Abstract
Rapid separation and analysis of trace quinolones (fleroxacin (FLRX), enoxacin (EN), norfloxacin (NOR), ciprofloxacin (CIP), enrofloxacin (ENRO), and lomefloxacin hydrochloride (LOME)) in real water samples were achieved by using a multi-templates molecularly imprinted polymer (MIP) based solid phase extraction (SPE) coupled with dispersive liquid-liquid microextraction (DLLME) followed by high performance liquid chromatography (HPLC). The MIP was prepared via surface molecular imprinting, using the selected quinolones as the templates and mesoporous silica modified magnetic graphene oxide as the carrier. The preparation and adsorption conditions were optimized. The MIP presented high adsorption capacity and wonderful selective recognition for the quinolones, with the adsorption capacities of 20.15, 20.88, 18.01, 20.01, 16.98, and 17.09 mg/g for FLRX, EN, NOR, CIP, ENRO, and LOME, respectively. Meanwhile, a SPE-DLLME-HPLC method for trace detection of FLRX, EN, NOR, CIP, ENRO, and LOME in real water samples was developed and showed outstanding applicability. The spiked recoveries and relative standard deviations (RSDs) were 89.67–100.5%, and 3.59–7.12%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quinolones were widely used to treat human and animal diseases (Lombardo-Agüí et al. 2015; Alcaráz et al. 2015). However, a large quantity of quinolones were emitted into the environment through human and animals’ excrement, causing environmental pollution because of their bacterial resistance, allergy, and toxicity (Junza et al. 2014; Hou et al. 2014). Therefore, extensive use of quinolones will pose harmful effect on ecological environment and human health (Moreno-González et al. 2014; Douros et al. 2015). Among quinolone antibiotics, fleroxacin (FLRX), enoxacin (EN), norfloxacin (NOR), ciprofloxacin (CIP), enrofloxacin (ENRO), and lomefloxacin hydrochloride (LOME) were often detected in environment waters (Lang et al. 2018; Zhang and Cheng, 2019). Currently, high performance liquid chromatography (HPLC) and gas chromatography mass-spectrometry (GC-MS) were frequently applied for simultaneous determination of these quinolones (Xie et al. 2015; Rocha et al. 2015). However, these methods faced a drawback that a rigorous pretreatment process was needed before analysis, which required great labor and time. Additionally, little work had been done to carry out simultaneous determination of multiple quinolones using a relatively simple and sensitive pretreatment method. In our previous study, a hyphenated technique using magnetic molecular imprinting polymers based solid phase extraction (SPE) coupled with dispersive liquid-liquid microextraction (DLLME) were frequently applied in the sample pretreatment procedures, which presented simplicity, selectivity, and high enrichment efficiency (Xie et al. 2019; Guo et al. 2019; Li et al. 2018).
Molecularly imprinted polymer (MIP) with template-created cavities has been widely used as molecular recognition material in various fields, such as solid phase extraction, drug delivery, biosensors, and protein crystallization (Li et al. 2015; Huang et al., 2015a, b, 2017; Liu et al. 2016, 2018, 2019; Wang et al. 2010; Lai et al. 2017). This could be attributed to their high selectivity, strong anti-interference ability, and good stability (Chang et al. 2012; Gu et al. 2015; Han et al. 2015; Pourjavadi et al. 2015). However, traditional synthesis methods of MIP such as precipitation polymerization, bulky organic co-polymerization, and so on have a disadvantage that plenty of the recognition sites in the particles are embedded in the interior area (Wang et al. 2010). On the contrary, surface molecular imprinting technology can overcome the above drawback and generate more affinity sites on the surface of MIP. In addition, to achieve high molecular recognition ability, an effective carrier of MIP is very important. Graphene oxide (GO) has high surface area and rich functional groups, and mesoporous silica (mSiO2) owns tunable pore sizes and large surface area; both of them have many applications in adsorption (Dreyer et al. 2014; Wang et al. 2019; Zhang and Cheng, 2019) Li et al. 2018). In our previous researches, mSiO2 supported on the surface of magnetic GO was used as the carrier of MIPs, and the MIPs showed excellent adsorption capacity, rapid kinetics, and high selectivity for analysis of organic pollutants in water (Xie et al. 2019; Guo et al. 2019; Li et al. 2018).
