Abstract
Biosolids are regarded as a major source of pharmaceutically active compounds (PhACs) in soil and may lead to their accumulation in plants and potential human risks through dietary intake. Using 14C labeling, we explored the effect of biosolids on the uptake and tissue distribution of carbamazepine (CAB) by three ready-to-eat vegetables (i.e., carrot, celery, and pak choi) under greenhouse conditions. The 14C-CAB was consistently detected in vegetables and plant tissues with bioconcentration factors in a range of 1.28–37.69, and it was easily translocated from root to leaf and/or stem with translocation factors > 1. The inhibition on the uptake and accumulation of 14C-labeled carbamazepine from soil by the addition of biosolids was consistently observed, and such inhibitory effect was related to the biosolid amendment rates, the category of vegetable, and the plant growth stages. The influence of biosolids on behavior of CAB and other emerging pollutants in the soil-plant system should be considered in their environmental risk assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceutically active compounds (PhACs) have raised great concern due to their undesired impact in environmental compartments, wildlife, and humans. Carbamazepine (CAB) is one of the most commonly occurring PhACs in the environment (Hossain et al. 2018; Ma et al. 2017; Ternes 1998; Williams and Kookana 2010). For instance, up to 6.3 μg/L of CAB was detected in treated wastewater (Ternes 1998). The concentrations of CAB were in the range of several hundreds of μg/kg in biosolids. For example, McClellan and Halden (2010) reported that CAB was present in biosolids with a concentration of up to 258 μg/kg. Previous studies have reported that CAB is highly recalcitrant to degradation in soil (Li et al. 2013; Durán-Álvarez et al. 2015). For instance, CAB was found to be persistent in soil with the estimated half-life (t1/2) values of > 120 days (Li et al. 2013). Wu et al. (2010a) also found that CAB was as high as 1.5 μg/g in agricultural soils receiving biosolid application. Several researchers have speculated that CAB at environmentally relevant concentrations could cause acute and chronic effects in non-target organisms (Álvarez-Muñoz et al. 2015; Carter et al. 2015; Contardo-Jara et al. 2011; Ferrari et al. 2004; Jarvis et al. 2014a; b; Li et al. 2011; Moreno et al. 2016). For instance, Martin-Diaz et al. (2009) found that the cAMP pathway, the mitoxantrone resistance–associated protein system, and the antioxidant enzyme system in Mediterranean mussels have been observed to be influenced by the exposure of CAB (0.1 g/L and 10 g/L). Clinical research has indicated the CAB-related teratogenic effects in infants (Kaneko et al. 1992).
Once in soil, CAB can be bioaccumulated in crops, especially in the edible portions, posing potential human health risks through dietary intake. It has been demonstrated that plants can take up and accumulate CAB (Holling et al. 2012; Hurtado et al. 2016; Malchi et al. 2014; Paz et al. 2016; Wu et al. 2010a; b; Wu et al. 2014). For example, trace levels (0.1–2.5 ng/g) of CAB were found in vegetables, including carrot, celery, spinach, lettuce, bell pepper, cabbage, tomato, and cucumber (Wu et al. 2014; Malchi et al. 2014). Holling et al. (2012) reported that up to 9727 ng/g CAB was accumulated in cabbage collected from soils with biosolid addition and 2.6 ng/g CAB. Wu et al. (2010a), b) reported that CAB was accumulated in soybean grown in biosolid-amended soils at 396.3 ng/g (dry weight).
The land application of biosolids is a common agricultural practice and considered a major exposure pathway of xenobiotics in the environment, as many xenobiotics are known to be persistent in the process of wastewater treatment and easily adsorbed to biosolids (Al-Rajab et al. 2009; Wu et al. 2009; Wu et al. 2015). Millions of tons of dry biosolids are produced every year, of which more than 50% are land applied in the USA and Europe (Langenkamp and Marmo 2000; U.S. EPA 1999; 2009). The chemical, physical, and biological properties of soil can be drastically affected once the biosolids are incorporated, and the environmental behavior of contaminants in the soil-plant system may thus be altered (Langenkamp and Marmo 2000; Wu et al. 2009). For example, biosolids are rich in organic matter (Kinney et al. 2006), which may enhance sorption of chemicals in soil and reduce their secondary contamination potential from soil (e.g., the plant uptake). On the other hand, the incorporated nutrients and exogenous microbial populations from biosolids could affect the soil microbial activity, which may also have an influence on fate of chemicals in the soil-plant system. The influence of biosolids on environmental fate of CAB has been previously investigated in soil (Li et al. 2013; Williams et al. 2006). For example, Li et al. (2013) reported that amendment of biosolids could inhibit the transformation of CAB with a half-life of 108 days, whereas it was 46 days in soil without biosolids. However, even though several studies have considered the uptake of PhACs (e.g., CAB) from soil with or without the addition of biosolids (Malchi et al. 2014; Wu et al. 2010a; b; Wu et al. 2012; Mordechay et al. 2018), few studies have been investigated to ascertain the influence of biosolids on plant uptake and translocation of the inherent and/or exogenous PhACs from soil and how biosolids affect such behavior. In this study, microcosm studies were conducted to explore the effect of biosolids on plant uptake and tissue distribution of 14C-CAB from soil. This investigation was an exploratory study for the purpose of better understanding the accumulation or human exposure of PhACs from the application of biosolids in agricultural fields.
