Abstract
Diesel engines are the most extensively used power source in automobiles and stationary power generation. The main drawback of using diesel engines is that it liberates a significant amount of NOx and PM emissions in the exhaust. NOx emission has a serious effect on the environment, and it has to be controlled effectively. SCR is the most widely used after-treatment technology to control NOx emission, but it has various disadvantages like ammonia slip and degradation of the catalyst. In this study, electrochemical decomposition of NOx is proposed for the simultaneous control of NOx, HC, and CO emissions in a diesel engine. In this work, ionically conducting ceramic electrochemical cells are investigated for control of diesel exhaust emissions. The electrochemical cell consisting of yttrium stabilized zirconia (YSZ) substrate plates as electrolyte and Ag-YSZ and NiO-YZS as an electrode material. The decomposition of NOx in an electrochemical cell is attained by passing electric current. A 2V supply of power was sufficient for effective operation of the electrochemical cell in all load conditions. All the experiments were conducted in a single-cylinder diesel engine. It is observed that the electrochemical cell shows high NOx decomposition rate of 80% at the exhaust temperatures between 350 and 400 °C. The HC reduction up to 65% and CO reduction up to 45% was observed with this technique. The power required to operate the electrochemical cell was low. The electrochemical NOx reduction is relatively simple technology with reduced complexity. From the experiment, it is observed that this concept works efficiently in the oxygen-rich diesel exhaust.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past few decades, humans are facing serious issues due to the drift in the climate change occurring globally (Quevauviller. 2011; Parthiban et al. 2017). Emissions from the transportation sector cause serious environmental pollution and climate change (IEA 2012; Sajjad et al. 2014; Mohankumar and Senthilkumar, 2017). Diesel engines are considered as the most efficient engine due to their low operating costs, energy efficiency, high durability, and reliability. The major drawbacks of the diesel engine are it liberated excessive amount of particulate matter (PM) and nitrogen oxides (NOx) emission from the exhaust (Subramaniam et al. 2016). Nitrogen oxides (NO and NO2) are collectively called as NOx emissions. During combustion process at higher temperature, oxidation of atmospheric nitrogen with oxygen produces NOx emission (Sharifi et al. 2019). Due to the lean operations, diesel engines produce high NOx emissions (Heywood, 1988). Based upon the operating conditions, NOx can be classified as Thermal NOx, Prompt NOx, and Fuel NOx (Zeldovich et al. 1947; Hill and Smoot, 2000; Kašpar et al., 2003; Sun et al. 2010; Hoekman and Robbins, 2012).
The other oxides of nitrogen such as N2O, NO3, and N2O5 formations are negligible and formed through low-temperature mechanisms (Sun et al., 2010; Hoekman and Robbins, 2012). NOx emissions cause serious health and environmental problems. In the atmosphere, NOx reacts with volatile organic compounds (VOCs) in the presence of heat and sunlight to form ozone (İlkiliç and Aydin, 2012). High levels of NOx can have a negative impact on vegetation, including leaf damage and reduced plant growth. Therefore, it is necessary to control the excessive liberation of NOx emissions from diesel engines (Vallapudi et al. 2018). The excess oxygen concentration in diesel exhaust makes it more difficult to design catalytic systems to reduce NOx (Theis et al. 2015). Selective catalytic reduction (SCR) is a conventional after treatment technique for NOx reduction. However, SCR has several drawbacks as it requires additional urea tank, complex electronic, and mechanical attachments. There is also the possibility of ammonia slip as secondary pollution (Fang and DaCosta, 2003). The lean NOx trap (LNT) is an emerging after-treatment technology for controlling NOx emission, but it is expensive because of the requirement of Noble metal catalyst and requires regeneration by switching the engine operation to lean and rich (Maurer et al. 2017). The several demerits stated with the existing NOx control technology are eliminated in electrochemical NOx decomposition technique. It is a novel method to reduce NOx emission effectively in ceramic electrochemical cells. In this technique, only a supply of voltage is required to operate a simple electrochemical cell to perform NOx reduction (Hansen, 2018; Kammer, 2005).