In this study, a new multi-templates MIP, with FLRX, EN, NOR, CIP, ENRO, and LOME as the templates, was successfully synthesized for simultaneous preconcentration and determination of quinolones in water. In the synthesis process, mesoporous silica modified magnetic graphene oxide was utilized as the carrier. The adsorption behaviors of MIP for these quinolones were investigated. Besides, a coupling of the MIP-based SPE with dispersive liquid-liquid microextraction (DLLME), another efficient sample pretreatment technique (Gao and Ma 2011; Ebrahim et al. 2015; Zhang and Cheng, 2019), was developed, and the conditions for SPE and DLLME were optimized. Finally, the SPE-DLLME-HPLC method was used to separate and detect trace amount of FLRX, EN, NOR, CIP, ENRO, and LOME in real water samples.
Materials and methods
Chemicals
Natural flake graphite (China National Medicine Corporation Ltd), fleroxacin (FLRX, 98 wt%), enoxacin (EN, 98 wt%), norfloxacin (NOR, 99 wt%), ciprofloxacin (CIP, 99 wt%), enrofloxacin (ENRO, 99 wt%), lomefloxacin (LOME, 98 wt%), ofloxacin (98 wt%), and gatifloxacin (99 wt%), 4-vinylbenzoic acid (98 wt%), 2,2′-azobisisobutyronitrile (AIBN, 98 wt%), ethylene glycol dimethacrylate (EGDMA, 98 wt%), tetraethyl orthosilicate (TEOS, 98 wt%), vinyltrimethoxysilance (VTTS, 98 wt%), cetyl trimethylammonium bromide (CTAB, 98 wt%), and HPLC-grade tetrachloroethylene, chlorobenzene, tetrachloroethane, carbon tetrachloride, methanol, and formic acid were bought from Aladdin Reagent (Shanghai, China).
Analytical methods
High performance liquid chromatography (HPLC, PDA, C18 column (250 × 4.6 mm, 5 μm), Shimadzu Corporation, Japan) was applied to detect the quinolone antibiotics. Other analytical condition were as follows: PDA detection at 270 nm; column temperature at 40 °C; injection volume of 10 μL; elution rate at 1 mL min−1 with water/formic acid (9/1, V:V) as eluent A and methanol as eluent B, from 80% B to 70% B in 0–3 min, 70% B to 60% B in 3–8 min, 60% B to 55% B in 8–13 min, 55% B to 60% B in 13–17 min, and 60% B in 17–20 min. Under these test conditions, the retention times of FLRX, EN, NOR, CIP, ENRO, and LOME were 10.9, 11.9, 12.8, 13.4, 13.6, and 14.1 min, respectively, and the chromatogram was showed in Fig. S1.
Preparation of multi-templates MIP
The synthesis procedures of magnetic graphene oxide embellished with mesoporous silica (MGO@mSiO2) and (MGO@mSiO2) modified with vinyl groups (VTTS-MGO@mSiO2) were introduced in detail in our previous work (Xie et al. 2019; Guo et al. 2019; Li et al. 2018). The synthesis steps of MIP were as follows: first, 30 mg FLRX, 26 mg EN, 26 mg NOR, 27 mg CIP, 29 mg ENRO, and 31 mg LOME (1:1:1:1:1:1, molar ratio) blended with 221 mg 4-vinylbenzoic were dispersed in 50-mL toluene and undergo an ultrasound for 30 min, then reacting under air bath shaking table for 4 h at 15 °C. After that, 300 mg VTTS-MGO@mSiO2, 50 mg AIBN, and 471 μL EGDMA were added to the mixture and then the nitrogen was used to deoxygenate under ice-cooling. The reaction was processed for 24 h at 60 °C. To completely remove templates, the obtained MIP was eluted with the mixtures of methanol and acetic acid (9:1, V:V) until no templates were detected using HPLC. Meanwhile, the non-imprinted polymer (NIP) was synthesized using the abovementioned ways in the lack of the target templates.
Batch adsorption experiments
The influences of pH and initial concentrations of quinolones on the adsorption performance of the MIP (or NIP) were investigated. A total of 20 mg MIP or NIP was dispersed in methanol/water (4:1, V: V) solutions of FLRX, EN, NOR, CIP, ENRO, and LOME with pH values from 3 to 9, and shaken for 1 h. The sorption isotherm tests were performed at three constant temperatures (298 K, 308 K, and 318 K). Then, the saturated solutions and MIP (or NIP) were separated by an external magnet. Next, the residual concentrations of FLRX, EN, NOR, CIP, ENRO, and LOME were detected using HPLC system. The adsorption capacity was calculated as
where Qe (mg/g) represents the adsorption capacity of the MIP (or NIP), Co and Ce stand for the initial and equilibrium concentration (mg/L), and V (L) and M (mg) represent the solution volume and adsorbent mass, respectively.