Materials and methods
Chemicals
14C-Carbonyl-labeled CAB (50 mCi mmol−1, radiochemical and chemical purity > 99%; see Fig. S1 for structure and 14C labeling position). A spiking solution of 14C-CAB (5000 mg/L, 0.1654 mCi/mmol) was freshly prepared by mixing the labeled and non-labeled CAB in methanol. The specific recipe for the scintillation cocktail can be found in our previous study (Li et al. 2013b). All chemicals or organic solvents were of AR or HPLC grade and used as received.
Soil, plants, and biosolids
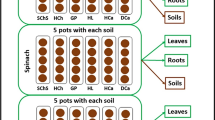
The tested plants were pak choi (Brassica campestris ssp. chinensis), carrot (Daucus carota L.), and celery (Apium graveolens L.), which were used as the representatives of leafy vegetable, root vegetable, and stem vegetable. Seedlings of pak choi, carrot, and celery with 3 to 4 leaves were purchased from Fengqi Bird-Flower Market in Hangzhou, China. Soils which were not cultivated or did not previously receive biosolids or wastewater were collected from the top 10 cm of the soil in an experimental station of Zhejiang University, China. Soil parameters are provided as follows: pH (6.95), organic matter (33.5 mg/g), cation exchange capacity (10.65 cmol/kg), clay (7.9%), silt (20. 8%), and sand (71.3%). A sample of biosolids was obtained from a full-scale activated sludge treatment plant located in Hangzhou. The organic matter content of biosolids was 229.8 mg/g. After sampling, the soil and biosolids were air-dried and sieved with a 2-mm sieve. The biosolids collected from WWTP is a complex matrix and may contain organic pollutions (e.g., pharmaceutical residues) and other exogenous substances. This study was therefore initiated by 14C labeling as 14C labeling can track the uptake and fate of 14C (CAB and/or its potential metabolites) in plant tissues and eliminate potential interferences. The biosolids without the addition of 14C-CAB were included as control.
Soil-plant microcosms and treatments
The experiments were conducted in a greenhouse from November 2016 to January 2017 in Zhejiang University Huajiachi Agricultural Experiment Station, Hangzhou, China. The average light time was 10–12 h, and the temperatures were 20 ± 10 °C. To revive the microbial activity in soil, the moisture content of the soil was adjusted to approximately 40% WHC. To explore the influence of biosolids on the plant uptake and tissue distribution of 14C-CAB in soil, different amendment ratios (0%, 5%, and 10%) of biosolids in soils were used and abbreviated as BS 0%, BS 5%, and BS 10%, respectively. According to the USEPA guidance on biosolid application, 5–20 tons/acre (dry weight) would be representative rates for many agricultural lands (U.S. EPA 2000). In this study, 5% and 10% of biosolid amendment rates are roughly equal to a field application rate of 6.45 dry tons per acre and 12.9 dry tons per acre, respectively, which are also common rates when biosolids are used for restoration (Sopper 1993). For soils without biosolids, a subsample of 200 g soil (dry weight) was spiked with 3.86 mL 14C-CAB stock solution (2.7 × 107 dpm) in a glass beaker and mixed with a glass rod in a fume hood. After the solvent was evaporated, the subsample soil was mixed with 2500 g untreated soil and homogenized by mixing thoroughly. The final soil moisture content was adjusted to 60% WHC. For the BS 5% and BS 10% treatments, an aliquot of 5.29 mL 14C-CAB stock solution was firstly spiked drop-wise to the biosolids (185 g for BS 5% treatment and 370 g for BS 10% treatment). The fortified biosolids were then homogenized with the soil. The total biosolid-amended soils were 3700 g (n = 3). The specific radioactivity of 14C-CAB in all treatments was 9919 ± 457 dpm/g (dry soil) with an initial concentration of 7.62 ± 0.35 μg/g (dry soil). The homogenized soils or biosolid-amended soils were divided and transferred to different soil pots.
The 14C homogeneity in all treated soil samples was confirmed with a relative standard deviation (RSD) of 4.6% by combustion of soil samples (n = 3) on a biological oxidizer (900 °C, 4 min; OX501, R. J. Harvey Instruments, USA). Firstly, soils (200 g, dry weight) containing 14C-CAB were weighed into plastic cups (6 cm/9 cm/14 cm, bottom diameter/top diameter/height). Each vegetable seedling with 3 to 4 leaves was carefully transferred to a soil cup with free drainage. A holed cup lid was used to support the seedling, maintain the humidity, and avoid the foliar uptake or contamination of 14C from soil. The water content of each soil pot was checked every 2 days or 3 days, and deionized water was added to each soil pot when necessary. Six pots with soil and seedling were transferred into a polymethyl methacrylate chamber (50 cm × 30 cm × 35 cm, l × w × h). A slow air stream was continuously passed through the chamber. To capture 14CO2 from mineralization of 14C-CAB, each chamber was connected with a conical flask (25 mL) filled with 10 mL NaOH solution (0.2 M). The conical flask was replaced by fresh NaOH solution at each sampling time. Spiked soils without plants were set as plant-free control, whereas non-spiked soils with plants were included as the CAB-free blank. The test systems allowed us to establish a mass balance for the 14C-CAB.