In this experimental investigation, a ceramic-based electrochemical cell is developed for the effective reduction of NOx and simultaneous oxidation of HC and CO emissions. Figure 1 shows the schematic representation of an electrochemical cell reaction. In this technique by applying a suitable potential difference over the cathode, NOx molecules are reduced to oxygen ions (O2−) and nitrogen (N2). The decomposed O2 is ionically diffused between the electrolytes and oxidizes the soot particles deposited on the anode (Bredikhin et al., 2001a). Pancharatnam et al. first proposed NO decomposition using Y-ZrO2 electrochemical cells by supplying voltage (Pancharatnam et al. 1975). Later, electrochemical cells using zirconia as a substrate was developed to perform NO reduction (Gür and Huggins, 1979). The performance of electrochemical cell was studied (Bredikhin et al., 2002; Bredikhin et al., 2001b) for effective NO conversion. The results showed NO conversion was improved due to the nanometer structure formed in the NiO/YSZ layers by increasing the reactive sites. The addition of NO adsorption layers in the electrochemical cell exhibited higher NO reduction due to the increased adsorption sites over the surface of the electrochemical cells (Li and Vernoux, 2005; Hamamoto et al. 2007; Xi wang, 2019).
In this work, an attempt was made to control diesel engine emission by developing ceramic electrochemical cell. The electrochemical cell consists of YSZ electrolyte and NiO-YSZ, Ag-YSZ electrodes. The electrode coatings (anode and cathode) are given over the electrolyte. The YSZ is an ionically conducting ceramic which has the tendency to oxidize the soot emissions (Hernández et al. 2013). YSZ is also most widely used as an electrolyte in solid oxide fuel cell (SOFC) (Yoshinobu et al. 2010;18). In the electrochemical cells, by the supply of electric current, the reduction takes place at the cathode and oxidation at the anode (Yoshinobu et al. 2010; Awano et al. 2004; Park et al. 2004; Hamamoto et al. 2006). The voltage polarity between the cathode and the anode is changed by the Electronic control module (ECM) so that the continuous regeneration in soot oxidation is achieved in this technique. In the previous work of the author (Muthiya and Pachamuthu, 2018), a circular shape 3 to 5 mm thickness electrochemical cell was used and its maximum NOx reduction was 80%. In this investigation square shape, electrochemical cells with 1.5 mm thickness were used and the NOx reduction was further improved in this work.
Experimental section
Electrochemical cell preparation
In the present experimental investigation, the electrolyte plate made up of (ZrO2) (Y2O5) YSZ (CSIR NAL, India) in a square (80 x 80 mm) with the thickness of 1.5 mm is used. YSZ is an inherently better oxygen diffusion material (Gür and Huggins, 1979; Yoshinobu et al. 2010). The cathode coating is given over the electrolyte with 55% of NiO and 45% of YSZ. The NiO and YSZ are made into slurry by adding polyvinyl alcohol as binder and is brush coated on the YSZ plate (Cheng et al. 2006). The anode coating is given over the electrolyte with 60% of silver (Ag) and 40% of YSZ slurry, layered coated over the electrolyte. Figure 2 shows the electrochemical cells before and after electrode coating. The fabricated electrochemical cell is tested in scanning electron microscopy (SEM). SEM shows the high-resolution images of the size and surface texture of the electrochemical cell. It offers the ability to visually inspect the other surface materials/deposits and detection of pores (Driese and Foreman, 1991). Figure 3 shows the SEM image of cross-section of the porous electrolyte and electrodes. It is observed from Fig. 3 that the electrode coating is finely dispersed over the electrolyte plate.
Experimental setup and testing
Engine setup
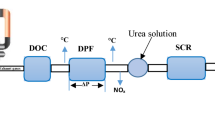
The experimental test bench involves a constant speed compression ignition engine single cylinder of Kirloskar (KOEL) connected to an eddy current dynamometer which is open-loop water-cooled. Figure 4 shows the complete experimental setup of the engine test setup. The detailed specification of the test engine is tabulated in Table 1. In this experiment, the engine testing was done by using diesel fuel for all load conditions. Figure 5 a shows the electrochemical cell device (ECD), and the specifications of the ECD are given in Table 2. Figure 5 b shows a complete view of electrochemical cell stack positioned inside the ECD. The ECD was connected to the tailpipe of the engine along with the external power supply. ECD is connected to a benchtop power supply that maintains 3.5V DC irrespective of current loading, and an electronic control module (ECM) is used in turn to control the power. The regulation of voltage supplied to the cell setting has been made in the benchtop power supply. The ECM is used to switch the polarity every 10 ms. The entire experiments were carried out using diesel as fuel for all loading conditions.