Adsorption selectivity and regeneration experiments
Ofloxacin and gatifloxacin (the retention times of ofloxacin and gatifloxacin were 11.9 and 15.8 min, respectively, and the chromatogram was showed in Fig. S2.) were chosen to prove the selectivity of MIP toward FLRX, EN, NOR, CIP, ENRO, and LOME. In total, 20 mg MIP or NIP was dispersed in methanol/water (4:1, V:V) solutions of FLRX, EN, NOR, CIP, ENRO, and LOME and shaken for 1 h to adsorption equilibrium with adsorption. Then the residual concentration of FLRX, EN, NOR, CIP, ENRO, and LOME was detected using HPLC. Meanwhile, the used MIP (or NIP) was washed in methanol/acetic acid to entirely remove the adsorbed quinolones, and the above steps were repeated five times to investigate the reusability. And the static distribution coefficient Kd, selectivity coefficient, and relative selectivity coefficients ko were calculated as
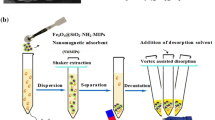
SPE-DLLME procedures
SPE and DLLME were applied as pretreatment ways to enrich the trace amounts of quinolones in real water samples. The procedures were as follows: first, 20 mg MIP as adsorbent was immersed into 50 mL water sample in a round-bottom flask, and then was stirred for 10 min. Next, the MIP was gathered with an external magnet and washed by deionized water. After that, the templates FLRX, EN, NOR, CIP, ENRO, and LOME were eluted by methanol. Finally, the obtained methanol eluent was used as the dispersant and tetrachloroethane as the extractant for the following DLLME. The formed emulsion was centrifuged and the sedimentary phase was gathered and then detected using HPLC.
Results and discussion
Preparation of multi-templates MIP
The amount of cross-linker and 4-vinylbenzoic acid for the preparation of MIP were optimized in the orthogonal design, and the results were presented in Table S1). The results showed that when 4-vinylbenzoic acid was 221 mg and cross-linker was 471 μL, the synthesized MIP had high adsorption capacity.
Adsorption study
Effects of pH on adsorption
The influence of the solution pH in the chemisorption process was tested in this study. The Fig. 1 showed that the adsorption capacity of MIP for FLRX, EN, NOR, CIP, ENRO, and LOME increased with the increased solution pH and reached to the highest at pH 6, which could be ascribed to the hydrogen bond and π-π interaction between quinolones and MIP. Thus, the solution pH 6 was selected in the following experiments.
Adsorption isotherm analysis
The adsorption isotherms for six quinolones at three constant temperatures (298 K, 308 K, and 318 K) were shown in Fig. 2. The results indicated that the adsorption capacity of MIP raised fast and then maintained flat with the initial concentrations improving, suggesting that the recognition sites for FLRX, EN, NOR, CIP, ENRO, and LOME reached a saturation. Meanwhile, the NIP showed the similar trend but with lower adsorption capacity. The Langmuir, Freundlich, and Tempkin models were chosen for subsequent analysis and the relevant fitting parameters were shown in Table 1 and Figs. 3, 4, and 5. The results showed that the correlation coefficients (R2) for Langmuir model (> 0.98) were significantly higher than those of both the Freundlich (> 0.44) and Tempkin models (> 0.91). Obviously, the Langmuir model could well describe the adsorption system, indicating that the adsorption process acted up to the monolayer sorption mechanism.
The Langmuir, Freundlich, and Tempkin models are shown as follows:
where Ce (mg/L), Qe (mg/g), and Qm (mg/g) stand for the equilibrium concentration, equilibrium, and maximum adsorption capacities, respectively; KF (mg/g) represents the Freundlich binding coefficient; and a, b, and n represent the constants.
Adsorption thermodynamics
The temperature influence on the adsorption of MIP for FLRX, EN, NOR, CIP, ENRO, and LOME was shown in Table 2 and Fig. 6. The results showed that the adsorption capacity of MIP was higher at a lower temperature, and the values of △G0 and of △H0 were all negative, implying that the adsorption process was a spontaneous endothermic reaction, which had good agreement with the isotherm experiments.
The adsorption process was investigated through the following equations:
where R, T, and b stand for the universal gas constant (8.314 J mol−1 K−1), Kelvin temperature (K), and the affinity constants, respectively; M represents the relative molecular mass of FLRX, EN, NOR, CIP, ENRO, and LOME, respectively; ΔH (KJ mol−1), ΔS (KJ mol−1), and ΔG represent the standard enthalpy changes, the entropy changes, and the Gibbs free energy changes, respectively.