Sampling and analysis
At 10 days, 20 days, 30 days, 40 days, 50 days, and 60 days after planting, celery and carrot were harvested, while pak choi was sampled (n = 3) at 10 days, 20 days, 30 days, and 40 days. At each sampling time, soil samples were analyzed for 14C residues. The plants were washed with deionized water to remove the residual soil. To understand how different parts of a plant might accumulate 14C-CAB, celery and pak choi were separated into components such as roots, leafs, and stems, and carrot was divided into roots and shoots. The wet weight and length of each part were immediately measured. All samples (soil, root, stem, and leaf) were dried at 70 °C until nearly constant weight and stored at − 20 °C before analysis.
The oxidizer efficiency was analyzed following the same protocol as detailed by Li et al. (2013) and was determined to be 91.93 ± 3.6% (n = 3). The released 14CO2 from combustion was analyzed using a liquid scintillation counter (LSC) (Perkin-Elmer Inc., USA). An Quatalus-1220 ultralow level LSC (ULLSC) spectrometer (PerkinElmer, Finland) was used to determine the radioactivity in the NaOH solutions. The blank was sampled as one pot at each time point.
Data analysis and statistics
All treatments were performed in triplicates. Non-spiked soils and plants were included as treatment blanks. The concentrations of 14C in different tissues of each carrot and celery were used to determine the dry weight of the samples. All data represent the mean and standard deviation of triplicates. A simple comparison of means was performed after analysis of variance using the t test, and multiple data sets (uptake, distribution, transport, and accumulation in celery and carrot for CBZ) were analyzed by analysis of variance (ANOVA). Duncan’s test was used to compare the differences at p < 0.05. Statistical analysis was performed using the SPSS 19.0 software for Windows 7.0. Regression analysis was performed using Origin Pro 9.0 (v.9.0; OriginLab, Northampton, MA).
The 14C bioconcentration factors in plants or different plant tissues were calculated as Eq. (1)
where BCF is the bioconcentration factor, Ca is the 14C concentration in the plants or in the tissue and in the test soils, and Cs indicates the 14C concentration in the test soils.
The 14C translocation in the plants was measured using translocation factor (TF) which is given below
where Ct and Cr are the 14C activities in the aboveground (leaf and stem, or shoots) and root of plants, respectively.
Results and discussion
Mass balance
The total 14C recovered from soil and plant tissue combustion and mineralization in each chamber was 90–101%, 85–103%, and 86–112% for the carrot, celery, and pak choi treatment, respectively (Table 1). In all soil treatments with or without biosolids, more than 97% of the radioactivity applied still remained in the soil (Table S1), and uptake of 14C (0.1–2.9%) by plants was low. ULLSC analysis showed that no radioactivity was detected or the radioactivity was below the detection limit in the 14CO2-trapping tubes for all treatments during the cultivation, indicating that mineralization of CAB in the soil-plant system was rather limited. The limited mineralization of CAB in soil-plant systems was likely due to the recalcitrance of CAB for biodegradation in soil or plant. Li et al. (2013) previously reported that lower than 1.2% of 14C-CAB was mineralized in aerobic soils or biosolid-amended soils within 120 days.
Influence of biosolids on uptake of 14C in vegetables
The 14C concentrations in different plants grown in different soil treatments are shown in Fig. 1. In general, 14C was detected in all plants. The detected 14C increased over the exposure time in celery and pak choi (Fig. 1a, c). For example, the amount of 14C in celery planted in unamended soils was 76.06 ± 6.90 dpm/mg and 256.09 ± 45.37 dpm/mg on day 10 and day 60, respectively, and the amount of 14C in pak choi was 41.94 ± 39.78 dpm/mg and 401.56 ± 22.78 dpm/mg on day 10 and day 40, respectively, indicating that the 14C was gradually taken up by celery and pak choi. The concentration of 14C in carrot first increased and subsequently decreased over the exposure time during the experiments (Fig. 1b). The highest concentration of 14C in carrot was 247.40 ± 82.54 dpm/mg in the soils without biosolids at 40 days. At the end of experiments (60 days), the level of 14C in carrot decreased to 129.03 ± 29.22 dpm/mg. The increase of 14C within 40 days was likely due to the continuous uptake of 14C in carrot from soils, and the following decrease in the 14C concentration may be attributed to the root bulking with increased weight as the biomass significantly increased after 40 days of incubation (Fig. S2C). The different uptake behaviors of 14C by different plants suggested that the uptake of 14C-CAB is mainly determined by plant physiological traits.