Emission measurement
In this experimental testing, a chemiluminescent type SIGNALS 4000VM Heat vacuum NOx analyzer is used to measure NOx emission. An AVL DiGas 444N analyzer is used to measure the HC, CO, and CO2 emissions for different load conditions. All the instruments were tested and warmed up to attain thermal equilibrium at all conditions. By connecting the emission analyzer probe at the outlet sections of the ECD the emission measurement was carried out.
Experimental procedure
The eddy current dynamometer is observed at no-load operation to tally the readings to the loaded condition for the absolute values. To reach nominal engine operating temperature 10 to 15 min lead time is provided before performing the test. Staged loading (0%, 25%, 50%, 75%, and 100%) was done by controlling the current supply to the eddy current dynamometer. By the increase of load the rate of quantity fuel injection increases which leads to a high concentration of emissions such as HC, CO, CO2, NOx, and PM. The temperature of the exhaust was measured for every set of load characteristics. Table 3 shows the mass flow rate of exhaust with respect to emission concentration.
Uncertainty analysis
The uncertainty analysis is carried out to maintain the accuracy in the measuring instruments. Uncertainties and errors may occur in the experimentations because of the calibration, observation, selection of instruments, calibration, working conditions, and environmental conditions. The main reason for the uncertainty occurrence is due to the random or fixed errors caused by the different manufacturing techniques of the instruments. The uncertainties in the measured parameters were estimated by using analytical method. Table 4 shows the analyzed uncertainties of the measured quantities. The uncertainty is calculated based on Kline and McClintock method (Kline and McClintock, 1953; Holman and Walter, 2001). The formula used in calculating the uncertainty of measured parameter is the following:
where xi is the average mean and σi is the standard deviation.
Before beginning the test, the following factors were noted down: steady-state conditions, ambient pressure, ambient air temperature, coolant temperature, lubricating oil, and fuel level. Initially, the engine is led to idle for 10–15 min to attain steady-state conditions. Staged loading (0%, 25%, 50%, 75%, and 100%) was done by controlling the current supply to the eddy current dynamometer. The ECD was tested in diesel engine exhaust for evaluating its emission characteristics. For evaluating the ECD for better accuracy, the values taken by conducting the experiments are averaged over five repetitions at given load. Table 4 shows the standard deviation of errors in the measured values.
Results and discussion
Electrochemical reactions
In this investigation, an electrochemical device consists of 10 individual electrochemical cells is placed in the exhaust stream. In ECD, NOx decomposition is achieved by passing electric current. The excess oxygen concentration in diesel exhaust initiates the ionization of oxygen molecule and the O2 reduction over the electrolyte and is given in the reaction (1).
By supplying power supply to the electrochemical cells, the polarization of the YSZ solid electrolyte takes place. Oxygen vacancies in higher concentrations are generated inside the three-dimensional network of YSZ particles by passing an electric current. NOx reduction takes place in the cathode layer which is indicated by the below reaction (2). Reactions (2)–(4) are the NO decomposition reactions in electrode by the supply of electric current followed by a change of voltage polarity. During positive cycle, NO reacts with Ni to form Ni-NO and by changing to negative polarity Ni-NO decomposes to form 2NiO + N2. The N2 is liberated at the end of the negative cycle. By further change in positive polarity, the NiO is decomposed into Ni and oxygen. When the NO molecules come in to contact with Ni-YSZ electrodes, the ionization takes place near the surface regions of Ni grains into the NiO. As an outcome, the nanosize nickel grain formations take place in the near YSZ/Ni interface boundary region (Awano et al. 2004). By switching the external voltage, the oxidation of Ni grains to NiO grains occurs due to the penetration of NOx and O2 gases. By switching the voltage polarity, NiO is reduced into Ni (Awano et al. 2004). As a result, N2 and O2 molecules are liberated out. The liberated O2 is further ionized into 2O2− as indicated by the reaction (5) (Hansen, 2018).