Adsorption selectivity and regeneration
Ofloxacin and gatifloxacin were chosen to investigate the selectivity of MIP toward FLRX, EN, NOR, CIP, ENRO, and LOME. The results shown in Table 3 and Fig. 7 indicated that the adsorption amounts of MIP toward FLRX, EN, NOR, CIP, ENRO, and LOME were evidently higher than those of loxacin and gatifloxacin. On the contrary, the NIP represented similar adsorption capacity to the competitive molecules, suggesting that the specific recognition sites on the surface of MIP were successfully synthesized. Meanwhile, the adsorption capacity of VTTS-MGO@mSiO2 was investigated to demonstrate the imprinting influence of MIP. The results showed that the adsorption capacity of VTTS-MGO@mSiO2 was much lower than MIP.
Besides, the regeneration capacity of MIP was also studied. As shown in Fig. 8, after five regeneration cycles, the adsorption capacity of MIP toward FLRX, EN, NOR, CIP, ENRO, and LOME is still maintained at a high level, suggesting that MIP had good reusability.
Optimization of SPE-DLLME conditions
The conditions of SPE and DLLME were optimized, and the results were shown in Fig. 9 and 10. As can be seen in SPE, 50 mL solution at pH 6 was tested and the adsorption arrived at equilibrium after 12 min. A total of 3-mL methanol was applied to elute the adsorbed quinolones for 6 min. In DLLME, 50-μL tetrachloroethane was used as the extractant and the eluent of SPE as the dispersant. Then, the recovery and enrichment coefficient of FLRX, EN, NOR, CIP, ENRO, and LOME were acquired under the above optimal parameters.
Environmental water analysis
The developed SPE-DLLME-HPLC method was used to separate and detect trace FLRX, EN, NOR, CIP, ENRO, and LOME in real water samples (a lake water sample, a well water sample, and a Pearl River water sample). The results were shown in Table S2. As can be seen, in the lake water and river water, NOR, CIP, and ENRO were detected with a concentration of 1.21, 1.52, and 1.64 and 0.96, 1.26, and 1.34 μg/L, respectively. And CIP and ENRO were found at a concentration of 1.01 and 0.88 μg/L in well water, respectively. In addition, the recoveries of the quinolones were 89.67–100.5%, the RSDs of the results were 3.59–7.12%, and the limits of detections (LODs) for FLRX, EN, NOR, CIP, ENRO, and LOME were 0.013, 0.012, 0.011, 0.012, 0.015, and 0.015 μg/L, respectively, indicating that the SPE-DLLME-HPLC method represented outstanding applicability for the rapid detection of trace FLRX, EN, NOR, CIP, ENRO, and LOME in real water samples.
Comparison with other methods
The comparison with other reported papers was presented in Table 4 (Tang et al. 2018; Zhu et al. 2019; Lian and Wang, 2016; David et al. 2014; Francisco et al. 2019). The MIP synthesized in this study could rapidly and simultaneously separate and detect trace six quinolones in real water. Meanwhile, as we can see, the method showed a lower linear range and LOD, a better extraction efficiency, and absorbing capacity toward FLRX, EN, NOR, CIP, ENRO, and LOME than those researches, meaning the sensitivity and feasibility of the suggested method possessed pretty good analytical performance.
Conclusions
An effective multi-templates molecularly imprinted polymer has been successfully prepared, with mesoporous silica supported onto the surface of magnetic graphene oxide as the carrier, to simultaneously extract and detect quinolones (FLRX, EN, NOR, CIP, ENRO, and LOME) in real water samples. The MIP presented high selectivity and adsorption capacity for the quinolones. Meanwhile, the SPE-DLLME-HPLCMIP method was used to separate and detect trace FLRX, EN, NOR, CIP, ENRO, and LOME in real water samples, and satisfactory results were obtained.