In addition, statistical analysis showed that uptake of 14C-CAB into plant was significantly inhibited by biosolid CAB (p < 0.05), and it was related to the biosolid amendment rates. More biosolid addition leads to decreased 14C in plants. For instance, the concentration of 14C was 187.69 ± 32.12 dpm/mg, 82.31 ± 16.32 dpm/mg, and 56.47 ± 22.17 dpm/mg in celery grown in BS 0%, BS 5%, and BS 10%, respectively. Similarly, the level of 14C also followed the order of BS 0% > BS 5% > BS 10% in carrot and pak choi. Recently, Fu et al. (2016) found that biosolid amendment could inhibit uptake of triclosan and triclocarban by carrot and radish. Such inhibition effect caused by the addition of biosolids reflected that CAB became less bioavailable in biosolid-amended soils. Chefetz et al. (2008) indicated that as a neutral substance, CAB is quite likely to interact with the soil organic matter and a higher organic carbon (OC) content could result in higher CAB retardation in soil. In a previous study, a significant increase of TOC was observed after biosolids were incorporated in soil and sorption of CAB was subsequently increased with a Kd value of 19.8 and 12.6 in biosolid-amended soils and unamended soil, respectively (Williams et al. 2006). The OC content of biosolids (229.8 mg/g) used in this study was approximately 7-fold that in unamended soil (33.5 mg/g), resulting in the enhanced sorption of 14C residues in soil and reduced their bioavailability. Williams et al. (2006) also speculated that the higher salinity in biosolid-amended soil was also one of the main factors influencing CAB sorption. Moreover, Li et al. (2013) have previously reported that biodegradation played a critical role in dissipation of CAB in soil, with an estimated half-life of > 120 days in non-sterile soil, as compared to 46.2 days in soils after sterilization. The reduced bioavailability of 14C-CAB for plant uptake was also likely due to the exogenous microorganisms from biosolids that could enhance the biodegradation of CAB to the incomplete transformation products with structures unfavorable for plant uptake.
Effect of biosolids on distribution of 14C in vegetables
Once a chemical is taken up by roots, posterior translocation, which is driven by the transpiration process (Carter et al. 2014; Chefetz et al. 2008; Dodgen et al. 2013), the amounts of 14C in different plant components were analyzed individually and are shown in Fig. 2. Generally, 14C was taken up by all plant tissues. The 14C followed the order of leaf > root > stem in celery. For example, in the BS 5% treatment, the amount of 14C was 235.42 ± 7.59 dpm/mg, 122.04 ± 17.77 dpm/mg, and 64.54 ± 5.42 dpm/mg in the celery leaf, root, and stem on day 60, respectively. The different accumulations in root, stem, and leaf could be attributed to their different component characteristics and physiological features (e.g., lipid and phloem contents) between different tissues (Wu et al. 2012). The amount of 14C in celery root and stem almost remained unchanged during the experiments, while the amount of 14C in celery leaves increased continuously. It is likely due to 14C was continuously translocated from roots while the roots were taking up 14C and the stems may serve as the duct for the transport of 14C from roots to leaves by mass flow, and thus, the 14C was continuously concentrated and accumulated to the largest extent in leaves and it kept almost unchanged in root and stem. In addition, for all plant tissues of celery, the amount of 14C was found to be significantly higher in biosolid-amended soil than the unamended soils. For example, the concentration of 14C in the leaves were 380.47 ± 39.20 dpm/mg, 235.42 ± 7.59 dpm/mg, and 137.62 ± 22.28 dpm/mg in the 0%, 5%, and 10% biosolid-amended soils, respectively (Fig. 2a). Likewise, the concentration of 14C in roots was 212.09 ± 20.48 dpm/mg, 122.04 ± 17.77 dpm/mg, and 108.55 ± 37.70 dpm/mg in the 0%, 5%, and 10% biosolid-amended soils on day 60, respectively, indicating that biosolids had a significant inhibitory effect on accumulation of 14C in the celery root. The accumulation of 14C decreased in all tissues, especially in leaves, of celery with the increasing biosolid amendment rate.
These results are in agreement with findings from a previous study that the biosolid amendments inhibited the accumulation of triclosan and triclocarban in the root of carrot and radish (Fu et al. 2016). Similar inhibitory effects by biosolids were also observed in carrot and pak choi. However, there was no significant difference between different biosolid amendment rates at 60 days, and the concentration of 14C was 80.03 ± 3.08 dpm/mg, 64.54 ± 5.42 dpm/mg, and 51.32 ± 14.72 dpm/mg in the carrot roots grown in the 0%, 5%, and 10% biosolid-amended soils, respectively, indicating that the inhibitory effect of biosolids on accumulation of 14C-CAB was also associated with the plant growth period. In addition, the concentration of 14C in carrot roots at 60 days was significantly lower than that detected on day 10 in different soil treatments. This may be attributed to the root bulking with increased weight or the translocation of 14C from root to shoots. Data suggested that the concentration of 14C in carrot shoots increased with time during the experiments. For instance, the concentration of 14C detected in shoots of carrot planted in the 0%, 5%, and 10% biosolid-amended soils at 10 days was 26.15 ± 9.40 dpm/mg, 23.86 ± 6.45 dpm/mg, and 12.85 ± 2.58 dpm/mg, respectively. The concentration of 14C reached 270.84 ± 55.56 dpm/mg, 185.75 ± 67.29 dpm/mg, and 101.94 ± 3.13 dpm/mg at 60 days, respectively, in shoots of carrot harvested from the 0%, 5%, and 10% biosolid-amended soils, respectively. The amount of 14C increased by 10.35, 7.84, and 7.93 times in the shoot samples harvested on day 10, respectively, indicating that the 14C residues were gradually translocated from root.
