1016/j.ecolind.2015.09.023
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag 217:56–70. https://doi.org/10.1016/j.jenvman.2018.03.077
Jafarabadi AR, Bakhtiyari AR, Toosi AS, Jadot C (2017) Spatial distribution, ecological and health risk assessment of heavy metals in marine surface sediments and coastal seawaters of fringing coral reefs of the Persian Gulf, Iran. Chemosphere 185:1090–1111
Jafarabadi AR, Bakhtiari AR, Maisano M, Pereira P, Cappello T (2018) First record of bioaccumulation and bioconcentration of metals in Scleractinian corals and their algal symbionts from Kharg and Lark coral reefs (Persian Gulf, Iran). Sci Total Environ 640:1500–1511
Jha B, Reddy C, Thakur MC, Rao MU (2009) Seaweeds of India: the diversity and distribution of seaweeds of Gujarat coast, vol 3. Springer Science & Business Media,
Jitar O, Teodosiu C, Oros A, Plavan G, Nicoara M (2015) Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol 32(3):369–378
Jones L, Sullivan T, Kinsella B, Furey A, Regan F (2017) Occurrence of selected metals in wastewater effluent and surface water in Ireland. Anal Lett 50(4):724–737
Kafaei R, Tahmasbi R, Ravanipour M, Vakilabadi DR, Ahmadi M, Omrani A, Ramavandi B (2017a) Urinary arsenic, cadmium, manganese, nickel, and vanadium levels of schoolchildren in the vicinity of the industrialised area of Asaluyeh, Iran. Environ Sci Poll Res 24(30):23498–23507. https://doi.org/10.1007/s11356-017-9981-6
Kafaei R, Tahmasebi R, Ravanipour M, Vakilabadi DR, Ahmadi M, Farzaneh MR, Ramavandi B (2017b) Data on metals biomonitoring in the body of schoolchildren in the vicinity of a heavily industrialized site. Data in Brief 12:405–408. https://doi.org/10.1016/j.dib.2017.04.027
Kamala-Kannan S, Batvari BPD, Lee KJ, Kannan N, Krishnamoorthy R, Shanthi K, Jayaprakash M (2008) Assessment of heavy metals (Cd, Cr and Pb) in water, sediment and seaweed (Ulva lactuca) in the Pulicat Lake, South East India. Chemosphere 71(7):1233–1240
Khaled A, Hessein A, Abdel-Halim AM, Morsy FM (2014) Distribution of heavy metals in seaweeds collected along Marsa-Matrouh beaches, Egyptian Mediterranean Sea. Egypt J Aquat Res 40(4):363–371. https://doi.org/10.1016/j.ejar.2014.11.007
Kobzar A, Khristoforova N (2015) Monitoring heavy-metal pollution of the coastal waters of Amursky Bay (sea of Japan) using the brown alga Sargassum miyabei Yendo, 1907. Russ J Mar Biol 41(5):384–388
Malea P, Kevrekidis T (2014) Trace element patterns in marine macroalgae. Sci Total Environ 494-495:144–157. https://doi.org/10.1016/j.scitotenv.2014.06.134
Manavi PN (2013) Heavy metals in water, sediment and macrobenthos in the interdidal zone of Hormozgan province, Iran. Mar Sci 3(2):39–47
McNeill J, Barrie F, Buck W, Demoulin V, Greuter W, Hawksworth D, Herendeen P, Knapp S, Marhold K, Prado J (2012) International code of nomenclature for algae, fungi and plants. Regnum Vegetabile 154
Nadal M, Schuhmacher M, Domingo JL (2011) Long-term environmental monitoring of persistent organic pollutants and metals in a chemical/petrochemical area: human health risks. Environ Poll 159(7):1769–1777. https://doi.org/10.1016/j.envpol.2011.04.007
Nasrolahi A, Smith BD, Ehsanpour M, Afkhami M, Rainbow PS (2014) Biomonitoring of trace metal bioavailabilities to the barnacle Amphibalanus amphitrite along the Iranian coast of the Persian Gulf. Mar Environ Res 101:215–224. https://doi.org/10.1016/j.marenvres.2014.07.008
Neyestani MR, Bastami KD, Esmaeilzadeh M, Shemirani F, Khazaali A, Molamohyeddin N, Afkhami M, Nourbakhsh S, Dehghani M, Aghaei S, Firouzbakht M (2016) Geochemical speciation and ecological risk assessment of selected metals in the surface sediments of the northern Persian Gulf. Mar Poll Bull 109(1):603–611. https://doi.org/10.1016/j.marpolbul.2016.05.024
Niamaimandi N, Bahmyari Z, Sheykhsagha N, Kouhgardi E, Vaghei RG (2017) Species diversity and biomass of macroalgae in different seasons in the northern part of the Persian Gulf. Reg Stud Mar Sci 15:26–30. https://doi.org/10.1016/j.rsma.2017.07.001