More oxygen vacancies are created in the YSZ lattice by passing an electric current. This can be explained by the tendency of the YSZ electrolyte to have higher ionic conductivity with the increase in temperatures. The ionized oxygen molecules will travels through to the electrolyte to the electrodes. The liberated oxygen further oxidizes the HC and CO emissions in the tailpipe.
Electrochemical cell characterization
In this work, the electrochemical cell characterization is carried out by means of SEM and energy-dispersive X-ray (EDX) spectroscopy. Energy-dispersive X-ray spectroscopy is a technique, which works based on the collection and energy dispersion of X-rays generated when electrons bombard on the sample surface (Wei et al. 2006). The intensities of the X-rays generated were measured in SEM, and it is coupled to an energy-dispersive X-ray. SEM with EDX can determine the main atomic composition of the material. The intensity of accelerating voltage is 15.0 kV. Figure 6 shows the EDX results of the electrochemical cell before testing in the exhaust. The existence of elements in the electrochemical cell was identified in EDX analysis. The graph below shows the qualitative elemental analysis data obtained in EDX analysis. From this analysis, elements presence is confirmed, which was used during the fabricated electrochemical cells. This proved that the electrochemical cell can be used in the ECD for effective NOx decomposition.
Figure 7 shows the EDX results of the electrochemical cell after testing in the diesel exhaust. From the EDX analysis graph, it is observed that no change in the existing electrochemical material takes place after testing the diesel exhaust. It is observed that carbon atom is newly added to the electrochemical cells which are due to the existence of carbon soot particles in the diesel exhaust. And it is also observed that the presence of carbon soot was minimal in the electrochemical cells due to the rapid oxidation process in the electrochemical cells.
Oxides of nitrogen emissions
The high combustion temperature and residence time in the high-temperature environment is an important factor for higher NOx formation in a diesel engine (Aggarwal et al. 2016). Figure 8 shows the NOx emission in the electrochemical cells with respect to brake power. By increasing the engine load, more amount of fuel will be taking part in combustion. This increases the in-cylinder temperatures and increased NO emission, which also results in increased exhaust temperature. Generally, YSZ material requires a high activation temperature for the initiation of electrochemical reactions (Hamamoto et al. 2006). In this method, thin YSZ substrate plates are used as an electrolyte material. By increasing the voltage, the ionic conductivity also increases with the thin YSZ substrate. The electrochemical plates are stacked inside the ECD to ensure maximum contact with the incoming exhaust gases. It is observed from Fig. 8 that the NOx emission reduced with the increase in voltage; it is due to the increase in ionic conductivity over the YSZ electrolyte. NOx reduction of up to 80% was achieved with a supply of 2 V at a current of 1.2 amps. It is observed from the experiment that the electrochemical cells worked effectively at 2V supply of the voltage at the temperature between 350 and 400 °C. The low voltage activation of the electrochemical cell is due to YSZ inherent increased ionic conductivity at high temperatures (Awano et al. 2004; Hernández et al. 2013). Further increases in voltage result in a blackening of YSZ material. Blackening is evidence of degradation of an electrochemical material (Casselton, 1974; Janek and Korte, 1999; Schefold, et al., 2009). Further increase in voltage reduces the emission reduction efficiency. The reduction of NOx in the ECD device is calculated by using the following formula:
where NOx without ECD and NOx with ECD are the concentrations of NOx before connecting ECD and after connecting the ECD, and the same method is followed for the calculation of HC and CO emission. In electrochemical decomposition of NOx, molecules like N2O and N2 are not measured. But it is liberated in electrochemical reaction during NOx conversion (Awano et al. 2004).