Effect of biosolids on bioconcentration of 14C in plant tissues
To better evaluate the effect of biosolids on uptake of 14C-CAB from soil, the BCFs were analyzed by dividing the 14C amounts in plant tissues by the measured 14C amounts in the test soils at each sampling time (Fig. 3). The BCFs of 14C in roots of celery kept almost unchanged during the experiment (Fig. 3a). The BCFs in stems showed a slight increase during the first 40 days of cultivation and a subsequent decrease until 60 days (Fig. 3b), while in leaves, the BCFs increased with the cultivation time. The BCF of 14C in carrot root firstly increased (10–30 days) and subsequently decreased (30–60 days) (Fig. 3d), and it increased with the experiment time for carrot shoots (Fig. 3e). For pak choi, the BCFs of 14C in pak choi root and leaf increased with time. For example, after 40 days of cultivation, the BCF for pak choi leaf in the unamended soil was 14.28 times that on day 10 (Fig. 3h). However, the 14C BCFs in the stem of pak choi were relatively stable (Fig. 3g). In general, the BCFs of 14C in celery followed the order of leaves > roots > stems. It was higher in pak choi root than in leaf and stem, which was in agreement with observations by Herklotz et al. (2010) that CAB had a lower accumulation potential in cabbage leaves than in roots. For carrot, the 14C BCF was generally lower in root than in leaf. These results suggested that the bioaccumulation of 14C-CAB showed a different pattern between different plant tissues and different plant species. Recently, Malchi et al. (2014) indicated that the different lipid contents in different plant species or plant tissues can have a significant impact on fate of chemicals. Wu et al. (2012) found that concentration of CAB in root was positively correlated with root lipid content and indicated that plant lipid content was one of the variables that had an influence on the bioaccumulation factors of CAB. Among the different plant tissues analyzed, common vegetables grown under greenhouse conditions were capable of accumulating CAB or its potential metabolites into their edible parts at different levels. The BCF in edible sample for celery (i.e., stem) and pak choi (i.e., leaf) was found to be lower than that for other parts; however, root of carrot was found to readily accumulate 14C residues from CAB.
The BCFs of 14C-CAB in plant tissues are shown in Fig. 3. In most cases, 14C BCFs in plant tissues harvested in soils without biosolids were higher than those in biosolid-amended soils, and as the amendment rates of biosolids increased, the BCFs decreased. For example, the BCF values in the celery leaf was 37.69 ± 3.57 in the unamended soil on day 60, which decreased to 23.41 ± 1.47 and 9.47 ± 6.32 in the 5% and 10% biosolid-amended soils, respectively. Similar observation was also found in plant tissues of pak choi and carrot. For instance, the BCF of 14C in carrot root was 30.79 ± 3.16, 18.57 ± 8.24, and 10.19 ± 0.43 in the 0%, 5%, and 10% biosolid-amended soils at 60 days, respectively. BCFs in pak choi leaf was 46.46 ± 4.59 in the unamended soil on day 60, which decreased significantly to 21.12 ± 8.18 in the 5% amended soil (p < 0.05) and then to 17.51 ± 0.89 in the 10% biosolid-amended soil, suggesting a 2.19–2.65-fold reduction. These findings indicated that amendment of biosolids could inhibit the bioaccumulation of 14C-CAB and/or its intermediates in plant tissues. Fu et al. (2016) reported a reduction of BCFs for triclosan in radish and carrot grown in soils with the incorporation of biosolids and suggested that TOC played a profound influence on bioaccumulation of triclosan and triclocarban and biosolids likely increased the soil TOC and lead to stronger sorption, resulting in a decreased plant uptake due to the reduced chemical bioavailability (Chefetz et al. 2008).
Influence of biosolids on tissue distribution of 14C residues in vegetables
TF was obtained by dividing the 14C activity in the aboveground (leaf and stem, or shoots) of plants to that in root to investigate the translocation of 14C residues from the roots to other tissues in soil or soil-biosolid mixture.
The TFs for 14C-CAB were generally greater than 1 in all three vegetables (Fig. 4). For example, in the unamended soils, the TFs of 14C-CAB were 2.32 ± 0.27, 9.46 ± 2.71, and 1.41 ± 0.55 in celery, carrot, and pak choi on day 60, respectively, suggesting that CAB was appreciably translocated to stems and/or leaves after being taken up by roots. Similar observations were reported for translocation of CAB in soybean, cucumber, ryegrass, and pea (Shenker et al. 2011; Tanoue et al. 2012; Winker et al. 2010; Wu et al. 2010a; b; Wu et al. 2013; Wu et al. 2014). Previous studies have demonstrated that non-ionic PCs (e.g., CAB) could easily cross cellular membranes, then move from xylem to phloem and be transferred throughout the stems via the transpiration stream, resulting in relatively higher level in leaves (Malchi et al. 2014; Goldstein et al. 2014). The TFs increased with the cultivation time during the first 30 days and kept almost unchanged thereafter (Fig. 4a), suggesting that the 14C-CAB was more readily translocated to the aboveground of celery during its early stages of the growth. For carrot, the TFs increased with time, especially after 30 days of cultivation. For example, the mean TF of CAB was 0.46 ± 0.19 in the unamended soil on day 10, which was 9.47 ± 2.71 at the end of cultivation, reflecting a 20-fold increase (Fig. 4b). The TFs for CAB were in the range of 1.41–2.06 in soils without biosolids (Fig. 4c), which was lower than that in celery and carrot, indicating that CAB was more preferentially accumulated in the pak choi root as compared to celery and carrot.