References
Alcaráz MR, Bortolato SA, Goicoechea HC, Olivieri AC (2015) A new modeling strategy for third-order fast high-performance liquid chromatographic data with fluorescence detection. Quantitation of fluoroquinolones in water samples. Anal Bioanal Chem 407:1999–2011
Chang LM, Chen SN, Li X (2012) Synthesis and properties of core-shell magnetic molecular imprinted polymers. J Appl Sur Sci 258:6660–6664
Douros A, Grabowski K, Stahlmann R (2015) Safety issues and drug–drug interactions with commonly used quinolones. Expert Opin Drug Met 11:25–39
David MG, Francisco JL, Laura GG, Ana MG (2014) Molecularly imprinted polymer as in-line concentrator in capillary electrophoresis coupled with mass spectrometry for the determination of quinolones in bovine milk samples. J Chromatogr A 1360:1–8
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2014) The chemistry of graphene oxide. J Chem Soc Rev 43:228–240
Ebrahim K, Poursafa P, Amin M (2015) Development of a simple and valid method for the trace determination of phthalate esters in human plasma using dispersive liquid-liquid microextraction coupled with GC-MS. J Sep Sci 40:4403–4410
Francisco B, Beatriz A, JoséLT AME (2019) Molecularly imprinted polymer-hollow fiber microextraction of hydrophilic fluoroquinolone antibiotics in environmental waters and urine samples. J Chromatogr A 1587:42–49
Gao ZB, Ma XG (2011) Speciation analysis of mercury in water samples using dispersive liquid–liquid microextraction combined with high-performance liquid chromatography. Anal Chim Acta 702:50–55
Guo LH, Ma XG, Xie XW, Huang RF, Zhang MY, Li J, Zeng GL, Fan YM (2019) Preparation of dual-dummy-template molecularly imprinted polymers coated magnetic graphene oxide for separation and enrichment of phthalate in water. Chem Eng J 2019:245–255
Gu FB, Liang M, Han DM, Wang ZH (2015) Multifunctional sandwich-like mesoporous silica-Fe3O4-graphene oxide nanocomposites for removal of methylene blue from water. RSC Adv 5:39964–39972
Hou XL, Chen G, Zhu L, Yang T, Zhao J, Wang L, Wu YL (2014) Development and validation of an ultra high performance liquid chromatography tandem mass spectrometry method for simultaneous determination of sulfonamides, quinolones and benzimidazoles in bovine milk. J Chromatogr B 962:20–29
Huang DL, Wang RZ, Liu YG, Zeng GM, Lai C, Xu P, Lu BA, Xu JJ, Wang C, Huang C (2015a) Application of molecularly imprinted polymers in wastewater treatment: a review. Environ Sci Pollut Res 22:963–977
Huang YH, Wang YZ, Pan Q, Wang Y (2015b) Magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein. Anal Chim Acta 877:90–99
Huang RF, Ma XG, Li X, Guo LH, Xie XW, Zhang MY, Li J (2017) A novel ion-imprinted polymer based on graphene oxide-mesoporous silica nanosheet for fast and efficient removal of chromium (vi) from aqueous solution. J Colloid Interface Sci 514:544–553
Han S, Li X, Wang Y, Chen SN (2015) Multifunctional imprinted polymers based on CdTe/CdS and magnetic graphene oxide for selective recognition and separation of p-t-octylphenol. Chem Eng J 271:87–95
Junza A, Dorival-García N, Zafra-Gómez A, Barrón D, Ballesteros O, Barbosa J, Navalón A (2014) Multiclass method for the determination of quinolones and β-lactams, in raw cow milk using dispersive liquid-liquid microextraction and ultra high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1356:10–22
Lombardo-Agüí M, García-Campana AM, Cruces-Blanco C, Gámiz-Gracia L (2015) Determination of quinolones in fish by ultra-high performance liquid chromatography with fluorescence detection using QuEChERS as sample treatment. Food Control 50:864–868
Lang L, Dong XQ, Di JB (2018) Determination of 11 antibiotics in Harbin section of Songhua river by SPE-HPLC. China Water Wastewater 34(20):114–118
Li X, Ma XG, Huang RF, Xie XW, Guo LH, Zhang MY (2018) Synthesis of a molecularly imprinted polymer on mSiO2@ Fe3O4 for the selective adsorption of atrazine. J Sep Sci 4:2837–2845
Li XJ, Wang XJ, Li LL, Duan HM, Luo CN (2015) Electrochemical sensor based on magnetic graphene oxide@gold nanoparticles molecular imprinted polymers for determination of dibutyl phthalate. Talanta 131:354–360