Hydrocarbon emission
The hydrocarbon (HC) emission increases in the diesel engine due to the incomplete combustion of diesel fuel. At low-engine loads, amount of fuel injected is less and HC emission was high, which is due to the poor fuel distribution, a large amount of excess air and low in-cylinder temperature. At high engine load, the HC emission decreases because of the increased in-cylinder temperature which results in incomplete combustion. Figure 9 shows the HC emission reduction in an electrochemical cell with respect to brake power. It is observed that the HC emission reduces with the electrochemical cell which is due to the oxidation process inside the electrochemical cells. The rates of oxidation of hydrocarbons are much faster in the electrochemical cell due to the liberated oxygen molecules inside the sites of electrodes. NOx decomposes into N2 and O2 that liberate O2 that can be utilized to oxidize the HC molecules. Ag is also an oxidizing catalyst which as a good tendency to oxides the harmful pollutants (Zhang et al. 2015). In the electrochemical cell, Ag plays a role in the oxidation of HC and CO molecules. HC emission reduction is observed at the 2V supply of power at high exhaust temperatures. 2V is found to be an optimum voltage range for the electrochemical cells. The temperature of the exhaust also increases at maximum load; the increases in temperature activate the YSZ substrate for electrochemical reactions.
Carbon monoxide emission
Figure 10 shows the CO emission with respect to engine brake power. The CO emission increases in the diesel engine due to the deficiency of oxygen during the combustion of fuels. The electrochemical cell shows a reduction in CO emission due to the oxidation of CO2. NOx decomposition liberates O2 that can be utilized to oxidize the CO molecules also. Ag in the electrochemical cell also supports in the oxidation of CO molecules. It is observed that 2V supply of power is the optimum voltage range for the electrochemical cells for oxidation of CO.
Exhaust temperature
Figure 11 shows the variation of NOx reduction with respect to the temperature of the exhaust. The reduction percentage was calculated using the following formula:
where NOx without ECD and NOx with ECD are the concentrations of NOx before connecting ECD and after connecting the ECD. The exhaust temperature was measured using a temperature sensor fixed to the exhaust line. The temperature of the exhaust is a significant factor for the activation of the electrochemical cells. In higher engine loads, an additional amount of fuel will be taking part in combustion. This increases the in-cylinder temperatures which result in increased exhaust temperature. It is observed from the experiment that the electrochemical cells worked effectively at 2V supply of the voltage at the temperature between 350 and 400°C. This is due to YSZ which has increased ionic conductivity at high temperatures. It is observed that the ionic conductivity increases in the YSZ electrolyte with an increase in temperature and voltage.
The oxygen concentration in the exhaust increases or decreases depends upon the rate of combustion of the fuel. Figure 12 shows the graph of oxygen concentrations with respect to all load conditions at the variable supply of voltage. It is observed that the oxygen concentration increases in ECD by about 2% when compared with baseline. The increase in oxygen concentration is due to the following factors: NO decomposition to N2 and O2 and the oxidation of CO into CO2 and O2. The future increase in oxygen is may be due to the dilution of atmospheric oxygen inside the ECD. It is observed that with all load condition the oxygen concentration with ECD is increased. At 0V–0.5V supply of power, there is no much change in the oxygen concentration. This is because at a minimum voltage, the NOx decomposition is observed less and the ionic transfer of O2 is low in the electrochemical cells. By the supply of 1V–3V power, the oxygen concentration observed increasing. The reason being is with the increase in voltage the NOx decomposition gets increases and further breakdown oxygen with the incoming atmospheric oxygen inside the ECD tends to increase the O2 concentration. The current consumption in the electrochemical cell is shown in Fig. 13. It is observed that at 2V supply, the maximum 1.2 amps were the total current consumption for the operation of electrochemical cells. The power consumption in the ECD will be 2.4 W.
Conclusion
In this work, a trial of plate type electrochemical cells is developed and tested for emission reduction in diesel engines. Based on their experimental result, the finding is summarized as
-
1.
The plate type YSZ electrochemical cell showed better results in NOx decomposition in higher load conditions. And a simultaneous reduction of HC and CO was achieved with this system.
-
2.
It is observed from the results that a supply of 2 V and 1.2A shows the best NOx reduction. In this investigation, 80% reduction of NOx, 65% of HC, and 45% of CO emissions are achieved at the effective exhaust temperatures of between 350 and 400 °C.
-
3.
In this method, high NOx reduction was observed in the oxygen-rich conditions of diesel engines which make it much suitable to use as a retrofit for existing diesel engines.
-
4.
The results obtained from this work shows that the electrochemical decomposition of NOx and simultaneous control of HC and CO is a preferred technique for diesel engine emission control.