The amendment of biosolids inhibited the translocation of 14C-CAB in carrot, and as the biosolid amendment rate increased, the TFs decreased. For example, the TF for 14C-CAB in carrot planted in 5% and 10% biosolid-amended soils was 4.99 ± 2.42 and 3.04 ± 1.20, respectively, as compared to 9.47 ± 2.72 in the unamended soil (60 days), suggesting a 1.89–3.15-fold reduction (Fig. 4b). Similarly, TF was also decreased in pak choi. The average TF for CAB in pak choi planted in unamended soils was 1.62 ± 0.67, which generally decreased to < 1 after the addition of biosolids in soil. The TFs kept almost unchanged when the amendment rate of biosolids was increased from 5 to 10% (p > 0.05), which was likely due to the fact that translocation of 14C-CAB or the system already approached a relatively stable balance at the lower amendment rate of biosolids. For celery, 10% biosolid amendment showed a significant inhibition (p < 0.05) on TF of 14C-CAB and a similar effect was not observed for 5% biosolid-amended soils. For example, at the end of experiments (60 days), TF was 0.32 ± 0.27 and 1.58 ± 0.41 in the unamended soils and soil amended with 10% biosolids, indicating that the inhibition effect occurred in celery when the amendment rate increased to a certain value. Thus, the inhibitory effect on translocation by biosolids was likely due to the complicated interaction and factors among soil, biosolids, plant physiology, and microorganisms (soil and/or biosolids), and it was related to the biosolid amendment rate, the category of vegetable, and the plant growth stages. In addition, effect of biosolids on vegetable growth was also examined by biomass and root length in this study. The biomass of celery followed the order of BS 5% > BS 10% > BS 0% (Fig. S2A). For example, the dry weight of celery was 0.20 ± 0.04 g, 0.29 ± 0.04 g, and 0.25 ± 0.03 g in the BS 0%, BS 5%, and BS 10% treatment at 60 days, respectively. However, the root length of celery followed the order of BS 0% > BS 5% > BS 10% (Fig. S2B). For example, the root length of celery was 14.07 ± 4.08 cm, 9.2 ± 3.03 cm, and 3.17 ± 1.24 cm in the BS 0%, BS 5%, and BS 10% at 60 days, respectively. In general, the biomass and root length of carrots were lower in the pots with biosolids than those without biosolids (Fig. S2C and D). For example, the dry weight of carrot was 2.87 ± 0.34 g in the soil without biosolids, and it decreased to 1.14 ± 0.15 g and 0.86 ± 0.32 g in the BS 5% and BS 10% at 60 days, respectively. It is likely due to the fact that even though biosolids contain macronutrients and micronutrients from which plants can benefit, there are non-essential elements such as cadmium and lead or excessive trace elements (e.g., cobalt, copper, manganese, and zinc essential for plant growth), causing harmful effects on the growth of carrot (Hirpassa and Codling 2018). However, no significant impacts on the biomass and root length of pak choi by biosolids were observed (Fig. S2E and F). These results indicated that a variety of pollutants, nutrients, and microorganisms from biosolids could be introduced into the soil and lead to physiological, biochemical, and molecular changes within the plant during plant development. Such biochemical process changes may also be involved in transportation and distribution of 14C-CAB within inner vegetables. Furthermore, some other chemicals in the biosolids may also be taken up by vegetable and have competitive transportation with CAB. The underlying mechanism of the inhibited uptake and translocation of 14C-CAB by biosolids should be further elucidated.
Conclusions
In this study, plant uptake of 14C-CAB by three edible vegetables (i.e., carrot, celery, and pak choi) was performed under greenhouse conditions. The 14C was detected in all vegetable tissues analyzed, and the edible tissues were found to readily accumulate 14C-CAB. In addition, appreciable translocation of 14C-CAB from roots to other plant tissues was observed. Bioaccumulation and translocation of 14C-CAB in soil were significantly inhibited by the incorporation of biosolids, and as the amendment rates of biosolids increased, the amount of 14C in plants or individual plant tissues decreased, indicating that benefits in terms of CAB bioaccumulation reduction were obtained where biosolids were used as soil amendments, leading to a potential mitigation of CAB bioaccumulation into edible vegetables grown in contaminated soil or a reduced human health effects of CAB and/or its metabolites through the food chain. Additionally, biosolid inhibition on uptake and translocation of 14C-CAB in vegetables is likely attributed to many factors, such as different biosolid amendment rates, vegetable characteristics, and chemical properties, and even growing periods of plants may produce 14C-CAB accumulation discrepancies in the different tissues. The underlying mechanism of the inhibited uptake and translocation of 14C-CAB by biosolids should be further elucidated. Biosolid application is known to be a major route for introduction of PhACs into soil, and it is of great significance to explore the influence of biosolids on uptake, bioaccumulation, and tissue distribution of PhACs for assessing their health and environmental risks. Given that PhACs have various physicochemical properties/chemical structures, the effect of biosolids on the plant uptake should be examined for the other commonly used and frequently detected PhACs under greenhouse or field conditions. Determination of trace levels of CAB in plants showed great challenges due to matrix interferences by the high contents of pigments and fatty materials, thus using dpm (or radioactivity) to report the results are appropriate in this study. It is noteworthy that the measurement of 14C represents the CAB and its metabolites in the present study, and further study is needed on the identification of metabolites and the kinetic evaluation of CAB and the individual metabolite.