Liu SC, Pan JM, Zhu HJ, Pan GQ, Qiu FG (2016) Graphene oxide based molecularly imprinted polymers with double recognition abilities: the combination of covalent boronic acid and traditional non-covalent monomers. Chem Eng J 290:220–231
Lai C, Liu XG, Qin L, Zhang C, Zeng GM, Huang DL, Cheng M, Xu P, Yi H, Huang DW (2017) Chitosan-wrapped gold nanoparticles for hydrogen-bonding recognition and colorimetric determination of the antibiotic kanamycin. Microchim Acta 184:2097–2105
Liu XG, Huang DL, Lai C, Zeng GM, Qin L, Zhang C, Yi H, Li BS, Deng R, Liu SY, Zhang YJ (2018) Recent advances in sensors for tetracycline antibiotics and their applications. Tr Ana Chem 109:260–274
Liu XG, Huang DL, Lai C, Zeng GM, Qin L, Wang H, Yi H, Li BS, Liu SY, Zhang MM, Deng R, Fu YK, Li L, Xue WJ, Chen S (2019) Recent advances in covalent organic frameworks (COFs) as a smart sensing material. Chem Soc Rev 48:5266–5302
Lian ZR, Wang JT (2016) Determination of ciprofloxacin in Jiaozhou Bay using molecularly imprinted solid-phase extraction followed by high-performance liquid chromatography with fluorescence detection. Mar Pollut Bull 111:411–417
Moreno-González D, Lara FJ, Gámiz-Gracia L, García-Campata AM (2014) Molecularly imprinted polymer as in-line concentrator in capillary electrophoresis coupled with mass spectrometry for the determination of quinolones in bovine milk samples. J Chromatogr A 1360:1–8
Pourjavadi A, Shakerpoor A, Tehrani ZM (2015) Magnetic graphene oxide mesoporous silica hybrid nanoparticles with dendritic pH sensitive moieties coated by PEGylated alginate-co-poly (acrylic acid) for targeted and controlled drug delivery purposes. J Polym Res 22:1–13
Rocha DG, Santos FA, Silva JC, Augusti R, Faria AF (2015) Multiresidue determination of fluoroquinolones in poultry muscle and kidney according to the regulation 2002/657/EC. A systematic comparison of two different approaches: liquid chromatography coupled to high-resolution mass spectrometry or tandem mass spectrometry. J Chromatogr A 1379:83–91
Tang YW, Liu H, Gao JW, Liu XY, Gao X, Lu XN, Fang GZ, Wang JP, Li JR (2018) Upconversion particle@Fe3O4@molecularly imprinted polymer with controllable shell thickness as high-performance fluorescent probe for sensing quinolones. Talanta 181:95–103
Wang ZM, Wang WD, Coombs N, Soheilnia N, Ozin GA (2010) Graphene oxide-periodic mesoporous silica sandwich nanocomposites with vertically oriented channels. J Acs Nano 4:437–7450
Wang R, Ma XG, Zhang XJ, Li X, Li DP, Dang YF (2019) C8-modified magnetic graphene oxide based solid-phase extraction coupled with dispersive liquid-liquid microextraction for detection of trace phthalate acid esters in water samples. Ecotox Environ Safe 170:789–795
Xie J, Peng T, Zhu A, He J, Chang Q, Hu X, Chen H, Fan C, Jiang W, Chen M, Li J (2015) Multi-residue analysis of veterinary drugs, pesticides and mycotoxins in dairy products by liquid chromatography-tandem mass spectrometry using low-temperature cleanup and solid phase extraction. J Chromatogr B 1002:19–29
Xie XW, Ma XG, Guo LH, Fan YM, Zeng GL, Zhang MY, Li J (2019) Novel magnetic multi-templates molecularly imprinted polymer for selective and rapid removal and detection of alkylphenols in water. Chem Eng J 357:56–65
Zhang ZC, Cheng HF (2019) Recent development in sample pretreatment and detection methods for the determination of quinolones in environmental matrices. Environ Chem 38(1):1–22
Zhu GF, Cheng GH, Wang PY, Li WW, Wang YC, Fan J (2019) Water compatible imprinted polymer prepared in water for selective solid phase extraction and determination of ciprofloxacin in real samples. Talanta 200:307–315
Funding
This work was supported by the National Natural Science Foundation of China (No. 41272262), the Science and Technology Planning Project of Guangdong Province, China (No. 2016A040403112), and the Major Projects (Natural Science) of Education Department of Guangdong Province, China (261555101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 296 kb)
Rights and permissions
About this article
Cite this article
Fan, Y., Zeng, G. & Ma, X. Multi-templates surface molecularly imprinted polymer for rapid separation and analysis of quinolones in water. Environ Sci Pollut Res 27, 7177–7187 (2020). https://doi.org/10.1007/s11356-019-07437-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07437-4