This method is a cost-effective and easier adaptation in existing diesel engines without major modifications. By implementing such technique, harmful pollutants from engine can be effectively controlled.
Abbreviations
- NOx:
-
oxides of nitrogen
- HC:
-
hydrocarbon
- CO:
-
carbon monoxide
- PM:
-
particulate matter
- YSZ:
-
yttrium stabilized zirconia
- NiO:
-
nickel oxide
- Ag:
-
silver
- ECM:
-
electronic control module
- SEM:
-
scanning electron microscopy
- EDX:
-
energy-dispersive X-ray spectroscopy
- ECD:
-
electrochemical cell device
References
Aggarwal SK, Fu X, Wijeyakulasuriya S (2016) Effects of fuel reactivity and injection timing on diesel engine combustion and emissions. International Journal of Green Energy 13(5):431–445
Awano M, Bredikhin S, Aronin A, Abrosimova G, Katayama S, Hiramatsu T (2004) NOx decomposition by electrochemical reactor with electrochemically assembled multilayer electrode. Solid State Ionics 175(1–4):605–608
Bredikhin S, Maeda K, Awano M (2001a) Electrochemical cell with two layers cathode for NO decomposition. Ionics 7(1–2):109–115
Bredikhin S, Maeda K, Awano M (2001b) NO decomposition by an electrochemical cell with mixed oxide working electrode. Solid State Ionics 144(1–2):1–9
Bredikhin S, Maeda K, Awano M (2002) Low current density electrochemical cell for NO decomposition. Solid State Ionics 152:727–733
Casselton REW (1974) Blackening in yttria stabilized zirconia due to cathodic processes at solid platinum electrodes. J Appl Electrochem 4(1):25–48
Cheng S, Liu H, Logan BE (2006) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8(3):489–494
Driese SG, Foreman JL (1991) Traces and related chemical changes in a Late Ordovician paleosol, Glossifungites ichnofacies, southern Appalachians, USA. Ichnos: An International Journal of Plant & Animal 1(3):207–219
Fang HL, DaCosta HF (2003) Urea thermolysis and NOx reduction with and without SCR catalysts. Appl Catal B Environ 46(1):17–34
Gür TM, Huggins RA (1979) Decomposition of nitric oxide on zirconia in a solid-state electrochemical cell. J Electrochem Soc 126(6):1067–1075
Hamamoto K, Fujishiro Y, Awano M (2006) Simultaneous removal of nitrogen oxides and diesel soot particulate in nano-structured electrochemical reactor. Solid State Ionics 177(26–32):2297–2300
Hamamoto K, Fujishiro Y, Awano M (2007) NOx decomposition using electrochemical reactor. In Meeting Abstracts (No. 11, pp. 800-800). In: The Electrochemical Society
Hansen KK (2018) Electrochemical removal of NOx using oxide-based electrodes-a review. Int J Electrochem Sci 13(10):9273–9280
Hernández WY, Hadjar A, Klotz M, Leloup J, Princivalle A, Tardivat C et al (2013) NOx storage capacity of yttria-stabilized zirconia-based catalysts. Appl Catal B Environ 130:54–64
Heywood, J. B. (1988). Internal combustion engine fundamentals
Hill SC, Smoot LD (2000) Modeling of nitrogen oxides formation and destruction in combustion systems. Prog Energy Combust Sci 26(4–6):417–458
Hoekman SK, Robbins C (2012) Review of the effects of biodiesel on NOx emissions. Fuel Process Technol 96:237–249
Holman JP, Walter J (2001) Experimental methods for engineers. McGraw-Hill, New York
IEA (2012) Emission from fuel combustion. Internal Energy Agency 13:2895–2902
İlkiliç C, Aydin H (2012) The harmful effects of diesel engine exhaust emissions. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 34(10):899–905
Janek J, Korte C (1999) Electrochemical blackening of yttria-stabilized zirconia–morphological instability of the moving reaction front. Solid State Ionics 116(3–4):181–195
Kašpar J, Fornasiero P, Hickey N (2003) Automotive catalytic converters: current status and some perspectives. Catal Today 77(4):419–449