References
Al-Rajab AJ, Sabourin L, Scott A, Lapen DR, Topp E (2009) Impact of biosolids on the persistence and dissipation pathways of triclosan and triclocarban in an agricultural soil. Sci Total Environ 407:5978–5985
Álvarez-Muñoz D, Rodríguez-Mozaz S, Maulvault AL, Tediosi A, Fernández-Tejedor M, Van den Heuvel F, Kotterman M, Marques A, Barceló D (2015) Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ Res 143:56–64
Carter LJ, Harris E, Williams M, Ryan JJ, Kookana RS, Boxall AB (2014) Fate and uptake of pharmaceuticals in soil-plant systems. J Agric Food Chem 62:816–825
Carter LJ, Williams M, Bottcher C, Kookana RS (2015) Uptake of pharmaceuticals influences plant development and affects nutrient and hormone homeostases. Environ Sci Technol 49:12509–12518
Chefetz B, Mualem T, Ben-Ari J (2008) Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 73:1335–1343
Contardo-Jara V, Lorenz C, Pflugmacher S, Nutzmann G, Kloas W, Wiegand C (2011) Exposure to human pharmaceuticals carbamazepine, ibuprofen and Bezafibrate causes molecular effects in Dreissena polymorpha. Aquat Toxicol 105:428–437
Dodgen LK, Li J, Parker D, Gan JJ (2013) Uptake and accumulation of four PPCP/EDCs in two leafy vegetables. Environ Pollut 182:150–156
Durán-Álvarez JC, Prado B, González D, Sánchez Y, Jiménez-Cisneros B (2015) Environmental fate of naproxen, carbamazepine and triclosan in wastewater, surface water and wastewater irrigated soil—results of laboratory scale experiments. Sci Total Environ 538:350–362
Ferrari B, Mons R, Vollat B, Fraysse B, Paxeus N, Lo GR, Pollio A, Garric J (2004) Environmental risk assessment of six human pharmaceuticals: are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ Toxicol Chem 23:1344–1354
Fu Q, Wu X, Ye Q, Ernst F, Gan J (2016) Biosolids inhibit bioavailability and plant uptake of triclosan and triclocarban. Water Res 102:117–124
Goldstein M, Shenker M, Chefetz B (2014) Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables. Environ Sci Technol 48:5593–5600
He Y, Nie E, Li C, Ye Q, Wang H (2016) Uptake and subcellular distribution of triclosan in typical hydrophytes under hydroponic conditions. Environ Pollut 200:400–406
Herklotz PA, Gurung P, Vanden HB, Kinney CA (2010) Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 78:1416–1421
Hirpassa WD, Codling EE (2018) Growth and metal uptake of edamame [Glycine max (L.) Merr.] on soil amended with biosolids and gypsum. Commun Soil Sci Plan 49:2793–2801
Holling CS, Bailey JL, Vanden HB, Kinney CA (2012) Uptake of human pharmaceuticals and personal care products by cabbage (Brassica campestris) from fortified and biosolids-amended soils. J Environ Monit 14:3029–3036
Hossain A, Nakamichi S, Habibullah-Al-Mamun M, Tani K, Masunaga S, Matsuda H (2018) Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ Res 165:258–266
Hurtado C, Trapp S, Bayona JM (2016) Inverse modeling of the biodegradation of emerging organic contaminants in the soil-plant system. Chemosphere 156:236–244
Jarvis AL, Bernot MJ, Bernot RJ (2014a) The effects of the psychiatric drug carbamazepine on freshwater invertebrate communities and ecosystem dynamics. Sci Total Environ 496:461–470
Jarvis AL, Bernot MJ, Bernot RJ (2014b) Relationships between the psychiatric drug carbamazepine and freshwater macroinvertebrate community structure. Sci Total Environ 496:499–509
Kaneko S, Otani K, Kondo T, Fukushima Y, Nakamura Y, Ogawa Y, Kan R, Takeda A, Nakane Y, Teranishi T (1992) Malformation in infants of mothers with epilepsy receiving antiepileptic drugs. Neurology 42:68–74
Kinney CA, Furlong ET, Zaugg SD, Burkhard MR, Werner SL, Cahill JD, Jorgensen GR (2006) Survey of organic wastewater contaminants in biosolids destined for land application. Environ Sci Technol 40:7207–7215
Langenkamp H, Marmo L (2000) Proceedings of the workshop on “Problems around sludge”. European Commission Joint Research Centre, Stresa
Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, Kolarova J, Li P, Randak T (2011) Acute toxicity of carbamazepine to juvenile rainbow trout (Oncorhynchus mykiss): effects on antioxidant responses, hematological parameters and hepatic EROD. Ecotoxicol Environ Saf 74:319–327
Li JY, Dodgen L, Ye Q, Gan J (2013) Degradation kinetics and metabolites of carbamazepine in soil. Environ Sci Technol 47:3678–3684
Li J, Zhang JB, Li C, Wang W, Yang Z, Wang HY, Gan J, Ye QF, Xu XY, Li Z (2013b) Stereoisomeric isolation and stereoselective fate of insecticide Paichongding in flooded paddy soils. Environ Sci Technol 47:12768–12774