Noronha-D’Mello CA, Nayak GN (2016) Assessment of metal enrichment and their bioavailability in sediment and bioaccumulation by mangrove plant pneumatophores in a tropical (Zuari) estuary, west coast of India. Mar Poll Bull 110(1):221–230. https://doi.org/10.1016/j.marpolbul.2016.06.059
Perry H, Isphording W, Trigg C, Riedel R (2015) Heavy metals in red crabs, Chaceon quinquedens, from the Gulf of Mexico. Mar Poll Bull 101(2):845–851
Qiu Y-W (2015) Bioaccumulation of heavy metals both in wild and mariculture food chains in Daya Bay, South China. Estuar Coast Mar Sci 163:7–14
Rahmanpour S, Ghorghani NF, Ashtiyani SML (2014) Heavy metal in water and aquatic organisms from different intertidal ecosystems, Persian Gulf. Environ Monit Assess 186(9):5401–5409
Ramachandra T, Sudarshan P, Mahesh M, Vinay S (2018) Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J Environ Manag 206:1204–1210
Ranjbar Jafarabadi A, Riyahi Bakhtiari A, Maisano M, Pereira P, Cappello T (2018) First record of bioaccumulation and bioconcentration of metals in Scleractinian corals and their algal symbionts from Kharg and Lark coral reefs (Persian Gulf, Iran). Sci Total Environ 640-641:1500–1511. https://doi.org/10.1016/j.scitotenv.2018.06.029
Safari M, Ramavandi B, Sanati AM, Sorial GA, Hashemi S, Tahmasebi S (2018) Potential of trees leaf/ bark to control atmospheric metals in a gas and petrochemical zone. J Environ Manag 222:12–20. https://doi.org/10.1016/j.jenvman.2018.05.026
Sharifinia M, Taherizadeh M, Namin JI, Kamrani E (2018) Ecological risk assessment of trace metals in the surface sediments of the Persian Gulf and Gulf of Oman: evidence from subtropical estuaries of the Iranian coastal waters. Chemosphere 191:485–493. https://doi.org/10.1016/j.chemosphere.2017.10.077
Shiber J (1980) Trace metals with seasonal considerations in coastal algae and molluscs from Beirut, Lebanon. Hydrobiologia 69(1–2):147–162
Singh N, Raghubanshi A, Upadhyay A, Rai U (2016) Arsenic and other heavy metal accumulation in plants and algae growing naturally in contaminated area of West Bengal, India. Ecotoxicol Environ Safe 130:224–233
Soltanieh M, Zohrabian A, Gholipour MJ, Kalnay E (2016) A review of global gas flaring and venting and impact on the environment: case study of Iran. Int J Greenh Gas Con 49:488–509
Teimouri A, Esmaeili H, Foroutan R, Ramavandi B (2018) Adsorptive performance of calcined Cardita bicolor for attenuating Hg(II) and As(III) from synthetic and real wastewaters. Korean J Cheml Eng 35(2):479–488. https://doi.org/10.1007/s11814-017-0311-y
Topcuoğlu S, Güven K, Balkıs N, Kırbaşoğlu Ç (2003) Heavy metal monitoring of marine algae from the Turkish coast of the Black Sea, 1998–2000. Chemosphere 52(10):1683–1688
Yümün ZÜ (2017) The effect of heavy metal pollution on foraminifera in the Western Marmara Sea (Turkey). J Afr Earth Sci 129:346–365. https://doi.org/10.1016/j.jafrearsci.2017.01.023
Zhang Y, Chu C, Li T, Xu S, Liu L, Ju M (2017) A water quality management strategy for regionally protected water through health risk assessment and spatial distribution of heavy metal pollution in 3 marine reserves. Sci Total Environ 599-600:721–731. https://doi.org/10.1016/j.scitotenv.2017.04.232
Funding
The authors are thankful to the Bushehr University of Medical Sciences for providing a grant (Grant No.: BPUMS-1296-3) to conduct this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vedula VSS Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghshenas, V., Kafaei, R., Tahmasebi, R. et al. Potential of green/brown algae for monitoring of metal(loid)s pollution in the coastal seawater and sediments of the Persian Gulf: ecological and health risk assessment. Environ Sci Pollut Res 27, 7463–7475 (2020). https://doi.org/10.1007/s11356-019-07481-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07481-0