Kammer K (2005) Electrochemical DeNOx in solid electrolyte cells—an overview. Appl Catal B Environ 58(1–2):33–39
Kline SJ, McClintock FA (1953) Describing uncertainties in single-sample experiments. Mech Eng 75(1):3–8
Li X, Vernoux P (2005) A new NOx storage-reduction electrochemical catalyst. Appl Catal B Environ 61(3–4):267–273
Maurer, M., Holler, P., Zarl, S., Fortner, T., & Eichlseder, H. (2017). Investigations of lean NOx trap (LNT) regeneration strategies for diesel engines (no. 2017-24-0124). SAE Technical Paper
Mohankumar S, Senthilkumar P (2017) Particulate matter formation and its control methodologies for diesel engine: a comprehensive review. Renew Sust Energ Rev 80:1227–1238
Muthiya SJ, Pachamuthu S (2018) Electrochemical NOx reduction and oxidation of HC and PM emissions from biodiesel fuelled diesel engines using electrochemically activated cell. International journal of green energy 15(5):314–324
Pancharatnam S, Huggins RA, Mason DM (1975) Catalytic decomposition of nitric oxide on zirconia by electrolytic removal of oxygen. J Electrochem Soc 122(7):869–875
Park S, Song HS, Choi HJ, Moon J (2004) NO decomposition over the electrochemical cell of lanthanum stannate pyrochlore and YSZ composite electrode. Solid State Ionics 175(1–4):625–629
Parthiban K, Pazhanivel K, Muthiya SJ (2017) Emission control in multi-cylinder spark ignition engines using metal-oxide coated catalytic converter. International Journal of Vehicle Structures & Systems 9(2):134
Quevauviller P (2011) Adapting to climate change: reducing water-related risks in Europe–EU policy and research considerations. Environ Sci Pol 14(7):722–729
Sajjad F, Noreen U, Zaman K (2014) Climate change and air pollution jointly creating nightmare for tourism industry. Environ Sci Pollut Res 21(21):12403–12418
Schefold J, Brisse A, Zahid M (2009) Electronic conduction of yttria-stabilized zirconia electrolyte in solid oxide cells operated in high temperature water electrolysis. J Electrochem Soc 156(8):B897–B904
Subramaniam, M., Pachamuthu, S., Arulanandan, J., & Muthiya, J. (2016). Simultaneous reduction of HC, NO x and PM by using active regeneration technique (no. 2016-01-0912). SAE Technical Paper
Sun J, Caton JA, Jacobs TJ (2010) Oxides of nitrogen emissions from biodiesel-fuelled diesel engines. Prog Energy Combust Sci 36(6):677–695
Theis JR, Kim J, Cavataio G (2015) TWC + LNT/SCR systems for satisfying tier 2, bin 2 emission standards on lean-burn gasoline engines. SAE International Journal of Fuels and Lubricants 8(2):474–486
Vallapudi DR, Makineni HK, Pisipaty SK, Venu H (2018) Combined impact of EGR and injection pressure in performance improvement and NOx control of a DI diesel engine powered with tamarind seed biodiesel blend. Environ Sci Pollut Res 25(36):36381–36393
Xi Wang. (2019) Effects of the Ba impregnation on Pt electrode on NO electrochemical reduction mechanism. International Journal of Economy, Energy and Environment, 4 (2) 24-32. Dio:https://doi.org/10.11648/j.ijeee.20190402.11
Yoshinobu Y, Tsuda Y, Ueda H, Nakanishi Y, Gong J (2010) Simultaneous reduction of NOx and PM in diesel exhaust based on electrochemical reaction. SAE International Journal of Fuels and Lubricants 3(1):50–60
Zhang J, Li Y, Zhang Y, Chen M, Wang L, Zhang C, He H (2015) Effect of support on the activity of Ag-based catalysts for formaldehyde oxidation. Sci Rep 5:12950
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solomon, J.M., Pachamuthu, S., Arulanandan, J.J. et al. Electrochemical decomposition of NOx and oxidation of HC and CO emissions by developing electrochemical cells for diesel engine emission control. Environ Sci Pollut Res 27, 32229–32238 (2020). https://doi.org/10.1007/s11356-019-07327-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07327-9