Ma R, Wang B, Yin L, Zhang Y, Deng S, Huang J, Wang Y, Yu G (2017) Characterization of pharmaceutically active compounds in Beijing, China: occurrence pattern, spatiotemporal distribution and its environmental implication. J Hazard Mater 323:147–155
Malchi T, Maor Y, Tadmor G, Shenker M, Chefetz B (2014) Irrigation of root vegetables with treated wastewater: evaluating uptake of pharmaceuticals and the associated human health risks. Environ Sci Technol 48:9325–9333
Martin-Diaz L, Franzellitti S, Buratti S, Valbonesi P, Capuzzo A, Fabbri E (2009) Effects of environmental concentrations of the antiepilectic drug carbamazepine on biomarkers and cAMP-mediated cell signaling in the mussel Mytilus galloprovincialis. Aquat Toxicol 94:177–185
McClellan K, Halden RU (2010) Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res 44:658–668
Mordechay EB, Tarchitzky J, Chen Y, Shenker M, Chefetz B (2018) Composted biosolids and treated wastewater as sources of pharmaceuticals and personal care products for plant uptake: a case study with carbamazepine. Environ Pollut 232:164–172
Moreno GR, Rodriguez MS, Huerta B, Barcelo D, Leon VM (2016) Do pharmaceuticals bioaccumulate in marine molluscs and fish from a coastal lagoon? Environ Res 146:282–298
Paz A, Tadmor G, Malchi T, Blotevogel J, Borch T, Polubesova T, Chefetz B (2016) Fate of carbamazepine, its metabolites, and lamotrigine in soils irrigated with reclaimed wastewater: sorption, leaching and plant uptake. Chemosphere 160:22–29
Shenker M, Harush D, Ben-Ari J, Chefetz B (2011) Uptake of carbamazepine by cucumber plants—a case study related to irrigation with reclaimed wastewater. Chemosphere 82:905–910
Sopper WE (1993) Municipal sludge use in land reclamation. CRC Press LLC, Boca Raton
Tanoue R, Sato Y, Motoyama M, Nakagawa S, Shinohara R, Nomiyama K (2012) Plant uptake of pharmaceutical chemicals detected in recycled organic manure and reclaimed wastewater. J Agric Food Chem 60:10203–10211
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
U.S. EPA (1999) Biosolids generation, use, and disposal in the United States. Washington, D.C.
U.S. EPA (2000) Biosolids technology fact sheet: land application of biosolids. Washington, D.C.
U.S. EPA (2009) Targeted national sewage sludge survey. Washington, D.C.
Williams M, Kookana R (2010) Isotopic exchangeability as a measure of the available fraction of the human pharmaceutical carbamazepine in river sediment. Sci Total Environ 408:3689–3695
Williams CF, Williams CF, Adamsen FJ (2006) Sorption-desorption of carbamazepine from irrigated soils. J Environ Qual 35:1779–1783
Winker M, Clemens J, Reich M, Gulyas H, Otterpohl R (2010) Ryegrass uptake of carbamazepine and ibuprofen applied by urine fertilization. Sci Total Environ 408:1902–1908
Wu C, Spongberg AL, Witter JD (2009) Adsorption and degradation of triclosan and triclocarban in soils and biosolids-amended soils. J Agric Food Chem 57:4900–4905
Wu C, Spongberg AL, Witter JD, Fang M, Ames A, Czajkowski KP (2010a) Detection of pharmaceuticals and personal care products in agricultural soils receiving biosolids application. Clean Soil Air Water 38:230–237
Wu C, Spongberg AL, Witter JD, Fang M, Czajkowski KP (2010b) Uptake of pharmaceutical and personal care products by soybean plants from soils applied with biosolids and irrigated with contaminated water. Environ Sci Technol 44:6157–6161
Wu C, Spongberg AL, Witter JD, Sridhar BB (2012) Transfer of wastewater associated pharmaceuticals and personal care products to crop plants from biosolids treated soil. Ecotoxicol Environ Saf 85:104–109
Wu X, Ernst F, Conkle JL, Gan J (2013) Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ Int 60:15–22
Wu X, Conkle JL, Ernst F, Gan J (2014) Treated wastewater irrigation: uptake of pharmaceutical and personal care products by common vegetables under field conditions. Environ Sci Technol 48:11286–11293
Wu X, Dodgen LK, Conkle JL, Gan J (2015) Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review. Sci Total Environ 536:655–666
Funding
This study was financially supported from the National Natural Science Foundation of China (Grants Nos. 21777104 and 21477105), the Natural Science Foundation of Guangdong Province (No. 2017A030313226), and the Shenzhen Science and Technology Project (Grant No. JCYJ20170818142823471).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(DOC 526 kb)
Rights and permissions
About this article
Cite this article
Li, M., Ding, T., Wang, H. et al. Biosolids inhibit uptake and translocation of 14C-carbamazepine by edible vegetables in soil. Environ Sci Pollut Res 27, 8323–8333 (2020). https://doi.org/10.1007/s11356-019-07429-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07429-4